Abstract
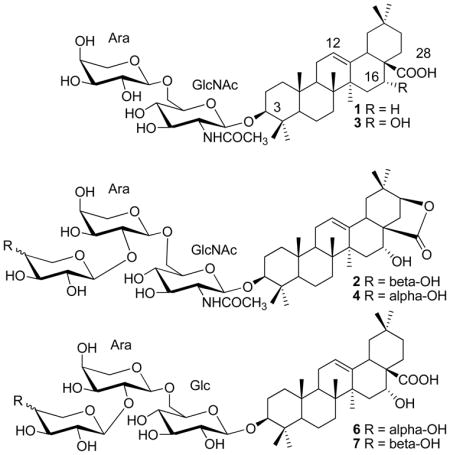
Bioassay-guided fractionation of a CH2Cl2-MeOH extract of the aerial parts of Albizia inundata resulted in the isolation of two new natural oleanane-type triterpene saponins {3-O-[α-L-arabinopyranosyl( 1→6)]-2-acetamido-2-deoxy-β-D-glucopyranosyl oleanolic acid (1) and 3-O-[α-L-arabinopyranosyl(1→2)-α-L-arabinopyranosyl(1→6)]-2-acetamido-2-deoxy-β-D-glucopyranosyl acacic acid lactone (2)} along with seven known saponins {3-O-[α-L-arabinopyranosyl(1→6)]-2-acetamido-2-deoxy-β-D-glucopyranosyl echinocystic acid (3), 3-O-[β-D-xylopyranosyl (1→2)-α-L-arabinopyranosyl (1→6)]-2-acetamido-2-deoxy-β-Dglucopyranosyl acacic acid lactone (concinnoside D) (4), 3-O-[β-D-glucopyranosyl (1→2)]-β-D-glucopyranosyl oleanolic acid (5), 3-O-[α-L-arabinopyranosyl (1→2)-α-L-arabinopyranosyl (1→6)]-β-D-glucopyranosyl oleanolic acid (6), 3-O-[β-D-xylopyranosyl (1→2)-α-L-arabinopyranosyl (1→6)]-β- D-glucopyranosyl oleanolic acid (7), 3-O-[α-L-arabinopyranosyl- (1→2)-α-L-arabinopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→2)]-β-D-glucopyranoside echinocystic acid (8) and 3-O-[β-D-xylopyranosyl-(1→2)-α-L-arabinopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→2)]-β-D-glucopyranoside echinocystic acid (9)}. The structures of 1 and 2 were established on the basis of extensive 2D NMR (1H-1H COSY or DQF-COSY, HSQC, HMBC, TOCSY, and HSQC-TOCSY) spectroscopic, ESIMS, and chemical methods. Saponins 1, 3, 6, and 7 showed cytotoxicity against human head and neck squamous cells (JMAR, MDA1986) and melanoma cells (B16F10, SKMEL28) with IC50 values in the range of 1.8–12.4 μM, using the MTS assay.
The genus Albizia (Fabaceae) is composed of about 150 species widely distributed in the tropics, with the greatest diversity encountered in Africa, and Central and South America.1 Triterpene saponins from several species (A. grandibracteata, A. gummifera, A. julibrissin) have been reported to exhibit cytotoxic activity in vitro against different cancer cell lines.2–5 As part of a continuing investigation to identify bioactive compounds from natural sources, a library of 200 plant extracts from Latin America was screened for cytotoxic activity in vitro against two human head and neck squamous cell carcinoma (HNSCC) cell lines as well as two melanoma cell lines. Albizia inundata (Martius) Barneby & Grimes, a perennial tree from Argentina, was one of the most active plant samples in the MTS [3-(4,5-dimethylthiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H tetrazolium] assay. The CH2Cl2-MeOH (1:1) extract of the aerial parts of this species showed cytotoxicity to JMAR and MDA1986 HNSCC cells and B16F10 and SKMEL28 melanoma cells with IC50 values in the range 1.0–3.8 μg/mL. This extract was selected for bioassay-guided fractionation, purification, and isolation of its active constituents. We report herein the details of the isolation, structure elucidation, and cytotoxicity of two new natural oleanane-type saponins (1, 2) and seven previously reported saponins (3–9). This is the first report of a bioassay-guided phytochemical study of this species.
The active crude CH2Cl2-MeOH extract of A. inundata (80 g) was divided into fractions soluble in hexane (5 g), EtOAc (17 g), n-BuOH (40 g), and water (12 g), by a liquid-liquid partitioning process. Bioassay results showed that the n-BuOH fraction showed the highest activity of all fractions tested, with cytotoxicity against the four cancer cell lines used (B16F10, JMAR, MDA1986, and SKMEL28) with IC50 values in the range of 0.1–1.0 μg/mL. The n-BuOH fraction (36 g) was further separated into six fractions by MCI gel CHP20P column chromatography, eluted by 100% water (Fraction 1), 20% MeOH (Fraction 2), 40% MeOH (Fraction 3), 60% MeOH (Fraction 4), 85% MeOH (Fraction 5), and 100% MeOH (Fraction 6). Fraction 5 (16 g), showed the highest activity against the four cancer cell lines tested with IC50 values in the range of 0.1–0.9 μg/mL. This fraction was further separated on a Si gel column eluted by CH2Cl2-MeOH-H2O (from 20:1:0.1 to 4:1:0.1). Further purification of the most active fraction [eluted by CH2Cl2-MeOH-H2O (7:1:0.1), with IC50 values in the range of 0.4–2.5 μg/mL against the above four cancer cell lines] by semi-preparative HPLC (mobile phase CH3CN:H2O), resulted in the isolation of two new natural saponins (1 and 2) and seven known saponins (3–9). The 1H and 13C NMR data indicated that compounds 1–9 are oleanene-type saponins. The absolute configuration of the sugar residues obtained by acidic hydrolysis of Fraction 5 was determined as L-arabinose, D-glucose, and D-xylose.
Compound 1, obtained as an amorphous powder, was found to have the molecular formula C43H69NO12 as determined through HRESIMS (positive mode, [M+H]+ peak at m/z 792.4869, calcd for C43H70NO12, 792.4898; [M+Na]+ peak at m/z 814.4697, calcd for C43H69NO12Na, 814.4717) and NMR experiments. Its 1H NMR spectrum (Table 1) showed the presence of eight methyl groups at δ 0.79, 0.97, 0.98, 1.00, 1.04, 1.15, 1.28, 2.16 (each 3H, s). Further features were signals observed at δ 3.17 (1H, dd, J = 12.5, 4.7 Hz, typical for an axial proton attached to a hydroxylate carbon), and at δ 4.95 (1H, d, J = 6.6 Hz), δ 4.98 (1H, d, J = 8.3 Hz), δ 5.46 (1H, brt, J = 3.0 Hz, assignable to a vinylic proton), and at δ 8.94 (1H, d, J = 9.1 Hz). The 13C NMR spectrum (DEPT) of 1 (Table 1) displayed a quaternary carbon signal at δ 180.7 (suggesting the presence of a COOH group), a quaternary carbon signal at δ 170.5 (ester or amide carbon), two olefinic carbon signals (one quaternary at δ 145.2 and one methine at δ 123.1, suggesting the presence of a double bond). The spectrum also showed two signals at δ 105.8 (CH) and 105.6 (CH) assignable to anomeric carbons of two sugar units. On the basis of these data, 1 was determined to be a triterpene diglycoside with a triterpene acid moiety of an oleanene skeleton. The assignments of the 1H and 13C signals of the triterpene moiety were secured by HSQC, 1H-1H COSY, and HMBC experiments, which allowed for the identification of the aglycon as oleanolic acid. The presence of an oleanolic acid moiety in 1 was further supported by 1H and 13C NMR data, which were similar to those obtained for the other triterpene saponins isolated in this study, including 3-O-[β-D-glucopyranosyl (1→2)]-β-D-glucopyranosyl oleanolic acid (5),6,7 3-O-[α-L-arabinopyranosyl (1→2)-α-L-arabinopyranosyl (1→6)]-β-D-glucopyranosyl oleanolic acid (6),8 and 3-O-[β-D-xylopyranosyl (1→2)-α-L-arabinopyranosyl (1→6)]-β-D-glucopyranosyl oleanolic acid (7).8 The structure of 1 was confirmed by a complete acid hydrolysis of this compound which afforded the aglycon oleanolic acid as identified by co-TLC with an authentic sample available in our laboratory.
Table 1.
1H and 13C NMR Data for Saponins 1 and 2 in Pyridine-d5
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 1 | 1.49, 0.95 | 39.0 CH2 | 1.56, 1.08 | 39.0 CH2 |
| 2 | 2.37, 1.84 | 27.0 CH2 | 2.30, 1.87 | 27.1 CH2 |
| 3 | 3.17 dd (12.5, 4.7) | 89.7 CH | 3.39 (11.5, 4.3) | 89.3 CH |
| 4 | 39.7 C | 39.8 C | ||
| 5 | 0.72 d (12.0) | 56.1 CH | 0.85 (10.6) | 56.2 CH |
| 6 | 1.49, 1.29 | 19.0 CH2 | 1.52, 1.31 | 18.9 CH2 |
| 7 | 1.47, 1.29 | 33.6 CH2 | 1.44, 1.36 | 33.0 CH2 |
| 8 | 40.2 C | 40.8 C | ||
| 9 | 1.61 t (8.8) | 48.4 CH | 1.59 | 47.7 CH |
| 10 | 37.4 C | 37.4 C | ||
| 11 | 1.99, 1.86 | 24.1 CH2 | 1.96, 1.80 | 24.2 CH2 |
| 12 | 5.46 t (3.0) | 123.1 CH | 5.32 t (2.7) | 125.1 CH |
| 13 | 145.2 C | 140.5 C | ||
| 14 | 42.6 C | 43.8 C | ||
| 15 | 2.16, 1.20 | 28.8 CH2 | 2.31, 1.45 | 38.7 CH2 |
| 16 | 2.12, 1.87 | 24.1 CH2 | 4.5 m | 67.1 CH2 |
| 17 | 47.1 C | 50.4 C | ||
| 18 | 3.64 dd (4.2, 14.1) | 42.4 CH | 2.79 | 42.2 CH |
| 19 | 2.87 dd (13.0,14.0), 1.44 m | 47.8 CH2 | 1.83, 1.36 | 43.4 CH2 |
| 20 | 31.4 C | 34.6 C | ||
| 21 | 34.7 CH2 | 4.26 d (5.0) | 83.8 CH | |
| 22 | 2.07, 1.86 | 33.6 CH2 | 2.78, 2.29 | 27.6CH2 |
| 23 | 1.15 (s) | 28.6 CH3 | 1.22 (s) | 28.4 CH3 |
| 24 | 1.00 (s) | 17.4 CH3 | 1.01 (s) | 17.4 CH3 |
| 25 | 0.79 (s) | 15.8 CH3 | 0.79 (s) | 16.7 CH3 |
| 26 | 0.98 (s) | 17.8 CH3 | 0.79 (s) | 16.1 CH3 |
| 27 | 1.28 (s) | 26.6 CH3 | 1.38 (s) | 29.3 CH3 |
| 28 | 180.7 C | 181.7 C | ||
| 29 | 1.04 (s) | 24.2 CH3 | 0.93 (s) | 29.0 CH3 |
| 30 | 0.97 (s) | 33.8 CH3 | 1.08 (s) | 24.7 CH3 |
| NHCOCH3 | 2.16 (s) | 24.2 CH3 | 2.18 (s) | 24.2 CH3 |
| NHCOCH3 | 8.94 d (9.1) | 170.5 C | 8.94 d (8.8) | 170.5 C |
| Glc-NAc | ||||
| 1′ | 4.98 d (8.3) | 105.6 CH | 5.06 d (8.6) | 105.5 CH |
| 2′ | 4.58 ddd (8.3, 9.1, 10.0) | 58.3 CH | 4.60 m | 58.3 CH |
| 3′ | 4.38 m | 76.5 CH | 4.34 m | 76.2 CH |
| 4′ | 4.08 m | 73.2 CH | 4.16 m | 73.3 CH |
| 5′ | 4.12 m | 77.2 CH | 4.07 m | 76.6 CH |
| 6′ | 4.88 dd (1.7, 10.9); 4.24 m | 70.4 CH2 | 4.66 dd (10.4, 4.0), 4.22 d (10.4) | 69.9 CH2 |
| Ara | ||||
| 1″ | 4.95 d (6.6) | 105.8 CH | 5.14 d (5.1) | 102.9 CH |
| 2″ | 4.48 m | 72.8 CH | 4.57 m | 80.1 CH |
| 3″ | 4.20 dd (8.5, 3.3) | 74.8 CH | 4.37 m | 73.3 CH |
| 4″ | 4.36 m | 69.6 CH | 4.39 m | 68.2 CH |
| 5″ | 4.32 m, 3.78 d (10.3, 2.0) | 66.9 CH2 | 4.31 dd (11.0, 5.0), 3.76 dd (11.0, 2.0) | 65.0 CH2 |
| Ara | ||||
| 1‴ | 5.07 d (6.5) | 106.2 CH | ||
| 2‴ | 4.51 m | 73.3 CH | ||
| 3‴ | 4.15 m | 74.8 CH | ||
| 4‴ | 4.28 m | 69.4 CH | ||
| 5‴ | 4.43 dd (11.0, 5.0), 3.69 dd (11.0, 2.0) | 67.1 CH2 | ||
Glycosylation of the alcoholic function at C-3 in the triterpene moiety was indicated by the low frequency chemical shift value of this carbon resonance (δ 89.7) and HMBC correlations between H-3 (δ 3.17, dd, J = 12.5, 4.7 Hz) and one anomeric carbon at δ 105.6 (C-1′), between one anomeric proton at δ 4.98 (H-1′, d, J = 8.3 Hz, which correlated to the anomeric carbon C-1′ at δ 105.6 in the HSQC spectrum) and C-3 (δ 89.7). From the anomeric proton in each sugar unit, the other protons in 1 were assigned using 1H-1H COSY and TOCSY spectra, after which the glycosidic carbons were identified from an analysis of the HSQC spectra (Table 1). In the diglycosidic moiety, one sugar was identified as an α-arabinopyranosyl unit, and the other as a N-acetylated amino-β-glycopyranosyl unit. The presence of a N-acetamido group was suggested by IR absorption peaks (1630 and 1560 cm−1), and the detection of signals at δH 8.94 (1H, d, J = 9.1 Hz, NHCOCH3), δH 2.16 (3H, s, NHCOCH3), δC 24.2 (NHCOCH3) and 170.5 (NHCOCH3) as well as the HMBC correlations between δH 2.16 (NHCOCH3) and δC 170.5 (NHCOCH3), between δH 4.58 (H-2 ′, 1H, ddd, J = 8.3, 9.1, 10.0 Hz) and δC 170.5 (NHCOCH3), and between δH 8.95 (NHCOCH3) and δC 170.5 (NHCOCH3). The proton resonating at δH 8.95 (NHCOCH3) showed a correlation in the 1H-1H COSY spectrum with the signal at δH 4.58 (H-2 ′, attached to C-2 ′ at δ 58.3 in the HSQC spectrum). The shielding of C-2′ at δC 58.3 is characteristic for the presence of a 2-deoxy-2-acetamidoglycosyl unit. The large proton coupling constant of H-2′ indicated the trans-diaxial orientation of H-2′ of the N-acetylglucosamine. Furthermore, the HMBC correlations between δH 4.32, 3.78 (H2-6′ in Glc-NAc) and δC 105.8 (C-1″ in Ara), between δH 4.95 (H-1″ in Ara) and δC 70.4 (C-6′ in Glc-NAc) allowed for the determination of the sequence of the glycosidic chains linked to C-3. Finally, the 13C NMR data obtained for the diglycosidic chain were superimposable with those obtained for 3-O-[α-L-arabinopyranosyl(1→6)]-2-acetamido-2-deoxy-β-D-glucopyranosyl echinocystic acid (3), 7 also isolated in this study. From these observations, 1 was determined as 3-O-[α-L-arabinopyranosyl(1→6)]-2-acetamido-2-deoxy-β-D-glucopyranosyl oleanolic acid. Although the structure of 1 was previously reported as a result of a partial acid hydrolysis of oleanolic acid 3-O-glycosides from the stems of A. subdimidiata,1 we report here for the first time its 1H and 13C NMR data based on 2D NMR spectroscopic analyses.
The structure determination of 2 was performed in a similar manner as described for 1. Its molecular formula of C48H75NO17 was determined through HRESIMS (positive mode, [M+H]+ peak at m/z 938.5061, calcd for C48H76NO17, 938.5113; [M+Na]+ peak at m/z 960.4890, calcd for C48H75NO17Na, 960.4933) and NMR experiments. The IR absorption peak at 1760 cm−1 suggested the presence of a γ-lactone ring. The 1H NMR spectrum of 2 (Table 1) showed the presence of eight methyl groups at δ 0.79 (6H, s), 0.93, 1.01, 1.08, 1.22, 1.38, 2.18 (each 3H, s), an axial proton attached to a hydroxylated carbon at δ 3.39 (1H, dd, J = 11.5, 4.3 Hz), three anomeric protons at δ 5.06 (1H, d, J = 8.6 Hz), δ 5.07 (1H, d, J = 6.5 Hz), and δ 5.14 (1H, d, J = 5.1 Hz), a vinylic proton at δ 5.32 (1H, brt, J = 2.7 Hz), and an amide proton δ 8.94 (1H, d, J = 8.8 Hz). The 13C NMR spectrum (APT) of 2 (Table 1) displayed quaternary carbon signals at δ 181.7 (lactone ester carbon), 170.5 (amide carbon), 140.5 (double bond), one olefinic methine at δ 125.09, and three anomeric carbon signals at δ 106.2, 105.5, and 102.9. Compound 2 was also determined to be a triterpene triglycoside with an oleanene skeleton. The assignments of the 1H and 13C NMR signals of the triterpene moiety were secured by HSQC, DQF-COSY, and HMBC experiments, allowing for the identification of the aglycon as acacic acid lactone. The identification of the aglycon of 2 was supported by its 1H and 13C NMR data which were superimposable to those obtained for the triterpene moiety, 3-O-[β-D-xylopyranosyl (1→2)-α-L-arabinopyranosyl (1→6)]-2-acetamido-2-deoxy-β-D-glucopyranosyl acacic acid lactone (concinnoside D) (4),9–12 also isolated in this study.
The chemical shift at δ 89.3 observed for C-3 in the triterpenoid ring system of 2, as well as the presence of three anomeric carbons indicated that the three sugars in the molecule are attached to C-3. Starting from the three anomeric proton signals attached to the three anomeric carbons in HSQC, the three sugars were identified as two α-arabinopyranosyl units (Ara) and one N-acetylated amino-β-glycopyranosyl unit (Glc-NAc), using DQF-COSY, TOCSY, and HSQC-TOCSY techniques, evaluation of the coupling constants, and from the chemical shifts. This observation was confirmed by acid hydrolysis of 2, which afforded the sugar components, 2-amino-2-deoxy-glucose and arabinose. The sugar unit with an anomeric signal at δH 5.06 (d, J = 8.6 Hz) was assigned to 2-acetamido-2-deoxy-β-glucose. This was further supported by the detection of signals at δH 8.94 (1H, d, J = 8.8 Hz, NHCOCH3), δH 2.18 (3H, s, NHCOCH3), δC 24.2 (NHCOCH3) and 170.5 (NHCOCH3), and by the HMBC correlations between δH 2.18 (NHCOCH3) and δC 170.5 (NHCOCH3), between δH 4.60 (H-2′) and δC 170.5 (NHCOCH3), and between δH 8.94 (NHCOCH3) and δC 170.5 (NHCOCH3) as well as the COSY correlation between the proton at δH 8.94 (NHCOCH3) with that at δH 4.60 (H-2′, attached to C-2′ at δ 58.3 in the HSQC spectrum). The sugar units with anomeric proton signals at δH 5.14 (H-1″, d, J = 5.1 Hz, attached to C-1″ at δC 102.9) and δH 5.07 (H-1‴, d, J = 6.5 Hz, attached to C-1‴ at δC 106.2) were attributed to two α-arabinose units. The connectivity of the sugar residues in the trisaccharide chain were determined by a glycosylation shift of the carbon signals and by HMBC correlations. The absence of any 13C NMR glycosylation shift for one arabinopyranosyl unit (δ 106.2, 73.3, 74.8, 69.4 and 67.1) suggested that this sugar is a terminal unit. Glycosylation shifts were observed for C-6′ (δ 69.9) of the Glc-NAc unit, and for C-2″ (δ 80.1) and C-1″ (δ 102.9) of the Ara unit. The HMBC correlations between H-3 (δ 3.39) in the aglycon and C-1′ (δ 105.5) in Glc-NAc, between H-1′ (δ 5.06) in Glc-NAc and C-3 (δ 89.3) indicated that the Glc-NAc unit is linked directly to C-3 of the triterpenoid moiety. The HMBC correlations between H2-6′ (δ 4.66, 4.22) in Glc-NAc and C-1″ (δ 102.9) in one Ara, between H-1″ (δ 5.14) in one Ara and C-6′ (δ 69.9) in Glc-NAc, between H-2″ (δ 4.57) in one Ara and the C-1‴ (δ 106.2) in the terminal Ara, between H-1‴ (δ 5.07) in the terminal Ara and C-6″ (δ 65.0) in the other Ara, allowed the determination of the sequence of the glycosidic chain linked to C-3. Finally, the 13C NMR data for the triglycosidic chain were superimposable with those obtained for 3-O-[α-L-arabinopyranosyl-(1→2) α-L-arabinopyranosyl-(1→6)]-2-acetamido-2-deoxy-β-D-glucopyranosyl echinocystic acid isolated from A. procera.8 Therefore, 2 was determined as 3-O-[α-L-arabinopyranosyl-(1→2)-α-L-arabinopyranosyl-(1→6)]-2-acetamido-2-deoxy-β-D-glucopyranosyl acacic acid lactone.
Saponins 2 and 4, 6 and 7, and 3-O-[α-L-arabinopyranosyl-(1→2)-α-L-arabinopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→2)]-β-D-glucopyranoside echinocystic acid (8) 11 and 3-O-[β-D-xylopyranosyl-(1→2)-α-L-arabinopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→2)]-β-D-glucopyranoside echinocystic acid (9) 11,13 were determined to be three pairs of isomers. The only difference between these pairs is that the terminal sugar in 2, 6, and 8 is α-arabinose with a 4β-OH group while that in 4, 7, and 9 is α-xylose with a 4α-OH group. 13C NMR data (especially the chemical shift value difference of C-2 due to the γ-gauche effect, and C-3 and C-4 due to an inductive effect) could be used effectively to differentiate between the two terminal sugars. For example, the chemical shifts observed for C-2, 3, 4 in the terminal arabinose were δc 73.3, 74.8, 69.4 in 2, δc 73.4, 74.8, 69.4 in 6, and δc 73.2, 74.8, 69.5 in 8 while those observed for the terminal xylose were δc 76.0, 78.4, 71.4 in 4, 7, and 9.
Compounds 1, 3, 6, and 7 showed cytotoxicity against B16F10, JMAR, MDA1986, and SKMEL28 cells with IC50 values in the range of 1.8–12.4 μM (Table 2) while saponins 2, 4, 5, 8, and 9 were inactive when tested at the higher concentration of 20 μM. Although 1 and 3 share the same sugar moiety, the cytotoxic activity was increased when the oleanolic acid aglycon in 1 was replaced by the echinocystic (16-hydroxyoleanolic) acid aglycon in 3. While 1, 5, 6, and 7 have oleanolic acid as their aglycon, 1, 6, and 7 showed activity, with 5 being inactive. This observation suggests the importance of the presence of a C-2′ functional group (-OH or - NHCOCH3) in the glucose sugar unit attached to the C-3 position of the aglycon unit as in 1, 6, and 7. This suggestion was supported by comparing the activities of saponins 3, 8, and 9. Compounds 3, 8, and 9 share the same echinocystic aglycon, with 3 active with a C-2′–NHCOCH3 group in the glucose unit attached to C-3 of the aglycon, while saponins 8 and 9 with a C-2′–Oglc group proved to be inactive.
Table 2.
| Saponin | B16F10 | JMAR | MDA1986 | SKMEL28 |
|---|---|---|---|---|
| 1 | 4.7 | 8.7 | 7.3 | 12.4 |
| 3 | 5.1 | 2.8 | 1.8 | 9.2 |
| 6 | 6.9 | 10.1 | 9.0 | 8.6 |
| 7 | 6.3 | 11.5 | 8.2 | 8.1 |
For cell lines used, see text.
Compounds 2, 4, 5, 8, and 9 are inactive for all cell lines used (IC50 > 20 μM).
Saponins, at concentrations tested, exhibited differential cytotoxicity against cancer lines. For example, compound 3 was more potent against head and neck squamous carcinoma [IC50 1.8 and 2.8 uM for MDA1986 and JMAR cells, respectively] while it was less potent against melanoma cell lines [IC50 5.1 and 9.2 uM for B16F10 and SKMEL28 cells, respectively], indicating at concentrations tested, these compounds display specific cytotoxic effect rather than nonspecific saponin-mediated membrane permeabilization. Murine melanoma cells B16F10 and human melanoma cells SKMEL28 both carry BRAF V600E mutation, whereas, SKMEL28 cells carry additional PTEN mutation which may explain increased resistance to compound 3. In addition, phase-contrast microscopic examination of cells treated with increasing concentrations of saponins did not show membrane disintegration.
Experimental Section
General Experimental Procedures
Optical rotations were measured with a Rudolph Research Analytical Autopol IV automatic polarimeter using a 100 mm glass microcell. IR spectrometry data were obtained with a Thermo Nicolet Avatar 360 FT-IR spectrometer. NMR spectra were recorded with a Bruker AV-500 instrument with a cryoprobe for 1H, 13C (or APT), DEPT135, COSY (or DQF-COSY), TOCSY, HSQC, HMBC, and HSQC-TOCSY. Chemical shift values are given in δ (ppm) using the peak signals of the solvent pyridine-d5 as reference and coupling constants are reported in Hz. ESIMS were measured with an Agilent 1200 Series LC-MS/MS ion trap 6300 mass spectrometer. HRESIMS data were collected with a LCT Premier time of flight mass spectrometer (Waters Corp., Milford, MA). Semi-preparative HPLC was performed on a Agilent 1200 unit with DAD detector, using a Lichrospher RP-18 column (250 × 10 mm, 5 μm) or a Eclipse XDB-C18 column (150 × 4.6 mm, 5 μm). Normal-phase silica gel G TLC plates (w/UV 254) and reverse-phase C18 TLC plates (w/UV 254) (Sorbent Technologies, Atlanta, GA) were used for fraction detection. The spots were visualized using UV light 254 nm and spraying with 10% EtOH-sulfuric acid reagent.
Cytotoxicity Bioassay
The in vitro antitumor cytotoxicity assays were performed as previously described.14 In general, five concentrations ranging from 0.1 ug/mL to 10 ug/mL were tested for the extracts, and five concentrations ranging 50 nM to 20 uM were tested for pure compounds. Statistical analysis was carried out by one way ANOVA on ranks test using GraphPad Prism 5 (GraphPad Software, San Diego, CA). IC50 values were obtained from Cell viability plots fitted with a sigmoidal dose response function with variable slope using GraphPad Prism 5 software.
Plant Material
Aerial parts of A. inundata were collected by R. Fortunato and A. Cabral along highway RN 86, 2 km north east of Primavera, Department Patino, Province of Formosa, Argentina (lat. 27°S, long. 58°W) on August 11, 1999. A voucher specimen (No. ARP 645) identified by R. Fortunato was deposited in the Herbarium (BAB) of the Institute of Biological Resources, National Institute of Agricultural Technology (INTA), Castelar, Buenos Aires, Argentina. Intellectual Property Rights Agreements for plant collections and interdisciplinary research have been executed between the collaborating institutions in the U.S. and Argentina.
Extraction and Isolation
Dried, ground biomass (870 g) was extracted at room temperature with CH2Cl2-MeOH (50:50, 2 L) three times. The concentrated extract (80 g) was active with an IC50 value less than 2 μg/mL. The extract (79 g) was suspended in 300 mL H2O, followed by partition with n-hexane, EtOAc, and n-butanol (3 × 500 mL). The n-butanol fraction (40 g) was the most active one with an IC50 value range of 0.1–1.0 μg/mL (see text), while the hexane fraction was inactive and the EtOAc fraction showed an IC50 value range of 1.1–2.5 μg/mL. The n-butanol extract (36 g) extract was then subjected to MCI gel CHP20P column chromatography (500 g, particle size 75–150 μm, Sigma-Aldrich Co., St. Louis, MO), eluted with a mixture of H2O-MeOH (100:0, 80:20, 60:40, 40:60, 85:15, 0:100), in order of increasing concentrations of methanol. The 85% MeOH fraction (Fraction 5, 13.6 g) was subjected to passage over a silica gel column (300 g, particle size 10–25 μm), eluted with CH2Cl2-MeOH-H2O (20:1:0, 20:1:0.1, 12:1:0.1, 7:1:0.1, 4:1:0.1) with increasing amounts of MeOH and water. The fraction (3.2 g) eluted with CH2Cl2-MeOH-H2O (7:1:0.1) was subjected to reverse-phase C18 Si gel column chromatography (200 g, particle size 40–63 μm), eluted by MeOH-H2O (40:60, 50:50, 60:40, 65:35), then the fractions were subjected to semi-preparative HPLC, with the mobile phase CH3CN-H2O (26:74; 28:72), to afford compounds 1 (4.2 mg), 2 (9.0 mg), 3 (20.1 mg), 4 (9.7 mg), 5 (4.5 mg), 6 (4.4 mg), 7 (5.0 mg), 8 (19.0 mg), and 9 (17.0 mg).
Acid Hydrolysis of the Saponin Mixture of Fraction 5
An aliquot of the saponin mixture (Fraction 5, 2.8 g) was dissolved in 100 mL of 0.02 N H2SO4-6.5% HClO4 (1:1) and refluxed for 5 h. The mixture was left to cool, diluted with H2O, filtered, and the aqueous acid solution was neutralized with 1 N KOH and then dried. The sugar mixture was purified by preparative TLC and the sugars isolated were compared with standard sugars in EtOAc-isopropanol-Me2CO-H2O (20:10:7:6) for xylose or in CHCl2-MeOH-H2O (10:5:1) for arabinose and glucose. The optical rotations of the purified sugars were measured for D-glucose [α]25 D +13 (c 0.1, H2O), D-xylose [α]25 D +22 (c 0.1, H2O), and L-arabinose [α]25 D +70 (c 0.1, H2O).
3-O-[α-L-arabinopyranosyl(1→6)]-2-acetamido-2-deoxy-β-D-glucopyranosyl oleanolic acid (1)
[α]26 D +25 (c 0.1, MeOH); IR (film) νmax 3363 (OH), 2940, 1650 (COOH), 1640, 1540 (NHCO) cm−1; 1H NMR and 13C NMR, see Table 1; ESIMS (in positive-ion mode) m/z 792 (M+H, 100), 660 (M-Ara+H, 4), 336 (80); HRESIMS m/z 792.4869 [M+H]+ calcd for C43H70NO12, 792.4898; m/z 814.4697 [M+Na]+ calcd for C43H69NO12Na, 814.4717.
3-O-[α-L-arabinopyranosyl(1→2)-α-L-arabinopyranosyl(1→6)]-2-acetamido-2-deoxy-β- D-glucopyranosyl acacic acid lactone (2)
[α]26 D −6 (c 0.1, MeOH); IR (film) νmax 3370 (OH), 2940, 1760, 1620, 1534 cm−1; 1H NMR and 13C NMR, see Table 1; ESIMS (in positive-ion mode) m/z 938 (M+H, 13), 806 (M-Ara+H, 7), 674 (M-2Ara+H, 4), 468 (100); HRESIMS m/z 938.5061 [M+H]+ calcd for C48H76NO17, 938.5113; m/z 960.4890 [M+Na]+ calcd for C48H75NO17Na, 960.4933.
Supplementary Material
Acknowledgments
The authors are thankful to the late Enrique Suarez (INTA) for the direction of the ICBG Project in Argentina (1995–2005); Renee Fortunato (INTA) for the collection and identification of the plant material. This field work was supported by the ICBG “Bioactive Agents from Dryland Biodiversity in Latin America” grant 5 UO1 TW 00316-10 from the National Institutes of Health (NIH) Fogarty International Center (FIC) to B.N.T. whereas the chemical work was supported by KU 2506014-910/099 to B.N.T. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH/FIC. The authors would like to acknowledge the University of Kansas Center for Cancer Experimental Therapeutics NIH-COBRE P20 RR015563 (PI: B.N.T., project award PI: M.S.C.), for generous support of the in vitro experiments for this project.
Footnotes
Dedicated to Dr. Koji Nakanishi of Columbia University for his pioneering work on bioactive natural products.
Supporting Information Available: HRESIMS, 1D and 2D NMR spectra of compounds 1 and 2, 1H and 13C NMR data of 3, bioassay data of the crude extracts, fractions, and the compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Abdel-Kader M, Hoch J, Berger JM, Evans R, Miller JS, Wisse JH, Mamber SW, Dalton JM, Kingston DGI. J Nat Prod. 2001;64:536–539. doi: 10.1021/np000295u. [DOI] [PubMed] [Google Scholar]
- 2.Cao SG, Norris A, Miller JS, Ratovoson F, Razafisalama J, Andriantsiferana R, Rasamison VE, TenDyke K, Suh T, Kingston DGI. J Nat Prod. 2007;70:361–366. doi: 10.1021/np060506g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad M, Laurens V, Lacaille-Dubois M. Bioorg Med Chem. 2006;12:4725–4734. doi: 10.1016/j.bmc.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Krief S, Thoison O, Sevenet T, Wrangham RW, Lavaud C. J Nat Prod. 2005;68:897–903. doi: 10.1021/np049576i. [DOI] [PubMed] [Google Scholar]
- 5.Ikeda T, Fujiwara S, Araki K, Kinjo J, Nohara T, Miyoshi T. J Nat Prod. 1997;60:102–107. doi: 10.1021/np960556t. [DOI] [PubMed] [Google Scholar]
- 6.Nagao T, Tanaka R, Iwase T, Hanazono H, Okabe H. Chem Pharm Bull. 1991;39:599–606. doi: 10.1248/cpb.39.599. [DOI] [PubMed] [Google Scholar]
- 7.Carpani G, Orsini F, Sisti M, Verotta L. Phytochemistry. 1989;28:863–866. [Google Scholar]
- 8.Nigam SK, Gopal M, Uddin R, Yoshikawa K, Kawamot M, Arihara S. Phytochemistry. 1997;44:1329–1334. doi: 10.1016/s0031-9422(96)00725-x. [DOI] [PubMed] [Google Scholar]
- 9.Melek FR, Miyase T, Ghaly NS, Nabil M. Phytochemistry. 2007;68:1261–1266. doi: 10.1016/j.phytochem.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa K, Satou Y, Tokunaga Y, Tanaka M, Arihara S, Nigam SK. J Nat Prod. 1998;61:440–445. doi: 10.1021/np970538r. [DOI] [PubMed] [Google Scholar]
- 11.Yoshikawa K, Suzaki Y, Tanaka M, Arihara S, Nigam SK. J Nat Prod. 1997;60:1269–1274. doi: 10.1021/np9703555. [DOI] [PubMed] [Google Scholar]
- 12.Gafur MA, Obata T, Kiuchi F, Tsuda Y. Chem Pharm Bull. 1997;45:620–625. doi: 10.1248/cpb.45.620. [DOI] [PubMed] [Google Scholar]
- 13.Orsini F, Pelizzoni F, Verotta L. Phytochemistry. 1991;30:4111–4115. doi: 10.1016/0031-9422(91)83477-3. [DOI] [PubMed] [Google Scholar]
- 14.Samadi AK, Tong XQ, Mukerju R, Zhang HP, Timmermann BN, Cohen MS. J Nat Prod. 2010;73:1476–1481. doi: 10.1021/np100112p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


