Abstract
The ternary complex comprising MutS, MutL, and DNA is a key intermediate in DNA mismatch repair. We used chemical cross-linking and fluorescence resonance energy transfer (FRET) to study the interaction between MutS and MutL and to shed light onto the structure of this complex. Via chemical cross-linking, we could stabilize this dynamic complex and identify the structural features of key events in DNA mismatch repair. We could show that in the complex between MutS and MutL the mismatch-binding and connector domains of MutS are in proximity to the N-terminal ATPase domain of MutL. The DNA- and nucleotide-dependent complex formation could be monitored by FRET using single cysteine variants labeled in the connector domain of MutS and the transducer domain of MutL, respectively. In addition, we could trap MutS after an ATP-induced conformational change by an intramolecular cross-link between Cys-93 of the mismatch-binding domain and Cys-239 of the connector domain.
Keywords: ATPases, Cysteine-mediated Cross-linking, DNA Repair, Fluorescence Resonance Energy Transfer (FRET), Ultracentrifugation
Introduction
The DNA mismatch repair system (MMR)2 plays an important role in maintaining genomic stability and avoidance of mutations in the DNA sequence (1). Major landmark studies in MMR have been the reconstitution of the system from purified proteins both for Escherichia coli and humans (2–4). The key MMR proteins MutS and MutL are present in bacteria and eukaryotes, and the initial steps of MMR are conserved (5). Whereas bacterial MutS and MutL proteins are homodimeric proteins, the eukaryotic homologues are heterodimeric proteins; e.g. MutSα is composed of MutS homologues 2 and 6 (MSH2/MSH6), and MutLα consists of MLH1 and PMS2 (6, 7). Bacterial MutS exists in a dimer-tetramer equilibrium, but the dimeric form of the protein is sufficient for DNA mismatch repair (8, 9). MutS is composed of seven domains (mismatch-binding, connector, core, lever, clamp, ATPase, and dimerization/tetramerization domains) (see Fig. 1) (9–12). MutL has been dissected into a 40-kDa N-terminal half (LN40) and a 20-kDa C-terminal dimerization domain (LC20) (13–16). LN40 comprises the ATPase domain and transducer domain and is connected by a non-conserved linker to the LC20 domain (13, 14).
FIGURE 1.
Cys-93 in mismatch-binding domain of MutS cross-links to Cys-131 in ATPase domain of MutL. A, structure and range of the thiol-specific chemical cross-linker BM[PEO]4 (50). B, crystal structure of MutS (residues 1–800) bound to GT DNA (Protein Data Bank code 1e3m (10)). The C-terminal dimerization/tetramerization domain (residues 801–853) is missing. Subunit A is denoted in gray. Domains of subunit B are colored as follows: mismatch-binding domain (residues 1–115), blue; connector domain (residues 116–266), cyan; core domain and levers (residues 267–443 and 504–567), red; clamp domain (residues 444–503), orange; ATPase and helix-turn-helix domain (residues 568–800), green. Cysteine residues are shown as yellow spheres. Residues that have been shown to display reduced solvent accessibility upon MutL binding are indicated as cyan (residues 205–213) and green (residues 678–688) spheres (32). C, nucleotide and DNA dependence of the cross-linking reaction between MutS and MutL. MutS (wild type or single cysteine MutS-93C; 400 nm) was incubated with single cysteine MutL-131C (1000 nm) in the presence or absence of a GT heteroduplex DNA (100 nm GT484 or 500 nm GT42), the indicated nucleotide (1 mm), and the chemical cross-linker BM[PEO]4 (50 μm) for 2 min at 37 °C. Reaction mixtures were analyzed by SDS-PAGE (6%) and colloidal Coomassie Blue staining. Note that the electrophoretic mobilities of the MutS-MutL cross-link (S x L) (verified after in-gel trypsin digestion and mass spectrometry) observed with MutS wild type and MutS-93C are identical. Cross-link formation of MutL-MutL (L x L) has been described before (37). Efficient cross-link formation of MutS and MutL was only observed in the presence of ATP (lanes 2, 5, and 8) or AMPPNP (lanes 4, 7, and 10) and long GT DNA. It was not observed in the presence of ADP (lanes 3, 6, and 9), with short DNA (lanes 5–7), or in the absence of DNA (lane 11). No cross-link formation was observed when the cross-linker was omitted or when GT DNA was replaced by a corresponding homoduplex control DNA (supplemental Fig. S1).
In crystal structures, an asymmetric complex of MutS encircling the heteroduplex DNA was observed. In this asymmetric complex, only the conserved Phe-X-Glu-motif of the mismatch-binding domain of one subunit is in direct contact with the mismatch, resulting in a 45–60° kink at the mismatch (10, 11, 17–19). In E. coli, the mismatched base is stacked onto Phe-36 and hydrogen-bonded to Glu-38, and stacking of Phe-36 between the bases is important for mismatch recognition and prevents MutS from sliding on the DNA (20). A hallmark of the mismatch recognition process by MutS is the ATP-induced transition from a stationary clamp bound to the mismatched DNA to a long lived mobile sliding clamp (21–23). For the formation of the sliding clamp, conformational changes involving movement of the mismatch-binding domain have been proposed (24). Computational studies using normal mode analysis and molecular dynamics have supported these models (25, 26).
ATP and Mg2+ binding was shown to be crucial not only for the formation of a sliding clamp but also for the formation of a dynamic ternary complex comprising DNA-MutS-MutL (27, 28). This ternary complex coordinates all subsequent steps in DNA repair (e.g. strand discrimination and DNA unwinding) and is also involved in signaling the DNA mismatch/damage to other cellular responses (29). Several lines of evidence suggest that MutS undergoes substantial conformational changes during this transition; however, until recently few structural details were known (30, 31).
A recent study with yeast MutSα suggested that ternary complex formation and sliding clamp formation are distinct steps involving different nucleotide states of MutS. Moreover, it was suggested that the ternary complex precedes sliding clamp formation (30). Attempts to get high resolution structural information on the ternary complex or the sliding clamp, e.g. by x-ray crystallography, have been hampered by the dynamic nature of the ternary complex, the mobility, and lack of DNA sequence specificity of the MutS sliding clamp. Mutational analyses and hydrogen/deuterium exchange mass spectrometry analyses suggested that the connector domain of MutS contains residues critical for the interaction with MutL (32).
Here, we used Cys-mediated cross-linking to shed light onto these two key events in DNA mismatch repair. To this end, we generated a set of cysteine variants of MutS and MutL and tested the proteins for cross-linking using the highly specific and reactive maleimide cross-linking chemistry. In addition, we used site-specifically labeled MutS and MutL single cysteine variants to monitor the ternary complex formation in solution using fluorescence resonance energy transfer (FRET). Based on our results and data from the literature, we provide a model of the MutS-MutL-MutH complex and its implication for the MMR process.
EXPERIMENTAL PROCEDURES
DNA Substrates
Linear DNA substrates (484 bp with or without a GT mismatch) were generated from two 484-bp PCR products amplified by Pfu DNA polymerase with a 5′-phosphate at the top or the bottom strand, respectively, using plasmids pET-15b-XhoI (with a GC base pair) and/or pET-15b-HindIII (with an AT base pair), respectively, with primers (BBseq A302/pA302, 5′-ATC TTC CCC ATC GGT GAT GTC-3′; and BBseq B111/p111, 5′-TCA TCC TCG GCA CCG TCA C-3′) essentially as described previously (33). The phosphorylated strands were digested by λ-exonuclease, and the top strand (containing a G at position 385) was annealed to the bottom strand (containing a C or a T at position 385, respectively) to give either a 484-bp homoduplex (GC484) or heteroduplex (GT484), respectively. A 42-bp GT heteroduplex (GT42) corresponding to positions 365–406 of GT484 was generated by annealing the two oligonucleotides TAT TAA TTT CGC GGG CTC GAG AGC TTC ATC CTC TAC GCC GGA and TCC GGC GTA GAG GAT GAA GCT TTC GAG CCC GCG AAA TTA ATA (the underlined bases indicate the position of the GT mismatch). The generation of circular DNA substrates (described in detail in the supplemental information) containing a single GT mismatch within a hemimethylated Dcm site at position 169 and a hemimethylated GATC site at position 356 (3315 bp with GT mismatch) was performed essentially as published before (34).
Site-directed Mutagenesis
Plasmids encoding the gene for a cysteine-free MutS (MutS-CF) and dimeric MutS-CF/D835R have been described before (8). Single cysteine MutS variants (Table 1 and supplemental Table S1) were generated by site-directed mutagenesis using a modification of the QuikChange protocol (35, 36). E. coli XL1-blue MRF′ cells were transformed with the full-length PCR product, and marker-positive clones were inoculated and grown overnight in LB medium with ampicillin. Plasmid DNA was isolated using QIAprep Spin Miniprep (Qiagen) or Wizard® Plus Miniprep (Promega), and the entire mutS gene was sequenced to confirm the mutation.
TABLE 1.
Summary of cross-linking analyses for MutS variants tested
| MutS variant | Domaina | Percent cross-link to MutL-131Cb in presence of ATP | In vivo activity,c median (range) |
|---|---|---|---|
| None | N.D. | 156 (100–490) | |
| WT | 40 ± 12 (182) | 1 (0–15) | |
| CF | No | 2 (0–5) | |
| 8C | MBD | 28 ± 8 (12) | 3 |
| 78C | MBD | 26 ± 7 (9) | 1 (0–3) |
| 93C | MBD | 46 ± 10 (20) | 3 (0–6) |
| 93/239C | MBD and connector | 48 ± 8 (6) | 4 (2–9) |
| 103C | MBD | 30 ± 13 (8) | 1 |
| 162C | Connector | 16 ± 3 (5) | 1 (0–17) |
| 239C | Connector | 9 ± 2 (2) | 4 (1–8) |
| 246C | Connector | 62 ± 5 (12) | 3 (3–13) |
| 246C/D835R | Connector | 55 ± 8 (28) | 5 (3–18) |
| 284C | Core | <5 | 0 (0–6) |
| 297C | Core | 11 ± 9 (3) | 2 (1–4) |
| 427C | Lever | 38 ± 2 (3) | 2 (0–8) |
| 449C | Clamp | 21 ± 1 (2) | 1 (0–20) |
| 483C | Clamp | 14 ± 1 (3) | 1 (0–1) |
| 526C | Lever | 24 ± 1 (2) | 3 (0–3) |
| 569C | ATPase | <5 (4) | 1 (0–3) |
| 686C | ATPase | <5 (2) | 1 (0–9) |
| 711C | ATPase | <5 (2) | 5 (1–18) |
a Domain containing the cysteine residues: mismatch-binding domain (MBD; residues 1–115), connector domain (residues 116–266), core domain and levers (residues 267–443 and 504–567), clamp domain (residues 444–503), ATPase domain (residues 568–800) (10).
b Cross-linking with BM[PEO]4 was performed with 400 nm MutS variant and 1000 nm MutL-131C in the presence of a GT mismatch DNA (100 nm). In parentheses are the number of independent experiments. N.D., not determined.
c In vivo activity was scored from E. coli TX2929 transformed with the expression plasmid coding for the indicated MutS variant (38). The numbers of rifampicin-resistant clones plated from 1 ml of overnight culture are given (see Ref. 8 for details). Median and ranges are from at least five independent experiments.
Protein Expression and Purification
Recombinant His6-tagged proteins (MutS and MutL) and variants thereof were expressed and purified by nickel-nitrilotriacetic acid chromatography and size exclusion chromatography essentially as described elsewhere (8, 36–38). MutS and MutL proteins were stored in 10 mm HEPES/KOH (pH 7.9), 200 mm KCl, and 1 mm EDTA (for MutS proteins 10% glycerol was added). MutS and MutL were snap frozen in liquid nitrogen and stored at −80 °C. Protein concentrations were determined from UV absorbance spectra using the theoretical molar extinction coefficients (MutS, 68,000 m−1 cm−1; or MutL, 54,800 m−1 cm−1) and are given in monomeric equivalents (39).
Mismatch-provoked Activation of MutH Endonuclease
Variants of MutS and MutL were tested for their ability to activate the MutH endonuclease using a covalently closed circular heteroduplex DNA substrate (3315 bp) containing a GT mismatch and a hemimethylated GATC site separated by 186 bp. DNA (15 nm) was incubated with 200 nm MutH, 400 nm MutL, and 400 nm MutS in 100 μl of 10 mm Tris-HCl (pH 7.9), 5 mm MgCl2, 1 mm ATP, and 125 mm KCl buffer at 37 °C. Aliquots (10 μl) were taken after different time points (0, 10, 20, 30, 60, 120, 300, and 600 s), and the reactions were stopped with 2 μl of 250 mm EDTA, 25% sucrose, 1.25% SDS, 0.1% bromphenol blue, and 1 μl of 10 units/μl proteinase K. Samples were subjected to gel electrophoresis (1% agarose in Tris-phosphate-EDTA containing 0.5 μg/ml ethidium bromide). MutH activity was monitored by the conversion of covalently closed circular DNA to nicked DNA (open circular). The intensity of the ethidium bromide-stained DNA bands was quantified using TotalLab v2.01 software and analyzed with Origin8.5 software. A single exponential functional was used to fit the time course to yield apparent first order rate constants (kobs).
Chemical Cross-linking
The homobifunctional maleimide cross-linkers of varying length, 1,11-bismaleimidotetraethylene glycol (BM[PEO]4) and bismaleimidoethane, and the methanethiosulfonate cross-linker were obtained from Pierce and Toronto Research Chemicals, respectively. Stock solutions of 10 mm were made in water or DMSO. MutL (10 μm) variants were incubated with 5 mm nucleotide (ADP, ATP, or AMPPNP) and MutS (0.57 μm) variants with homo- or heteroduplex DNA on ice for 25 min in buffer HK (20 mm HEPES/KOH (pH 7.5), 5 mm MgCl2, and 0.01 mm EDTA) and 125 or 150 mm KCl. MutS and MutL were mixed with final concentrations as indicated. After 10-min incubation at room temperature followed by 2 min at 37 °C, 50 μm cross-linker was added to the samples (at least 50-fold molar excess over thiol groups) and incubated for 1–2 min at 37 °C. The reaction was quenched by adding DTT (50 mm final concentration for maleimide cross-linker) or N-ethylmaleimide (50 mm final concentration for methanethiosulfonate cross-linker). Samples were subjected to 6% SDS-PAGE and stained with colloidal Coomassie Blue (AppliChem). Gels were analyzed with a video documentation system (Bio-Rad). The intensity of the stained protein bands was quantified using TotalLab v2.01 software. Cross-linking yields were calculated as
 |
where I (S x L) is the intensity of the MutS-MutL band, I (S x S) is the intensity of the MutS-MutS band, and I (S) is the intensity of the MutS band. Mr,S = 97,000 and Mr,L = 70,000, i.e. the Mr (molecular weight) of MutS and MutL, respectively.
Labeling Proteins with Fluorophores
MutS and MutL variants were labeled with Alexa Fluor 488 or Alexa Fluor 594 in a 1:3 or 1:4 molar ratio, respectively. After 30-min incubation on ice, the samples were twice purified over Zeba Desalt Spin Columns (Pierce, Thermo Scientific) followed by the measurement of the concentration using absorbance spectroscopy,
 |
where A280 is the absorbance at 280 nm, Amax is the absorbance of the Alexa Fluor 594 or Alexa Fluor 488, CF is the correction factor for the used dye (CFA488 = 0.12 and CFA594 = 0.57), and ϵprot is the theoretical molar extinction coefficients of the respective protein at 280 nm. The degree of labeling (DOL) was determined using the equation
 |
where ϵmax is the molar extinction coefficients of Alexa Fluor 488 (71,000 m−1 cm−1) or Alexa Fluor 594 (73,000 m−1 cm−1), respectively.
Analytical Ultracentrifugation
Sedimentation velocity experiments were performed in a Beckman Coulter ProteomeLab XL-I analytical ultracentrifuge equipped with a fluorescence detection system (Aviv Biomedical) using an An50Ti rotor at 20 °C and speeds from 22,000 to 33,000 rpm. The concentration profiles were measured using the analytical ultracentrifuge equipped with a fluorescence detection system with an excitation wavelength of 488 nm, and emission was detected through a pair of long pass (>505-nm) dichroic filters. Programming of the centrifuge and data recording were performed using the AOS software (Aviv Biomedical) on a computer attached to the centrifuge. Special cell housings (Nanolytics) were used that allow the placement of standard 3-mm double sector centerpieces directly beneath the upper window of the cell. The cells were filled with 100 μl of sample. The experiments were performed in buffer HKT (20 mm HEPES (pH 7.5), 125 mm KCl, 5 mm MgCl2, 0.01 mm EDTA, and 0.05% (v/v) Tween 20 to prevent protein adsorption to surfaces). For MutS-449C and MutL-297C, the Alexa Fluor 488-labeled proteins were examined at concentrations between 100 nm and 1 μm. The experiments for MutS-246C-Alexa Fluor 488 were done at a concentration of 100 nm. To test the influence of bound nucleotide on the sedimentation behavior, the experiments were performed in the presence of 1 mm ADP, ATP, or AMPPNP, respectively. To check whether the fluorescently labeled MutS variants are still able to bind to heteroduplex DNA, a 100 nm concentration of the respective protein was incubated in the presence of 1 mm ADP with 500 nm GT42 and subjected to sedimentation velocity analysis. The measured concentration profiles were evaluated using the program package SEDFIT (40), which provides a model for diffusion-corrected differential sedimentation coefficient distributions (c(s) distributions). For hydrodynamic analyses, measured s values were corrected to s20,w using the partial specific volumes calculated from amino acid composition (41). Because the partial specific volume of complexes of different macromolecules with unknown composition cannot be calculated, such a correction could not be performed, and uncorrected sedimentation coefficients (sexp) are given in these cases.
Fluorescence Spectroscopy
Fluorescence emission spectra were obtained using a FluoroMax-4 (HORIBA Jobin Yvon) with excitation wavelength λex at 470 or 575 nm and band width set to 1.8 nm. Emission spectra were recorded at 20 °C in a total volume of 100 μl in buffer HKT. Spectra were normalized to the maximum of fluorescence intensity at 515 nm.
Protein-Protein Docking
Docking runs were performed with the Hex software to automatically get thousands of configurations (42). In a first docking run, we used the mismatch-binding and connector domains of MutS (Protein Data Bank code 1e3m, chain B, residues 2–266) and the N-terminal domains of MutL (Protein Data Bank code 1b63, chain A and B, residues 1–331), and in a second docking run, the N-terminal domains of MutL and MutH (Protein Data Bank code 2azo, chain B) (37) were used. Docking solutions were used to generate structural models of the full-length MutS with MutL and MutH. These models were filtered using the experimentally derived distance constraints (this work and Ref. 37). For details of the procedure, see the supplemental information.
RESULTS
Cys-93 in Mismatch-binding Domain of MutS Cross-links to Cys-131 in ATPase Domain of MutL
In a previous report, we demonstrated that the single cysteine MutL variant (MutL-131C) modified with benzophenone at Cys-131 in the ATPase domain could be photo-cross-linked to MutS (43). In addition, using the thiol-specific bismaleimide reagent BM[PEO]4 (Fig. 1A), we were able to obtain a MutS-MutL cross-link in the presence of a GT mismatch DNA and ATP (43). This indicated that one or more of the six endogenous cysteine residues of MutS are in proximity to the N-terminal domain of MutL (Fig. 1B). Here we investigated the cross-link formation between MutS and MutL-131C in more detail. In the presence of ATP, the formation of a cross-link was dependent on the length of the DNA and was observed with a long 484-bp GT-containing DNA (Fig. 1C, lane 2) but was almost absent when the DNA was only 42 bp long (Fig. 1C, lane 5). Moreover, the cross-link formation was mismatch-dependent as we only observed the MutS-MutL cross-link in the presence of heteroduplex (GT) DNA but not with the corresponding homoduplex (GC) DNA (supplemental Fig. S1). To test whether ATP binding or ATP hydrolysis is required to form the cross-link, we replaced ATP by ADP or the non-hydrolyzable analogue AMPPNP. It has been noticed before that MutS is not able to recognize mismatches when ATP is bound but cannot be hydrolyzed, e.g. in the absence of Mg2+ or by using non-hydrolyzable ATP analogue ATPγS or AMPPNP (44–46). As expected from the DNA dependence of the cross-link formation, no MutS-MutL cross-link was observed when MutS was incubated with AMPPNP prior to the addition of DNA and MutL (data not shown). In contrast, when MutS was bound to DNA prior to the addition of MutL and AMPPNP, MutS-MutL cross-link formation was as efficient as with ATP, indicating that ATP binding but not hydrolysis is required for cross-link formation (Fig. 1C, lanes 2 and 4). No cross-link formation was observed in the presence of ADP (Fig. 1C, lane 3). Moreover, using MutS-E694A, which is defective in ATP hydrolysis (47, 48), cross-link formation was observed only when MutS was first bound to DNA followed by the addition of ATP, MutL, and the cross-linker (data not shown).
To identify the cysteine residue involved in the chemical cross-linking reaction, we tested variants in which one or more of the endogenous cysteine residues were replaced by other amino acid residues (supplemental Table S2). The six non-conserved cysteine residues in MutS are located in the mismatch-binding domain (Cys-93), the connector domain (Cys-235 and Cys-239), the core domain (Cys-297), and the ATPase domain (Cys-569 and Cys-711) (Fig. 1B). In the crystal structure of MutS in complex with mismatched DNA and bound ADP, only three of these cysteine residues (235, 297, and 569) are solvent-exposed (49). By replacing all six cysteine residues, we generated a cysteine-free variant of MutS that is fully functional in vitro and in vivo, indicating that none of the cysteine residues are essential for MutS function (8). None of the three solvent-exposed cysteine residues (235, 297, and 569) were essential for chemical cross-link formation with MutL because replacement of these residues with serine did not abolish cross-link formation with MutL-131C (supplemental Table S2). Moreover, the single cysteine MutS variants MutS-297C and MutS-569C did not form a cross-link to MutL-131C (supplemental Table S2). In contrast, all MutS variants still harboring Cys-93, which is located in the mismatch-binding domain, were able to form the chemical cross-link with MutL-131C. Therefore, we generated a single cysteine variant, MutS-93C, containing only Cys-93 and compared the MutL cross-linking reactions with that with wild type MutS (Fig. 1C). MutS-93C displayed the same nucleotide and DNA dependence as wild type MutS; i.e. efficient cross-link formation was observed only in the presence of DNA and ATP or AMPPNP but not ADP (Fig. 1C, lanes 8–10). Finally, efficient cross-linking between MutS-93C and MutL-131C was even observed using the shorter cross-linker bismaleimidoethane, which has a calculated sulfur to sulfur distance range of about 6.3–10.5 Å (data not shown) (50). In summary, our data revealed that the chemical cross-linking between Cys-93 of the mismatch-binding domain of MutS and Cys-131 of the N-terminal domain of MutL occurs only under conditions that are required for ternary complex formation as observed in other biochemical assays (51). Given the length of the cross-linker used, our cross-linking data indicate that in the ternary complex the mismatch-binding domain of MutS is in close proximity (less than 10 Å) to the ATPase domain of MutL.
Cys-246 of Connector Forms Cross-link to Cys-131 of MutL
To obtain additional distance restraints and to get further insights into structure of the ternary complex, we generated a series of single cysteine MutS variants (supplemental Fig. S2). The positions were chosen on the basis of two criteria. First, the residues to be replaced should be maximally solvent-exposed to allow optimal reaction with the cross-linker. Second, the residues should not be conserved to minimize negative effects of the amino acid exchange. A list of the variants tested is shown in Table 1 (see supplemental Fig. S2 for the position of cysteine residues in MutS tested in the present study). Indeed, almost all plasmid-borne MutS variants were able to complement a mutS mutator phenotype in vivo, indicating that the proteins were functional and still able to interact with MutL. Next we tested the purified MutS variants for their ability to form a cross-link with MutL-131C. Interestingly, only six variants with a single cysteine either in the mismatch-binding domain (MutS-8C, MutS-78, MutS-93C, and MutS-103), the connector domain (MutS-246C), or the lever domain (MutS-427C) resulted in efficient (>25%) cross-link formation in the presence of ATP (Table 1) (32). Next we investigated the kinetics of cross-link formation for two variants (MutS-93C and MutS-246C) with MutL-131C in greater detail. Interestingly, kinetics of the cross-linking reaction revealed that the formation of the MutS-MutL cross-link was much faster (within 10 s) with MutS-246C compared with MutS-93C (Fig. 2, A–C). Another MutS variant with a single cysteine in the connector domain (MutS-162C) was tested further for cross-linking to another MutL variant (MutL-135C). For this variant, cross-linking was not observed with MutL-131C but exclusively with MutL-135C that formed cross-links only with a reagent having a long linker arm (Fig. 2, D and E). Of note, both cysteine residues (Cys-162 and Cys-246) are close to peptides in MutS that had been shown by hydrogen/deuterium exchange mass spectrometry to become protected in the presence of MutL (32) (see below).
FIGURE 2.

Cys-246 in connector domain forms cross-link to Cys-131 of MutL-131C. A and B, time course of chemical cross-linking in the presence of ATP using single cysteine MutS variants with a cysteine residue in the mismatch-binding domain (MutS-93C) (A) or in the connector domain (MutS-246C) (B). The samples were analyzed by 6% SDS-PAGE and stained with colloidal Coomassie Blue. The cross-linking reaction and the concentrations used are the same as described in Fig. 1. Note that the electrophoretic mobility of the MutS-MutL complex (S x L) is decreased for the MutS-246C-MutL-131C as the branching point in the cross-linked complex moved closer toward the center of the MutS protein sequence. C, quantitative analysis of the cross-link reaction kinetics (see “Experimental Procedures” for details). D, cross-linking of MutS-162C to MutL-135C is dependent on the range of the cross-linker. Cross-linking experiments were carried out essentially as described under “Experimental Procedures” with the exception that BM[PEO]4 was replaced with methanethiosulfonate cross-linkers (MxM) of varying spacer arm length, and the reaction was stopped with N-ethylmaleimide. Note that cross-link yield between MutS-162C and MutL-135C (S x L) was only high in the presence of DNA and with the cross-linker M17M with the longest spacer arm (lane 3). No band corresponding to the MutS-MutL cross-link (S x L) was observed in the absence of either MutS-162C (lane 1) or MutL-135C (lane 2). The cross-link yield of the MutS-MutL cross-link was much reduced when the length of the cross-linker was shortened (lanes 4 and 5) or DNA was omitted from the reaction (lane 6). Less cross-link formation was observed when using BM[PEO]4 or when variants MutS-686C or MutS-449C were used instead of MutS-162C (data not shown). E, chemical structures of the methanethiosulfonate cross-linkers used.
N-terminal Half of MutL Is Sufficient for Cross-link Formation with MutS
Because bacterial MutS exist in dimer-tetramer equilibrium, the interpretation of the cross-linking data shown in Table 1 is ambiguous, i.e. which subunit of a MutS tetramer is cross-linked to MutL. Previously, we described the generation of a fully functional cysteine-free MutS variant that is unable to form tetramers. In this variant, Asp-835 in the dimerization/tetramerization domain was replaced by an arginine residue (8). We generated the single cysteine variant MutS-246C/D835R and tested this dimeric MutS for cross-linking with MutL-131C. Indeed, cross-linking yields were comparable with that obtained for MutS-246C (i.e. about 50%; Table 1), and cross-linking was dependent on the presence of DNA (supplemental Fig. S1B). This suggests that MutL is cross-linked to only one subunit of the MutS dimer and not to both subunits of one dimer in a MutS tetramer. To address whether the N-terminal half comprising the ATPase domain of MutL is sufficient for ternary complex formation, we generated a 40-kDa single cysteine N-terminal fragment of MutL (residues 1–331; LN40-131C). In the absence of nucleotide or in the presence of ADP, this fragment has been shown to exist predominantly in the monomeric form, whereas ATP or AMPPNP binding supports the formation of dimers (13, 52) (Fig. 3A). When tested for chemical cross-linking to MutS-246C/D835R, we observed high yield cross-linking between MutS and LN40 only in the presence of ATP and AMPPNP but much less cross-linking (<10%) with ADP (Fig. 3B, lanes 2–4). In the presence of AMPPNP, an additional band corresponding to the LN40 dimer was observed, consistent with the reported stable dimer formation of LN40 under these conditions (Fig. 3B, lane 3). This band was absent or less pronounced in the presence of ATP or ADP (Fig. 3B, lanes 2, 4, and 5). LN40 can form dimers in the presence of ADP or ATP that are not stable (13). In such dimers, Cys-131 can either cross-link within the LN40 dimer (Fig. 3B, lanes 4 and 5) or, in the presence of MutS-246C, form a cross-link to MutS (Fig. 3B, lane 2). Under this competitive situation (Fig. 3B, lane 2 versus lane 5), the cross-link was formed preferentially to MutS, whereas in the absence of MutS, the LN40-LN40 cross-link was observed. In the presence of AMPPNP, stable dimers of LN40 are formed (13). As expected, the efficiency for LN40-LN40 cross-link formation was increased (Fig. 3B, lane 3).
FIGURE 3.
LN40 is sufficient for complex formation and cross-linking with MutS. A, ATPase/dimerization cycle of the N-terminal fragment of MutL (LN40). Upon ATP binding (black sphere), LN40 undergoes a conformational change (indicated by dark gray) that allows dimerization. After ATP hydrolysis (light gray), the subunits dissociate and release ADP (gray sphere) (13, 52). B, the N-terminal fragment of MutL containing a single cysteine at position 131 (LN40-131C) was tested for complex formation with dimeric MutS-246C/D835R using chemical cross-linking in the presence of the indicated nucleotides essentially as described in Fig. 1. The samples were analyzed by 6% SDS-PAGE and stained with colloidal Coomassie Blue. Note that the monomeric form of LN40 has run out of the gel. S, MutS.
MutS and MutL Variants Are Proficient in MMR in Vivo and in Vitro
The MMR proficiency of the MutS and MutL mutants used in the study was tested in vivo (Table 1 and Ref. 37). All MutS and MutL variants tested in this study showed MMR activity in vivo (>95 or > 90%, respectively). In addition, the variants MutS-WT, MutS-246C/D835R, and MutL-131C, which showed the highest cross-linking yields, were tested in an in vitro mismatch-provoked MutH activation assay using circular DNA containing a GT mismatch and a hemimethylated GATC site (see “Experimental Procedures” for details). We observed similar apparent nicking rates (kobs) of 4.9 and 3.4 min−1 for MutL-WT in combination with MutS-WT and MutS-246C/D835R, respectively (Table 2). The activities with MutL-131C both with MutS-WT and MutS-246C/D835R were reduced (1.8 and 0.7 min−1) but still significantly higher than in the absence of MutL (<0.1 min−1). These data suggest that the interaction between these single cysteine MutS and MutL variants is in principle still functional.
TABLE 2.
Mismatched-provoked activation of MutH by MutS and MutL variants
MutH incision of a 3.3-kbp DNA containing a GT mismatch and one hemimethylated GATC site was monitored using 15 nm DNA, 200 nm MutH, 400 nm MutL, and 400 nm MutS at 37 °C. kobs ± 1 S.E. from global fits are shown (n = 2–4). Background incision rates (in the absence of a mismatch, MutS, or MutL) were <0.1 min−1.
| Apparent first order rate constants, kobs |
||
|---|---|---|
| MutL-WT | MutL-131C | |
| min−1 | ||
| MutS-WT | 4.9 ± 0.9 | 1.8 ± 0.3 |
| MutS-246C/D835R | 3.4 ± 0.6 | 0.7 ± 0.1 |
Modification of MutS and MutL with Fluorescent Dyes
Because Cys-246 of MutS could be cross-linked to MutL-131C in the ternary complex, this position must be accessible to allow modification with the cross-linking reagent. To obtain additional information on the MutL binding of MutS, we modified MutS-246C/D835R and for comparison MutS-449C/D835R using the fluorescent dyes Alexa Fluor 488- and Alexa Fluor 594-maleimide. Both proteins could be modified to >95% as quantified using absorption spectroscopy after removal of excess dye by size exclusion chromatography (data not shown). SDS-PAGE analysis followed by fluorescence imaging and Coomassie Blue staining confirmed the covalent modification of the protein with the dye (data not shown). Similarly, we could modify the single cysteine MutL variant MutL-297C, a functional single cysteine MutL variant that had been studied before (37), with Alexa Fluor 488 to >95%.
Sedimentation Velocity Analysis of Fluorophore-modified MutS and MutL
To determine whether the fluorophore modification had an influence on the quaternary structure of the proteins, we analyzed the proteins modified with Alexa Fluor 488 using sedimentation velocity analysis monitoring the fluorescence of the Alexa Fluor 488 dye in the analytical ultracentrifuge. In the presence of ADP, the c(s) distributions (40) yielded about 90% of the total protein sedimenting with a sedimentation coefficient s20,w of 8.2 S for MutS-246C/D835R-Alexa Fluor 488 and 8.3 S for MutS-449C/D835R-Alexa Fluor 488, respectively, indicative of the formation of MutS dimers (Table 3 and supplemental Fig. S3). In the presence of ATP, s20,w increased slightly to 8.4 S, indicating a slightly more compact form of the ATP-bound proteins as has been observed by other methods (53). Addition of a 42-bp GT heteroduplex DNA (GT42) to the protein in the presence of ADP resulted in an increase of the uncorrected sedimentation coefficient from 7.9 or 8.0 to 8.9 S, showing the formation of a complex between MutS and DNA. These data demonstrate that chemical modification of position 246 or 449 in MutS did not affect the quaternary structure of the protein and was not inducing aggregation. Moreover, the protein retained its ability to interact with DNA. Similarly, we performed a sedimentation velocity analysis with MutL-297C modified with Alexa Fluor 488 in the presence of different nucleotides. For the modified MutL-297C in the presence of AMPPNP, the c(s) analysis yielded about 90% of the protein sedimenting in a single boundary with a sedimentation coefficient s20,w of 6.1 S, indicating the formation of MutL dimers under these conditions with a frictional ratio f/f0 = 1.53. (Table 2 and supplemental Fig. S3). In the presence of ATP, the protein sedimented more slowly (s20,w of 5.8. S and f/f0 of 1.62), suggesting that the complex is less compact as compared with the one formed with AMPPNP. In the presence of ADP, the c(s) distributions were much broader compared with those of the other nucleotides and showed several peaks. SDS-PAGE analysis of the samples after centrifugation showed substantial degradation of the modified MutL-297C protein in the presence of ADP only, indicating that the protein is less stable in the presence of ADP (data not shown). Taken together, these data suggest that the modified MutL-297C is not aggregating and still able to form stable dimers in the presence of AMPPNP and ATP as has been observed before by size exclusion chromatography (14) or for the eukaryotic homologues by scanning force microscopy (54).
TABLE 3.
Sedimentation velocity analyses of MutS and MutL variants labeled with Alexa Fluor 488
| Variant |
s20,w (frictional ratio f/f0 of the dimer) |
sexp |
|||
|---|---|---|---|---|---|
| ADP | ATP | AMPPNP | +ADP | +ADP +GT42 | |
| MutS-246C/D835R | 8.2 S (1.43) | 8.4 S (1.40) | 7.9 S | 8.9 S | |
| MutS-449C/D835R | 8.3 S (1.42) | 8.4 S (1.40) | 8.4 Sa (1.40) | 8.0 S | 8.9 S |
| MutL-297C | N.D.b | 5.8 S (1.62) | 6.1 S (1.53) | ||
a Measurement was done in 25 mm Tris (instead of HEPES) (pH 7.5), 125 mm KCl, 5 mm MgCl2, 0.01 mm EDTA, and 1 μm BSA.
b Not determined.
Ternary Complex Formation of DNA, MutS, and MutL Monitored by FRET
To obtain additional information on the structure of the MutS-MutL complexes, we tested whether ternary complex formation can be monitored by FRET using MutS-246C/D835R modified with Alexa Fluor 594 and MutL-297C modified with Alexa Fluor 488. Alexa Fluor 488 and Alexa Fluor 594 form a FRET pair with a theoretical R0 of 57 Å (55). Incubation of 200 nm MutS with 200 nm MutL in the presence of ATP did not result in any significant FRET (SR/SG ratio of 0.08) (Fig. 4A). This is in agreement with previous observations that MutS does not interact with MutL in the absence of DNA. Upon addition of 50 nm 484-bp DNA containing a single GT mismatch, we observed a strong decrease of the donor fluorescence with a concomitant increase in acceptor fluorescence indicative of FRET (SR/SG ratio of 0.36). Increasing the concentration of KCl to 300 mm, which results in dissociation of MutS from DNA, led to a rise in donor and drop in acceptor fluorescence back to the values obtained in the absence of DNA (SR/SG ratio of 0.07). Similar DNA-induced FRET between MutS-449C/D835R-Alexa Fluor 594 and MutL-297C-Alexa Fluor 488 was observed; albeit the extent of FRET was reduced (Fig. 4B). These results show that it is possible to monitor the ternary complex formation between DNA, MutS, and MutL using FRET. Moreover, the differences in the FRET efficiency suggest that position 297 in MutL is closer to position 246 than to position 449 in MutS and that the distance has to be in the range of R0.
FIGURE 4.
Ternary complex formation between MutS and MutL monitored using FRET. A and B, fluorescence emission spectra (excitation at 470 nm; normalized to the emission at 515 nm) were recorded with MutL-297C (200 nm) labeled with Alexa Fluor 488 in the presence of 1 mm ATP in HT buffer (25 mm HEPES/KOH (pH 7.5), 0.05% Tween 20, 125 mm KCl, and 5 mm MgCl2) (green line). Next 200 nm MutS-246C (A) or MutS-449C (B), respectively, labeled with Alexa Fluor 594 were added (red) followed by the addition of 50 nm 484-bp GT heteroduplex DNA (orange) and finally KCl to a final concentration of 300 mm (black dashed line). The inset shows the ratio (SR/SG) between red signal (SR; emission at 615 nm) and green signal (SG; emission at 515 nm). Averages and standard deviations (error bars) from two independent experiments are plotted.
MutS Forms Internal Cross-link between Cys-93 in Mismatch-binding Domain and Cys-239 in Connector Domain
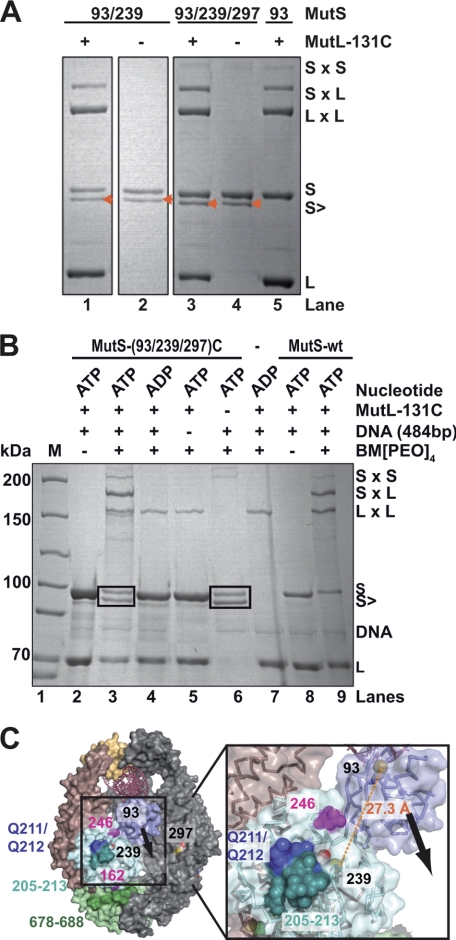
In the process of identifying the cysteine residue in wild type MutS that cross-links to MutL-131C (see above), we noticed that with some MutS variants an additional band with higher electrophoretic mobility than uncross-linked MutS was observed after cross-linking (Fig. 5A, lanes 1–4, and B, lanes 3 and 6, and supplemental Fig. S4). The formation of this cross-link was dependent on the presence of ATP and DNA (Fig. 5B, lanes 3–6) and was observed both in the absence or presence of MutL (Fig. 5A, lanes 1–4). Inspection of available crystal structures of MutS revealed that only several pairs of cysteine residues are in a distance compatible with the length of the cross-linker used (297 and 569, 19 Å; and 235 and 239, 7 Å). However, this cross-link band was observed also with two triple cysteine variants that were without these pairs, i.e. MutS-(93/239/297)C and MutS-(93/239/711)C. Distances between cysteine 93 of the mismatch-binding domain and cysteine 239 of the connector domain are 24 and 28 Å (within subunit A or B), respectively, whereas the other pairs show larger distances (38–49 Å) (Fig. 5C). To answer whether the observed cross-link can be formed between Cys-93 and Cys-239, we generated the double cysteine variant MutS-(93/239)C, which, similar to the triple cysteine variant MutS-(93/239/297)C, formed not only a cross-link to MutL-131C but also the internal cross-link (Fig. 5A, lane 1). No internal cross-link formation was observed in any of the single cysteine variants, e.g. MutS-93C (Figs. 1C and 5A, lane 5) or MutS-239C (data not shown). Of note, the formation of the internal cross-link was ATP-dependent as no internal cross-link formation was observed when ATP was replaced by ADP (Fig. 5B, lane 4), suggesting that an ATP-induced conformational change is required to allow cross-link formation. This agrees with the fact that the distance between Cys-93 and Cys-239 (≈27.3 Å) observed in the co-crystal structure of MutS in complex with mismatched DNA in the presence of ADP is larger than the range (≈17 Å) of the BM[PEO]4 cross-linker (Fig. 1A).
FIGURE 5.
ATP and DNA induce conformational change in MutS bringing mismatch-binding domain closer to connector domain. A, chemical cross-linking of variants MutS-93C, MutS-(93/239)C, and MutS-(93C/239C/297)C in the presence (lanes 1, 3, and 5) and absence (lanes 2 and 4) of MutL-131C and 484-bp GT heteroduplex DNA (GT484). Cross-linking was carried out in the presence of 1 mm ATP essentially as described in Fig. 1 (see “Experimental Procedures” for details). A band (marked by an orange arrowhead) migrating slightly faster than MutS was observed only with MutS-(93/239)C and MutS-(93/239/297)C. B, internal cross-link formation in MutS is ATP- and DNA-dependent. Cross-linking between MutL-131C (1 μm) and the triple cysteine variant MutS-(93/239/297)C (0.4 μm) (lanes 3–6), MutS-WT (lane 9), and without MutS (lane 7) in the presence of 100 nm heteroduplex DNA (GT484), 1 mm ATP, and 125 mm KCl with BM[PEO]4 (see “Experimental Procedures” for details). The reactions in lanes 2 and 8 were done in the absence of BM[PEO]4 with the triple cysteine MutS variant and MutS-WT. Reactions were analyzed by 6% SDS-PAGE followed by colloidal Coomassie Blue staining. The black boxes in lanes 3 and 6 mark the MutS double band. Lane 1, molecular mass markers. C, positions of cysteine residues in MutS. The subunits of MutS are colored in gray (subunit A) or according to the domains (subunit B) as in Fig. 1B. Cysteine residues are shown as yellow spheres. In addition, positions of cysteine residues of two single cysteine variants in the connector domain (MutS-162C and MutS-246C) are shown in magenta. The peptides 205–213 and 674–688 are shown in dark cyan and dark green, respectively. The residues Gln-211/Gln-212 (shown in dark blue) have been shown to be important for the interaction with MutL (32). Note that the distance between the sulfur atoms of Cys-93 and Cys-239 is 27.3 Å, which is 10 Å larger than the maximum span of the BM[PEO]4 cross-linker (17.8 Å). The arrow indicates the direction of movement of the mismatch-binding domain toward the connector domain upon ATP binding required to allow internal cross-link formation. L, MutL; S, MutS.
Models for MutS-MutL Complex
The cross-linking data presented here and available information from literature (32, 37) were used to generated a coarse grained structural model using the mismatch-binding and connector domains of MutS and the N-terminal domain of MutL (see supplemental data). The top cluster containing nine models for the complex are shown in Fig. 6A. After reconstruction of MutS, the previously published docking model of the MutL-MutH complex (37) was superimposed, and DNA was added from the Haemophilus influenzae MutH-DNA crystal structure (56) (Fig. 6B). The C-terminal domain of MutL had been omitted from modeling because the flexible linker between the N-terminal domain of MutL and the C-terminal domain of MutL makes it difficult to determine the position of the C-terminal domain of MutL in this model. In these models, the distance between position 246 of MutS and position 297 of MutL (chain A or chain B) is <40 Å, whereas the distance between position 449 of MutS and position 297 of MutL is >50 Å. This fits well to the higher FRET efficiency observed with Alexa Fluor 594-labeled MutS-246C/D835R with Alexa Fluor 488-labeled MutL-297C compared with the experiment with Alexa Fluor 594-labeled MutS-449C/D835R with Alexa Fluor 488-labeled MutL-297C (Fig. 4). Of note, although not used for filtering during docking, residues 205–213, especially Gln-211 and Gln-212, of MutS are in close contact to MutL (Fig. 6B). In summary, the model for the MutS-MutL complex is in agreement with the experimental data presented in this work and data published before (32, 37).
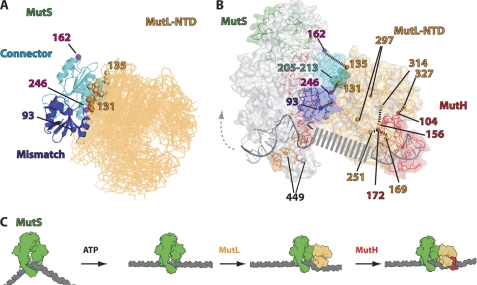
FIGURE 6.
Model of complex between MutS, MutL, and MutH on DNA. A, models for the MutS-MutL complex were generated using the Hex program, and docking decoys were scored using the constraints obtained from the cross-linking data presented in this work (see “Experimental Procedures” and supplemental information for details). The top nine docking solutions of residues 9–266 of MutS (mismatch-binding (blue) and connector domains (cyan)) with the N-terminal domain of MutL (MutL-NTD) shown in orange are superimposed. The position of residues 93 (blue), 162, and 246 (both magenta) of MutS and positions of residues 131 (orange) and 135 (light orange) of MutL are indicated as spheres. B, a structural model for the MutS, MutL, and MutH complex was generated based on the crystal structures of E. coli MutS with a GT mismatch (green; Protein Data Bank code 1e3m) superimposed to one of the docking models shown in A and the published complex between the N-terminal domain of E. coli MutL in complex with AMPPNP (orange; Protein Data Bank code 1b63) and E. coli MutH (red; Protein Data Bank code 2azo, chain B) (37). The DNA bound to MutH was taken from the structure of H. influenzae MutH in complex with DNA containing a hemimethylated GATC site (gray; Protein Data Bank code 2aor) (56). Coloring for MutS is identical to that in Fig. 5. Residues making chemical cross-links are indicated by their number and connected by black dashed lines. In this model, the distance between position 297 (chain A or chain B) of MutL to position 246 (chain B) in MutS is 35 Å. In contrast, the distances to position 449 (chain A or chain B) in MutS are 68 and 52 Å, respectively. Note that the DNA and the mismatch-binding domain(s) are likely to change their conformation upon ATP binding and complex formation with MutL (indicated by the gray arrow). A gray dashed line indicates how the DNA could run from MutS via MutL to MutH. C, model for the formation of a complex between MutS-MutL and MutH scanning the DNA for the strand discrimination signal. After mismatch binding by MutS, ATP triggers the formation of a long lived sliding clamp. MutL can bind to this clamp. Binding of MutH to MutL leads to the formation of a quaternary complex in which MutS and MutH scan the DNA for a strand discrimination signal, i.e. a hemimethylated GATC site.
DISCUSSION
The complex between MutS and MutL formed on mismatched DNA in the presence of ATP is an important coordinator of DNA mismatch repair. Despite numerous efforts, little is known about its quaternary structure/arrangement. The dynamic nature of this complex and the requirement of long DNA for its formation have significantly hampered structural analyses in the past. Using site-directed chemical cross-linking and FRET, we were able to identify regions in MutS and MutL that are in proximity in the complex. Moreover, cross-linking enabled us to trap the dynamic complex, which should allow more detailed structural analyses in the future.
In a previous study, we demonstrated that the N-terminal ATPase domain of MutL is in proximity to MutS (43). A MutL variant with a single cysteine at position 131 formed a photo- and a chemical cross-link to MutS. Here we showed that the thiol-specific bismaleimide reagent BM[PEO]4 cross-linked Cys-93 in the mismatch-binding domain of MutS to Cys-131 in the ATPase domain of MutL (Fig. 1). To our knowledge, this is the first time that a link between the mismatch-binding domain and MutL has been shown. The lack of efficient cross-link formation in the absence of mismatched DNA or ATP (Fig. 1) suggests that this cross-link reaction is indicative for the ternary complex formation. These conclusions are corroborated by our cross-linking experiments with a MutS variant containing a cysteine residue in the connector domain (MutS-162C and MutS-246C) (Table 1 and Fig. 2). Interestingly, cross-linking of MutL-131C to Cys-246 in the connector domain of MutS was much faster compared with the reaction with Cys-93 in the mismatch-binding domain of MutS (Fig. 2C). This could be explained by the higher solvent accessibility of Cys-246 compared with the partially buried Cys-93. However, it might also reflect that the mismatch-binding domain becomes mobile after the ATP-induced conformational change as was proposed before (24, 25). In contrast, Cys-246 remains in the same spatial position to the primary interaction site in the connector domain, i.e. Gln-211/Gln-212 (Fig. 5B and Ref. 32). A mobile mismatch-binding domain also explains why several variants with single cysteine residues in this domain were able to form an ATP- and mismatch-dependent cross-link to MutL-131C (Table 1). Moreover, ATP-induced mobilization of the mismatch-binding domain is consistent with the internal cross-link formation between Cys-93 of the mismatch-binding domain and Cys-239 in the connector domain that otherwise are too far separated for cross-linking when MutS is bound to a mismatch in the absence of ATP (Fig. 5).
Further experiments showed that the N-terminal fragment of MutL (LN40-131C) was sufficient for cross-linking with MutS, although it cannot bind to DNA alone (14). This corroborates earlier data showing that DNA binding of MutL is not required for complex formation with MutS (57). Although full-length MutL has been shown to exist in the presence of ADP predominantly in an open conformation, it can also adopt a closed conformation (up to 30%) (14, 52, 54).
In the presence of ATP or AMPPNP, the closed conformation is the dominant form of MutL, and cross-linking to MutS was enhanced in the presence of these nucleotides (Figs. 1 and 2). In contrast, LN40 was not able to form stable dimers in the presence of ADP and ATP (reduced LN40-LN40 cross-link formation; Fig. 3B, lanes 2 and 4) due to the missing LC20 domain, which links the two N-terminal domains in the full-length MutL (13). However, in the presence of ATP, high yield cross-link formation between MutS-246C/D835R and LN40-131C was observed, suggesting that the monomeric form of MutL is sufficient for the interaction with MutS (Fig. 3B, lane 2). In the presence of the non-hydrolyzable ATP analogue AMPPNP, LN40 forms dimers (13), which could interact with and cross-link to MutS (Fig. 3B, lane 3). Our results are in agreement with the observation that several mutants of MutS defective in either ATP binding, ATP hydrolysis, or dimerization are still capable of forming a ternary complex with MutL on mismatched DNA (58).
Taken together, our results suggest that the ATPase domain of MutL is in proximity to both the mismatch-binding and connector domains of MutS. These conclusions are corroborated by the FRET analysis using Alexa Fluor 594-labeled MutS-246C and with Alexa Fluor 488-labeled MutL-297C, indicating that these two positions are at a distance of ∼50–60 Å (Fig. 4A) and that the observed proximity between these two domains is not an artifact of the cross-linking reaction. In contrast, the lower FRET between Alexa Fluor 488-labeled MutL-297C and Alexa Fluor 594-labeled MutS-449C suggests a longer distance between the ATPase domain and the clamp domain. In summary, our data are in agreement with the recent study on the ternary complex formation of E. coli MutS/MutL and yeast MutSα/MutLα (32). Using time-resolved hydrogen/deuterium exchange monitored by mass spectrometry, the authors could demonstrate that two regions in E. coli MutS displayed decreased exchange rates in the presence of MutL, i.e. residues 205–213 of the connector domain and residues 674–688 of the ATPase domain, which are close to residues 246 and 162, respectively (Fig. 5C and Ref. 32). Moreover, the authors could demonstrate that the variant MutS-211,2 (Q211S,Q212S) was strongly impaired in MutL binding (Fig. 5C).
Notably, cross-linking yields in our experiments were often close to 50% even under conditions using a molar excess of MutL over MutS. This observation suggests that only one mismatch-binding domain of the homodimeric MutS is close to the N-terminal domain of MutL (Fig. 2 and Table 1). The cross-linking yields between Cys-93 of the mismatch-binding domain and Cys-239 of the connector domain also were close to 50%, suggesting, but not proving, that only one subunit is able to undergo the ATP-induced conformational change required for formation (Fig. 5B). These results fit to the observation that in yeast MutSα MSH2 but not MSH6 interacts with MutLα, leading to the conclusion that one subunit of MutS directly recognizes the mismatch, and the second subunit recruits MutL (32). It is tempting to speculate that for E. coli MutS subunit B is in contact with MutL, whereas subunit A recognizes the mismatch. Based on comparative normal mode analysis, a movement of the mismatch-binding domain, primarily of subunit A (or MSH6 in eukaryotic MutSα), similar to that shown in Fig. 5B, has been proposed (26). However, without additional experiments, we cannot distinguish whether one or both subunits are able to form the internal cross-link that indicates a mismatch and ATP-induced movement of the mismatch-binding domain.
We were able to generate a coarse grained structural model of the MutS-MutL complex that is based on experimental data (Fig. 6). This model is in agreement with the proposed interaction surface of MutL for MutS (52) and with the identification of the connector domain of MutS being important and sufficient for the interaction with MutL (32). We are aware that more experimental data are needed to refine this model and to determine additional states of the MutS-MutL complex during the MMR processes. For example, in our model, we did not take into account the conformational changes in MutS that had been observed experimentally (Fig. 5 and Refs. 24, 30, and 59) and modeled using normal mode analysis and molecular dynamics simulations (25, 26). These changes, which are likely to influence the mode of MutS-DNA interaction, may involve the transition from a bent to an unbent DNA conformation as proposed before (60) and indicated in Figs. 5C and 6B. Despite this limitation, it is possible to suggest a coarse grained model for the MutS, MutL, and MutH complex on DNA (Fig. 6B) using the previous published model for the MutL-MutH complex (37). According to the model presented, it seems likely that a complex comprising MutS, MutL, and MutH can assemble on the DNA without the formation of an intervening DNA loop, thereby enabling the complex to scan the DNA for the strand discrimination signal (Fig. 6C). This complex would be affected by protein roadblocks on the DNA as has been observed experimentally (61).
CONCLUSIONS
The biochemical analyses of the interaction of several MutS variants with MutL carried out in this study and the data from literature support the following conclusions. First, in the ternary complex, the mismatch-binding and connector domains of MutS are in proximity to the ATPase domain of MutL. Second, the interaction between MutS and MutL does not depend on the formation of the closed form of MutL. Third, ATP-induced sliding clamp formation is accompanied by an ATP-induced movement of the mismatch-binding domain toward the connector domain. This movement is independent of the binding of MutL to MutS. Finally, the structural model presented for the MutS-MutL-MutH complex fits well to models for the coupling of the mismatch recognition and strand discrimination process involving the formation of an active MutS clamp that recruits MutL and MutH scanning the DNA for the strand discrimination signal (i.e. in E. coli a hemimethylated GATC site (58, 61)).
Supplementary Material
Acknowledgments
We thank Lidia Litz for excellent technical assistance and Flora Groothuizen for critically reading the manuscript.
This work was supported by European Community Seventh Framework Program FP7/2007-2013 under Grant HEALTH-F4-2008-223545 (to P. F., I. W., and R. J. H.), Deutsche Forschungsgemeinschaft Grant GRK 1384 “Enzymes and Multienzyme Complexes Acting on Nucleic Acids,” and Russian Foundation for Basic Research Grant RFBR-DFG 08-04-91973 (to P. F. and A. D. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4, Tables S1 and S2, and additional experimental procedures.
- MMR
- mismatch repair system
- AMPPNP
- 5′-adenylyl-β,γ-imidodiphosphate
- BM[PEO]4
- 1,11-bismaleimidotetraethylene glycol
- ATPγS
- adenosine 5′-O-(thiotriphosphate).
REFERENCES
- 1. Modrich P., Lahue R. (1996) Annu. Rev. Biochem. 65, 101–133 [DOI] [PubMed] [Google Scholar]
- 2. Lahue R. S., Su S. S., Modrich P. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 1482–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y., Yuan F., Presnell S. R., Tian K., Gao Y., Tomkinson A. E., Gu L., Li G. M. (2005) Cell 122, 693–705 [DOI] [PubMed] [Google Scholar]
- 4. Constantin N., Dzantiev L., Kadyrov F. A., Modrich P. (2005) J. Biol. Chem. 280, 39752–39761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Larrea A. A., Lujan S. A., Kunkel T. A. (2010) Cell 141, 730. [DOI] [PubMed] [Google Scholar]
- 6. Drummond J. T., Li G. M., Longley M. J., Modrich P. (1995) Science 268, 1909–1912 [DOI] [PubMed] [Google Scholar]
- 7. Li G. M., Modrich P. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 1950–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manelyte L., Urbanke C., Giron-Monzon L., Friedhoff P. (2006) Nucleic Acids Res. 34, 5270–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendillo M. L., Putnam C. D., Kolodner R. D. (2007) J. Biol. Chem. 282, 16345–16354 [DOI] [PubMed] [Google Scholar]
- 10. Lamers M. H., Perrakis A., Enzlin J. H., Winterwerp H. H., de Wind N., Sixma T. K. (2000) Nature 407, 711–717 [DOI] [PubMed] [Google Scholar]
- 11. Obmolova G., Ban C., Hsieh P., Yang W. (2000) Nature 407, 703–710 [DOI] [PubMed] [Google Scholar]
- 12. Warren J. J., Pohlhaus T. J., Changela A., Iyer R. R., Modrich P. L., Beese L. S. (2007) Mol. Cell 26, 579–592 [DOI] [PubMed] [Google Scholar]
- 13. Ban C., Yang W. (1998) Cell 95, 541–552 [DOI] [PubMed] [Google Scholar]
- 14. Guarné A., Ramon-Maiques S., Wolff E. M., Ghirlando R., Hu X., Miller J. H., Yang W. (2004) EMBO J. 23, 4134–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kosinski J., Steindorf I., Bujnicki J. M., Giron-Monzon L., Friedhoff P. (2005) J. Mol. Biol. 351, 895–909 [DOI] [PubMed] [Google Scholar]
- 16. Pillon M. C., Lorenowicz J. J., Uckelmann M., Klocko A. D., Mitchell R. R., Chung Y. S., Modrich P., Walker G. C., Simmons L. A., Friedhoff P., Guarné A. (2010) Mol. Cell 39, 145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Malkov V. A., Biswas I., Camerini-Otero R. D., Hsieh P. (1997) J. Biol. Chem. 272, 23811–23817 [DOI] [PubMed] [Google Scholar]
- 18. Lebbink J. H., Georgijevic D., Natrajan G., Fish A., Winterwerp H. H., Sixma T. K., de Wind N. (2006) EMBO J. 25, 409–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schofield M. J., Brownewell F. E., Nayak S., Du C., Kool E. T., Hsieh P. (2001) J. Biol. Chem. 276, 45505–45508 [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto A., Schofield M. J., Biswas I., Hsieh P. (2000) Nucleic Acids Res. 28, 3564–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gradia S., Acharya S., Fishel R. (2000) J. Biol. Chem. 275, 3922–3930 [DOI] [PubMed] [Google Scholar]
- 22. Jeong C., Cho W. K., Song K. M., Cook C., Yoon T. Y., Ban C., Fishel R., Lee J. B. (2011) Nat. Struct. Mol. Biol. 18, 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gradia S., Subramanian D., Wilson T., Acharya S., Makhov A., Griffith J., Fishel R. (1999) Mol. Cell 3, 255–261 [DOI] [PubMed] [Google Scholar]
- 24. Lamers M. H., Georgijevic D., Lebbink J. H., Winterwerp H. H., Agianian B., de Wind N., Sixma T. K. (2004) J. Biol. Chem. 279, 43879–43885 [DOI] [PubMed] [Google Scholar]
- 25. Mukherjee S., Law S. M., Feig M. (2009) Biophys. J. 96, 1707–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mukherjee S., Feig M. (2009) Biophys. J. 96, L63–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mendillo M. L., Mazur D. J., Kolodner R. D. (2005) J. Biol. Chem. 280, 22245–22257 [DOI] [PubMed] [Google Scholar]
- 28. Lebbink J. H., Fish A., Reumer A., Natrajan G., Winterwerp H. H., Sixma T. K. (2010) J. Biol. Chem. 285, 13131–13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Polosina Y. Y., Cupples C. G. (2010) BioEssays 32, 51–59 [DOI] [PubMed] [Google Scholar]
- 30. Mendillo M. L., Putnam C. D., Mo A. O., Jamison J. W., Li S., Woods V. L., Jr., Kolodner R. D. (2010) J. Biol. Chem. 285, 13170–13182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hargreaves V. V., Shell S. S., Mazur D. J., Hess M. T., Kolodner R. D. (2010) J. Biol. Chem. 285, 9301–9310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mendillo M. L., Hargreaves V. V., Jamison J. W., Mo A. O., Li S., Putnam C. D., Woods V. L., Jr., Kolodner R. D. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 22223–22228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas E., Pingoud A., Friedhoff P. (2002) Biol. Chem. 383, 1459–1462 [DOI] [PubMed] [Google Scholar]
- 34. Heinze R. J., Giron-Monzon L., Solovyova A., Elliot S. L., Geisler S., Cupples C. G., Connolly B. A., Friedhoff P. (2009) Nucleic Acids Res. 37, 4453–4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirsch R. D., Joly E. (1998) Nucleic Acids Res. 26, 1848–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toedt G. H., Krishnan R., Friedhoff P. (2003) Nucleic Acids Res. 31, 819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giron-Monzon L., Manelyte L., Ahrends R., Kirsch D., Spengler B., Friedhoff P. (2004) J. Biol. Chem. 279, 49338–49345 [DOI] [PubMed] [Google Scholar]
- 38. Feng G., Winkler M. E. (1995) BioTechniques 19, 956–965 [PubMed] [Google Scholar]
- 39. Pace C. N., Vajdos F., Fee L., Grimsley G., Gray T. (1995) Protein Sci. 4, 2411–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schuck P. (2000) Biophys. J. 78, 1606–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Durchschlag H. (1986) in Thermodynamic Data for Biochemistry and Biotechnology (Hinz H. J. ed) pp. 45–128, Springer-Verlag, Berlin [Google Scholar]
- 42. Ritchie D. W., Kemp G. J. (2000) Proteins 39, 178–194 [PubMed] [Google Scholar]
- 43. Plotz G., Welsch C., Giron-Monzon L., Friedhoff P., Albrecht M., Piiper A., Biondi R. M., Lengauer T., Zeuzem S., Raedle J. (2006) Nucleic Acids Res. 34, 6574–6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacobs-Palmer E., Hingorani M. M. (2007) J. Mol. Biol. 366, 1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhai J., Hingorani M. M. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 680–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Blackwell L. J., Wang S., Modrich P. (2001) J. Biol. Chem. 276, 33233–33240 [DOI] [PubMed] [Google Scholar]
- 47. Baitinger C., Burdett V., Modrich P. (2003) J. Biol. Chem. 278, 49505–49511 [DOI] [PubMed] [Google Scholar]
- 48. Junop M. S., Obmolova G., Rausch K., Hsieh P., Yang W. (2001) Mol. Cell 7, 1–12 [DOI] [PubMed] [Google Scholar]
- 49. Natrajan G., Lamers M. H., Enzlin J. H., Winterwerp H. H., Perrakis A., Sixma T. K. (2003) Nucleic Acids Res. 31, 4814–4821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Green N. S., Reisler E., Houk K. N. (2001) Protein Sci. 10, 1293–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iyer R. R., Pluciennik A., Burdett V., Modrich P. L. (2006) Chem. Rev. 106, 302–323 [DOI] [PubMed] [Google Scholar]
- 52. Ban C., Junop M., Yang W. (1999) Cell 97, 85–97 [DOI] [PubMed] [Google Scholar]
- 53. Kato R., Kataoka M., Kamikubo H., Kuramitsu S. (2001) J. Mol. Biol. 309, 227–238 [DOI] [PubMed] [Google Scholar]
- 54. Sacho E. J., Kadyrov F. A., Modrich P., Kunkel T. A., Erie D. A. (2008) Mol. Cell 29, 112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stevens N., Dyer J., Martí A. A., Solomon M., Turro N. J. (2007) Photochem. Photobiol. Sci. 6, 909–911 [DOI] [PubMed] [Google Scholar]
- 56. Lee J. Y., Chang J., Joseph N., Ghirlando R., Rao D. N., Yang W. (2005) Mol. Cell 20, 155–166 [DOI] [PubMed] [Google Scholar]
- 57. Junop M. S., Yang W., Funchain P., Clendenin W., Miller J. H. (2003) DNA Repair 2, 387–405 [DOI] [PubMed] [Google Scholar]
- 58. Acharya S., Foster P. L., Brooks P., Fishel R. (2003) Mol. Cell 12, 233–246 [DOI] [PubMed] [Google Scholar]
- 59. Lamers M. H., Winterwerp H. H., Sixma T. K. (2003) EMBO J. 22, 746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang H., Yang Y., Schofield M. J., Du C., Fridman Y., Lee S. D., Larson E. D., Drummond J. T., Alani E., Hsieh P., Erie D. A. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14822–14827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pluciennik A., Modrich P. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 12709–12713 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.