Abstract
Plasmodium falciparum invades erythrocytes through multiple ligand-receptor interactions, with redundancies in each pathway. One such alternate pathway is the trypsin-resistant pathway that enables P. falciparum to invade trypsin-treated erythrocytes. Previous studies have shown that this trypsin-resistant pathway is dependent on glycophorin B, as P. falciparum strains invade trypsin-digested glycophorin B-deficient erythrocytes at a highly reduced efficiency. Furthermore, in a recent study, the P. falciparum 7G8 strain did not invade glycophorin B-deficient erythrocytes, a finding that was not confirmed in the present study. To analyze the degree of dependence on glycophorin B for invasion by P. falciparum through the trypsin-resistant pathway, we have studied the invasion phenotypes of five parasite strains, 3D7, HB3, Dd2, 7G8, and Indochina I, on trypsin-treated normal and glycophorin B-deficient erythrocytes. Invasion was variably reduced in glycophorin B-deficient erythrocytes. Four strains, 3D7, HB3, Dd2, and Indochina I, invaded trypsin-treated erythrocytes, while invasion by the 7G8 strain was reduced by 90%. Among the four strains, invasion by 3D7, HB3, and Dd2 of trypsin-digested glycophorin B-deficient erythrocytes was further reduced. However, Indochina I invaded trypsin-digested glycophorin B-deficient erythrocytes at the same efficiency as its invasion of trypsin-digested normal erythrocytes. This strongly suggests that the Indochina I strain of P. falciparum is not dependent on glycophorin B to invade through a trypsin-resistant pathway as are the strains 3D7, HB3, and Dd2. Thus, P. falciparum is able to invade erythrocytes through a glycophorin B-independent, trypsin-resistant pathway.
The asexual erythrocytic phase in the life cycle of malarial parasites produces the clinical symptoms and pathology associated with infection. During the erythrocytic phase, merozoites released from schizont-infected erythrocytes invade uninfected erythrocytes. The invasion process is highly dependent on specific molecular interactions between parasite ligands on the merozoite and host receptors on the erythrocyte membrane. However, these molecular interactions are not completely defined. Plasmodium vivax is totally dependent on two receptors for erythrocyte invasion: the Duffy blood group antigen (29) and an unknown receptor on reticulocytes (16). In contrast, P. falciparum probably invades through the same basic series of receptor-ligand interactions as P. vivax, but P. falciparum differs in that it has redundancy or alternate invasion pathways.
The alternate invasion pathways of P. falciparum have been recognized on the basis of invasion and binding studies on genetically deficient erythrocytes (deficient in one or more surface molecules) as well as on erythrocytes treated with different enzymes, such as neuraminidase and trypsin (5, 28, 30, 34, 35). Glycophorins are glycoproteins expressed on the erythrocyte surface with sialic acid residues attached to the O-linked oligosaccharides (7, 8) and are known to play a role as erythrocyte receptors in invasion by P. falciparum (31-33). Erythrocytes deficient in glycophorin A and B, En(a−) and S−s−U−, respectively, were observed to be resistant to invasion by P. falciparum (31-33). The sialic acid residues play a role in invasion, as neuraminidase treatment of the erythrocytes reduces or eliminates invasion depending on the P. falciparum strain studied (5, 28, 30, 34, 35). The erythrocyte binding protein EBA-175 of P. falciparum binds specifically to glycophorin A, and this binding is neuraminidase and trypsin sensitive (40). Similarly, BAEBL, a homologue of EBA-175 in P. falciparum strain Dd2/Nm, binds glycophorin C, which is neuraminidase and trypsin sensitive (23, 25-27). These ligand-receptor interactions define a sialic acid-dependent, trypsin-sensitive invasion pathway. Glycophorin B is resistant to trypsin treatment but is neuraminidase sensitive (44) and appears to define a trypsin-resistant pathway (13). Certain P. falciparum strains invade neuraminidase-treated erythrocytes in a sialic acid-independent manner, using a neuraminidase-resistant, trypsin-sensitive receptor(s) whose identity is unknown and which is designated receptor X (13). Because of redundancy in P. falciparum, ligands may exist that require a particular receptor, but invasion could occur independent of that receptor. For example, sialic acid and the peptide backbone on glycophorin A are required for binding of EBA-175 (40), yet the invasion may be sialic acid independent (Dd2/Nm) because of redundancy (12).
Many P. falciparum strains invade trypsin-treated erythrocytes at an invasion rate of 30 to 70% compared to that for untreated erythrocytes (13, 37). The invasion of trypsin-digested erythrocytes has been attributed to the presence of glycophorin B on their surfaces (13, 44). To date, parasite strains that have been observed to invade trypsin-digested erythrocytes were also shown to be dependent on glycophorin B, as their invasion of trypsin-digested glycophorin B-deficient (S−s−U−) erythrocytes was greatly reduced (13, 37). Thus, it appeared that in the absence of glycophorin B, invasion through the trypsin-resistant pathway occurred at a low level, supporting the notion that glycophorin B is a critical factor for invasion through the trypsin-resistant pathway (37). It was also observed that the 7G8 strain of P. falciparum did not invade glycophorin B-deficient erythrocytes (37), indicating an absolute dependence for invasion on that erythrocyte molecule for this strain.
For this study, we analyzed the invasion phenotypes of trypsin-treated normal and glycophorin B-deficient (S−s−U−) erythrocytes by several P. falciparum strains. This enabled us to understand whether invasion through the trypsin-resistant pathway is totally dependent on glycophorin B or if there are glycophorin B-independent, trypsin-resistant pathways. We show that P. falciparum Indochina I has the ability to invade erythrocytes through pathways that are completely independent of the involvement of glycophorin B and that 7G8 is able to invade untreated glycophorin B-deficient erythrocytes.
MATERIALS AND METHODS
P. falciparum parasites.
P. falciparum 7G8 (6) was obtained from Thomas Wellems (Laboratory of Malaria and Vector Research [LMVR], National Institutes of Health [NIH], Bethesda, Md.) and from the Malaria Research and Reference Reagent Resource Center (American Type Culture Collection, Manassas, Va.). The parasites HB3 (3) and Dd2 (47) were obtained from Xin-zhuan Su (LMVR, NIH). The Indochina I strain (10) was obtained from Sanjay Desai (LMVR, NIH). The 3D7 strain (46) was obtained from the Malaria Research and Reference Reagent Resource Center (American Type Culture Collection). The correct identity of each parasite strain (data not shown) was confirmed by P. falciparum rifin repetitive microsatellite (PfRRM) fingerprinting as described previously (42). PfRRM fingerprinting is a PCR typing method that is based on fragment length polymorphisms of a microsatellite (PfRRM) within a known multicopy PfRR, or rif, repetitive element of P. falciparum (42).
Erythrocytes.
Normal erythrocytes were obtained from the Department of Transfusion Medicine, NIH. The serum and leukocytes were removed and the erythrocytes were washed three times in RPMI 1640 incomplete medium, pH 6.7 (GIBCO, Grand Island, N.Y.), that contained 24 mM HEPES and 360 μM hypoxanthine, but not NaHCO3. The cells were stored at a 50% hematocrit in RPMI 1640 incomplete medium at 4°C. S−s−U− erythrocytes that lack glycophorin B were obtained from the New York Blood Center (New York, N.Y.) and the LifeSouth Community Blood Center (Gainesville, Fla.). The erythrocytes were washed in RPMI 1640 incomplete medium and stored at a 50% hematocrit in RPMI 1640 incomplete medium at 4°C. S−s−U+var/He+ erythrocytes expressing a variant of glycophorin B on their surfaces were obtained from the Department of Transfusion Medicine, NIH. These cells were shown to be U+ by serological testing at the New York Blood Center. The erythrocytes were washed in RPMI 1640 incomplete medium and stored at a 50% hematocrit in RPMI 1640 incomplete medium at 4°C.
Serological and molecular analysis of glycophorin B on erythrocyte samples.
Due to heterogeneity in the U antigen and variable reactivities of several samples of anti-U sera with S−s− erythrocytes, as described previously (4, 11, 20, 21, 41), the phenotypes of the erythrocyte samples were checked first by serological testing. This was done by the hemagglutination protocol described previously (38). Serological typing showed that five of the six samples were of the S−s−U− phenotype and that one blood sample had the S−s−U+var/He+ phenotype (with weak expression of the U antigen). S−s−U− and Henshaw positive (He+) are African phenotypes (2, 9, 14, 15, 24, 36, 39). As described earlier (38, 41), detection of He and U antigens on the S−s− erythrocytes provides evidence for the presence of altered glycophorin B molecules on the membranes of the erythrocytes from the two blood samples. The phenotypes of the erythrocyte samples were further confirmed by molecular analysis of the glycophorin B gene. The molecular analysis protocol was to amplify glycophorin B exon 5 from genomic DNA with gene-specific primers glycophorin B 4/5 (5′-CTGTCTTATTTTTCTATTGCTATG-3′) and glycophorin B IVS5 (5′-CTGTTTCTCTTTTGAGTTTAACTG-3′). The PCR mixture contained 5 μl of 10×X PCR buffer, 3 μl of 25 mM MgCl2, 1 μl of 10 mM deoxynucleoside triphosphates, 1 μl of each primer (100 ng/μl), 1 μl of Rh control primer (100 ng/μl), 0.25 μl of Taq polymerase (0.5 U), and H2O to 48 μl. After mixing the contents of each tube, 2 μl of template DNA (100 ng/μl) was added. The mixtures were denatured at 94°C for 15 min, cycled at 94°C, 55°C, and 72°C for 30 s each, for 30 cycles, and incubated at 72°C for 7 min. An internal amplification control (Rh primer mix) was included. Molecular analysis of four of the five S− s− U− samples showed that the glycophorin B exon 5 was not amplified (data not shown), thus confirming them to be of the deletion type (Table 1), with exons 2 to 6 of the glycophorin B gene deleted from the genome (19, 43). DNA for the fifth sample was not available.
TABLE 1.
Invasion assay for P. falciparum strains 7G8 and Indochina I on erythrocytes expressing different glycophorin B variants
| Erythrocyte donor no. | Phenotype | Condition of sample | Invasion rate (%) by
P. falciparum strain (no. of
expts)a
|
|
|---|---|---|---|---|
| 7G8 | Indochina I | |||
| 1726 | S−s−U− | Fresh | 72 ± 6 (5) | 60 ± 6 (5) |
| 2570 | S−s−U− | Fresh | 74 ± 8 (5) | 84 ± 6 (5) |
| 3585 | S−s−U− | Fresh | 51 ± 3 (4) | 44 ± 1 (3) |
| 4137 | S−s−U− | Fresh | 40 ± 1 (3) | 55 ± 2 (3) |
| 5114 | S−s−U− | Frozen | 87 ± 7 (3) | 102 ± 4 (3) |
| 6111 | S−s−U+var/He+ | Fresh | 74 ± 6 (3) | 74 ± 5 (3) |
Invasion rates are expressed as percentages of invasion of test erythrocytes compared to invasion in normal erythrocytes. Data are means ± standard errors.
Enzymatic treatment of erythrocytes. (i) Trypsin.
Erythrocytes (108 per ml of RPMI 1640 incomplete medium, pH 6.7) with 1 mg of tosylsulfonyl phenylalanyl chloromethyl ketone-treated trypsin (Sigma, St. Louis, Mo.) per ml were incubated, with rocking, at 37°C for 1 h and then washed with 10 packed cell volumes of RPMI 1640 incomplete medium. The erythrocytes were then treated with 1 mg of soybean trypsin inhibitor (Sigma) per ml at room temperature for 10 min. The cells were washed twice with 10 packed cell volumes of RPMI 1640 incomplete medium and then stored in RPMI 1640 incomplete medium at 4°C for a maximum of 1 day.
(ii) Neuraminidase.
Erythrocytes (2.5 × 109) in 5 ml of RPMI 1640 incomplete medium, pH 6.7, were incubated with 0.037 U of Vibrio cholerae neuraminidase (Calbiochem, San Diego, Calif.) at 37°C for 1 h, with rocking, and then washed twice with 10 packed cell volumes of RPMI 1640 incomplete medium.
Invasion assays.
Schizont-stage-infected erythrocytes were purified by centrifugation on a 40%-70% percoll-sorbitol gradient (1, 22). The parasites were washed with 10 cell volumes of RPMI 1640 incomplete medium, counted in a hemocytometer, and mixed with target erythrocytes in in vitro cultures. Schizont-infected erythrocytes (8 × 105) were mixed with 4 × 107 target erythrocytes in 200 μl of complete medium, pH 7.2 (RPMI 1640, 24 mM HEPES, 360 μM hypoxanthine, 24 mM NaHCO3, 10 μg of gentamicin per ml, 5% Albumax [GIBCO]), gassed with 5% CO2-5% O2-90% N2, and incubated for 16 to 20 h at 37°C in a modular incubator chamber (45). Experiments were performed in duplicate in flat-bottomed microtiter plates. Rhesus monkey erythrocytes, which are refractory to invasion by P. falciparum, were included in the assay as a control to estimate invasion into uninfected normal erythrocytes that may be present along with the purified parasite-infected erythrocytes. Following the incubation period, thin smears were made and stained with Giemsa. Ring-stage parasites were counted to determine the rate of invasion. The slides were counted in a blind manner. At least 50 ring-stage parasites were counted per assay, and if the invasion rates were <1% rings, at least 100 fields of the smear were counted. The percentage invasion of the test and control erythrocytes was corrected by subtracting the percentage invasion of rhesus erythrocytes.
RESULTS
Invasion by P. falciparum of erythrocytes expressing glycophorin B variants.
Invasion by P. falciparum 7G8 and Indochina I strains was studied in five S−s−U− samples and one S−s−U+var/He+ erythrocyte sample (Table 1). P. falciparum strains 7G8 and Indochina I invaded the five S−s−U− (glycophorin B deficient) erythrocyte samples at invasion rates of 40 to 87% and 44 to 102%, respectively, compared to invasion of normal erythrocytes (Table 1). These results showed that P. falciparum invaded S−s−U− erythrocytes, but at a reduced efficiency, as published previously. Similarly, the two P. falciparum strains, 7G8 and Indochina I, invaded the S−s−U+var/He+ erythrocytes at a rate of 74% compared to invasion of normal erythrocytes (Table 1). One S−s−U− erythrocyte sample (donor 5114) was obtained as a frozen sample, because a fresh blood sample could not be obtained. This was the same S−s−U− sample that was used for a previous study (37). We felt that it was important to include this sample in our study in order to exclude any unique properties in this S−s−U− sample that might affect its invasion by P. falciparum. The invasion rate of this frozen S−s−U− sample by the P. falciparum strain 7G8 was comparable to that of the fresh S−s−U− erythrocytes (Table 1). Importantly, P. falciparum 7G8, whether originating at NIH or Centers for Disease Control, invades glycophorin B-deficient (S−s−U−) erythrocytes.
Invasion of enzymatically treated normal and glycophorin B-deficient erythrocytes by various strains of P. falciparum.
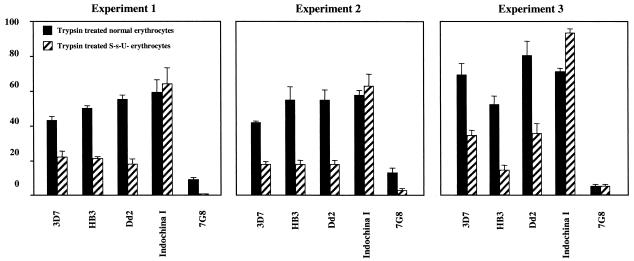
We studied invasion by five P. falciparum strains, 3D7, Indochina I, Dd2, HB3, and 7G8, of neuraminidase- and trypsin-treated normal and glycophorin B-deficient erythrocytes in three independent experiments. The complete results of experiment 1 (representative of the results for all three experiments) are shown in Table 2, and the results of trypsin treatment for all three experiments are shown in Fig. 1.
TABLE 2.
Invasion by P. falciparum strains of enzyme-treated normal and glycophorin B-deficient (S−s−U−) erythrocytes
| Strain | Invasion
of erythrocytes by P.
falciparuma
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Normal
|
S−s−U−
|
|||||||
| U | N | T | N + T | U | N | T | N + T | |
| 3D7 | 77 | 113 | 43 | 3 | 69 | 80 | 22 | 1 |
| HB3 | 74 | 82 | 50 | 12 | 53 | 86 | 21 | 0 |
| Dd2 | 51 | 4 | 55 | 0 | 40 | 5 | 18 | 0 |
| Indochina I | 128 | 61 | 59 | 9 | 96 | 52 | 64 | 9 |
| 7G8 | 73 | 88 | 9 | 0 | 37 | 68 | 0 | 0 |
For untreated erythrocytes, data are numbers of ring-stage parasites per 1,000 erythrocytes. For treated erythrocytes, data are percentages compared to untreated erythrocytes. U, untreated erythrocytes; N, neuraminidase-treated erythrocytes; T, trypsin-treated erythrocytes; N + T, neuraminidase- and trypsin-treated erythrocytes.
FIG. 1.
Comparison of the effect of trypsin treatment of normal and glycophorin B-deficient (S−s−U−) erythrocytes on invasion by different P. falciparum strains in three independent experiments. The black bars represent percent invasion of trypsin-treated normal erythrocytes compared to that of untreated normal erythrocytes. The diagonally hatched bars represent percent invasion of trypsin-treated glycophorin B-deficient (S− s−U−) erythrocytes compared to that of untreated glycophorin B-deficient (S−s−U−) erythrocytes. Experiments 1 and 2 were done with S−s− U− erythrocytes from the same donor (no. 2570), while experiment 3 was done with S− s− U− erythrocytes from a different donor (no. 1726). The error bars show the standard deviations for each invasion assay performed in duplicate.
Four P. falciparum strains, 3D7, Indochina I, HB3, and 7G8, efficiently invaded neuraminidase-treated normal erythrocytes at invasion rates of 61 to 113% compared to those with untreated normal erythrocytes (Table 2). Only for the Dd2 strain was there a marked reduction in invasion upon neuraminidase treatment of the erythrocytes (Table 2), as described previously (13, 30). Four P. falciparum strains (3D7, Indochina I, Dd2, and HB3) invaded trypsin-treated normal erythrocytes at invasion rates of 43 to 59% compared to invasion of untreated normal erythrocytes (Table 2). However, the invasion rate of the trypsin-digested erythrocytes by the 7G8 strain averaged only 9% compared to that for normal cells, which is a ≥90% reduction in invasion (Table 2). As reported in previous studies (13, 30, 37), these results show that P. falciparum invades erythrocytes through alternate pathways that could be sialic acid dependent or independent and trypsin sensitive or resistant.
Glycophorin B is known to be present on the surfaces of trypsin-digested erythrocytes, as it is resistant to trypsin treatment. As noted earlier, glycophorin B is the only defined erythrocyte receptor that is known to be involved in a trypsin-resistant invasion pathway, which suggests that the 7G8 strain does not possess the parasite ligand that binds to glycophorin B or another molecule crucial for invading trypsin-digested erythrocytes (13).
To analyze the degree of dependence of the different P. falciparum strains on glycophorin B as a receptor in the trypsin-resistant pathway, we further studied the invasion by the five parasite strains of trypsin-treated glycophorin B-deficient (S−s−U−) erythrocytes. The strains HB3, 3D7, and Dd2 invaded trypsin-digested S−s−U− erythrocytes at rates of 18 to 22% compared to invasion of untreated S−s−U− erythrocytes (Table 2). These P. falciparum strains invaded trypsin-treated normal erythrocytes at invasion rates of 43 to 59% compared to untreated normal erythrocytes (Table 2). Hence, the absence of glycophorin B reduced invasion of trypsin-digested erythrocytes by 50 to 60%, suggesting that glycophorin B plays a crucial role as an erythrocyte receptor in the trypsin-resistant pathway used by these three strains. A similar phenomenon was observed in the other two independent experiments (Fig. 1). In previous studies (13, 37), the invasion rates of the parasite strains HB3, Dd2, and 3D7 on trypsin-digested S−s−U− erythrocytes were lower than the 18 to 22% that we report here. In these previous reports, the invasion rates were calculated by comparing invasion of trypsin-digested S−s−U− erythrocytes with that of untreated normal erythrocytes. For our study, we calculated invasion rates by comparing invasion of trypsin-digested S−s−U− erythrocytes with that of untreated S−s−U− erythrocytes, that is, their own untreated control cells. Since in our studies the absolute invasion rate of S−s−U− erythrocytes is lower than that of normal erythrocytes, comparison with untreated S−s−U− cells results in a relatively higher invasion rate.
It is interesting that the absence of glycophorin B did not affect invasion of the Indochina I strain of P. falciparum through the trypsin-resistant pathway. Indochina I invaded trypsin-digested normal and S−s−U− erythrocytes at a similar rate relative to their respective untreated controls (Table 2, Fig. 1), indicating that Indochina I can invade through a trypsin-resistant pathway independent of glycophorin B. Further, the invasion of neuraminidase- and trypsin-treated S−s−U− erythrocytes by Indochina I was reduced to 9% of the control (Table 2), suggesting that this particular glycophorin B-independent, trypsin-resistant pathway is sialic acid dependent.
DISCUSSION
We have demonstrated a new pathway through which the Indochina I strain of P. falciparum invades erythrocytes. This pathway allows the parasite to invade trypsin-digested erythrocytes independent of glycophorin B. Previously, experiments with several strains of P. falciparum indicated that the invasion of trypsin-digested erythrocytes by P. falciparum was apparently dependent on glycophorin B. Strains 3D7, HB3, and Dd2 had greatly reduced invasion rates for trypsin-digested S−s− U− cells compared to those for trypsin-digested normal erythrocytes, as previously described (13). These three P. falciparum strains appear to have a very limited ability to invade through the glycophorin B-independent, trypsin-resistant pathway, which accounts for their 20% invasion of trypsin-digested glycophorin B-deficient erythrocytes. We now show that Indochina I invades trypsin-digested S−s−U− erythrocytes at the same relative efficiency as trypsin-digested normal erythrocytes, indicating the presence of a glycophorin B-independent pathway. This unique characteristic of Indochina I allows for its use as a reagent for the study of this invasion pathway.
The parasite ligand and erythrocyte receptor molecules that mediate invasion through the trypsin-resistant pathway are not completely known. Glycophorin B is the only known trypsin-resistant erythrocyte receptor involved in invasion by P. falciparum. The parasite ligand that binds to glycophorin B still remains unknown. Recently, several parasite molecules, such as a polymorphic variant of BAEBL/EBA-140 in the clone E12 (27, 44), PfNBP-1, a P. falciparum ortholog of the P. vivax reticulocyte binding protein (PvRBP1) (37), and another EBA-175 paralogue, JESEBL/EBA-180 (17), have been reported to bind to neuraminidase-sensitive, trypsin-resistant erythrocyte receptors. Although these receptor characteristics are similar to those of glycophorin B, these parasite molecules do not bind to glycophorin B and their erythrocyte receptors are yet unknown. The unknown receptors for P. falciparum NBP-1 and JESEBL (EBA-180) are denoted receptor Y (37) and E (17), respectively. Also, a recent study demonstrated that band 3 acts as a trypsin-resistant host receptor for the P. falciparum ligand MSP-1 (18). It is difficult to predict whether each of these molecules defines an independent trypsin-resistant pathway or if they are all involved in a common trypsin-resistant pathway. In a previous report, it was hypothesized that glycophorin B and receptor Y have overlapping roles in a common trypsin-resistant pathway (37). Here we have shown that at least one trypsin-resistant pathway does not involve glycophorin B. However, it is also possible that the Indochina I strain of P. falciparum can invade through more than one glycophorin B-independent, trypsin-resistant pathway. Our results show that this glycophorin B-independent, trypsin-resistant pathway is sensitive to neuraminidase treatment and thus is sialic acid dependent. Therefore, it appears that another sialic acid-containing erythrocyte receptor is involved in this pathway. This erythrocyte receptor may be a glycolipid or sialoglycoprotein other than glycophorin B which is trypsin resistant. The parasite ligand that mediates this glycophorin B-independent, trypsin-resistant invasion pathway may be JESEBL, BAEBL, or another merozoite molecule.
It was previously reported that the 7G8 strain of P. falciparum cannot invade S−s−U− erythrocytes, while the FVO, Camp, and 3D7 strains invade at rates of 73 to 79% of those for normal erythrocytes (37). Contrary to this result, the invasion studies reported here show that the P. falciparum 7G8 strain does invade S−s−U− erythrocytes. Thus, no human erythrocyte is known to be completely refractory to invasion by P. falciparum.
It is well established that P. falciparum invades erythrocytes through several alternate pathways. Until now, P. falciparum was observed to be dependent on glycophorin B for invading trypsin-digested erythrocytes. Here we have demonstrated for the first time that P. falciparum is capable of invading erythrocytes through a glycophorin B-independent, trypsin-resistant pathway. Several parasite molecules have been identified that bind to trypsin-resistant erythrocyte receptors, and it would be interesting to elucidate the parasite molecules and host receptors that mediate this glycophorin B-independent, trypsin-resistant pathway exhibited by the Indochina I strain of P. falciparum.
Acknowledgments
We thank Joann Moulds (Drexel University School of Medicine, Philadelphia, Pa.) and the Life South Community Blood Center (Gainesville, Fla.) for providing S−s−U− erythrocyte samples. We thank Thomas Wellems, Sanjay Desai, and Xinzhuan Su (LMVR, NIH, Bethesda, Md.) for providing the parasites used in this study. The P. falciparum 3D7 strain was obtained from D. J. Carucci through the Malaria Research and Reference Reagent Resource Center, Division of Microbiology and Infectious Diseases, NIAID, NIH. We give special thanks to Xin-zhuan Su, Tetsuya Furuya, Xiaorong Feng, and Anna Liu for helping us to fingerprint the parasites.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aley, S. B., J. A. Sherwood, and R. J. Howard. 1984. Knob-positive and knob-negative Plasmodium falciparum differ in expression of a strain-specific malarial antigen on the surface of infected erythrocytes. J. Exp Med. 160:1585-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allbrook, D., N. A. Barnicot, N. Dance, S. D. Lawler, R. Marshall, and J. Mungai. 1965. Blood groups, haemoglobin and serum factors of the Karamojo. Hum. Biol. 37:217-237. [PubMed] [Google Scholar]
- 3.Bhasin, V. K., and W. Trager. 1984. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 33:534-537. [DOI] [PubMed] [Google Scholar]
- 4.Booth, P. B. 1978. Two Melanesian antisera reacting with SsU components. Vox Sang. 34:212-220. [DOI] [PubMed] [Google Scholar]
- 5.Breuer, W. V., H. Ginsburg, and Z. I. Cabantchik. 1983. An assay of malaria parasite invasion into human erythrocytes. The effects of chemical and enzymatic modification of erythrocyte membrane components. Biochim. Biophys. Acta 755:263-271. [DOI] [PubMed] [Google Scholar]
- 6.Burkot, T. R., J. L. Williams, and I. Schneider. 1984. Infectivity to mosquitoes of Plasmodium falciparum clones grown in vitro from the isolate. Trans. R. Soc. Trop. Med. Hyg. 78:339-341. [DOI] [PubMed] [Google Scholar]
- 7.Cartron, J. P., and C. Rahuel. 1992. Human erythrocyte glycophorins: protein and gene structure analyses. Transfus. Med. Rev. 6:63-92. [DOI] [PubMed] [Google Scholar]
- 8.Cartron, J. P., and C. Rahuel. 1995. MNSs and major glycophorins of human erythrocytes. Transfus. Clin. Biol. 2:251-258. [DOI] [PubMed] [Google Scholar]
- 9.Cavalli-Sforza, L. L., L. A. Zonta, F. Nuzzo, L. Bernini, W. W. de Jong, P. Meera Khan, A. K. Ray, L. N. Went, M. Siniscalco, L. E. Nijenhuis, E. van Loghem, and G. Modiano. 1969. Studies on African Pygmies. I. A pilot investigation of Babinga Pygmies in the Central African Republic (with an analysis of genetic distances). Am. J. Hum. Genet. 21:252-274. [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, W. E., C. C. Campbell, J. C. Skinner, W. Chin, P. Nguyen-Dinh, and A. Y. Huong. 1983. Studies on the Indochina I/CDC strain of Plasmodium falciparum in Colombian and Bolivian Aotus monkeys and different anophelines. J. Parasitol. 69:186-190. [PubMed] [Google Scholar]
- 11.Dahr, W. 1986. Immunochemistry of sialoglycoproteins in human red blood cell membranes, p. 23-65. In V. Vengelen-Tyler and W. J. Judd (ed.), Recent advances in blood group biochemistry. American Association of Blood Banks, Arlington, Va.
- 12.Dolan, S. A., L. H. Miller, and T. E. Wellems. 1990. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J. Clin. Investig. 86:618-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolan, S. A., J. L. Proctor, D. W. Alling, Y. Okubo, T. E. Wellems, and L. H. Miller. 1994. Glycophorin B as an EBA-175 independent Plasmodium falciparum receptor of human erythrocytes. Mol. Biochem. Parasitol. 64:55-63. [DOI] [PubMed] [Google Scholar]
- 14.Field, S. P., E. Hempelmann, B. V. Mendelow, and A. F. Fleming. 1994. Glycophorin variants and Plasmodium falciparum: protective effect of the Dantu phenotype in vitro. Hum. Genet. 93:148-150. [DOI] [PubMed] [Google Scholar]
- 15.Fraser, G. R., E. R. Giblett, and A. G. Motulsky. 1966. Population genetic studies in the Congo. III. Blood groups (ABO, MNSs, Rh, Jsa). Am. J. Hum. Genet. 18:546-552. [PMC free article] [PubMed] [Google Scholar]
- 16.Galinski, M. R., C. C. Medina, P. Ingravallo, and J. W. Barnwell. 1992. A reticulocyte-binding protein complex of Plasmodium vivax merozoites. Cell 69:1213-1226. [DOI] [PubMed] [Google Scholar]
- 17.Gilberger, T. W., J. K. Thompson, T. Triglia, R. T. Good, M. T. Duraisingh, and A. F. Cowman. 2003. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J. Biol. Chem. 278:14480-14486. [DOI] [PubMed] [Google Scholar]
- 18.Goel, V. K., X. Li, H. Chen, S. C. Liu, A. H. Chishti, and S. S. Oh. 2003. Band 3 is a host receptor binding merozoite surface protein 1 during the Plasmodium falciparum invasion of erythrocytes. Proc. Natl. Acad. Sci. USA 100:5164-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, C. H., K. Johe, J. J. Moulds, P. D. Siebert, M. Fukuda, and O. O. Blumenfeld. 1987. Delta glycophorin (glycophorin B) gene deletion in two individuals homozygous for the S-s-U- blood group phenotype. Blood 70:1830-1835. [PubMed] [Google Scholar]
- 20.Issitt, P. D. 1990. Heterogeneity of anti-U. Vox Sang. 58:70-71. [DOI] [PubMed] [Google Scholar]
- 21.Issitt, P. D., W. L. Marsh, M. R. Wren, M. Theuriere, and K. Mueller. 1989. Heterogeneity of anti-U demonstrable by the use of papain-treated red cells. Transfusion 29:508-513. [DOI] [PubMed] [Google Scholar]
- 22.Jensen, J. B. 1978. Concentration from continuous culture of erythrocytes infected with trophozoites and schizonts of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 27:1274-1276. [DOI] [PubMed] [Google Scholar]
- 23.Lobo, C. A., M. Rodriguez, M. Reid, and S. Lustigman. 2003. Glycophorin C is the receptor for the Plasmodium falciparum erythrocyte binding ligand PfEBP-2 (baebl). Blood 101:4628-4631. [DOI] [PubMed] [Google Scholar]
- 24.Lowe, R. F., and P. P. Moores. 1972. S− s− U−red cell factor in Africans of Rhodesia, Malawi, Mozambique and Natal. Hum. Hered. 22:344-350. [DOI] [PubMed] [Google Scholar]
- 25.Maier, A. G., M. T. Duraisingh, J. C. Reeder, S. S. Patel, J. W. Kazura, P. A. Zimmerman, and A. F. Cowman. 2003. Plasmodium falciparum erythrocyte invasion through glycophorin C and selection for Gerbich negativity in human populations. Nat. Med. 9:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayer, D. C., O. Kaneko, D. E. Hudson-Taylor, M. E. Reid, and L. H. Miller. 2001. Characterization of a Plasmodium falciparum erythrocyte-binding protein paralogous to EBA-175. Proc. Natl. Acad. Sci. USA 98:5222-5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer, D. C., J. B. Mu, X. Feng, X. Z. Su, and L. H. Miller. 2002. Polymorphism in a Plasmodium falciparum erythrocyte-binding ligand changes its receptor specificity. J. Exp. Med. 196:1523-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, L. H., J. D. Haynes, F. M. McAuliffe, T. Shiroishi, J. R. Durocher, and M. H. McGinniss. 1977. Evidence for differences in erythrocyte surface receptors for the malarial parasites, Plasmodium falciparum and Plasmodium knowlesi. J. Exp. Med. 146:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, L. H., S. J. Mason, D. F. Clyde, and M. H. McGinniss. 1976. The resistance factor to Plasmodium vivax in blacks. The Duffy-blood-group genotype, FyFy. N. Engl. J. Med. 295:302-304. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell, G. H., T. J. Hadley, M. H. McGinniss, F. W. Klotz, and L. H. Miller. 1986. Invasion of erythrocytes by Plasmodium falciparum malaria parasites: evidence for receptor heterogeneity and two receptors. Blood 67:1519-1521. [PubMed] [Google Scholar]
- 31.Pasvol, G., and M. Jungery. 1983. Glycophorins and red cell invasion by Plasmodium falciparum. Ciba Found. Symp. 94:174-195. [DOI] [PubMed] [Google Scholar]
- 32.Pasvol, G., M. Jungery, D. J. Weatherall, S. F. Parsons, D. J. Anstee, and M. J. Tanner. 1982. Glycophorin as a possible receptor for Plasmodium falciparum. Lancet ii:947-950. [DOI] [PubMed] [Google Scholar]
- 33.Pasvol, G., J. S. Wainscoat, and D. J. Weatherall. 1982. Erythrocytes deficient in glycophorin resist invasion by the malarial parasite Plasmodium falciparum. Nature 297:64-66. [DOI] [PubMed] [Google Scholar]
- 34.Perkins, M. 1981. Inhibitory effects of erythrocyte membrane proteins on the in vitro invasion of the human malarial parasite (Plasmodium falciparum) into its host cell. J. Cell Biol. 90:563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perkins, M. E., and E. H. Holt. 1988. Erythrocyte receptor recognition varies in Plasmodium falciparum isolates. Mol. Biochem. Parasitol. 27:23-34. [DOI] [PubMed] [Google Scholar]
- 36.Race, R., and R. Sanger. 1975. Blood groups in man, 6th ed., p. 92-138. Blackwell Scientific Publications, Oxford, England.
- 37.Rayner, J. C., E. Vargas-Serrato, C. S. Huber, M. R. Galinski, and J. W. Barnwell. 2001. A Plasmodium falciparum homologue of Plasmodium vivax reticulocyte binding protein (PvRBP1) defines a trypsin-resistant erythrocyte invasion pathway. J. Exp. Med. 194:1571-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reid, M., J. R. Storry, H. Ralph, O. O. Blumenfeld, and C. H. Huang. 1986. Expression and quantitative variation of the low-incidence blood group antigen He on some S− s− red cells. Transfusion 36:719-724. [DOI] [PubMed] [Google Scholar]
- 39.Reid, M. E., C. Lomas-Francis, G. L. Daniels, V. Chen, J. Shen, Y. C. Ho, V. Hare, R. Batts, M. Yacob, E. Smart, et al. 1995. Expression of the erythrocyte antigen Henshaw (He; MNS6): serological and immunochemical studies. Vox Sang. 68:183-186. [DOI] [PubMed] [Google Scholar]
- 40.Sim, B. K., C. E. Chitnis, K. Wasniowska, T. J. Hadley, and L. H. Miller. 1994. Receptor and ligand domains for invasion of erythrocytes by Plasmodium falciparum. Science 264:1941-1944. [DOI] [PubMed] [Google Scholar]
- 41.Storry, J. R., and M. E. Reid. 1996. Characterization of antibodies produced by S− s− individuals. Transfusion 36:512-516. [DOI] [PubMed] [Google Scholar]
- 42.Su, X. Z., D. J. Carucci, and T. E. Wellems. 1998. Plasmodium falciparum: parasite typing by using a multicopy microsatellite marker, PfRRM. Exp. Parasitol. 89:262-265. [DOI] [PubMed] [Google Scholar]
- 43.Tate, C. G., M. J. Tanner, P. A. Judson, and D. J. Anstee. 1989. Studies on human red-cell membrane glycophorin A and glycophorin B genes in glycophorin-deficient individuals. Biochem. J. 263:993-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson, J. K., T. Triglia, M. B. Reed, and A. F. Cowman. 2001. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol. Microbiol. 41:47-58. [DOI] [PubMed] [Google Scholar]
- 45.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 46.Walliker, D., I. A. Quakyi, T. E. Wellems, T. F. McCutchan, A. Szarfman, W. T. London, L. M. Corcoran, T. R. Burkot, and R. Carter. 1987. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 236:1661-1666. [DOI] [PubMed] [Google Scholar]
- 47.Wellems, T. E., L. J. Panton, I. Y. Gluzman, V. E. do Rosario, R. W. Gwadz, A. Walker-Jonah, and D. J. Krogstad. 1990. Chloroquine resistance not linked to mdr-like genes in Plasmodium falciparum clones. Nature 345:253-255. [DOI] [PubMed] [Google Scholar]