Abstract
Immunization against arthritis-related protein (Arp) elicits antibody in mice that resolves arthritis but is not protective against challenge with Borrelia burgdorferi. In mice immunized against Arp, an unrelated 37-kDa protein (P37-42), outer surface protein A (OspA), or glutathione S-transferase (GT) and then challenged by syringe or tick, only OspA conferred protection. Passive transfer of Arp antiserum into infected SCID mice induced arthritis resolution, but antisera to P37-42, OspA, GT, or six overlapping Arp peptide fragments did not. Results suggest that the arthritis-resolving immunogenicity is specific to Arp, but the relevant epitopes may be conformational.
The agent of Lyme disease, Borrelia burgdorferi, disseminates in mice following syringe- or tick-borne infection,resulting in induction of arthritis and carditis, which then undergo immune-mediated resolution (1, 2, 3). Although disease resolves, infection persists and the infected mice develop strong protective and disease-resolving antibody responses, which can be measured by passive transfer. Transfer of immune serum from infected immunocompetent mice into naïve mice protects against syringe challenge with B. burgdorferi but not against tick challenge or against host-adapted spirochetes (4, 5, 7). Passive transfer of immune serum into SCID mice with existing infection also induces arthritis resolution but does not eliminate infection, thus mimicking events in immunocompetent mice (4, 5, 6).
Two proteins illustrate the concept that different antigenic targets may be involved in protective or arthritis-resolving immunity. Decorin binding protein A (DbpA) elicits protective immunity against syringe (but not tick)-borne challenge (10, 14-16, 18), yet does not induce disease-resolving immunity (10). In contrast, arthritis-related protein (Arp) elicits arthritis-resolving immunity but not protective immunity against syringe-borne challenge (9). Since these negative findings with syringe challenge could be attributed to the fact that Arp is expressed in vivo rather than under culture conditions and expression increases within ticks during feeding (17), a more thorough examination of protective immunity using both syringe and tick challenges was undertaken. The immunogenicity of Arp was compared to that induced by glutathione S-transferase (GT, negative control), P37-42 (similar-size protein, negative control), and OspA (positive control).
The arp gene, lacking the hydrophobic N-terminal leader region (amino acids 1 to 12), was amplified by PCR with oligonucleotide primers (9). Primers corresponded with nucleotides 37 to 73 and 951 to 975 of the arp gene (GenBank sequence accession no. N40-AF050212), which is nearly identical to B31 sequence bbf01 (13). Amplicons were cloned in frame with the GT gene into pMX, a pGEX-2T vector (Pharmacia, Piscataway, N.J.) with a modified polylinker (18). Recombinant P37-42 (GenBank sequence accession no. N40-AF035553), which is nearly identical to B31 open reading frame bbk47, was also generated without the leader peptide, by using primers corresponding with nucleotides 72 to 98 and 953 to 987 (9, 13). The PCR-amplified DNA sequences were confirmed by sequence comparison with the original insert. Recombinant proteins were purified free of their GT fusion partner as described previously (8). OspA was generated from Escherichia coli transformed with plasmid 197-OspA-N40 (11), which was provided by Erol Fikrig, Yale University School of Medicine. OspA was purified and cleaved of its GT fusion partner as described above.
Female C3H/HeN (Harlan Sprague-Dawley, Inc., Indianapolis, Ind.) mice were hyperimmunized subcutaneously with 20 μg of purified recombinant protein emulsified in 0.1 ml of complete Freund's adjuvant and boosted at 14 and 28 days with 10 μg of protein in incomplete Freund's adjuvant. To confirm effective immunization, mice were bled 2 weeks after the last boost, sera were tested by enzyme-linked immunosorbent assay with the respective recombinant proteins, and antibody reactivity was verified at serum dilutions of ≥1:100,000 (10). Groups of 10 C3H mice were immunized with Arp, P37-42 (negative control), OspA (positive control), and GT (negative control). Five mice in each group were then challenged intradermally in the dorsal thoracic midline with a clonal isolate of B. burgdorferi cN40, at a dose of 104 spirochetes in 0.1 ml of modified Barbour-Stoenner-Kelly medium (3), or with five B. burgdorferi cN40-infected nymphal Ixodes scapularis lymphal ticks placed on the thoracic dorsal midline. Adult ticks were field collected in southern Connecticut by Durland Fish, Yale University, New Haven, Conn. Infections were induced by feeding larval ticks upon experimentally infected mice, and infection status of nymphal ticks was verified as previously described (17). Mice were killed 2 weeks after challenge, and then urinary bladder and inoculation sites were cultured to determine infection status.
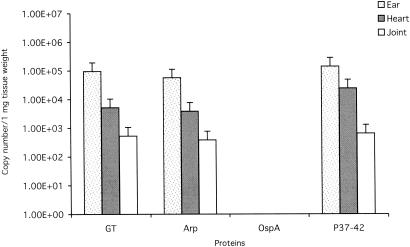
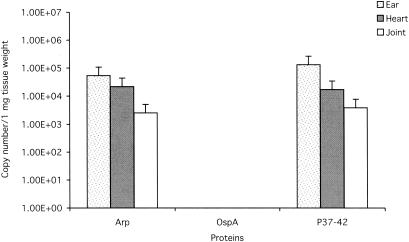
Only the mice immunized with OspA, and not with Arp, P37-42, or GT, were protected against challenge by syringe or tick (Table 1), confirming the effectiveness of the immunization protocol (OspA) but the nonprotective ability of Arp immunization. Furthermore, there were no significant differences in copy numbers of spirochetes in the ear, tibiotarsus, or heart among the infected groups of mice, regardless of mode of infection (Fig. 1 and 2). For quantitative analysis, ear, heart base, and left tibiotarsal tissues were analyzed, and copy numbers of flaB DNA target were expressed per unit weight of tissue (17). Multiple comparison analyses were made using one-way analysis of variance, followed by least squares difference post hoc tests. Calculated P values of <0.05 were considered significant.
TABLE 1.
Evaluation of protective immunity in C3H mice actively immunized with different recombinant proteins and challenged with syringe- or tick-borne B. burgdorferi
| Immunogen | No. of mice
positive/no. of mice testeda
|
|
|---|---|---|
| Syringe challenge | Tick challenge | |
| GT (negative control) | 5/5 | 5/5 |
| Arp | 5/5 | 5/5 |
| OspA (positive control) | 1/5 | 0/5 |
| P37-42 (negative control) | 5/5 | 5/5 |
Data are culture results of urinary bladder and inoculation site at 2 weeks after challenge with 104 B. burgdorferi cN40 cultured spirochetes or five B. burgdorferi cN40-infected ticks.
FIG. 1.
Numbers of B. burgdorferi flaB gene copies per milligram of ear, heart, or tibiotarsal joint tissue at 2 weeks after syringe challenge with B. burgdorferi in mice previously immunized with GT, Arp, OspA, or P37-42. Other than the OspA positive-control group, there were no differences in copy numbers among treatment groups compared with GT or P37-42 negative controls.
FIG. 2.
Numbers of B. burgdorferi flaB gene copies per milligram of ear, heart, or tibiotarsal joint tissue at 2 weeks after tick challenge with B. burgdorferi in mice previously immunized with Arp, OspA, or P37-42. Other than the OspA positive-control group, there were no differences in copy numbers among treatment groups compared with P37-42 negative controls.
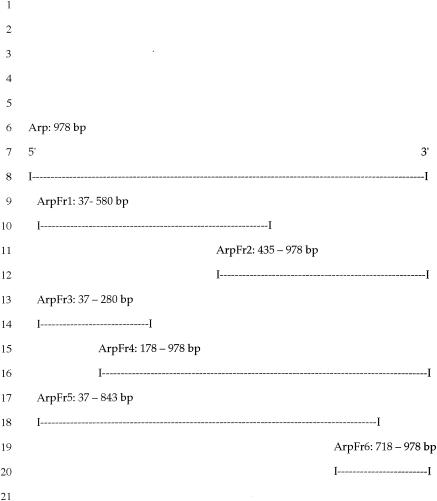
A previous study demonstrated that passive transfer of antiserum to Arp would induce resolution of arthritis in infected SCID mice (9), but a truncated form of Arp (ErpT), which is 50 amino acids shorter than Arp at the C terminus, did not induce arthritis-resolving immunity (12). Based upon this fact, we sought to confirm the lack of activity with truncated Arp and to attempt to identify the immunogenic region of Arp by creating overlapping polypeptide fragments, using methods described above. A series of six Arp peptide fragments were created (Fig. 3): ArpFr1, amino acids 13 to 194; ArpFr2, amino acids 145 to 326; ArpFr3, amino acids 13 to 94; ArpFr4, amino acids 60 to 326; ArpFr5, amino acids 13 to 281; and ArpFr6, amino acids 240 to 326. Immunoblots of full-length Arp and each of the six Arp fragments were incubated with serum from C3H mice that were actively infected with B. burgdorferi for 90 days and with hyperimmune serum prepared against each recombinant Arp. Immune serum reacted with Arp and ArpFr4 but not with ArpFr1, ArpFr2, ArpFr3, ArpFr5, or ArpFr6. Hyperimmune serum against Arp reacted with Arp, ArpFr1, ArpFr2, ArpFr4, and ArpFr5 but not ArpFr3 or ArpFr6, the two smallest peptide fragments (data not shown).
FIG. 3.
Relative size and relationships of full-length and fragments of arp gene sequences encoding Arp (ca. 35 kDa), ArpFr1 (ca. 20 kDa), ArpFr2 (ca. 20 kDa), ArpFr3 (ca. 9 kDa), ArpFr4 (ca. 30 kDa), ArpFr5 (ca. 30 kDa), and ArpFr6 (ca. 10 kDa).
We next generated hyperimmune antisera to Arp, P37-42, OspA, GT, and each of the six Arp peptide fragments and then tested these antisera for their ability to induce arthritis resolution following passive transfer into infected female C3H/Smn.CIcrHsd-Prkdcscid (C3H-scid) mice obtained from the Frederick Cancer Research Center, Frederick, Md. At 6 and 10 days after intradermal inoculation with 103 spirochetes (intervals at which arthritis is established and evolving), groups of four C3H-scid mice were injected subcutaneously with 0.3 ml of antisera to Arp, ArpFr1 to ArpFr6 peptide fragments, OspA, P37-42, or GT. Because of the number of mice needed to execute this experiment, up to four antisera were tested in a single experiment, but each experiment contained a group of GT antiserum-treated controls. Mice were necropsied 2 weeks later, tissues were cultured to confirm infection status, and tissues (joints and hearts) were examined by histology. Both knees and tibiotarsi from each mouse were examined for the presence of arthritis, and tibiotarsi were scored by blinded examination for arthritis on a scale of 0 (negative) to 3 (severe) (5, 6).
Compared to control mice treated with GT antiserum (mean severity score, 2.7 ± 0.4), no effect on arthritis was noted with OspA antiserum (mean severity score, 2.4 ± 0.6) or P37-42 antiserum (mean severity score, 2.6 ± 0.9), whereas Arp antiserum resulted in reduction of arthritis severity (mean severity score, 1.1 ± 0.3). None of the antisera to ArpFr1 through ArpFr6 caused arthritis resolution in passively immunized C3H-scid mice (data not shown). Furthermore, active immunization of C3H mice with these recombinant proteins did not induce protective immunity (data not shown).
The present study confirms previous findings that immunization with recombinant Arp elicits an antibody response that will reduce the severity of arthritis in actively infected SCID mice upon passive transfer (9). Furthermore, we found that antiserum generated against peptide fragments of Arp did not have this quality, which explains why ErpT, a truncated form of Arp, failed to induce arthritis-resolving immunity (12). Results suggest that the arthritis-resolving immunogenicity of the recombinant protein is likely to be conformational. In studies with recombinant OspC, it has been shown that the conformational nature of the recombinant protein is critically important in eliciting therapeutic immunity in SCID mice infected with B. burgdorferi ZS7 (20, 21), and the immunogenicity of DbpA has likewise been linked to conformational structure (19).
Arp serves as a prototype antigen to study host immune responses to an in vivo-expressed antigen that elicits disease-modulating responses during active infection. Although the biological activity and function of Arp have yet to be defined, it is presumably important to B. burgdorferi, as B. burgdorferi B31 encodes a gene product that is identical to that of B. burgdorferi N40 Arp on the protein level (9, 13). In addition, B. bissetti 25015, B. afzelii PKo, and B. garinii PBi all possess this gene and express Arp protein as well (9).
Acknowledgments
We thank Sara Mulinyawe and Amy Smith for technical support.
This work was supported by NIH grant AI26815 from the National Institute of Allergy and Infectious Diseases.
Editor: F. C. Fang
REFERENCES
- 1.Armstrong, A. L., S. W. Barthold, D. H. Persing, and D. S. Beck. 1992. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 47:249-258. [DOI] [PubMed] [Google Scholar]
- 2.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease following intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 3.Barthold, S. W., M. S. deSouza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:951-971. [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold, S. W., and L. K. Bockenstedt. 1993. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect. Immun. 61:4696-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold, S. W., S. Feng, L. K. Bockenstedt, E. Fikrig, and K. Feen. 1997. Protective and arthritis-resolving activity in serum from mice infected with Borrelia burgdorferi. Clin. Infect. Dis. 25:S9-S17. [DOI] [PubMed] [Google Scholar]
- 6.Barthold, S. W., M. deSouza, and S. Feng. 1996. Serum-mediated resolution of Lyme arthritis in mice. Lab. Investig. 74:57-67. [PubMed] [Google Scholar]
- 7.deSilva, A., E. Fikrig, E. Hodzic, S. R. Telford III, and S. W. Barthold. 1998. Immune evasion by tick-borne and host-adapted Borrelia burgdorferi. J. Infect. Dis. 177:395-400. [DOI] [PubMed] [Google Scholar]
- 8.Feng, S., S. Das, T. Lam, R. A. Flavell, and E. Fikrig. 1995. A 55-kilodalton antigen encoded by a gene on a Borrelia burgdorferi 49-kilobase plasmid is recognized by antibodies in sera from patients with Lyme disease. Infect. Immun. 63:3459-3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng, S., E. Hodzic, and S. W. Barthold. 2000. Lyme arthritis resolution with antiserum to a 37-kilodalton Borrelia burgdorferi protein. Infect. Immun. 68:4169-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng, S., E. Hodzic, B. Stevenson, and S. W. Barthold. 1998. Humoral immunity to Borrelia burgdorferi N40 decorin binding proteins during infection of laboratory mice. Infect. Immun. 66:2827-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fikrig, E., S. W. Barthold, F. S. Kantor, and R. A. Flavell. 1990. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science 250:553-556. [DOI] [PubMed] [Google Scholar]
- 12.Fikrig, E., M. Chen, S. W. Barthold, J. Anguita, W. Feng, S. R. Telford III, and R. A. Flavell. 1999. Borrelia burgdorferi erpT expression in the arthropod vector and murine host. Mol. Microbiol. 31:281-290. [DOI] [PubMed] [Google Scholar]
- 13.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, and R. D. Fleischmann. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 14.Hagman, K. E., X. Yang, S. K. Wikel, G. B. Schoeler, M. J. Caimano, J. D. Radolph, and M. V. Norgard. 2000. Decorin-binding protein A (DbpA) of Borrelia burgdorferi is not protective when immunized mice are challenged via tick infestation and correlates with the lack of DbpA expression by B. burgdorferi by ticks. Infect. Immun. 68:4759-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson, M. S., N. K. Patel, D. R. Cassatt, and N. D. Ulbrandt. 2000. Evidence for vaccine synergy between Borrelia burgdorferi decorin binding protein A and outer surface protein A in the mouse model of Lyme borreliosis. Infect. Immun. 68:6457-6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanson, M. S., D. R. Cassatt, B. P. Guo, N. K. Patel, M. P. McCarthy, D. W. Dorward, and M. Hook. 1998. Active and passive immunity against Borrelia burgdorferi decorin binding protein A (DbpA) protects against infection. Infect. Immun. 66:2143-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodzic, E., S. Feng, K. J. Freet, D. L. Borjesson, and S. W. Barthold. 2002. Borrelia burgdorferi population kinetics and selected gene expression at the host-vector interface. Infect. Immun. 70:3382-3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sears, J. E., E. Fikrig, T. Y. Nakagawa, K. Deponte, N. Marcantonio, F. S. Kantor, and R. A. Flavell. 2001. Molecular mapping of OspA-mediated immunity against Borrelia burgdorferi, the agent of Lyme disease. J. Immunol. 147:1995-2001. [PubMed] [Google Scholar]
- 19.Ulbrandt, N. D., D. R. Cassatt, N. K. Patel, W. C. Roberts, C. M. Bachy, C. A. Fazenbaker, and M. S. Hanson. 2001. Conformational nature of the Borrelia burgdorferi decorin binding protein A epitopes that elicit protective antibodies. Infect. Immun. 69:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong, W., T. Stehle, C. Museteanu, A. Siebers, L. Gern, M. Kramer, R. Wallich, and M. M. Simon. 1997. Therapeutic passive vaccination against chronic Lyme disease in mice. Proc. Natl. Acad. Sci. USA 94:12533-12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong, W., L. Gern, T. Stehle, C. Museteanu, M. Kramer, R. Wallich, and M. M. Simon. 1999. Resolution of experimental and tick-borne Borrelia burgdorferi infection in mice by passive, but not active immunization using recombinant OspC. Eur. J. Immunol. 29:946-957. [DOI] [PubMed] [Google Scholar]