Abstract
Multiple Escherichia coli isolates from four adults with extraintestinal infections underwent molecular phylotyping and virulence profiling. A patient with secondary peritonitis had two low-virulence E. coli strains from phylogenetic groups A and D. In contrast, three patients with invasive extraurinary infections (septic arthritis/pyomyositis, nontraumatic meningitis/hematogenous osteomyelitis, and pneumonia) each had a single high-virulence phylogenetic group B2 strain resembling typical isolates causing urinary infection and/or sepsis, i.e., extraintestinal pathogenic E. coli.
Urinary tract infection (UTI), sepsis, and neonatal meningitis are the most-studied extraintestinal infection syndromes caused by Escherichia coli (8, 9). The causative strains constitute a distinctive subset of the E. coli population characterized by a predominance of phylogenetic group B2 and diverse virulence factors (VFs), such as adhesins, iron sequestration systems, toxins, and polysaccharide coatings (8, 9).
E. coli from different syndromes has been regarded as syndrome specific, as reflected in the traditional designations uropathogenic E. coli, sepsis-associated E. coli, and meningitis-associated E. coli (8, 9). However, accumulating evidence suggests considerable overlap among these major syndromes with respect to the causative strains and their VFs (8, 9). Additionally, bacteremic pneumonia (7), spontaneous peritonitis (20), and even ascending cholangitis (21) are caused by E. coli strains resembling those that cause UTI, meningitis, and sepsis. This supports use of an inclusive designation such as extraintestinal pathogenic E. coli (ExPEC) for such strains (18). It also suggests the possibility of broadly active preventive measures (8, 9), which are sorely needed because of the tremendous morbidity, mortality, and increased costs associated with extraintestinal E. coli infections (17).
We recently reported three patients with invasive extraurinary infections due to E. coli clones classically associated with UTI, neonatal meningitis, and/or sepsis (9). The infections included community-acquired pneumonia, vertebral osteomyelitis with associated epidural/iliacus/psoas abscesses, and deep surgical wound infection (9). In the present study we further tested the hypothesis that ExPEC, which is established as the main cause of UTI and neonatal E. coli meningitis, also causes most other invasive extraintestinal E. coli infections.
Patients and isolates.
Patients 1 to 3 (2002 to 2003) and 4 (2001) were from the Veterans Affairs Medical Centers in Buffalo, N.Y., and Minneapolis, Minn., respectively. Isolates were retrieved from the hospital clinical microbiology laboratories after identification and susceptibility testing and were stored at −80 C.
Molecular methods.
Isolates were defined as representing the same or different strains according to randomly amplified polymorphic DNA (RAPD) analysis, which was done by using oligonucleotide 1254 (5′-CCGCAGCCAA-3′) (9). Isolates were assigned to one of four major E. coli phylogenetic groups (A, B1, B2, and D) by multiplex PCR (6) and were tested for 35 ExPEC-associated VFs and 13 alleles of papA (P fimbrial structural subunit) by PCR and dot blot hybridization (6). Six additional VFs, including F17 fimbriae (3), secreted autotransporter toxin (sat) (5), uropathogen-specific protein (usp) (1), afimbrial adhesin VIII (afaE8) (12), CS31A adhesins (clpG) (2), and enteroaggregative E. coli heat-stable toxin EAST1 (astA) (22), also were detected by PCR. Primers included, for F17, P7 and P9 (3); for sat, SatF (5′-GCAGCTACCGCAATAGGAGGT-3′) and SatR (5′-CATTCAGAGTACCGGGGCCTA-3′; 937-bp product); for usp, Usp-F and Usp-R (1); for afaE8, afaE8-f and afaE8-r (12); for clpG, clpG1 and clpG2 (2); and for astA, EAST12a and EAST12b (22). PCR conditions for the six added VFs were as described (6), except for a 58°C annealing temperature. Three previously reported ExPEC isolates from patients with invasive extraintestinal infections (9) also were tested for these six VFs, for comparison with the new isolates. Based on previous molecular epidemiological analyses, isolates were defined as ExPEC if positive for ≥2 of five key markers: papA and/or papC (P fimbriae), sfa/foc (S and F1C fimbriae), afa/dra (Dr-binding adhesins), iutA (aerobactin system), and kpsM II (6). The VF score was the number of unique VFs detected (6).
Statistical methods.
Comparisons of proportions were tested with Fisher's exact test. Comparisons of VF scores were tested with the Mann-Whitney U test. The criterion for statistical significance was a P of <0.05. Confidence intervals were calculated according to standard methods (13).
Case 1.
A 79-year-old man was admitted for elective repair of a Spigelian hernia. After 48 h he developed tachypnea, abdominal distension, right lower quadrant cellulitic changes, localized peritoneal signs, and leukocytosis. Ampicillin-sulbactam was given, and he was taken to laparotomy, which revealed peritonitis, purulent fluid, and a perforated retrocecal appendix without abscess formation. Intraoperative cultures yielded E. coli no. 1 (susceptible to all antibiotics tested), E. coli no. 2 (resistant only to cephalothin), Klebsiella pneumoniae, beta-hemolytic Streptococcus (not group A, B, C, F, or G), Bacteroides species, Clostridium species, and Prevotella species. Piperacillin-tazobactam was given for 10 days, and the patient recovered uneventfully.
Case 2.
A 56-year-old man was admitted with 3 days of fatigue, fever, and chills and 1 day of chest pain and dyspnea. Past history included diabetes mellitus, coronary disease, and chronic left shoulder pain status post-rotator cuff surgery. Abnormal physical findings included a temperature of 101.3°C. Myocardial infarction was confirmed by serial enzyme determinations and electrocardiography. The following day the patient developed leukocytosis and the admission urine and blood cultures were growing E. coli, susceptible to all antibiotics tested. Piperacillin-tazobactam therapy was instituted. A transthoracic echocardiogram revealed no valvular lesions.
The patient developed confusion, agitation, back pain, and an inflamed left shoulder and right ankle. Fluid from both joints showed an elevated white-blood-cell count but no crystals and grew E. coli susceptible to all antibiotics tested. Daily fevers persisted. Abdominal computed tomography (CT) showed emphysematous myositis of the right psoas and erector spinae muscles, without abscesses or vertebral osteomyelitis. Psoas fluid aspirated under CT guidance on day 14 yielded E. coli susceptible to all antibiotics tested. Antibiotic therapy was intensified. A transthoracic echocardiogram again revealed no distinct vegetations. Antibiotics were continued for 6 weeks, with full recovery.
Case 3.
A 72-year-old man with alcoholic cirrhosis presented with increasing confusion, nausea, vomiting, and diarrhea. Initial evaluation showed acute renal insufficiency, biochemical pancreatitis, and presumed hepatic encephalopathy. Abdominal ultrasonography and CT showed no ascites. Urinalysis revealed pyuria. Piperacillin-tazobactam was given for possible UTI. Admission urine and blood cultures grew E. coli susceptible to all antibiotics tested.
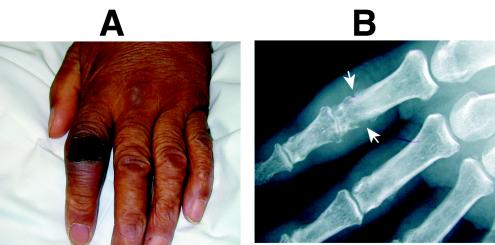
The patient remained unresponsive despite improving blood chemistries. Cerebrospinal fluid (CSF) from day 3 contained 9,450 white blood cells per μl (92% neutrophils), showed gram-negative bacilli on smear, and grew E. coli within 24 h. The antibiotic regimen was revised. The patient's mental status slowly improved over the next 2 weeks, but a new ecchymosis appeared over the left index finger and a roentgenogram showed evidence of acute osteomyelitis (Fig. 1). Aspiration was not attempted. Recovery continued slowly until the patient's death on day 28 from unrelated causes.
FIG. 1.
Clinical and radiographic evidence of hematogenous osteomyelitis (case 3). (A) Ecchymosis with associated swelling is evident over the proximal interphalangeal joint of the left index finger. (B) Underlying bony destruction consistent with osteomyelitis is apparent radiographically (white arrows).
Case 4.
A 67-year-old man was admitted from home with acute onset dyspnea, nausea, vomiting, abdominal cramps, and confusion. Past history included ankylosing spondylitis and Crohn's disease (both in remission), adrenal insufficiency with replacement therapy, mild chronic renal insufficiency, gout, stable coronary disease, moderate aortic stenosis, and chronic interstitial and restrictive lung disease. The patient lived independently and exercised thrice weekly at a fitness center.
The initial evaluation suggested septic shock. The chest roentgenogram showed chronic bilateral interstitial infiltrates. Expectorated sputum was grossly purulent and microscopically showed many neutrophils and gram-negative bacilli. Sputum culture yielded many E. coli bacteria susceptible to all antibiotics tested and many K. pneumoniae bacteria resistant only to ampicillin. Urinalysis showed pyuria, and urine culture yielded 10,000 colonies of Candida tropicalis per ml.
Despite fluid resuscitation and broad-spectrum antibiotic therapy, the patient remained hypotensive and anuric. He developed rapidly progressive bilateral alveolar infiltrates and worsening hypoxemia and required intubation, mechanical ventilation, bicarbonate infusions, and high-dose combination vasopressor therapy. Leukopenia developed. Echocardiography demonstrated a hyperdynamic left ventricle. A pretherapy blood culture grew only E. coli susceptible to all antibiotics tested. Because of refractory shock and respiratory failure despite maximal supportive measures, after discussion with the family, aggressive support was withdrawn. The patient died shortly thereafter.
Molecular typing results.
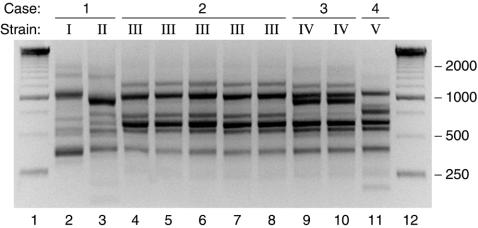
The two E. coli peritoneal isolates from patient 1 exhibited distinct RAPD profiles, hence representing two unique strains (Fig. 2). In contrast, the five isolates from patient 2, which were from blood (n = 1), psoas muscle (n = 2), left shoulder (n = 1), and right ankle (n = 1), all exhibited the same RAPD profile, hence representing a single strain. Likewise, the blood (n = 1) and CSF (n = 1) isolates from patient 3 exhibited the same RAPD profile, hence representing a single strain. (The initial urine isolate from patient 3 was not available for analysis.) From patient 4, only the blood isolate was available, but since its susceptibility profile matched that of the concurrent E. coli sputum isolate, these isolates were presumed to represent the same strain.
FIG. 2.
RAPD profiles of 10 Escherichia coli isolates from four patients with serious extraintestinal E. coli infections. Case and strain number are indicated above each sample lane (lanes 2 to 11). Lane numbers are shown at bottom. Lanes 1 and 12, 250-bp ladder.
The two strains from patient 1 were from E. coli phylogenetic groups D and A. Both failed to satisfy the molecular criteria for ExPEC and exhibited extremely sparse VF profiles, with only four and two VFs detected, respectively. In contrast, all three strains from patients 2 to 4 were from phylogenetic group B2 (Table 1). All met criteria for ExPEC and exhibited robust virulence genotypes, with from 9 to 11 unique VFs each (Table 1).
TABLE 1.
Characteristics of E. coli isolates from four patients with serious extraintestinal E. coli infectionsa
| Case | Site | Strain | Phylo | Presence or absence of:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adhesin
|
Toxin
|
Iron system
|
Capsule
|
Miscellaneous
|
|||||||||||||||||
| papA | papG | sfa/foc | sfaS | focG | fimH | hlyD | cnf1 | cdtB | astA | iroN | fyuA | kpsM II | K2 | traT | ompT | malX | usp | ||||
| 1 | Peritoneum | I | D | − | − | − | − | − | + | − | − | + | + | − | − | − | − | + | − | − | − |
| 1 | Peritoneum | II | B1 | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | + | − | − |
| 2 | Blood | III | B2 | F48 | III | + | + | − | + | + | + | − | − | + | + | + | − | + | − | + | + |
| 2 | Psoas no. 1 | III | B2 | F48 | III | + | + | − | + | + | + | − | − | + | + | + | − | + | − | + | + |
| 2 | Psoas no. 2 | III | B2 | F48 | III | + | + | − | + | + | + | − | − | + | + | + | − | + | − | + | + |
| 2 | Shoulder | III | B2 | F48 | III | + | + | − | + | + | + | − | − | + | + | + | − | + | − | + | + |
| 2 | Ankle | III | B2 | F48 | III | + | + | − | + | + | + | − | − | + | + | + | − | + | − | + | + |
| 3 | Blood | IV | B2 | − | − | + | − | + | + | + | − | − | − | + | + | + | + | + | + | + | + |
| 3 | CSF | IV | B2 | − | − | + | − | + | + | + | − | − | − | + | + | + | + | + | + | + | + |
| 4 | Blood | V | B2 | F12 | III | + | − | − | + | + | + | − | − | + | + | + | − | − | + | + | + |
Phylo, E. coli phylogenetic group (A, B1, B2, or D); plus and minus signs, presence and absence of genes, respectively. Isolates positive for an allele of papA or papG (P fimbrial major subunit or adhesin, respectively) were also positive for papC or papEF (P fimbrial assembly or minor subunits, respectively). Other virulence markers detected in at least one isolate: sfa/foc (S and F1C fimbriae), sfaS (S fimbriae), focG (F1C fimbriae), fimH (type 1 fimbriae), hlyD (hemolysin), cnf1 (cytotoxic necrotizing factor 1), cdtB (cytolethal distending toxin), astA (enteroaggregative E. coli stable toxin), iroN (siderophore receptor), fyuA (yersiniabactin system), kpsM II (group 2 capsule, as detected by probe hybridization), K2 (K2 kpsM II variant, as inferred from hybridization with the kpsM II probe but not the corresponding primers) (10), traT (serum resistance associated), ompT (outer membrane protein T), malX (marker for pathogenicity island from strain CFT073), and usp (uropathogen-specific protein). No isolate was positive for a papA allele other than F12 and F48, papG allele I or II, afa/dra (Dr-binding adhesins), iha (putative siderophore-adhesin), bmaE (M fimbriae), gafD (G fimbriae), F17 (F17a-G and F11 adhesions), clpG (CS31A adhesion), afaE8 (afimbrial adhesion VIII), sat (secreted autotransporter toxin), ireA (siderophore receptor), iutA (aerobactin system), the K1 kpsMT variant, kpsMT III (group 3 capsule), rfc (O4 lipopolysaccharide), cvaC (colicin V), ibeA (invasion of brain endothelium), iss (increased serum survival), or the H7 flagellar (fliC) variant.
The five strains from the present study were combined for statistical analysis with the three previously reported strains from patients with invasive extraurinary infections (i.e., community-acquired pneumonia, vertebral osteomyelitis with associated epidural/iliacus/psoas abscesses, and deep surgical wound infection) (9). A statistically significant difference was apparent in the proportion of E. coli strains qualifying as ExPEC in invasive infections versus secondary peritonitis (six of six, versus zero of two [P = 0.036, Fisher's exact test]). Likewise, VF scores were significantly higher among the six invasive strains than they were among the two peritonitis isolates (median score, 11 versus 3 [P = 0.046, Mann-Whitney U test]).
Implications of findings.
This phylogenetic and pathotypic analysis of E. coli isolates from four patients with serious extraurinary infections demonstrated that the causative strains in the first case were quite different from those in the other three cases. The findings are consistent with the hypothesis that ExPEC can cause serious disease at diverse extraintestinal sites even in noncompromised or moderately impaired hosts, whereas non-ExPEC E. coli causes such infections only when assisted by major host compromise, a large inoculum, and/or companion microbes (8, 9).
Case 1 involved secondary peritonitis due to intestinal perforation, in which setting bowel contents spill directly into the peritoneal space, producing an initially polymicrobial infection. Here seven different bacterial strains were identified. The two E. coli strains were of low inferred virulence and from phylogenetic groups D and A, the latter being associated with commensal status (8). Thus, they appeared to represent nonpathogens. Given that in most individuals the predominant intestinal E. coli strains are commensals rather than ExPEC (even though ExPEC also derives from the host's gut flora) (8), it is not surprising that only commensal-like E. coli bacteria were isolated immediately post-intestinal perforation. It will be interesting to determine if E. coli strains that exhibit prolonged survival within the peritoneal cavity (e.g., in abscesses) are more likely to be ExPEC.
In contrast, cases 2 to 4 involved diverse spontaneous invasive infections at sites removed from the intestinal tract, in each instance caused by a single E. coli strain of high inferred pathogenicity from virulence-associated phylogenetic group B2 (8). Notably, in case 3 bacteremia and meningitis developed in the setting of alcoholic liver disease. Meningitis due to E. coli and other enteric gram-negative bacteria is a rare event in the adult population. However, recent reports document an increased incidence of meningitis among cirrhotics (14, 16). Although the underlying mechanism for this association is unknown, a diminished hepatic capacity for bacterial clearance may cause an increased incidence and/or degree of bacteremia. If such bacteremia episodes are caused by pathogenic strains such as ExPEC, it is easy to envision metastatic spread to other sites such as the meninges or peritoneum.
The causative E. coli strains in cases 2 to 4 closely resembled typical UTI, neonatal meningitis, and sepsis isolates with respect to phylogenetic background and VF profiles (9). These characteristics, plus the strains' aggressive clinical behavior, identified them as true pathogens, i.e., ExPEC (18). These findings comport with previous data showing that E. coli isolates from diverse extraintestinal infection syndromes more commonly exhibit UTI-associated virulence traits or serotypes than do fecal controls (7, 20, 21). Cases 2 to 4 extend to six our series of consecutive patients in whom spontaneous invasive extraurinary E. coli infections were due to typical ExPEC strains (9). This allowed detection of a significantly higher prevalence of ExPEC and of significantly higher VF scores, among such strains compared with secondary peritonitis isolates. It also permits a revised calculation of 61% (cf. the previously calculated 29%) (9) for the lower 95% confidence limit for the proportion of such invasive infections caused by ExPEC, evidence that most such infections are due to ExPEC.
The present cases also are significant in that they add emphysematous pyomyositis, septic arthritis, spontaneous meningitis, and nonvertebral hematogenous osteomyelitis to the spectrum of extraintestinal infections caused by ExPEC in adults. These findings further illustrate the limitations of conventional syndrome-specific designations such as uropathogenic E. coli, sepsis-associated E. coli, and meningitis-associated E. coli and support the prediction that virulence analysis of ExPEC isolates from one clinical syndrome may yield insights also into the pathogenesis of anatomically distinct, yet pathophysiologically related, syndromes (8, 9). They thus encourage the hope that, as suggested by a growing body of evidence regarding the cross-syndrome commonality of ExPEC clones and VFs, broadly applicable protective interventions (4, 10, 11, 15, 19) may be developed against multiple extraintestinal syndromes due to E. coli.
Acknowledgments
This material is based upon work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (J.R.J., A.J.L., and T.A.R.); National Institutes of Health grants DK-47504 (J.R.J.), HL-69763 (T.A.R.), and AI-42059 (T.A.R.); the John R. Oishei Foundation (T.A.R.); and National Research Initiative Competitive Grants Program/U.S. Department of Agriculture grant 00-35212-9408 (J.R.J.).
Dave Prentiss helped prepare the figures. Ann Emery helped prepare the manuscript.
REFERENCES
- 1.Bauer, R. J., L. Zhang, B. Foxman, A. Siitonen, M. E. Jantunen, H. Saxen, and C. F. Marrs. 2002. Molecular epidemiology of 3 putative virulence genes for Escherichia coli urinary tract infection—usp, iha, and iroNE. coli. J. Infect. Dis. 185:1521-1524. [DOI] [PubMed] [Google Scholar]
- 2.Bertin, Y., C. Martin, J.-P. Girardieu, P. Pohl, and M. Conterpois. 1998. Association of genes encoding P fimbriae, CS31A antigen and EAST 1 toxin among CNF1-producing Escherichia coli strains from cattle with septicemia and diarrhea. FEMS Microbiol. Lett. 162:235-239. [DOI] [PubMed] [Google Scholar]
- 3.Bertin, Y., C. Martin, E. Oswald, and J.-P. Girardieu. 1996. Rapid and specific detection of F17-related pilin and adhesin genes in diarrheic and septicemic Escherichia coli strains by multiplex PCR. J. Clin. Microbiol. 34:2921-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox, F., and L. Taylor. 1990. Prevention of Escherichia coli K1 bacteremia in newborn mice by using topical vaginal carbohydrates. J. Infect. Dis. 162:978-981. [DOI] [PubMed] [Google Scholar]
- 5.Guyer, D. M., I. R. Henderson, J. P. Nataro, and H. L. T. Mobley. 2000. Identification of Sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53-56. [DOI] [PubMed] [Google Scholar]
- 6.Johnson, J. R., M. A. Kuskowski, K. Owens, A. Gajewski, and P. L. Winokur. 2003. Phylogenetic origin and virulence genotype in relation to resistance to fluoroquinolones and/or extended-spectrum cephalosporins and cephamycins among Escherichia coli isolates from animals and humans. J. Infect. Dis. 188:759-768. [DOI] [PubMed] [Google Scholar]
- 7.Johnson, J. R., T. T. O'Bryan, M. A. Kuskowski, and J. N. Maslow. 2001. Ongoing horizontal and vertical transmission of virulence genes and papA alleles among Escherichia coli blood isolates from patients with diverse-source bacteremia. Infect. Immun. 69:5363-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson, J. R., and T. A. Russo. 2002. Extraintestinal pathogenic Escherichia coli (ExPEC): the “other bad E. coli.” J. Lab. Clin. Med. 139:155-162. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, J. R., and T. A. Russo. 2002. “Uropathogenic” Escherichia coli as agents of diverse non-urinary-tract extraintestinal infections. J. Infect. Dis. 186:859-864. [DOI] [PubMed] [Google Scholar]
- 10.Kaijser, B., P. Larsson, S. Olling, and R. Schneerson. 1983. Protection against acute, ascending pyelonephritis caused by Escherichia coli in rats, using isolated capsular antigen conjugated to bovine serum albumin. Infect. Immun. 39:142-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langermann, S., S. Palaszynski, M. Barnhart, G. Auguste, J. S. Pinkner, J. Burlein, P. Barren, S. Koenig, S. Leath, C. H. Jones, and S. J. Hultgren. 1997. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276:607-611. [DOI] [PubMed] [Google Scholar]
- 12.Le Bougenec, C., L. Lalioui, L. du Merle, M. Jouve, P. Courcoux, S. Bouzari, R. Selvarangan, B. J. Nowicki, G. Y., A. Andremont, P. Gounon, and M.-I. Garcia. 2001. Characterization of AfaE adhesins produced by extraintestinal and intestinal human Escherichia coli isolates: PCR assays for detection of Afa adhesins that do or do not recognize Dr blood group antigens. J. Clin. Microbiol. 39:1738-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lentner, C., K. Diem, and J. Seldrup. 1982. Introduction to statistics. Statistical tables. Mathematical formulae, p. 89-103, 221-222. In C. Lentner, C. Lentner, and A. Wink (ed.), Geigy scientific tables, vol. 2. Medical Education Division, Ciba-Geigy Corp., West Caldwell, N.J.
- 14.Molle, I., A. M. Thulstrup, N. Svendsen, C. Schonheyder, and H. T. Sorensen. 2000. Risk and case fatality rate of meningitis in patients with liver cirrhosis. Scand. J. Infect. Dis. 32:407-410. [DOI] [PubMed] [Google Scholar]
- 15.O'Hanley, P. 1996. Prospects for urinary tract infection vaccines, p. 405-425. In H. L. T. Mobley and J. W. Warren (ed.), Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, D.C.
- 16.Pauwels, A., E. Pines, M. Abboura, I. Chiche, and V. G. Levy. 1997. Bacterial meningitis in cirrhosis: review of 16 cases. J. Hepatol. 27:830-834. [DOI] [PubMed] [Google Scholar]
- 17.Russo, T. A., and J. R. Johnson. 2003. Medical and economic impact of extraintestinal infections due to Escherichia coli: an overlooked epidemic. Microbes Infect. 5:449-456. [DOI] [PubMed] [Google Scholar]
- 18.Russo, T. A., and J. R. Johnson. 2000. A proposal for an inclusive designation for extraintestinal pathogenic Escherichia coli: ExPEC. J. Infect. Dis. 181:1753-1754. [DOI] [PubMed] [Google Scholar]
- 19.Somerville, J. E., L. Casiano, and R. P. Darveau. 1999. Escherichia coli msbB gene as a virulence factor and a therapeutic target. Infect. Immun. 67:6583-6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soriano, G., P. Coll, C. Guarner, J. Such, F. Sanchez, G. Prats, and F. Vilardell. 1995. Escherichia coli capsular polysaccharide and spontaneous bacterial peritonitis in cirrhosis. Hepatology 21:668-673. [PubMed] [Google Scholar]
- 21.Wang, M.-C., C.-C. Tseng, C.-Y. Chen, J.-J. Wu, and J.-J. Huang. 2002. The role of bacterial virulence and host factors in patients with Escherichia coli bacteremia who have acute cholangitis or upper urinary tract infection. Clin. Infect. Dis. 35:1161-1166. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto, T., and P. Echeverria. 1996. Detection of the enteroaggregative Escherichia coli heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coli strains pathogenic for humans. Infect. Immun. 64:1441-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]