Seizures and memory loss are common features of anti-LGI1 limbic encephalitis,1 a recently described autoimmune encephalitis, previously attributed to voltage-gated potassium channel antibodies (VGKC-ab).2,3
Abnormal involuntary movements are frequently seen in patients diagnosed with anti-LGI1 limbic encephalitis. However, to date it is not clear if these abnormal movements represent seizures or an extrapyramidal movement disorder. Here we describe 3 patients with anti-LGI1 limbic encephalitis, who presented with severe movement abnormalities that were associated with ictal EEG changes.
Methods.
We present 3 female patients (ages 80, 46, and 72) with similar features.
Clinical features.
All patients developed abnormal movements of face, shoulders, arms, or legs (videos 1–3 on the Neurology® Web site at www.neurology.org). These strong, massive movements were slightly slower than typical myoclonus, occurring 5 to 100 times per day. Their consciousness was preserved during the movements.
EEG.
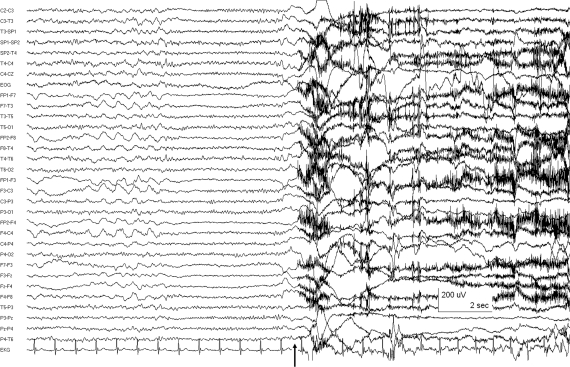
Continuous video-EEG recordings showed that the longer movements were clearly associated with generalized EEG electrodecremental events (EDEs), usually preceding the onset of movement by approximately 500 msec (figure), a pattern typical of epileptic tonic seizures. Identical movements of shorter duration were not associated with definite EEG changes.
Figure. Ictal EEG (patient 1) using extended 10–20 system with surface sphenoidal (zygomatic) electrodes, displaying a combination of circular and longitudinal bipolar montages.
The arrow indicates the onset of movement, which occurred after a generalized EEG electrodecremental event (EDE). The onset of EDEs could precede by as much as 500 msec the onset of the more prolonged abnormal movements, indicating that the motor events were indeed epileptic tonic seizures.
Interestingly, all 3 patients also developed frequent, repeated subclinical electrographic seizures of an entirely different sort, not associated with their tonic seizures. These subclinical seizures were recorded with a broad distribution, at times showing a regional maximum over the temporal (neocortical) regions. In contrast to the tonic seizures described here, this sort of subclinical electrographic seizure activity has been previously observed.4,5
Imaging.
Patients 1 and 2 developed hippocampal atrophy. Patient 3 had normal brain imaging.
Antibody testing.
Investigations in all 3 patients confirmed high anti-LGI1 antibody titers.
Response to treatment.
The tonic seizures improved significantly with a combination of lamotrigine and carbamazepine in patient 1, and phenytoin and levetiracetam in patient 2. Patient 3 had a poor response to carbamazepine and levetiracetam. She had a moderate and temporary response to IV immunoglobulin.
Results.
This is the first determination that the abnormal movements commonly seen in patients with anti-LGI1 limbic encephalitis (previously known as VGKC-ab limbic encephalitis) are tonic seizures. Though the motor movements were not absolutely typical of dyskinesias or myoclonus, in most instances ictal EEG recordings were unremarkable and, therefore, the movements could easily have been considered nonepileptic and not treated appropriately. It was only during some of the more prolonged events that an ictal EEG pattern was demonstrated, confirming that these were indeed tonic seizures. The ictal EDE pattern seen on scalp EEG, typical for tonic motor seizures, is consistent with remote onset and propagation of ictal activity. These seizures presumably originated in the interhemispheric frontal or frontoparietal structures. Due to the longer events captured with video-EEG an electrophysiologic diagnosis was possible, and treatment with antiepileptic drugs, with or without additional immunomodulation, was instituted.
Discussion.
Abnormal, involuntary movements have been described in up to 40% of patients with anti-LGI1 limbic encephalitis.1 These movements have been described as “twitches,”4 “myoclonus,”1 or “stereotyped brief monomorphic movements.”5 However, to date it was not known if these were seizures or a movement disorder. For instance, Irani and colleagues5 suggested that the highly stereotyped movements most likely represented extratemporal seizures. However, those authors noted that “without the gold-standard of video-EEG telemetry in all patients, potential nonepileptic generators of these jerk events, including startle disorders, paroxysmal nonkinesogenic dyskenesias, and negative myoclonus, form part of the differential diagnosis.” Barajas et al.4 also reported one patient with abnormal movements similar to those seen in our patients (synchronous twitches of arm and face) without a pathophysiologic diagnosis.
The unusual nature of these tonic seizures, when seen in the setting of an acute encephalopathy, and especially in patients with other, unrelated, temporal maximum ictal EEG abnormalities, should serve as a diagnostic clue to the possibility that the acute encephalopathy may be anti-LGI1 limbic encephalitis. It should be noted that these tonic seizures may present with abnormal movements similar to those described above, even without a definitive ictal EEG recording, as the latter may only be seen in association with the more prolonged seizures.
The abnormal myoclonic-like or spasm-like movements that are commonly seen in anti-GLI1 limbic encephalitis represent tonic seizures and not an extrapyramidal movement disorder. This is important as appropriate diagnosis of these movements may allow proper treatment with antiepileptic medications (with or without the addition of immunomodulation).
Supplementary Material
Footnotes
Supplemental data at www.neurology.org.
Disclosure: Dr. Andrade reports no disclosures. Dr. Tai has served on a scientific advisory board for Sepracor Inc. Dr. Dalmau has filed a patent application for the use of LGI1 as a diagnostic test; has received royalties from a patent re: Ma2 autoantibody test and has patents pending re: NMDA and GABAB receptor autoantibody tests (license fee payments received from EUROIMMUN AG); and receives research support from funding from EUROIMMUN AG and the NIH/NCI. Dr. Wennberg serves on the editorial board of Clinical Neurophysiology and serves as a consultant for Medtronic, Inc.
References
- 1. Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010;9:776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thieben MJ, Lennon VA, Boeve BF, Aksamit AJ, Keegan M, Vernino S. Potentially reversible autoimmune limbic encephalitis with neuronal potassium channel antibody. Neurology 2004;62:1177–1182 [DOI] [PubMed] [Google Scholar]
- 3. Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004;127:701–712 [DOI] [PubMed] [Google Scholar]
- 4. Barajas RF, Collins DE, Cha S, Geschwind MD. Adult-onset drug-refractory seizure disorder associated with anti-voltage-gated potassium-channel antibody. Epilepsia 51:473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Irani SR, Buckley C, Vincent A, et al. Immunotherapy-responsive seizure-like episodes with potassium channel antibodies. Neurology 2008;71:1647–1648 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.