Abstract
Listeria monocytogenes is a facultative intracellular bacterial pathogen that escapes from a phagosome and grows in the host cell cytosol. Escape of the bacterium from the phagosome to the cytosol is mediated by the bacterial pore-forming protein listeriolysin O (LLO). LLO has multiple mechanisms that optimize activity in the phagosome and minimize activity in the host cytosol. Mutants that fail to compartmentalize LLO activity are cytotoxic and have reduced virulence. We sought to determine why cytotoxic bacteria have attenuated virulence in the mouse model of listeriosis. In this study, we constructed a series of strains with mutations in LLO and with various degrees of cytotoxicity. We found that the more cytotoxic the strain in cell culture, the less virulent it was in mice. Induction of neutropenia increased the relative virulence of the cytotoxic strains 100-fold in the spleen and 10-fold in the liver. The virulence defect was partially restored in neutropenic mice by adding gentamicin, an antibiotic that kills extracellular bacteria. Additionally, L. monocytogenes grew more slowly in extracellular fluid (mouse serum) than within tissue culture cells. We concluded that L. monocytogenes controls the cytolytic activity of LLO to maintain its nutritionally rich intracellular niche and avoid extracellular defenses of the host.
Intracellular pathogens are responsible for an enormous amount of worldwide morbidity and mortality (41). The success and prevalence of intracellular pathogens suggest that the microenvironment of the intracellular space provides pathogens with a relatively nutrient-rich environment that is protected from the influence of the extracellular defenses of the host. Consequently, intracellular pathogens pose a serious challenge to the mammalian immune system, a challenge that is largely met by cell-mediated immunity (22).
Much of our understanding of immunity to intracellular pathogens is derived from the murine model of Listeria monocytogenes infection. L. monocytogenes is a facultative intracytosolic bacterial pathogen whose cell biology and determinants of pathogenesis have been well defined (39). Subsequent to internalization by macrophages, L. monocytogenes is either killed or escapes into the host cytosol. Escape from a phagosome is largely dependent on secretion of a cholesterol-dependent cytolysin called listeriolysin O (LLO). LLO− mutants are unable to grow in cells and are absolutely avirulent a murine model of infection (25). Once in the host cell cytosol, L. monocytogenes grows rapidly and expresses a surface protein called ActA that allows the bacteria to exploit the host actin cytoskeleton and spread from cell to cell without exposure to the extracellular milieu. Thus, L. monocytogenes is able to evade the humoral immune response by growing intracellularly and to evade the cell-mediated immune response by cell-to-cell spread (35).
Cell-mediated resistance to L. monocytogenes relies on the function of tissue macrophages, neutrophils, infiltrating monocytes, and eventually cytotoxic T lymphocytes (9, 22, 30). Early resistance to L. monocytogenes infection has been attributed primarily to the function of resident liver macrophages (Kupffer cells), which trap and destroy a majority of an initial intravenous inoculum (30). During the first 48 h postinfection, neutrophils infiltrate into the tissues and are eventually replaced by activated macrophages entering from the periphery (14, 30). Both neutrophils and activated macrophages act as vital effectors to limit the spread of the bacteria. It is not clear how these phagocytes function to control intracellular bacteria, but cell culture analysis suggests that L. monocytogenes may be transiently extracellular, which would expose the bacteria to phagocytic defenses (19). Cytotoxic T lymphocytes recognize and specifically lyse L. monocytogenes-infected host cells (22), thereby effectively forcing the intracellular pathogen into the extracellular space (2).
LLO is the primary determinant of L. monocytogenes pathogenesis. It acts as a vacuole-specific lysin to allow bacterial entrance into the host cytosol. However, LLO is a double-edged sword: it is essential, but it is potentially toxic. Accordingly, L. monocytogenes has evolved multiple mechanisms to control LLO activity and prevent cytotoxicity (13, 19). Mutations in LLO that affect compartmentalization of the activity lead to inappropriate activity in the cytosol, damage to the host cell, and decreased virulence. We have characterized two separate mechanisms which control the function of LLO and which distinguish LLO from other cholesterol-dependent cytolysins secreted by extracellular pathogens. First, LLO has an acidic pH optimum. Substitution of a threonine for the naturally occurring leucine at position 461 results in a mutant LLO protein that is active at neutral pH, and it damages the host's plasma membrane when the bacteria reside within the host cytosol, which has a neutral pH (19). Second, LLO has an N-terminal PEST-like sequence. Deletion of this sequence results in an extremely toxic phenotype that reduces virulence by 4 orders of magnitude (13). The mechanism by which the PEST-like sequence functions is under investigation, but deletion of it clearly leads to increased amounts of LLO in the host cell cytosol.
In this study, we constructed a series of mutants with changes in LLO that led to progressively more cytotoxic bacteria and asked why cytotoxic L. monocytogenes is less virulent. We addressed this question by using the cytotoxic L. monocytogenes strains in the mouse model of infection and by eliminating components of the mouse's immune system. We concluded that in L. monocytogenes, the essential (31) but potentially cytotoxic LLO protein evolved to act lytically primarily within the phagosome in order to maintain the integrity of the host cell and, consequently, to protect the bacterium from the extracellular defenses of the host.
MATERIALS AND METHODS
Strains, growth conditions, and reagents.
The wild-type L. monocytogenes strain used for this study was 10403S (5). L. monocytogenes strains with deletions of actA were constructed by allelic exchange as described previously (7, 38). Strain DP-L4017 (which produces LLO L461T) has been described previously (19). The strains used in this study are shown in Table 1. Bacteria were grown in 3 ml of brain heart infusion (BHI) broth (Becton Dickinson, Sparks, Md.) slanted without agitation in 15-ml conical tubes at 30°C overnight, unless otherwise noted.
TABLE 1.
L. monocytogenes strains used in this study
| Strain | Descriptiona | Reference |
|---|---|---|
| 10403S | Wild type | 5 |
| DP-L3903 | Wild type Ermr | 3 |
| DP-L2161 | ΔLLO | 25 |
| DP-L4017 | LLO L461T | 19 |
| DP-L4057 | LLO S44A | This study |
| DP-L4384 | LLO S44A L461T | This study |
| DP-L4157 | LLO L461T Ermr | This study |
| DP-L4382 | LLO S44A Ermr | This study |
| DP-L4385 | LLO S44A L461T Ermr | This study |
| DP-L3078 | ΔActA | 38 |
| DP-L4038 | ΔActA LLO L461T | This study |
| DP-L4396 | ΔActA LLO S44A | This study |
| DP-L4397 | ΔActA LLO S44A L461T | This study |
| DP-L4403 | ΔActA LLO L461T Ermr | This study |
| DP-L4399 | ΔActA LLO S44A Ermr | This study |
| DP-L4400 | ΔActA LLO S44A L461T Ermr | This study |
Ermr, erythromycin resistance; ΔLLO, the hly gene has an in-frame deletion in the open reading frame; ΔActA, the actA gene has an in-frame deletion in the open reading frame.
Tissue culture cells were grown in Dulbecco modified Eagle medium (DMEM) (Gibco-BRL) containing 7.5% heat-inactivated fetal bovine serum (Hy-Clone, Logan, Utah) and 2 mM glutamine (Gibco-BRL) at 37°C in the presence of 5% CO2, unless otherwise noted. All chemicals were purchased from Sigma-Aldrich, St. Louis, Mo., unless otherwise noted.
Unless otherwise noted, female C57BL/6 mice (Jackson Laboratory, Bar Harbor, Maine) that were 6 to 8 weeks old were used for infection and bone marrow isolation by using University of California, Berkeley animal use protocol R235-0701B. RB6-8C5 monoclonal antibody was produced by Strategic BioSolutions (Newark, Del.) from a hybridoma generously donated by Robert North and Ronald LaCourse of the Trudeau Institute (9). The ascites was harvested from nude mice and then partially purified by precipitation with 45% ammonium sulfate by using endotoxin-free conditions. The antibody was subsequently resuspended and dialyzed in phosphate-buffered saline (PBS).
Construction of LLO mutants.
Strain DP-L4057 (which produces LLO S44A) was produced by using splicing by overlap extension PCR (24) in order to change the serine at position 44 to alanine with the following oligonucleotides (Operon Technologies): DP-1569 (GGGTCGACTCCTTTGATTAGTATATTCCT; SalI site underlined), DP-1700 (TTTGGATAAGCTTGAGCATATT; HindIII site underlined), DP-3820 (GCACCACCAGCAGCTCCGCCTGCAAG), and DP-3821 (CTTGCAGGCGGAGCTGCTGGTGGTGC). DP-1569 was paired with DP-3821 and DP-3820 was paired with DP-1700 to produce 382- and 480-bp DNA fragments, respectively, by using Vent polymerase (New England Bioloabs, Beverly, Mass.) and genomic DNA from strain 10403S as the template. The two fragments were then spliced to form a 834-bp fragment that was cut with SalI and HpaI and ligated into a SalI/SmaI-cut pKSV7 plasmid for allelic exchange (7). Allelic exchange was performed in an L. monocytogenes strain containing an allele encoding LLO with the 26 N-terminal residues removed (LLO Δ26) (strain DP-L4042) (13). Exchange of the LLO S44A allele for the LLO Δ26 allele was monitored by using PCR to detect a 78-bp increase in the size of the N terminus of the hly gene, and subsequently the sequence was determined to verify the mutation. Strain DP-L4384 (which produces LLO S44A L461T) was produced by introducing the plasmid used to produce LLO L461T (p4005) into DP-L4057 for allelic exchange (19). Clones were screened for the loss of an NheI site, introduced by the L461T mutation, and subsequently verified by sequencing.
All of the mutant strains producing LLO were marked for the competitive index assay by transducing the gene for erythromycin resistance from strain DP-L3903 by using phage U153, as described by Auerbuch et al. (3) and Hodgson (23). Briefly, phage U153 isolated from DP-L3903 was added to the recipient strain while it was in the mid-log growth phase, 10 mM CaCl2 and 10 mM MgCl2 were added, and the bacteria were incubated at room temperature for 1 h with occasional mixing. After 1 h, 0.1 μg of erythromycin per ml was added and the preparation was incubated for 30 min, and then the mixture was spread on BHI agar plates containing 1 μg of erythromycin per ml and incubated at 37°C for 2 days. Transduction of the erythromycin resistance gene was verified by PCR analysis by using primers DP-4409 (CCCAAGCTTCTAAAGTTATGGAAATAAGAC) and DP-4410 (CCGAGCTCACGGATTTTGGTACTTGAT), which flank erm in Tn917-LTV3. Additionally, the newly isolated resistant strain was used in a competition assay along with the parental nonresistant strain in the mouse model to confirm that there was no alteration in virulence. The resulting strains are described in Table 1.
Phagosomal escape assay.
The percentage of bacteria that escaped from phagosomes was determined by indirect immunofluorescence as described previously (26). Briefly, bone marrow-derived macrophages (described below) on a coverslip were infected for 30 min and washed with PBS, and then 10 μg of gentamicin per ml was added at 60 min. At 90 min, the macrophages were fixed with 4% formalin-PBS. Before macrophage permeabilization, extracellular bacteria were bound with Bacto-Listeria O rabbit serum (Difco Laboratories) and visualized with aminomethylcoumarine acetate-conjugated donkey anti-rabbit secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, Pa.). Subsequently, the macrophages were permeabilized with Triton X-100 and stained with rhodamine phalloidin and Bacto-Listeria O rabbit serum. Bacto-Listeria O serum bound to intracellular bacteria that was not bound by aminomethylcoumarine acetate antibodies was visualized with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G serum. A minimum of 200 bacterium-associated macrophages from nine different coverslips were examined for each bacterial strain.
Plaque assay.
Plaque assays with L2 cell monolayers were performed as described previously (25), except that the methods of measurement were modified (38). Briefly, L2 cells were grown to confluence in six-well tissue culture dishes and then infected with bacteria for 1 h. Subsequently, DMEM agar containing 5 μg of gentamicin per ml was added, and plaques were grown for 3 days. Living cells were visualized by adding an additional DMEM agar overlay containing neutral red (Gibco-BRL) on day 3 and incubating the dishes overnight.
Cytotoxicity assays. (i) Growth in J774 macrophage-like cells.
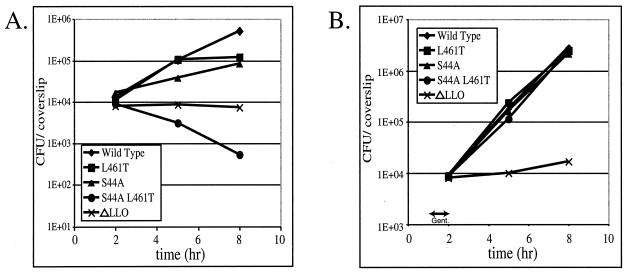
L. monocytogenes was grown intracellularly as described previously (26), with the following modification to the gentamicin treatment. The previously described protocol requires gentamicin to be present from 1 h postinfection until the termination of the experiment, as shown Fig. 1A. However, in the experiment whose results are shown in Fig. 1B, gentamicin was added at 1 h postinfection, and then at 2 h postinfection the gentamicin-containing medium was aspirated, the cells were washed with PBS, and antibiotic-free tissue culture medium was added for the remainder of the experiment.
FIG. 1.
Growth of the cytotoxic mutants in J774 macrophage-like cells. (A) Numbers of CFU in monolayers of J774 cells on 12-mm glass coverslips at different times in the presence of the extracellular antibiotic gentamicin added 1 h postinfection. The data are means derived from three coverslips. (B) Numbers of CFU in monolayers of J774 cells on 12-mm glass coverslips at different times after gentamicin (Gent.) treatment from 1 to 2 h postinfection (indicated by the arrow) followed by three washes in PBS. The data are means derived from three coverslips.
(ii) Flow cytometry.
Flow cytometry was performed by using cultures of bone marrow-derived macrophages from C57BL/6 mice prepared as previously described (32). The assay was performed like a previously described assay (19), with the following modifications. In brief, bone marrow-derived macrophage monolayers were infected with bacteria for 30 min and then washed with PBS and incubated at 37°C until 4 h postinfection. Unlike the previously described assay, this assay was performed in a shorter time, and no gentamicin was added because the most cytotoxic strains were adversely affected by addition of gentamicin. The cells were then removed from the dish, stained with propidium iodide, and analyzed by flow cytometry as described previously.
Mouse infections.
Cerus Pharmaceuticals (Concord, Calif.) determined 50% lethal doses by tail vein injection into C57BL/6 mice as previously described (32). Competitive indices for LLO mutants, marked with erythromycin resistance, compared with wild-type bacteria or single-strain infections were determined essentially as previously described (3), with the following modifications. Bacterial strains intended for injection into the mice were grown in BHI broth until they reached an optical density at 600 nm of 0.5, and then 1-ml samples were frozen at −80°C until they were used. The frozen samples were thawed and used to inoculate 10 ml of BHI broth, and the cultures were grown at 37°C until the optical density was 0.5. Wild-type mice were infected by tail vein injection of 5 × 105 CFU. RB6-8C5 monoclonal antibody-treated mice were infected with 5 × 103 CFU, since a dose of 5 × 105 CFU led to death before 48 h. In the ΔActA competitive index assay 1 × 107 CFU was injected so that sufficient CFU could be retrieved from the organs. The mutant bacteria were differentiated from the wild-type bacteria in the competitive index assay by treating organ lysates with 0.1 μg of erythromycin per ml for 30 min to induce the resistance gene and then plating the samples on Luria-Bertani agar plates and BHI agar plates containing 1 μg of erythromycin per ml to establish a ratio of sensitive (wild-type) bacteria to resistant (mutant) bacteria at each time. Mice that were treated with RB6-8C5 received 100 μg of the monoclonal antibody via the tail vein 6 h before bacterial infection. Gentamicin-treated mice received 1 mg of Garamycin (gentamicin sulfate; Schering Corporation, Kenilworth, N.J.) in PBS subcutaneously 6 h prior to organ harvest. Twelve hours after injection we found that the concentration of gentamicin was 5.6 μg/ml in the pooled serum of three mice (determined by Debra Randall, Stanford University Hospital Clinical Labs, Palo Alto, Calif.), which is sufficient to inhibit bacterial growth.
Bacterial growth in serum.
Mouse blood was removed by cardiac puncture from mice anesthetized with isofluorane (Abbott Laboratories, North Chicago, Ill.) and then allowed to clot overnight at 4°C. Each clot was removed, and the samples were centrifuged to separate the serum from any remaining solids. Then 1 × 103 bacteria were added to each sample of 50% serum in PBS, and time points were determined by plating dilutions on Luria-Bertani agar plates. Incubating the serum at 65°C for 30 min produced heat-deactivated serum.
RESULTS
Construction and characterization of cytotoxic strains in cell culture.
Four chromosomal alleles of the gene encoding LLO were used in this study (Table 1). Wild-type LLO has an acidic activity optimum and mediates escape from a vacuole with little observed cytotoxicity to the host during subsequent intracellular growth (18, 19). The previously characterized LLO L461T protein is active at neutral pH and exhibits some cytotoxicity resulting from activity at the neutral pH of the host cytosol (19). LLO S44A has an acidic activity optimum similar to that of wild-type LLO, but it increases cytotoxicity like the previously described LLO S44A S48A T51A protein that results in an increase in LLO found in the host cytosol (13). A double mutant, LLO S44A L461T, encoded by an allele having both of the point mutations, exhibits properties of each independent single mutant.
Each of the mutant strains displayed a growth defect in J774 macrophage-like cells over an 8-h period (Fig. 1A). This growth defect was not caused by an inability to escape from the phagosome (Table 2) and was eliminated by removal of the extracellular antibiotic gentamicin (Fig. 1B). Sensitivity to gentamicin was also observed when the strains were used to form plaques in cell culture monolayers (Table 2). As seen previously (19), the bacteria with the LLO L461T allele could form plaques whose size was equivalent to that of the plaques of wild-type bacteria after 3 days of growth at a low gentamicin concentration, but the plaque size decreased as the gentamicin concentration increased. Bacteria with the LLO S44A allele could form plaques whose diameter was 14% of the diameter of the plaques of wild-type bacteria, but they did so only at the lowest concentration of gentamicin, while the bacteria producing LLO S44A L461T were unable to form plaques at all.
TABLE 2.
Virulence and phagosomal escape efficiency of L. monocytogenes strains
| Strain | 50% Lethal dosea | Phagosomal escape (%)b | Plaque size (%)c |
|---|---|---|---|
| Wild type | 5 × 104 | 51 ± 15 | 100 |
| ΔLLOd | >1 × 109 | 0 | NDe |
| LLO L461T | 7.5 × 106 | 43 ± 9 | 100 ± 2f |
| LLO S44A | 7.5 × 107 | 63 ± 9 | 14 ± 5 |
| LLO S44A L461T | >1 × 108 | 58 ± 8 | ND |
The 50% lethal dose is the quantity of bacteria injected into the tail vein that leads to the death of 50% of C57BL/6 mice.
The percentage of phagosomal escape (mean ± standard deviation) is the percentage of actin-coated bacteria in all of the total bacteria at 90 min postinfection. A minumm of 200 bacterium-associated macrophages were counted.
The plaque size, expressed as a percentage of the wild-type plaque size, in L2 monolayers after 3 days of of bacterial growth with 5 μg of gentamicin per ml. The values are means ± standard deviations.
Strain DP-L2161 (ΔLLO) has been described previously by Jones and Portnoy (25).
ND, plaques are not measurable.
The plaque size of the LLO L461T strain is sensitive to the gentamicin concentration, as described by Glomski et al. (19).
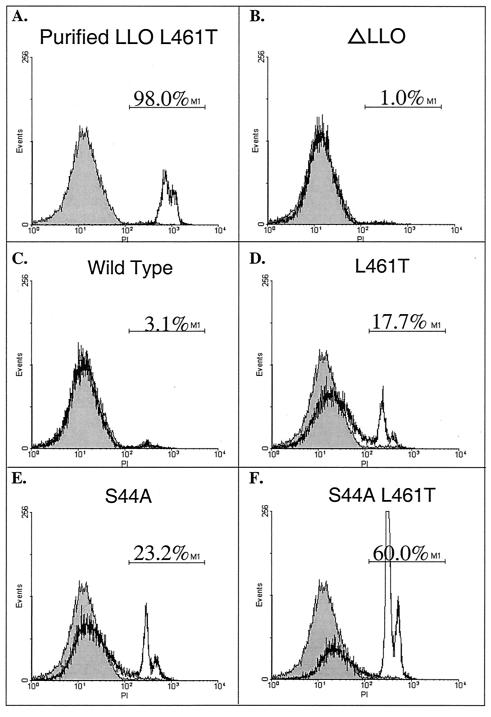
The gentamicin sensitivity of the mutant L. monocytogenes strains suggested that these strains damaged the host cell membrane and allowed gentamicin to enter and inhibit the growth of the intracellular bacteria. Thus, plasma membrane damage was assessed by infecting bone marrow-derived macrophages and monitoring host DNA staining with the membrane-impermeant dye propidium iodide (Fig. 2). Using flow cytometry to quantify staining, we found that 3.1% of the macrophages were permeabilized by wild-type bacteria, while 17.7, 23.2, and 60.0% of the macrophages were permeabilized in 4 h by bacteria secreting LLO L461T, LLO S44A, and LLO S44A L461T, respectively. We concluded from these observations that the L. monocytogenes strains carrying the LLO alleles described here represent a range of bacterial cytotoxicity. Starting with the least cytotoxic to the most cytotoxic, the strains can be placed in the following order: wild-type strain 10403S, LLO L461T strain, LLO S44A strain, and LLO S44A L461T strain.
FIG. 2.
LLO mutants permeabilize the plasma membrane. C57BL/6 bone marrow-derived macrophages were infected for 4 h without gentamicin and then stained with the membrane-impermeant dye propidium iodide (PI), which increases fluorescence when it passes through the membrane and interacts with host DNA. A total of 2.5 × 104 cells were examined by flow cytometry; the results for half of the cells are shown. The gray histogram represents uninfected cells. The fluorescence range for cells considered permeabilized, as indicated by the marker M1, was defined by adding 106 hemolytic units of purified LLO L461T to the macrophages, as shown in Fig. 1A. The infecting strain and the percentage of cells falling within the marker M1 range are indicated.
Greater cytotoxicity correlates with reduced virulence.
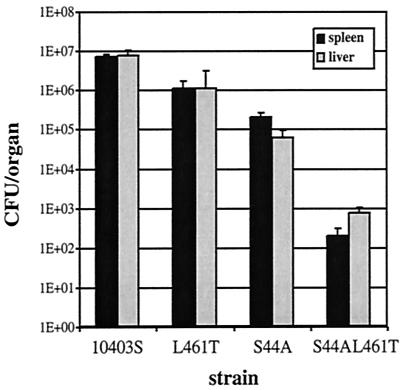
The more cytotoxic strains had higher 50% lethal doses in the mouse model of listeriosis (Table 2). Since monitoring host animal survival does not necessarily reveal the ability of bacteria to multiply inside a mouse, mice were infected for 24 h with each strain, and the numbers of CFU in both the liver and spleen were determined. We found that as the cytotoxicity of the strain increased, the numbers of bacteria recovered from both the spleen and the liver decreased (Fig. 3). We concluded that the more cytotoxic the strain of L. monocytogenes, the less virulent the strain is in the mouse model of listeriosis.
FIG. 3.
The greater the cytotoxicity, the less the cytotoxic bacteria grow in mice. A total of 1 × 105 CFU of each strain were injected into the tail veins of C57BL/6 mice. After 24 h the liver and spleen of each mouse were removed, homogenized, and plated to determine the number of CFU in each organ. The error bars indicate standard deviations for five mice.
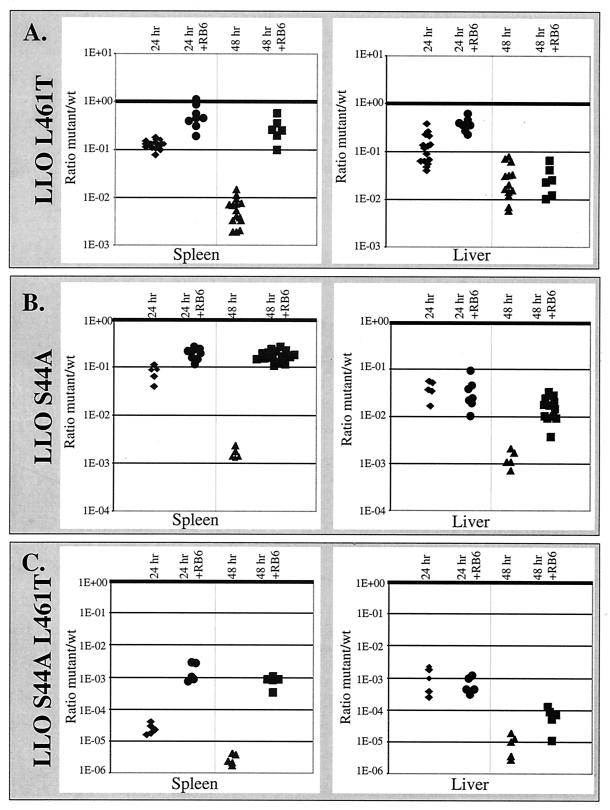
A competitive index assay was performed with each mutant strain to more accurately measure the mutants' virulence defects relative to the characteristics of the wild-type bacteria and to ensure that the virulence of a mutant was not affected by the presence of wild-type bacteria (3). In this assay, wild-type bacteria and erythromycin-resistant mutants at a ratio of 1:1 were coinjected into mice, and the ratios of wild-type bacteria to erythromycin-resistant (mutant) bacteria in the spleen and liver were established. We found that the trend for the defect in virulence was similar to the trend for the 50% lethal dose (Fig. 4); thus, the greater the cytotoxicity of the strain, the fewer bacteria were recovered compared to the number of wild-type bacteria.
FIG. 4.
The greater the cytotoxicity, the greater the virulence defect. A competitive index was established by injecting both wild-type bacteria (wt) and erythromycin resistance-marked mutants into C57BL/6 mouse tail veins. Competitive indices were determined for the spleen and liver by performing competition assays with wild-type and LLO L461T Erm strains (A), wild-type and LLO S44A Erm strains (B), and wild-type and LLO S44A L461T Erm strains (C). The y axis indicates the ratio of the number of mutant CFU to the number of CFU of wild-type bacteria isolated from the spleen or liver of mice at different times on a log scale. Therefore, the nearer the bottom of the graph, the fewer mutant bacteria were retrieved from a mouse compared to the number of wild-type bacteria. The ratio for each mouse is represented by a single marker for the spleen and a single marker for the liver. The +RB6 mice received an injection of the neutrophil-depleting monoclonal antibody RB6-8C5 6 h before infection with L. monocytogenes (9). The thick horizontal lines indicate a competitive index of 1.
Granulocytes are a major contributor to the cytotoxic mutants' growth defects in mice.
A number of previous studies have shown that neutrophils contribute to early resistance to L. monocytogenes infection (9, 10, 21). Indeed, neutrophils readily phagocytose and kill extracellular L. monocytogenes in vitro (12, 34). Therefore, since the cytotoxic strains were rapidly outcompeted by wild-type bacteria, we hypothesized that the reduced virulence observed for the cytotoxic mutants reflected sensitivity to neutrophils. To address this hypothesis, we eliminated neutrophil infiltration by introducing the anti-GR1 monoclonal antibody RB6-8C5 into mice 6 h before infection. RB6-8C5 has been shown to eliminate neutrophils from the circulation and prevent their infiltration into foci of L. monocytogenes infection (9). In neutropenic mice the relative virulence defects of the cytotoxic mutants were reduced by 99% in the spleen, which allowed the cytotoxic mutants to grow much more like the coinjected wild-type bacteria in the competitive index assay (Fig. 4). Less of an effect was observed in the liver, yet by 48 h the relative virulence of the more cytotoxic mutants (LLO S44A and LLO S44A L461T) increased 10-fold after elimination of the neutrophils. These data suggest that the cytotoxic mutants are more susceptible to neutrophil killing in immunocompetent mice.
A larger percentage of cytotoxic bacteria are extracellular.
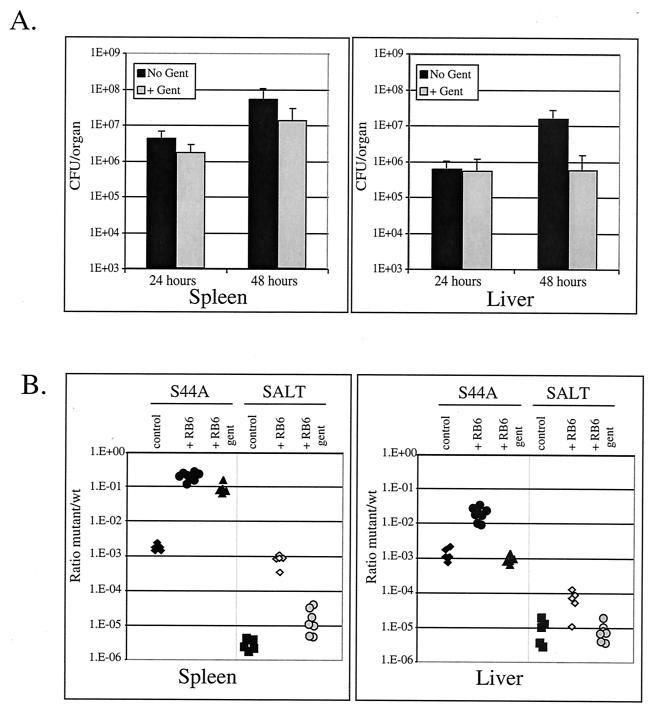
The data described above suggested that cytotoxic strains were exposed to the extracellular environment, where neutrophils could readily phagocytose and destroy the bacteria. To further explore this possibility, we injected the antibiotic gentamicin into infected mice. Gentamicin kills extracellular bacteria without affecting intracellular bacteria in cell cultures (15), has no significant effect on wild-type bacteria at 24 h postinfection in the mouse, and decreases the number of wild-type bacteria in the liver 10-fold at 48 h (Fig. 5A). The sensitivity of the cytotoxic mutants to gentamicin was examined in neutropenic mice because neutrophils would likely phagocytose and destroy many extracellular bacteria, thereby obscuring our ability to detect the effects of gentamicin on extracellular bacteria. Treatment of mice with gentamicin decreased the ratios of the LLO S44A and LLO S44A L461T mutants to the wild-type bacteria in the competitive index assay (Fig. 5B). By 48 h about 99% of the LLO S44A L461T bacteria in the spleen and liver were sensitive to gentamicin, whereas the LLO S44A mutant in the spleen and the LLO L461T mutant in both organs were less affected. However, addition of gentamicin did not completely reconstitute the resistance of the mice to the level seen in mice containing active neutrophils.
FIG. 5.
Cytotoxic mutants are more sensitive to gentamicin. (A) A total of 1 × 105 CFU wild-type bacteria were injected into the tail veins of C57BL/6 mice. Then 1 mg of gentamicin was injected subcutaneously, and at different times the livers and spleens were removed, homogenized, and plated to determine the number of CFU in each organ. The error bars indicate standard deviations for a minimum of seven mice. (B) Competitive indices were established at 48 h as described in the legend to Fig. 4. The +RB6 gent mice received an injection of the RB6-8C5 monoclonal antibody 6 h before infection, as well as a 1-mg gentamicin sulfate injection subcutaneously 6 h before organs were harvested. SALT, LLO S44A L461T strain; Gent, gentamicin; wt, wild type.
Virulence defects of cytotoxic mutants are not caused by defects in cell-to-cell spread.
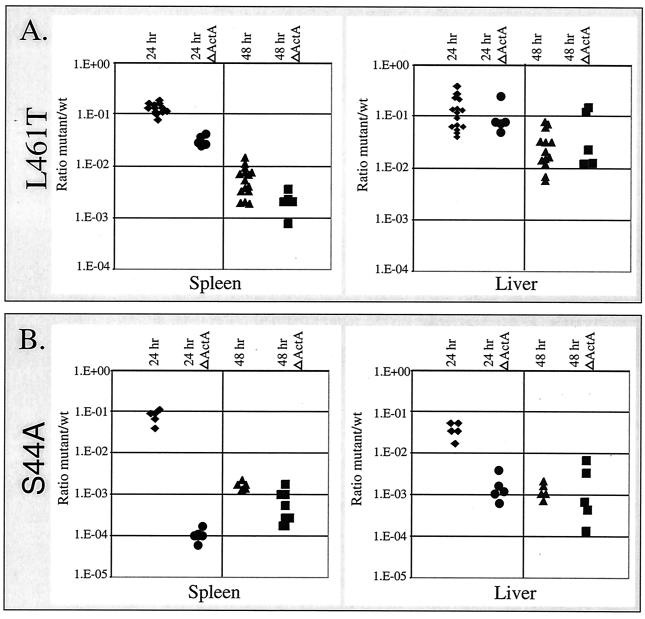
The defects observed in bacterial plaquing in cell culture monolayers raised the concern that the cytotoxic bacteria may have damaged their host cells to such a degree that the cytoskeletal dynamics were disrupted. The ability of bacteria to manipulate the host cytoskeleton is vital to virulence, since bacteria that are unable to form actin tails are 1,000-fold less virulent (6). Therefore, it was conceivable that the cytotoxic mutants were less virulent because they spread less efficiently from cell to cell. To address this question, we constructed an in-frame deletion in the actA gene (ΔActA) of each of the cytotoxic mutants to eliminate the influence of cell-to-cell spread and then repeated the competitive index assay by comparing the ΔActA LLO mutants with ΔActA wild-type LLO bacteria. ActA is the sole bacterial protein necessary for actin nucleation and actin-based motility in host cells (40). If cell-to-cell spread was the major factor contributing to the virulence defect of the cytotoxic mutants, then we predicted that elimination of ActA would result in mutants whose virulence was similar to that of the ΔActA bacteria secreting wild-type LLO. As such, the ratio of ΔActA bacteria to ΔActA bacteria with mutant LLO would approach 1:1 in the competitive index assay. We did not find this to be the case. In contrast, eliminating the ActA function had either little effect or increased the defect observed for the cytotoxic mutants (Fig. 6). Both ΔActA cytotoxic strains, the strain secreting LLO L461T and the strain secreting LLO S44A, competed less well against the ΔActA strain with wild-type LLO. The ΔActA LLO S44A L461T strain became so attenuated that there were insufficient numbers of bacteria in the liver or spleen to obtain reliable counts of CFU.
FIG. 6.
Virulence defects are not due to defects in cell-to-cell spread. Competitive indices were determined as described in the legend to Fig. 4. For the ΔActA data sets, all strains, including the reference strain secreting wild-type LLO, had an in-frame deletion in actA that eliminated actin-based motility. (A) 24 hr and 48 hr indicate the times of organ harvest. The data points are the ratios of the number of CFU of erythromycin-resistant LLO L461T bacteria to the number of CFU of wild-type bacteria in the spleen and liver. The 24 hr ΔActA and 48 hr ΔActA data points are the ratios of the number of erythromycin-resistant ΔActA LLO L461T bacteria to the number of wild-type LLO-secreting bacteria without the ActA gene recovered from the spleen and liver at 24 and 48 h, respectively. (B) Competitive indices established like those described for panel A, except that LLO S44A erythromycin-resistant mutants were used. wt, wild type.
Growth of L. monocytogenes in mouse serum.
Despite the fact that phagocytes were responsible for much of the growth defect of the cytotoxic bacteria in the mice, removal of these cells did not make the mutants grow as well as the wild-type bacteria under any of our experimental conditions. We therefore reasoned that cytotoxic bacteria might not divide extracellularly at the rate observed in the intracellular environment. Thus, we determined the doubling times of bacteria growing in mouse serum. We found that L. monocytogenes grew in mouse serum (native, heat deactivated, or derived from infected mice), but the maximum doubling time was 58 min. Thus, the growth rate was significantly lower than the previously described maximal intracellular growth rate (doubling time, 42 min) (19). Considering that bacterial growth is exponential, a 16-min difference in doubling time between the two environments could quickly lead to great differences in bacterial numbers and could thus account for some of the growth defects which we observed for the cytotoxic mutants. Interestingly, bacteria grew well in fetal bovine serum and mouse serum supplemented with BHI broth, with doubling times of 34 and 31 min, respectively, suggesting that mouse serum did not have an inhibitory effect but was more likely to be nutrient limiting.
DISCUSSION
It is commonly accepted that by adopting an intracellular lifestyle, pathogens gain a number of advantages, including protection from the immune system and a good source of nutrients (29). Obligate intracellular pathogens clearly need intact host cells for survival, and some viruses actively prevent death of their host cells (4). The case is not as clear for facultative intracellular pathogens like L. monocytogenes that can also grow extracellularly. However, L. monocytogenes has evolved mechanisms to grow and spread in host cells while minimizing cytotoxicity. For example, there are at least two different mechanisms that restrict the activity of the pore-forming protein, LLO, to an acidic vacuole (13, 19). In this study, we showed that there is a direct correlation between the degree of LLO cytotoxicity in vitro and the loss of virulence in the murine model of listeriosis. Indeed, the most cytotoxic mutants were almost as attenuated as mutants lacking LLO.
A number of reports have shown that neutrophils contribute to host resistance to L. monocytogenes and other facultative intracellular pathogens (2, 8, 9, 11). Indeed, mice rendered neutropenic with RB6-8C5 monoclonal antibody are 105-fold more sensitive to wild-type L. monocytogenes in the liver and 103-fold more sensitive in the spleen by 48 h postinfection (unpublished data). The results of a competitive index assay clearly showed that the virulence of the cytotoxic mutants was enhanced approximately 100- and 10-fold relative to the virulence of the wild type in the spleen and liver, respectively. Therefore, the primary reason that cytotoxic mutants are attenuated appears to be enhanced susceptibility to neutrophils, especially in the spleen. However, it should be noted that although for the most cytotoxic of the mutants the relative virulence increased 200-fold in the spleen of neutropenic mice, this strain was still 1,000-fold less virulent than the wild type. In this case, we believe that the bacteria were mostly extracellular because they were susceptible to gentamicin. Thus, the difference could be largely accounted for by our observation that L. monocytogenes grows approximately 30% faster intracellularly than in mouse serum. L. monocytogenes is naturally auxotrophic for several amino acids and vitamins (33). Therefore, it is not surprising that this bacterium does not replicate as well in mouse serum as in mammalian cytoplasm (27). Goetz et al. described the importance of a hexose phosphate transporter for intracytoplasmic growth of L. monocytogenes (20). This hexose phosphate transporter allows the bacteria to utilize glucose 1-phosphate, which is a breakdown product of glycogen in the liver. Thus, there is evidence that pathogens not only have evolved virulence factors to customize their pathogenic niches but also have tuned their metabolism to each of their niches.
We found that the cytotoxic mutants were less sensitive to the function of neutrophils in the liver than in the spleen. Based on the hypothesis that cytotoxic mutants are exposed to extracellular defenses, there are a number of explanations that may account for the differences observed in different tissues. One possibility is that hepatocytes are capable of coping with cytotoxic bacteria better than splenic cells. If hepatocytes repair damaged membranes or resist lysis, the cytotoxic mutants would persist in a protected environment longer than in cells that were more sensitive to the lytic activity of LLO. Indeed, the ability of the liver to rapidly recover from toxic insults and tissue damage is well documented (1). A second possibility is that after bacteria lyse the initial host cell, they can be internalized by neighboring cells more readily in the liver, thus reducing their extracellular residence time. Rapid phagocytosis in the liver may be aided by the function of the bacterial internalin proteins, which mediate the uptake of L. monocytogenes into hepatocytes and contribute to L. monocytogenes growth in the liver (39).
We did not directly examine the mechanism by which neutrophils preferentially eliminate cytotoxic mutants, but there are a number of defense strategies that could be functioning. It has been shown in previous studies that neutrophils are capable of killing L. monocytogenes in vitro (12) yet are incapable of killing intracellular bacteria within hepatocytes in tissue culture (34). Therefore, it is likely that neutrophils simply have greater access to the cytotoxic mutants because the bacteria are extracellular. However, the importance of neutrophils in controlling wild-type infections implies that wild-type bacteria have some degree of extracellular exposure as well. It is also possible that neutrophils selectively lyse L. monocytogenes-infected hepatocytes (9), although the mechanism, whether direct or indirect, has not been established.
The antibiotic gentamicin has been used in both tissue culture and in vivo as a means to eliminate extracellular bacteria (15, 19, 21). In this study we found that the two most cytotoxic mutants examined, one which secreted LLO S44A and one which secreted LLO S44A L461T, were particularly sensitive to gentamicin injected into infected mice. However, the gentamicin sensitivity was most pronounced when neutrophils were first eliminated. This suggests that the same population of bacteria that are sensitive to gentamicin is also sensitive to the activity of neutrophils. Interestingly, gentamicin treatment did not entirely restore the level of resistance in neutropenic mice to the level of resistance observed in immunocompetent mice. In agreement with the rest of our data, this indicates that the virulence defects observed for the cytotoxic mutants are multifactoral. The fact that gentamicin cannot completely replace the activity of neutrophils may indicate that neutrophils play a broader role in bacterial clearance than simply phagocytosing and destroying extracellular bacteria or that some extracellular bacteria are not accessible to gentamicin.
An alternate explanation for the virulence defects of the cytotoxic mutants is that they are a more obvious threat than the wild-type bacteria. By damaging their host cells prematurely, these bacteria may cause the liberation of inflammatory cytokines and thereby recruit more inflammatory cells to foci of infection, as well as activate the function of the infiltrates (37). Accordingly, a number of inflammatory cytokines have been proven to be vital for resistance to L. monocytogenes (28). Thus, the presence of more inflammatory cells, such as neutrophils, that are highly activated should then foster greater clearance of the bacteria from the foci of infection. Permeabilization of the host cell's plasma membrane may also allow the efflux of activated complement or bacterial components, such as formylated peptides, that are chemoattractants of neutrophils and have been shown to be important in murine resistance to L. monocytogenes (17, 36). In this scenario, the cytotoxic bacteria would be targeted for phagocytosis and destruction earlier than the wild-type bacteria are targeted, since they would emit chemotactic signals from within damaged cells. Exposure to the lytic functions of complement is unlikely to directly affect L. monocytogenes given our finding that the bacteria grow at similar rates in normal and heat-deactivated mouse serum. However, the opsonizing properties of complement may target the cytotoxic bacteria for more efficient phagocytosis (16).
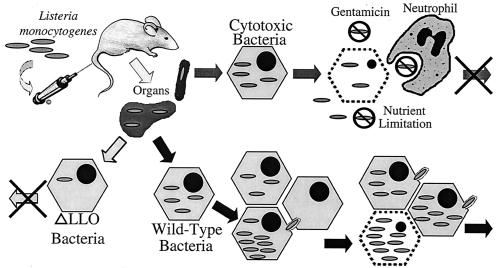
We propose a model to explain why L. monocytogenes needs to balance the activity of its cytolysin, LLO, between functionality and cytotoxicity (Fig. 7). When the cytolysin is inactive or absent, the bacteria are trapped and killed in the phagosome and thus cannot multiply (25). At the other extreme, bacteria that produce an overly active LLO (due to greater biochemical activity and/or greater cytosolic quantity) escape from the phagosome and begin to grow in the cytosol; however, these cytotoxic bacteria quickly damage the host cell and consequently expose themselves to the influence of extracellular defenses and nutrient limitation that contains or terminates the infection. The wild-type bacteria fall between these two extremes, striking a balance, controlling LLO activity to mediate the efficient lysis of the phagosome, and limiting the function of LLO to avoid damage to the host cell. This balance allows the wild-type bacteria to escape to the cytosol, multiply, and spread from cell to cell. The wild-type bacteria eventually cause enough damage to the host cell to expose them to the same environment that adversely affects the cytotoxic mutants (19), but this occurs at a time which is late enough so that a larger number of bacteria have grown and spread into new host cells. Thus, the wild-type bacteria can continue to spread the infection through host tissues and continue to multiply.
FIG. 7.
Model for the virulence defect associated with cytotoxic L. monocytogenes. When L. monocytogenes is injected into a mouse through the tail vein, the majority of the bacteria are found in the spleen and liver. In these organs the bacteria invade cells. Bacteria lacking LLO activity (ΔLLO) are unable to escape from the phagosome and thus are unable to multiply, which terminates the infection. Both wild-type and cytotoxic bacteria are able to escape from the phagosome and initiate growth in the cytosol. The wild-type bacteria are able to efficiently grow and spread to neighboring cells before the initial cell's integrity is compromised. This allows the wild-type bacteria to spread through the tissues and continue the infection. In contrast, the cytotoxic mutant damages the host cell soon after it enters the cytosol and exposes the bacteria to the extracellular milieu, which results in a reduction in the net bacterial growth and therefore a reduction in virulence.
Implicit in our proposed model and previously described models of listeriosis (35) is the importance of bacterial cell-to-cell spread in avoiding host defenses. For this reason we explored the possibility that the cytotoxic mutants' virulence defects were caused by disruption of the actin-based cell-to-cell spread process by elimination of cell-to-cell spread by deletion of ActA. If spreading from cell to cell were the primary mechanism by which the cytotoxic mutants caused growth defects, one would predict that when actin-based cell-to-cell spread was eliminated, the cytotoxic bacteria would grow more like the ΔActA bacteria secreting wild-type LLO. This was not the case. Growth of the bacteria was not affected or further decreased when ActA was deleted. Indeed, we observed a striking 3-log decrease in growth of the ΔActA LLO S44A mutant at 24 h compared to the growth of a ΔActA wild-type LLO strain. As determined in previous tissue culture assays (8, 22), bacteria that cannot nucleate actin are more cytotoxic, and thus the cytotoxicity of the mutants may be exacerbated by elimination of cell-to-cell spread. The simplest explanation for this observation is that preventing the migration of a portion of the bacteria from the initially infected cell into a new cell effectively increases the number of bacteria within the initial cell. Alternatively, the increased cytotoxicity of ActA-null bacteria may be more complicated than this simple hypothesis and may instead suggest that there is a link between ActA and LLO function.
In this paper we present data suggesting that maintenance of the cytoplasmic niche is vital to L. monocytogenes pathogenesis. In particular, we show that if this bacterium does not properly manage the lytic effects of the pore-forming cytolysin LLO, it compromises its ability to grow in the host due to pressures from host extracellular defenses. Similarly, if the host acts cytolytically on infected cells, bacterial clearance is also achieved (2). Indeed, the primary effector cells in adaptive immunity to L. monocytogenes are cytotoxic CD8+ T cells Thus, whether cytotoxicity is caused by the bacteria or by the host, the movement of L. monocytogenes from an intracellular compartment to an extracellular compartment reduces the ability of the bacteria to grow. To reduce its extracellular residence time, L. monocytogenes has developed a number of virulence factors to ensure that it becomes and remains primarily intracellular and that it thrives in this environment. These factors ensure uptake into cells (the internalins), escape from the primary and spreading vacuoles (LLO and phospholipases C), and spread from cell to cell in search of fresh host cells and to avoid cytotoxic lymphocytes (ActA) (2, 31). Without these functions the virulence of L. monocytogenes is reduced because, according to our model, there is a reduction in its intracellular residence time.
Acknowledgments
We thank Martin Giedlin and Cerus Pharmaceuticals (Concord, Calif.) for establishing the 50% lethal doses and Ronald LaCourse and Robert North (Trudeau Institute, Saranac Lake, N.Y.) for supplying the hybridoma and purification protocols for monoclonal antibody RB6-8C5. We also thank Richard Calendar for helping us develop the protocol used for phage transduction of the erythromycin gene in L. monocytogenes. Many thanks are extended to Victoria Auerbuch, Laurel Lenz, Kyung-Dall Lee, Luisa Cheng, Julie Viala, and Pamela Schnupf for their scientific input and reviews of the manuscript. We additionally thank Zuzana Ponca for her artistic design of Fig. 7.
This research was supported by National Institutes of Health grants AI27655 and AI29619 (to D. A. Portnoy) and by National Research Service Award postdoctoral fellowship AI10283 (to A. L. Decatur).
Editor: V. J. DiRita
REFERENCES
- 1.Alison, M., M. Golding, N. Lalani el, and C. Sarraf. 1998. Wound healing in the liver with particular reference to stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353:877-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelberg, R., A. G. Castro, and M. T. Silva. 1994. Neutrophils as effector cells of T-cell-mediated, acquired immunity in murine listeriosis. Immunology 83:302-307. [PMC free article] [PubMed] [Google Scholar]
- 3.Auerbuch, V., L. Lenz, and D. Portnoy. 2001. Developmment of a competitive index assay to evaluate the virulence of Listeria monocytogenes actA mutants during primary and secondary infection of mice. Infect. Immun. 69:5953-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedict, C. A., P. S. Norris, and C. F. Ware. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3:1013-1018. [DOI] [PubMed] [Google Scholar]
- 5.Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139:2005-2009. [PubMed] [Google Scholar]
- 6.Brundage, R. A., G. A. Smith, A. Camilli, J. A. Theriot, and D. A. Portnoy. 1993. Expression and phosphorylation of the Listeria monocytogenes ActA protein in mammalian cells. Proc. Natl. Acad. Sci. USA 90:11890-11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conlan, J. W. 1997. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect. Immun. 65:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conlan, J. W., and R. J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czuprynski, C. J., J. F. Brown, N. Maroushek, R. D. Wagner, and H. Steinberg. 1994. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J. Immunol. 152:1836-1846. [PubMed] [Google Scholar]
- 11.Czuprynski, C. J., J. F. Brown, R. D. Wagner, and H. Steinberg. 1994. Administration of antigranulocyte monoclonal antibody RB6-8C5 prevents expression of acquired resistance to Listeria monocytogenes infection in previously immunized mice. Infect. Immun. 62:5161-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czuprynski, C. J., P. M. Henson, and P. A. Campbell. 1984. Killing of Listeria monocytogenes by inflammatory neutrophils and mononuclear phagocytes from immune and nonimmune mice. J. Leukoc. Biol. 35:193-208. [DOI] [PubMed] [Google Scholar]
- 13.Decatur, A. L., and D. A. Portnoy. 2000. A PEST-like sequence in listeriolysin O essential for Listeria monocytogenes pathogenicity. Science 290:992-995. [DOI] [PubMed] [Google Scholar]
- 14.DiTirro, J., E. R. Rhoades, A. D. Roberts, J. M. Burke, A. Mukasa, A. M. Cooper, A. A. Frank, W. K. Born, and I. M. Orme. 1998. Disruption of the cellular inflammatory response to Listeria monocytogenes infection in mice with disruptions in targeted genes. Infect. Immun. 66:2284-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drevets, D. A., T. A. Jelinek, and N. E. Freitag. 2001. Listeria monocytogenes-infected phagocytes can initiate central nervous system infection in mice. Infect. Immun. 69:1344-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drevets, D. A., P. J. Leenen, and P. A. Campbell. 1996. Complement receptor type 3 mediates phagocytosis and killing of Listeria monocytogenes by a TNF-alpha- and IFN-gamma-stimulated macrophage precursor hybrid. Cell. Immunol. 169:1-6. [DOI] [PubMed] [Google Scholar]
- 17.Gao, J. L., E. J. Lee, and P. M. Murphy. 1999. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J. Exp. Med. 189:657-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geoffroy, C., J. L. Gaillard, J. E. Alouf, and P. Berche. 1987. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect. Immun. 55:1641-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glomski, I. J., M. M. Gedde, A. W. Tsang, J. A. Swanson, and D. A. Portnoy. 2002. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 156:1029-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetz, M., A. Bubert, G. Wang, I. Chico-Calero, J. A. Vazquez-Boland, M. Beck, J. Slaghuis, A. A. Szalay, and W. Goebel. 2001. Microinjection and growth of bacteria in the cytosol of mammalian host cells. Proc. Natl. Acad. Sci. USA 98:12221-12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregory, S. H., A. J. Sagnimeni, and E. J. Wing. 1996. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J. Immunol. 157:2514-2520. [PubMed] [Google Scholar]
- 22.Harty, J. T., A. R. Tvinnereim, and D. W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275-308. [DOI] [PubMed] [Google Scholar]
- 23.Hodgson, D. A. 2000. Generalized transduction of serotype 1/2 and serotype 4b strains of Listeria monocytogenes. Mol. Microbiol. 35:312-323. [DOI] [PubMed] [Google Scholar]
- 24.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 25.Jones, S., and D. A. Portnoy. 1994. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect. Immun. 62:5608-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, S., and D. A. Portnoy. 1994. Intracellular growth of bacteria. Methods Enzymol. 236:463-467. [DOI] [PubMed] [Google Scholar]
- 27.Marquis, H., H. G. Bouwer, D. J. Hinrichs, and D. A. Portnoy. 1993. Intracytoplasmic growth and virulence of Listeria monocytogenes auxotrophic mutants. Infect. Immun. 61:3756-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mocci, S., S. A. Dalrymple, R. Nishinakamura, and R. Murray. 1997. The cytokine stew and innate resistance to L. monocytogenes. Immunol. Rev. 158:107-114. [DOI] [PubMed] [Google Scholar]
- 29.Moulder, J. W. 1985. Comparative biology of intracellular parasitism. Microbiol. Rev. 49:298-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.North, R. J. 1970. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J. Exp. Med. 132:521-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Portnoy, D. A., T. Chakraborty, W. Goebel, and P. Cossart. 1992. Molecular determinants of Listeria monocytogenes pathogenesis. Infect. Immun. 60:1263-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Portnoy, D. A., P. S. Jacks, and D. J. Hinrichs. 1988. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J. Exp. Med. 167:1459-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Premaratne, R. J., W. J. Lin, and E. A. Johnson. 1991. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl. Environ. Microbiol. 57:3046-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers, H. W., M. P. Callery, B. Deck, and E. R. Unanue. 1996. Listeria monocytogenes induces apoptosis of infected hepatocytes. J. Immunol. 156:679-684. [PubMed] [Google Scholar]
- 35.San Mateo, L. R., M. M. Chua, S. R. Weiss, and H. Shen. 2002. Perforin-mediated CTL cytolysis counteracts direct cell-cell spread of Listeria monocytogenes. J. Immunol. 169:5202-5208. [DOI] [PubMed] [Google Scholar]
- 36.Schroder, J. M. 2000. Chemoattractants as mediators of neutrophilic tissue recruitment. Clin. Dermatol. 18:245-263. [DOI] [PubMed] [Google Scholar]
- 37.Shalaby, M. R., B. B. Aggarwal, E. Rinderknecht, L. P. Svedersky, B. S. Finkle, and M. A. Palladino, Jr. 1985. Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J. Immunol. 135:2069-2073. [PubMed] [Google Scholar]
- 38.Skoble, J., D. A. Portnoy, and M. D. Welch. 2000. Three regions within ActA promote Arp2/3 complex-mediated actin nucleation and Listeria monocytogenes motility. J. Cell Biol. 150:527-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vazquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Dominguez-Bernal, W. Goebel, B. Gonzalez-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welch, M. D., J. Rosenblatt, J. Skoble, D. A. Portnoy, and T. J. Mitchison. 1998. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science 281:105-108. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. 2002. The world health report 2002. World Health Organization, Geneva, Switzerland.