Abstract
Alzheimer's disease (AD) is an incurable neurodegenerative disorder clinically characterized by progressive cognitive impairment. A prominent pathologic hallmark in the AD brain is the abnormal accumulation of the amyloid-β 1–42 peptide (Aβ), but the exact pathways mediating Aβ neurotoxicity remain enigmatic. Endoplasmic reticulum (ER) stress is induced during AD, and has been indirectly implicated as a mediator of Aβ neurotoxicity. We report here that Aβ activates the ER stress response factor X-box binding protein 1 (XBP1) in transgenic flies and in mammalian cultured neurons, yielding its active form, the transcription factor XBP1s. XBP1s shows neuroprotective activity in two different AD models, flies expressing Aβ and mammalian cultured neurons treated with Aβ oligomers. Trying to identify the mechanisms mediating XBP1s neuroprotection, we found that in PC12 cells treated with Aβ oligomers, XBP1s prevents the accumulation of free calcium (Ca2+) in the cytosol. This protective activity can be mediated by the downregulation of a specific isoform of the ryanodine Ca2+ channel, RyR3. In support of this observation, a mutation in the only ryanodine receptor (RyR) in flies also suppresses Aβ neurotoxicity, indicating the conserved mechanisms between the two AD models. These results underscore the functional relevance of XBP1s in Aβ toxicity, and uncover the potential of XBP1 and RyR as targets for AD therapeutics.
INTRODUCTION
Alzheimer's disease (AD) is a progressive neurological disorder characterized by memory loss, cognitive decline and neuronal death mainly in the cerebral cortex and hippocampus. The typical pathological features of AD include an abundance of extracellular amyloid-β 1–42 (Aβ) deposits and intracellular neurofibrillary tangles (1). The revised amyloid hypothesis proposes that soluble Aβ aggregates (oligomers) play a leading role in the initial neurotoxic cascade that leads to synaptic impairment and neuronal loss in AD (2,3). Experimental observations have confirmed the ability of Aβ to induce Tau pathology, indicating the leading role of Aβ in launching the pathogenic cascade in AD (4). Therefore, investigating the molecular and cellular consequences of Aβ neurotoxicity will contribute to a better understanding and treatment of AD. Elevated cytosolic free calcium (Ca2+) has recently gained considerable support as a mediator of neurodegeneration (5,6). In particular, presenilin-1 (PS1) mutations associated with familial AD cause elevated levels of free Ca2+ in PC12 cells and hippocampal cells from the brain of transgenic mice, and lead to increased susceptibility to stressing agents, potentially explaining AD pathogenesis (7). Interestingly, the brain of early AD patients showed increased expression of endoplasmic reticulum (ER)-resident ryanodine Ca2+ channel receptors (RyR) (8), further linking AD to Ca2+ dyshomeostasis. Using cellular and animal models of AD, Mattson and colleagues found that mutant PS1 leads to elevated levels of the type 3 RyR (RyR3) in both PC12 cells and primary neurons from transgenic mice (9,10). Moreover, mutant PS1 also results in increased Aβ production, which could also be directly involved in intracellular Ca2+ dysregulation. In fact, only the RyR3 isoform was elevated in transgenic mice carrying three mutant AD genes [amyloid precursor protein (APP), PS1, Tau] (10,11) and in transgenic mice expressing triple mutant APP (12). Thus, Ca2+ dysregulation seems to play a key role as mediator of AD pathogenesis.
Protein misfolding and aggregation are common pathogenic mechanisms in a number of human diseases. Perturbations of the function or integrity of the ER, such as the accumulation of misfolded proteins in the ER lumen, results in a condition-termed ER stress. To avert this condition, cells activate an integrated array of adaptive intracellular signaling cascades known as the unfolded protein response (UPR) (13). During the UPR, the ER stress sensors PERK, ATF6 and IRE1 are activated, launching three independent pathways that coordinately inhibit protein translation and promote clearance of misfolded proteins in the ER. In particular, phosphorylation and dimerization of the transmembrane sensor IRE1 activates its cytoplasmic RNase domain, which cleaves the X-box binding protein 1 (XBP1) pre-mRNA in the cytoplasm. Under normal cell metabolism, the XBP1 pre-mRNA encodes for XBP1u (unspliced), which contains a DNA-binding domain, but no activation domain, suggesting that this isoform functions as a transcriptional repressor (14). Under ER stress, IRE1 induces the unconventional splicing of XBP1 pre-mRNA, which eliminates a 26-nucleotide intron (23 in flies) that changes the reading frame of the 3′ exon. The evolutionarily conserved cytoplasmic splicing of XBP1 results in a new protein with transcriptional activity called XBP1s (spliced) (15). XBP1s directly activates ER stress target genes to facilitate the refolding and degradation of misfolded proteins, including ER chaperones such as Grp78/BiP, Grp58, Grp94; ER-associated degradation (ERAD) components such as EDEM and HRD1, and lipid synthesis and ER biogenesis pathways (13). Indirect evidence has recently linked AD pathogenesis to ER alterations: the ER stress sensor PERK and its target the initiation factor 2α (eIF2α) are activated (phosphorylated) in brain neurons in AD (16,17); the levels of XBP1s transcripts and the ER chaperone Grp78/BiP are significantly elevated in the AD brain (17–19), and mutations in the ER transmembrane proteins PS1 and PS2 inhibit UPR signaling and increase vulnerability to ER stress (20,21). Despite all these data, the protective role of XBP1 and other ER stress-response pathways in AD has not been determined in vivo.
We show here that overexpression of the ER stress factor XBP1s rescues Aβ neurotoxicity in two models of AD: flies expressing human Aβ and rat PC12 cells exposed to Aβ oligomers. Investigating the potential molecular mechanisms mediating XBP1s neuroprotection, we found that XBP1s downregulates the expression of RyR3 Ca2+ channels and prevents the accumulation of intracellular Ca2+ in PC12 cells treated with Aβ oligomers. Moreover, reducing RyR activity in flies also suppressed Aβ neurotoxicity in the eye and increased viability. Thus, our studies identify novel pathways with a potential role in Aβ neurotoxicity that may provide new therapeutic opportunities for treating AD.
RESULTS
Generation of a new AD model in flies
To better understand the mechanisms underlying AD pathogenesis, we created transgenic flies expressing human amyloid-β 1–42 (Aβ) fused to a signal peptide for secretion from a new construct containing two tandem copies of Aβ under the control of UAS (22). This bi-cistronic construct was created for two reasons: one, to mimic the APP duplication associated with early onset familial AD (23) and, two, to express high levels of Aβ that would induce a strong phenotype in the eye. This strong phenotype would be critical for the identification of genetic suppressors of Aβ neurotoxicity. As predicted, expression of the bi-cistronic Aβ construct in all the cells of the developing eye led to small and disorganized eyes containing black, necrotic spots (Fig. 1A, B, F, G). The retinas of young flies (day 1 post-eclosion) were also thin and disorganized, with poorly differentiated photoreceptors and lenses (Fig. 1K and L). This phenotype was similar whether Aβ was expressed in all cell types of the eye with gmr-Gal4 (Fig. 1B and G) or only in photoreceptors using the pan-neural line, Elav-Gal4 (Supplementary Material, Fig. S1).
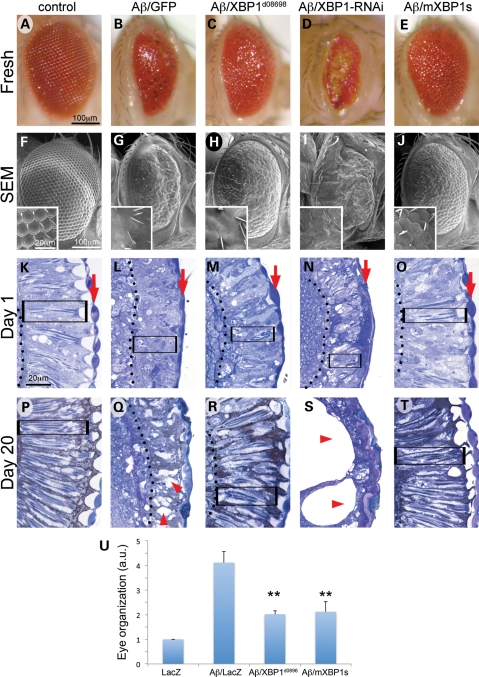
Figure 1.
XBP1 suppresses Aβ neurotoxicity in the fly eye. Fresh eyes (A–E), scanning electron micrographs (F–J) and frontal eye sections at day 1 (K–O) and day 20 (P–T) of control flies (gmr-Gal4/UAS–GFP; A, F, K, P), flies expressing Aβ (gmr-Gal4/UAS–Aβ/UAS–GFP; B, G, L, Q) or Aβ in combination with Drosophila XBP1 (gmr-Gal4/UAS–Aβ/XBP1d08698; C, H, M, R), dXBP1–RNAi (gmr-Gal4/UAS–Aβ/UAS–XBP1–RNAi; D, I, N, S) or mXBP1s (gmr-Gal4/UAS–Aβ/UAS–mXBP1s; E, J, O, T). Compared with control flies (A and F), the eyes of flies expressing Aβ alone are small, highly disorganized and contain black, necrotic spots (B and G). The retina at day 1 is thinner (from arrow to dotted line), the photoreceptors (boxes) are very disorganized and the lenses are poorly differentiated (arrows) (K and L). By day 20, the retinas of the Aβ flies are more disorganized and vacuolated (P and Q, arrowheads). The eyes of flies co-expressing Aβ and dXBP1 (C and H) or mXBP1s (E and J) are bigger, better organized and do not contain necrotic spots. At day 1, the retinas of these flies are deeper (arrow to dotted line), the photoreceptors are better differentiated (boxes) and the lenses (arrows) are partially rescued (M and O). By day 20, these retinas maintain their organization with clearly visible photoreceptors (R and T, boxes). Flies co-expressing dXBP1–RNAi exhibit very small, disorganized and depigmented eyes (D and I) and their retinas are very thin with poorly developed lenses (N, arrow) and photoreceptors (N, box). By day 20, the retinas are mostly vacuolated (arrowheads) and no photoreceptors are visible (S). (U) Quantitation of eye phenotypes (1 = normal, 5 = small, disorganized eyes) shows that both XBP1d08698 and mXBP1s significantly reduced Aβ neurotoxicity (n= 4, P< 0.01).
XBP1 suppresses Aβ neurotoxicity in the Drosophila eye
Following previous reports connecting neurodegeneration with ER stress and the activation of UPR, we decided to test the protective activity of XBP1, a key regulatory component of UPR. For this, we overexpressed XBP1 taking advantage of the XBP1d08698 insertion that introduces the Gal4-binding sequence (UAS) upstream of XBP1. The combination of Aβ and XBP1d08698 significantly improved both eye organization and size (Fig. 1C, H, U). The retinas of these flies were also wider and showed improved differentiation of photoreceptors and lenses (Fig. 1M). As control, we found that overexpression of XBP1 alone had no effect in eye organization (Supplementary Material, Fig. S2A, E, I). Next, we demonstrated that XBP1 overexpression mediated this protective activity, because flies in which the insertion was removed showed the same small and disorganized eyes as flies only expressing Aβ (Supplementary Material, Fig. S2C, G, L). Then, we asked if the physiologic activity of endogenous XBP1 was relevant for Aβ neurotoxicity. To test this, we reduced XBP1 activity in the eye by RNA interference (RNAi). Flies expressing only the XBP1–RNAi construct showed slightly irregular and depigmented eyes (Supplementary Material, Fig. S2D and H), but displayed mainly normal retinas (Supplementary Material, Fig. S2L). However, flies co-expressing XBP1–RNAi and Aβ exhibited very small and depigmented eyes (Fig. 1D and I) with extremely thin and disorganized retinas (Fig. 1N). The ability of XBP1 overexpression and loss-of-function alleles to modify Aβ neurotoxicity in the eye suggested that XBP1 plays a key role in Aβ pathobiology.
To further demonstrate that XBP1 overexpression was solely responsible for the suppression of Aβ neurotoxicity, we created transgenic flies expressing the active (spliced) version of murine XBP1 (mXBP1s). When mXBP1s was co-expressed with Aβ, the eyes were bigger, better-organized and lacked necrotic spots (Fig. 1E, J, U). Additionally, the retinas were thicker and the photoreceptors and lenses were better differentiated (Fig. 1O). However, overexpression of mXBP1s alone did not perturb eye development (Supplementary Material, Fig. S2B, F, J). Thus, expression of mammalian XBP1s isoform resulted in the same protective activity as overexpression of endogenous Drosophila XBP1.
To examine the ability of XBP1 to protect against the progressive degeneration of the retina, we prepared eye sections from flies aged 20 days. As expected, aged control flies showed no major changes in retina morphology (Fig. 1P), whereas expression of Aβ for 20 days induced further disorganization and vacuolation of the retina (Fig. 1Q). In contrast, flies co-expressing either dXBP1 or mXBP1s showed milder degenerative changes in the retina (Fig. 1R and T). Thus, XBP1 overexpression protected against the progressive degeneration of photoreceptors induced by Aβ. Moreover, in flies co-expressing Aβ and XBP1–RNAi aged 20 days, the retinas underwent dramatic degeneration, leaving large holes underneath the cornea (Fig. 1S). These results support a physiological role for XBP1 in the progressive neurodegenerative changes induced by Aβ.
Molecular confirmation of the XBP1d08698 insertion
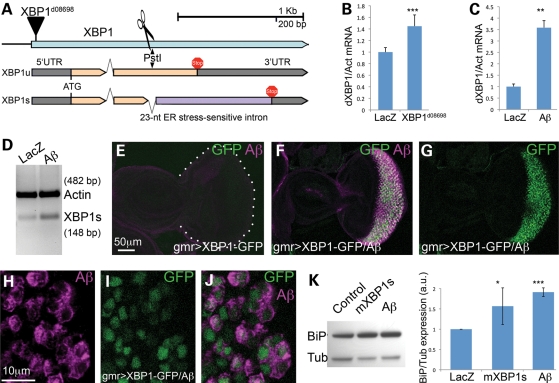
To confirm that the XBP1d08698 insertion was inducing XBP1 expression, we verified its insertion site and orientation. Inverse polymerase chain reaction (PCR) from genomic DNA confirmed that XBP1d08698 was inserted in the 5′ UTR (untranslated region) of XBP1, although the actual position of the transposon (chromosome 2: 17031199) was 117 bp 3′ to the previously reported location (17031082). Thus, XBP1d08698 is consistent with expression of XBP1 under the transcriptional control of Gal4 (Fig. 2A). To demonstrate that XBP1d08698 actually upregulated XBP1, we combined XBP1d08698 with da-Gal4 to induce ubiquitous overexpression of the target gene. Then, we extracted total RNA from these flies and control flies, and measured total XBP1 transcripts by conventional RT–PCR (data not shown) and quantitative RT–PCR (qPCR). These experiments showed that the XBP1d08698 insertion induces a 40% overexpression of XBP1 transcripts (Fig. 2B), thus confirming its regulation by UAS.
Figure 2.
Unconventional splicing of XBP1. (A) Genomic map of the XBP1 locus in Drosophila. The XBP1d08698 insertion that regulates XBP1 expression is located in the 5′ UTR. XBP1 produces two isoforms that differ in the inclusion (XBP1u) or exclusion (XBP1s) of a 23-nucleotide intron subjected to cytoplasmic splicing. The site for PstI digestion inside the 23 bp intron is also shown. (B) The XBP1d08698 insertion induces XBP1 expression. Combination of da-Gal4 with XBP1d08698 induces a 40% increase in total XBP1 mRNA compared with flies expressing LacZ (control) by qPCR. (C) Aβ induces XBP1 expression. Flies expressing Aβ under the control of da-Gal4 induce a 3.5-fold increase in total XBP1 transcripts compared with LacZ control flies. In (C) and (D), XBP1 expression was normalized to Actin. (D) Aβ induces XBP1 unconventional splicing. Flies expressing Aβ under the control of da-Gal4 accumulate higher amounts of the XBP1s isoform than control flies expressing LacZ. Actin is shown as loading control. (E–J) Aβ activates XBP1 splicing in vivo. (E) Control flies expressing the XBP1–GFP sensor in the eye (gmr-Gal4/UAS–XBP1–GFP) do not produce GFP. (F and G) Flies co-expressing Aβ and the sensor (gmr-Gal4/UAS–Aβ/UAS–XBP1–GFP) accumulate Aβ (magenta, F) and high levels of GFP (green, F and G) in the same territory of the developing eye. (H–J) All the cells expressing Aβ also accumulate GFP, although the two signals do not co-localize because Aβ is in the membrane and GFP is nuclear. (K) Both mXBP1s and Aβ induce Grp78/BiP upregulation. Heads from flies expressing a control transgene (LacZ), mXBP1s or Aβ were homogenized, resolved in western blot and incubated with BiP and Tubulin antibodies. BiP appears significantly upregulated in flies expressing mXBP1s and Aβ (n= 3).
Aβ induces unconventional XBP1 splicing in Drosophila neurons
Once we demonstrated that XBP1 overexpression is responsible for the protective activity of the XBP1d08698 insertion, the next question was to determine whether Aβ could induce XBP1 expression. During ER stress, a fragment of the ATF6 sensor translocates to the nucleus where it regulates the expression of ER stress-response genes, including XBP1 (13). To determine whether Aβ induced XBP1 transcription, we created flies expressing Aβ or LacZ ubiquitously under the control of da-Gal4, extracted total RNA from 1-day-old adult flies and measured total XBP1 by qPCR. Flies expressing Aβ significantly accumulated higher levels of endogenous XBP1 than control flies, with an average increase of 3.5-fold (Fig. 2C). Thus, Aβ expression induced ER stress and resulted in the transcriptional activation of XBP1.
Another consequence of ER stress is the cytoplasmic, unconventional splicing of the small intron of XBP1 by the ER stress sensor IRE1 (Fig. 2A) (13). Thus, we investigated the ability of Aβ to induce XBP1 splicing. Using the same flies described above that express either LacZ or Aβ ubiquitously, we specifically amplified XBP1s transcripts by conventional PCR using a primer straddling the ER-sensitive intron that cannot hybridize with XBP1u transcripts. Consistent with the finding that Aβ induces XBP1 expression, flies expressing Aβ accumulated higher levels of XBP1s transcripts than control flies (Fig. 2D). To further demonstrate that Aβ induces cytoplasmic splicing of XBP1, we used a sensor construct that produces green fluorescent protein (GFP) in the correct reading frame upon elimination of the 23-nt intron sensitive to ER stress, thus providing a dynamic readout of XBP1 activation (24). As predicted, the XBP1–GFP sensor produced no signal in the eye imaginal disc of control flies (Fig. 2E). In contrast, expression of Aβ in the eye resulted in strong GFP signal in the Aβ-expressing territory (Fig. 2F). In fact, all the cells expressing Aβ in the developing eye also accumulated GFP, suggesting that Aβ efficiently induced unconventional splicing of the XBP1 intron (Fig. 2G–J). Overall, these experiments demonstrated that Aβ induces both expression and unconventional splicing of XBP1, two indicators of ER stress.
Under ER stress, the ER chaperone BiP/Grp78, along with other chaperones and protein degradation factors, is directly upregulated by XBP1s. We monitored BiP expression in flies overexpressing either mXBP1s or Aβ by western blot and found that both conditions resulted in elevated levels of BiP (Fig. 2K), although Aβ induced higher levels than mXBP1s. These results demonstrated the transcriptional activity of the mXBP1s construct and the ability of Aβ to induce ER stress.
XBP1 does not affect Aβ accumulation and misfolding in fly neurons
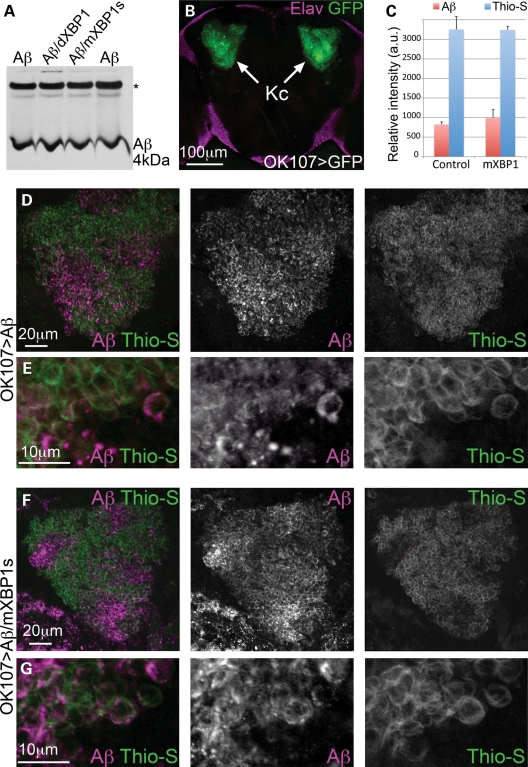
To better understand the mechanism by which XBP1 suppresses Aβ neurotoxicity, we analyzed its effects on total Aβ accumulation. For this, we collected head homogenates from flies expressing only Aβ and flies co-expressing Aβ with dXBP1 or mXBP1s in the mushroom bodies, and determined the levels of total Aβ by western blot. In all the samples analyzed, the levels of Aβ were very similar, suggesting that XBP1s did not promote Aβ degradation (Fig. 3A). Next, we detected Aβ by immunofluorescence and determined its aggregation state in the Kenyon cells, the brain neurons involved in memory and learning. These cells form two tight clusters of 2500 neurons that are easily found in the posterior brain (Fig. 3B). To visualize total Aβ and amyloid aggregates of Aβ, we co-stained whole-mount brains with an anti-Aβ antibody and thioflavine-S, a dye that fluoresces upon incorporation to amyloids. In flies expressing either Aβ alone or co-expressing mXBP1s, the anti-Aβ antibody showed the same expression levels of Aβ (Fig. 3C, D, F). This result, consistent with the result observed in western blot, suggested that mXBP1s did not affect Aβ accumulation. Thioflavine-S also showed the same intensity and distribution in flies expressing Aβ alone or co-expressing mXBP1s (Fig. 3C, D, F), suggesting that mXBP1s did not affect the amyloid aggregation of Aβ. Both anti-Aβ and thioflavine-S signals showed an irregular, patchy distribution through the Kenyon cell clusters regardless of mXBP1s expression, with the highest levels of Aβ located in areas of low thioflavine-S signal (Fig. 3D and F). The patchy appearance of the anti-Aβ staining may be due to the sequential incubation following thioflavine-S, which may result in the partial blocking of Aβ epitopes in areas of high retention of thioflavine-S (Fig. 3C and D). At higher magnification, we detected Aβ in the membrane and diffusing away from the membrane, whereas thioflavine-S labeled the cell contours more clearly (Fig. 3E). Expression of mXBP1s had no effect on the expression and distribution of Aβ and thioflavine-S (Fig. 3G). Overall, these results indicated that mXBP1s reduced Aβ neurotoxicity without altering Aβ levels and aggregation.
Figure 3.
XBP1 does not affect Aβ accumulation in brain neurons. (A) Western blot shows total Aβ accumulation in Drosophila brains expressing Aβ alone (lanes 1 and 4) or co-expressing dXBP1 (lane 2) or mXBP1s (lane 3). Levels of total Aβ are the same in the three conditions. *Indicates an unspecific band. (B) Z-axis projection of a 20 µm optical section of the posterior brain of Drosophila showing accumulation of GFP in the Kenyon cells (arrows, OK107-Gal4/CD8–GFP). The cortex was labeled with anti-Elav (magenta) to include the outline of the brain. (C) Quantification of fluorescent signal for Aβ and thioflavine-S in (D) and (F). The co-expression of mXBP1s did not significantly change the levels of Aβ (red bars) or thioflavine-S (blue bars). (D and F) Detail of the Kenyon cells showing total Aβ expression (magenta) and thioflavine-S fluorescence (green) in flies only expressing Aβ (D) or co-expressing mXBP1s (F). (E and G) At higher magnification, neither the distribution of Aβ (magenta) nor the intensity of thioflavine-S fluorescence (green) is affected by mXBP1 co-expression.
Aβ oligomers induce unconventional XBP1 splicing in mammalian cultured neurons
After observing that Aβ upregulates both XBP1 and BiP in transgenic flies, we were interested in reproducing these results in a simplified mammalian model of AD. For this, we used a cell culture system with differentiated rat pheochromocytoma (PC12) cells highly susceptible to exogenous Aβ oligomers. This cellular model provides additional experimental flexibility for investigating the molecular mechanisms of XBP1 neuroprotection. Before the treatment of the cells, we characterized the composition of the oligomeric Aβ preparation. A representative sample of the Aβ oligomers contained small, round aggregates (oligomers) and incipient protofibers under the electron microscope (Supplementary Material, Fig. S3A). Immunoreaction with the A11 anti-oligomer and the OC anti-fiber conformational antibodies (25,26) indicated that Aβ was highly enriched in oligomers and protofibers, but contained no mature fibers (Supplementary Material, Fig. S3B).
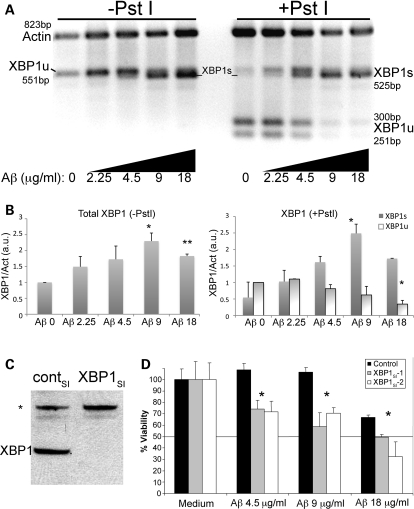
Then, we treated differentiated PC12 cells with these Aβ oligomers and determined their ability to induce XBP1 splicing. To detect the XBP1 transcripts, we performed RT–PCR with primers straddling the small intron that amplify the two isoforms. Then, half the PCR products were digested with PstI, a rare restriction site only present in the intron, allowing the diagnostic identification of XBP1s transcripts (resistant to PstI). Non-treated cells accumulated mostly XBP1u transcripts, which produced two small bands upon PstI digestion (Fig. 4A and B). Incubation for 6h with increasing concentrations of Aβ oligomers induced the transcriptional upregulation of total XBP1, with maximal levels found at 9 µg/ml (equivalent to 2 µm monomeric Aβ) (Fig. 4A and B, −PstI). Doubling the amount of Aβ to 18 µg/ml caused a reduction in the levels of total XBP1 possibly due to cell death. These results indicated that Aβ oligomers induced a 2-fold upregulation of XBP1 mRNA following a 6 h acute treatment. After PstI digestion to detect the XBP1s isoform (resistant to PstI), we detected very low levels of XBP1s in untreated cells, but its expression increased in response to Aβ (Fig. 4A and B, +PstI). The peak levels of XBP1s were similar to the highest levels of total XBP1 found at 9 µg/ml of Aβ, indicating that Aβ is an efficient activator of XBP1 splicing (Fig. 4B). In contrast, the XBP1u isoform (cleaved) decreased in the treatments with Aβ and almost disappeared at the highest concentrations of Aβ (Fig. 4A and B). These experiments indicated that short, acute treatments with Aβ induced potent ER stress and UPR. Moreover, low, subtoxic levels of Aβ (2.25 µg/ml) also induced unconventional XBP1 splicing, underscoring the strong ER toxicity of Aβ oligomers and the sensibility of XBP1 to the Aβ insult.
Figure 4.
Aβ induces accumulation of neuroprotective XBP1s in mammalian neurons. (A) Accumulation of XBP1 transcripts in PC12 cells treated for 6h with a gradient of Aβ oligomers. Half of the RT–PCR reaction was digested with PstI to cleave the XBP1u transcripts (right). Actin was also amplified as internal loading control. Untreated cells (0) accumulate very low levels of XBP1s, but in the presence of Aβ, the PstI-resistant XBP1s accumulates in a dose-dependent manner. (B) Quantitation of total XBP1, XBP1s and XBP1u from two independent gels. The levels of total XBP1 increase with the amount of Aβ, with a maximum corresponding to 9 µg/ml. The levels of XBP1s parallel the activation curve of total XBP1, while XBP1u levels decrease with increasing amounts of Aβ. (C and D) Endogenous XBP1 protects against Aβ cytotoxicity. (C) A siRNA against XBP1 (XBP1SI) eliminates the XBP1 protein in PC12 cell extracts by western blot, while a control siRNA (contSI) does not change XBP1 levels. An unspecific band (*) is detected with the anti-XBP1 antibody. (D) The dose-dependent toxicity of Aβ oligomers in WT cells is shown in black bars. Cell viability is compromised at 18 µg/ml in 6 h treatments. Silencing of XBP1 transcripts with two independent siRNAs (XBP1SI–1 [grey] and XBP1SI–2 [white]) results in significantly reduced cell viability in the presence of Aβ oligomers (n= 3), even at subtoxic Aβ treatments (4.5 and 9 µg/ml). *P< 0.05, **P< 0.01.
Endogenous XBP1 protects against Aβ neurotoxicity in cultured neurons
We wondered next whether endogenous XBP1 was necessary for neuroprotection in mammalian cultured neurons. For this, we analyzed cellular viability after endogenous XBP1 was silenced with small interfering siRNAs. First, we confirmed that specific siRNAs eliminated XBP1 in PC12 protein extracts (Fig. 4C) and confirmed that the siRNAs were not toxic in PC12 cells not treated with Aβ (Fig. 4D). Then, we found that, prior to siRNA treatment, PC12 cells resisted Aβ toxicity at 4.5 and 9 µg/ml, but produced significant cell death at 18 µg/ml after a short incubation of 6h (Fig. 4D, black bars). In contrast, pre-treatment with two independent siRNA against XBP1 resulted in a 30–40% decrease in cell viability at subtoxic Aβ concentrations (4.5 and 9 µg/ml) (Fig. 4D). At toxic concentrations of Aβ (18 µg/ml), XBP1 silencing further enhanced the cell death induced by Aβ alone. Thus, silencing endogenous XBP1 increased the sensitivity of PC12 cells to Aβ oligomers, indicating that, as seen in Drosophila, endogenous XBP1 prevented Aβ-dependent neuronal cell death.
XBP1 overexpression prevents Aβ neurotoxicity in cultured neurons
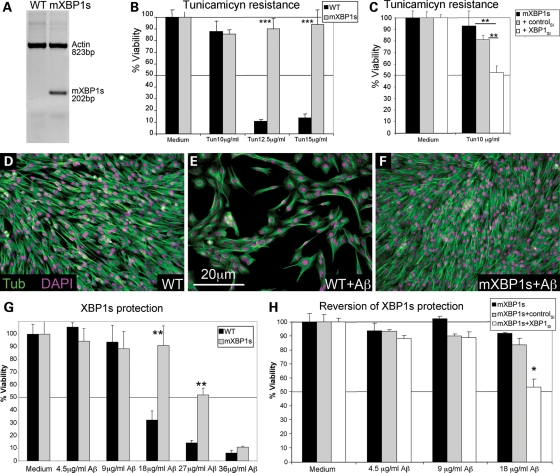
The next question was to determine whether XBP1s overexpression could protect mammalian cultured neurons against Aβ cytotoxicity. For this, we first generated stably transfected PC12 cells expressing mXBP1s and confirmed its expression in the new clones by using primers that specifically amplified mXBP1s (Fig. 5A). To demonstrate the ability of mXBP1s to protect against ER stress, we treated these cells with the ER stressor tunicamycin. Tunicamycin treatments above 10 µg/ml caused extensive cell death in control cells (Fig. 5B); however, cells expressing mXBP1s were completely protected against tunicamycin-mediated cell death (Fig. 5B). Moreover, the protection conferred by mXBP1s was reverted when mXBP1 was silenced by siRNA (Fig. 5C). Furthermore, both control and mXBP1s cells were equally sensitive to staurosporine, a known inducer of caspases and apoptosis, confirming the specific protection of mXBP1s against ER stressors (Supplementary Material, Fig. S4).
Figure 5.
XBP1s protects against Aβ cytotoxicity. (A) Detection of mXBP1s mRNA in PC12 cells stably transfected with CMV-mXBP1s using specific primers for mouse XBP1. (B and C) mXBP1s overexpression confers resistance to tunicamycin. mXBP1s cells (grey) survive tunicamycin concentrations that kill control cells (black) (B). The protective activity of mXBP1s against tunicamycin is reverted by pre-treatment with siRNA against XBP1 (C). (D–H) mXBP1s prevents cytotoxicity of Aβ oligomers. Representative fields show the effects of Aβ on cell viability: non-treated control (WT) cells (D), and control cells (E) and mXBP1s cells (F) treated with Aβ oligomers at 18 µg/ml. Cells are stained with anti-Tubulin (green) and DAPI (magenta). (G) Aβ causes a dramatic drop in viability at 18 µg/ml in control cells (black) after an 8 h incubation. However, mXBP1s (grey) completely protects PC12 neurons treated with Aβ at 18 µg/ml, and partially protects at higher concentrations. (H) The protective activity of mXBP1s (black bars) is reverted by pre-treatment with XBP1SI (white), but not by a control RNAi (grey). *P< 0.05, **P< 0.01, ***P< 0.001.
Then, we tested the protective activity of mXBP1s in PC12 cells exposed to Aβ oligomers. Differentiated naïve cells tolerated treatments of up to 9 µg/ml of Aβ oligomers for 8h (Fig. 5D and G). However, treatments of 18 µg/ml caused up to 70% of cell death (Fig. 5E and G) and higher concentrations of Aβ caused over 85% of cell death (Fig. 5G). Interestingly, cells expressing mXBP1s showed significant protection against Aβ cytotoxicity at all toxic concentrations (Fig. 5F and G). mXBP1s overexpression prevented cell death at 18 µg/ml of Aβ and conferred partial protection at higher Aβ concentrations (Fig. 5G). We also demonstrated that siRNA pre-treatment against XBP1 blocked the protective activity of mXBP1s (Fig. 5H). Control cells expressing mXBP1s and cells treated with an unrelated control siRNA survived treatments of up to 18 µg/ml Aβ oligomers; however, silencing of XBP1 reduced viability by 50% in the 18 µg/ml treatment (Fig. 5H). Overall, these results support the idea that increased expression of XBP1s prevents cell death induced by Aβ oligomers.
XBP1s overexpression prevents caspase activation
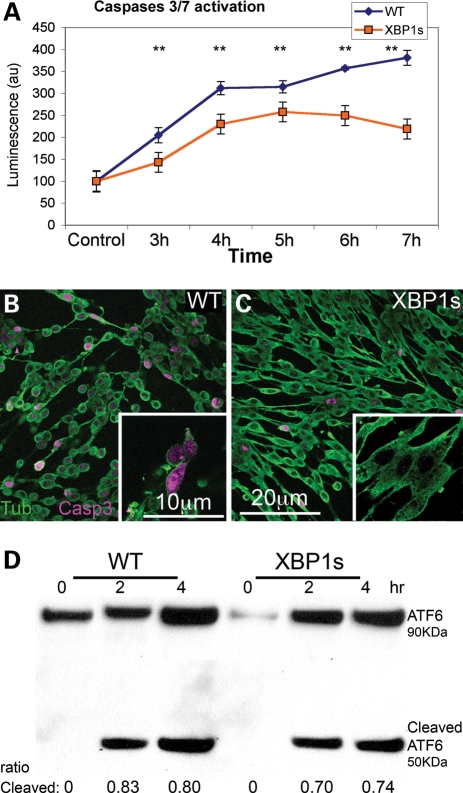
To better understand the dynamics of XBP1s protection from cell death, we studied the temporal activation of caspase-3 and -7 in response to Aβ oligomers. As expected, control cells treated with Aβ oligomers at 18 µg/ml progressively accumulated higher levels of activated caspases (Fig. 6A), consistent with the reduction in cell viability as described above. Cells expressing mXBP1s also activated caspase-3 and -7 up to 5h after exposure to Aβ at significantly lower levels than wild-type (WT) cells (Fig. 6A). Interestingly, in cells expressing mXBP1s, the activation of caspases leveled off at 5h and decreased 6 and 7h after Aβ exposure, when activation of caspases is still growing in control cells. We also visualized caspase activation in fixed cells by immunofluorescence with the anti-Act-Casp-3 antibody specific for the active form of caspase-3. Control cells treated with Aβ exhibited abnormal, round and small morphology, while over 50% of cells showed activation of Casp-3 (Fig. 6B). In contrast, cells expressing mXBP1s were larger and displayed normal morphology, although a few cells accumulated activated Casp-3 (Fig. 6C). Overall, mXBP1s did not prevent the activation of caspases, but it limited their activation, conferring the ability to better respond to the damaging stimuli of Aβ oligomers.
Figure 6.
XBP1s prevents Aβ-dependent caspase activation, but not ER stress. (A) Temporal analysis of caspase-3 and -7 activation in WT and mXBP1s cells treated with Aβ oligomers at 18 µg/ml. (A) WT cells (blue diamonds) progressively accumulate activated caspases over the course of the experiment. In mXBP1s cells (orange squares), activated caspase levels peak at 5 h, then decrease in the last 2h (n= 3, **P< 0.01). (B and C) Distribution of activated (act)-caspase-3 in PC12 cells treated with oligomeric Aβ at 18 µg/ml. WT cells appear small and round, and accumulate act-caspase-3 (B), whereas mXBP1s cells preserve their morphology and accumulate very little act-caspase-3 (C). (D) Western blot shows ATF6 activation in WT and mXBP1s cells treated with a subtoxic dose of Aβ oligomers (9 µg/ml). After a 4 h incubation, both WT (0.83 ± 0.0075 and 0.8 ± 0.03) and mXBP1s cells (0.7 ± 0.026 and 0.74 ± 0.023) accumulate cleaved ATF6. After a 4 h incubation, both WT and mXBP1s cells accumulate cleaved ATF6. There is no significant difference in the ratio of cleaved ATF6 between WT and mXBP1s, although mXBP1s accumulated less-activated ATF6 in two different time points.
XBP1s overexpression does not prevent ER stress
During UPR, XBP1s regulates the expression of a number of ER stress factors, including ER chaperones and ERAD components that restore normal ER physiology. Thus, we asked whether mXBP1s could revert the ER stress induced by Aβ. To detect ER stress independently of XBP1, we examined the activity of ATF6, one of the three sensors that respond to UPR. ATF6 encodes for a transcription factor normally anchored to the ER membrane. In response to unfolded proteins in the ER, ATF6 translocates to the Golgi, where it is cleaved, releasing a fragment with transcriptional activity (13). Before exposure to Aβ, both WT and mXBP1s cells accumulated only full-length ATF6 (Fig. 6D). After incubation with Aβ, however, control cells accumulated the smaller band indicative of cleaved ATF6 (Fig. 6D). mXBP1s cells also accumulated cleaved ATF6 at slightly lower levels. These differences are not statistically significant, suggesting that XBP1s neuroprotection may be mediated by downstream pathways that prevent the maladaptive consequences of ER stress.
XBP1s prevents calcium release into the cytosol
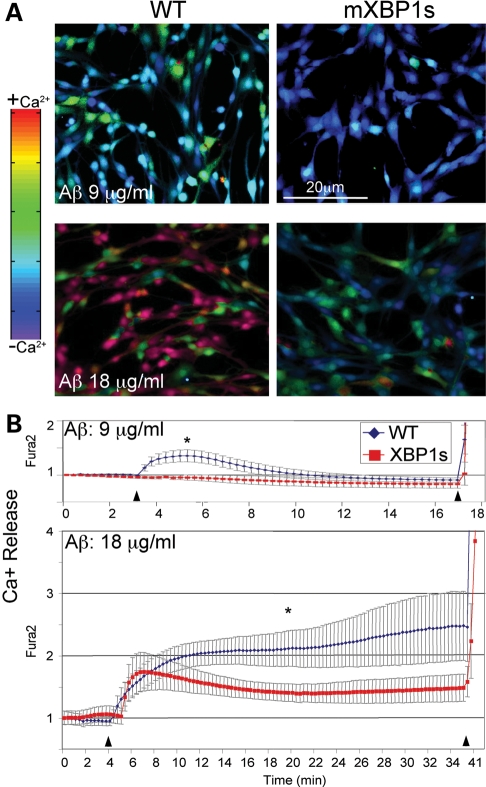
Strong evidence has suggested that accumulation of free Ca2+ in the cytosol is a key cellular mediator of Aβ cytotoxicity (5,6,27). Release of intraluminal Ca2+ stores into the cytosolic space, mainly from the ER, typically leads to cell death through the activation of Ca2+-dependent signaling molecules, such as the kinase CaMKII, the phosphatase calcineurin and the protease calpain. The activation of these molecules leads to further Ca2+ release from other organelles, including mitochondria, caspase activation and, finally, apoptosis (5,6). Given the role of XBP1s in ER homeostasis, we asked whether XBP1s interfered with the ability of Aβ to induce Ca2+ release from the ER. For this, we measured Ca2+ dynamics by Fura2 in WT PC12 cells and cells overexpressing mXBP1s treated with Aβ oligomers. Treatment of WT cells with subtoxic Aβ concentrations (9 µg/ml) induced moderate Ca2+ release in ∼50% of the control cells (Fig. 7A), leading to a peak signal 2min after exposure to Aβ (Fig. 7B). Then, Ca2+ levels slowly returned to normal values over the next few minutes (Fig. 7B and Supplementary Material, Movie S1). In contrast, cells overexpressing mXBP1s did not release Ca2+ into the cytosol over the course of the experiment (Fig. 7A and B and Supplementary Material, movie 2). Thus, mXBP1s completely prevented the Ca2+ release at subtoxic levels of Aβ, suggesting a direct link between XBP1s activity and intraluminal Ca2+ dynamics.
Figure 7.
XBP1s prevents Aβ-dependent calcium release. (A) Representative micrographs of luminal Ca2+ release measured by fura2 in PC12 cells treated with oligomeric Aβ. (B) Longitudinal analysis of Ca2+ release from the same samples. A subtoxic concentration of Aβ (9 µg/ml) elicits moderate Ca2+ release into the cytosol in WT cells (blue) that are reversible after a few minutes. On the other hand, mXBP1s cells (red) do not release Ca2+ at this concentration of Aβ. At toxic concentrations (18 µg/ml), Aβ oligomers induce high levels of cytosolic Ca2+ in normal cells that continue to rise until the end of the experiment. Fifty percent of mXBP1s cells also release Ca2+ early on, although at moderate levels, and this effect is reverted later on. In (B), the first arrowhead indicates the addition of Aβ at the start of the experiment. The second arrowhead indicates the addition of ionomycin to terminate the experiment by liberating all intraluminal Ca2+ stores. Two-way ANOVA shows significant differences between the WT and mXBP1s (**P< 0.05).
When we treated the cells with cytotoxic concentrations of Aβ (18 µg/ml), control cells released high amounts of ER Ca2+ (Fig. 7A, red cells). The increase in intracellular free Ca2+ started immediately after Aβ treatment and continued to climb for the duration of the experiment (Fig. 7B and Supplementary Material, movie 3). On the other hand, only ∼50% of the cells expressing mXBP1s showed moderate levels of cytosolic Ca2+ following Aβ exposure, while the rest showed low free Ca2+ levels (Fig. 7A). After the cells reached maximum levels of free Ca2+ 7min after initiation of the treatment, free Ca2+ levels slowly decreased over time and then stabilized at levels compatible with cell viability (Fig. 7B and Supplementary Material, movie 4). In summary, XBP1s conferred the ability to better regulate Ca2+ release after exposure to Aβ oligomers.
Regulation of ryanodine calcium channels by XBP1s
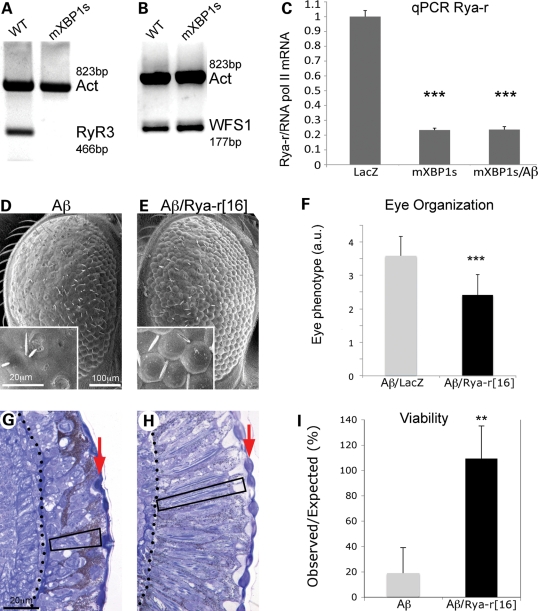
Since XBP1s prevented intracellular Ca2+ dysregulation in the presence of Aβ, we investigated a potential link with the ER Ca2+ channels that regulate Ca2+ dynamics. Over the past decade, several studies have shown that ryanodine receptors (RyR) are upregulated in cellular and rodent models of AD, as well as in the brain of early AD patients (8,9,11). Specifically, the RyR3 isoform appears upregulated in PC12 cells expressing mutant PS1 and in transgenic mice expressing mutant forms of PS1, APP or a triple combination PS1–APP–Tau (9,11,12). Thus, increased Ca2+ traffic through RyR3 seemed an ideal candidate to be implicated in the prevention of Ca2+ dysregulation as described above. We hypothesized that XBP1s could modulate Ca2+ dynamics by downregulating the expression of RyR3. To test this idea, we designed specific primers for rat RyR3, and then analyzed its expression in WT and mXBP1s cells by RT–PCR. Whereas RyR3 transcripts were easily amplified in WT cells using two different sets of primers (only one shown), RyR3 expression was dramatically reduced in mXBP1s cells to the point that we could not detect the amplicons (Fig. 8A). As a positive control for the transcriptional activity of mXBP1s we amplified WFS1 (the Wolfram disease gene), a known target of XBP1s (28). WFS1 was upregulated 1.5-fold in cells overexpressing mXBP1s (Fig. 8B), similarly to published data (28), suggesting that the transcriptional repression of RyR3 is specific. These results strongly supported our hypothesis that XBP1s may regulate Ca2+ dynamics through the transcriptional downregulation of RyR3.
Figure 8.
Reduced ryanodine calcium channels mediate XBP1 neuroprotection. (A) Expression of RyR3 is severely downregulated by XBP1s in PC12 cells. The RyR3 isoform is expressed in PC12 cells, but the RyR3 transcripts are almost undetectable in cells expressing mXBP1s. Actin was amplified as loading control. (B) WFS1, a known target of XBP1s, is upregulated 1.5-fold in cells expressing mXBP1s. (C) Analysis of Drosophila Rya-r transcripts by qPCR indicates that mXBP1s expression reduces Rya-r levels by 80% even in flies also expressing Aβ (n= 8, P< 0.001). (D–H) Reduced Rya-r activity rescues the eye degeneration induced by Aβ. Flies expressing Aβ display disorganized ommatidia (D) and small photoreceptors (G, box). In contrast, flies also carrying the null allele Rya-r16 have better-organized ommatidia (E), and deeper retinas, better differentiated lenses (arrow) and elongated photoreceptors (box) (H). (F) The distribution of eye phenotypes (1 = normal, 5 = small, disorganized eyes) shows a significant rescue of eye morphology in flies also carrying the Rya-r deletion (n= 4, P< 0.001). (I) Reduced Rya-r activity rescues the lethality induced by Aβ. Expression of Aβ reduces adult eclosion to 20% with respect to control siblings (grey, n= 6). Flies that also carrying the null allele Rya-r16 present normal viability (black, n= 3, P< 0.01). In (F) and (I), n is the number of vials analyzed, each producing at least 50 flies.
We, then, moved to confirm the relationship between XBP1s and RyR in transgenic flies expressing Aβ. A clear advantage of the compact genome of Drosophila is that flies only have one RyR gene, Rya-r. We designed experiments to compare Rya-r transcript levels in control flies and flies expressing mXBP1s by qPCR. Interestingly, flies expressing mXBP1s alone or co-expressing Aβ and mXBP1s accumulated dramatically reduced levels of Rya-r compared with LacZ control flies (Fig. 8C). These results further supported the idea that XBP1s may control intracellular Ca2+ through downregulation of ryanodine Ca2+ channels.
Reduced activity of ryanodine calcium receptor is neuroprotective
After showing the interplay between Aβ, XBP1s and RyRs in mammalian cells, we wanted to demonstrate the functional consequence of these interactions in vivo. The fly model provides complex assays for Aβ neurotoxicity with the added advantage of containing only one RyR gene for increased functional susceptibility. For this, we created flies expressing Aβ in the eye that also carried one copy of a Rya-r hypomorphic allele caused by a 6kb internal deletion. Homozygous Rya-r16 embryos are lethal and show slow feeding, locomotion and heart rate, suggesting that Rya-r plays a similar role in neuron and muscle physiology to vertebrate RyRs (29). Flies heterozygous for Rya-r16 had normal eyes and viability, suggesting that reducing the levels of Rya-r had no deleterious consequences (data not shown; 29). However, heterozygous Rya-r16 significantly suppressed eye degeneration induced by Aβ (Fig. 8D–F). Sixty percent of the flies expressing only Aβ showed a highly disorganized eye surface (Fig. 8D) and short retina with small photoreceptors (Fig. 8G). On the other hand, 62% of the flies also carrying the Rya-r deletion exhibited a mild disorganization of the eyes (Fig. 8E) with well-developed retinas and long photoreceptors (Fig. 8H). The quantitative analysis of eye phenotypes showed a significant improvement in eye organization in flies carrying the Rya-r mutation (Fig. 8F). Thus, removing one copy of Rya-r clearly protected against Aβ neurotoxicity in the eye.
Finally, we tested the ability of the Rya-r deletion to rescue the lethality induced by high levels of Aβ in the CNS. We developed an assay that resulted in 20% eclosion of adult flies (80% lethality) (Fig. 8I). In contrast, viability was completely restored in flies also carrying the strong Rya-r allele (Fig. 8I). Combined, these experiments underscore a key functional role for the Rya-r in Aβ neurotoxicity.
DISCUSSION
ER stress has been proposed as a key cellular response in a number of human maladies, including immune and inflammatory conditions, cancer, diabetes, ischemia, cardiovascular diseases and mental disorders (30,31). Activation of UPR is also prominent in neurodegenerative diseases, in which accumulation of misfolded proteins provide a direct link to the activation of cellular stress pathways. Different ER stress indicators—including the ER chaperones PDI, Grp94, Grp78/BiP and Grp58, the ER stress sensor PERK and its target eIF2α—are present in the brains of AD (16,17) and Parkinson's disease (PD) patients (32), and in the spinal cord of amyotrophic lateral sclerosis (ALS) patients (33,34). Interestingly, human prion disorders have shown contradictory results depending on the markers used: whereas Grp58, Grp78 and Grp94 were elevated in Creutzfeldt-Jacob disease, PERK and eIF2α were not significantly activated (35,36). So far, ER stress has served as a molecular marker of cellular dyshomeostasis, suggesting a close link with neurodegenerative diseases. But the causal relationship between UPR and neurodegeneration has not been fully explored.
The IRE1–XBP1 pathway plays a critical role in UPR by regulating the expression of target genes that refold and degrade misfolded substrates (13,37). Recent reports have shown that the XBP1s isoform is upregulated in the spinal cord of several mouse models of familial ALS (33,38), in the brain of mice inoculated with several strains of prions (39), and in a chemical mouse model of PD (40). These conformational disorders provide an easy causal link to ER stress: protein misfolding in different cellular compartments. Interestingly, XBP1s is also elevated in the muscles of myotonic dystrophy patients, a neuromuscular disease caused by a non-coding RNA expansion (41), suggesting that altered cellular pathways secondary to the RNA pathology also result in UPR. So far, the only report of unconventional XBP1 splicing in the human brain comes from the temporal cortex of AD patients, providing key evidence for the activation of XBP1 in vivo (19). In contrast, XBP1 is not activated in the cortex of Tg2576 mice, a mouse model of AD with plaque formation and memory deficit, but no significant neuronal degeneration (19). Thus, activation of the IRE1–XBP1 pathway seems to correlate better with neuronal degeneration than with deposition of misfolded Aβ, suggesting that XBP1s is a specific marker for ongoing pathogenesis in AD. Our report contributes to the recent literature by showing that Aβ induces unconventional splicing of XBP1 in two models of AD: transgenic flies expressing Aβ and differentiated rat PC12 cells treated with Aβ oligomers. As opposed to Tg2576 mice, these two AD models show overt neuronal degenerations and demonstrate that XBP1 is highly sensitive to Aβ neurotoxicity. In PC12 cells, XBP1 is activated at concentrations four to eight times lower than the toxic Aβ concentration, whereas in flies every eye cell expressing Aβ also accumulated spliced XBP1–GFP. Thus, XBP1 activation may be a sensitive disease marker that could potentially help detect presymptomatic disease stages. On the other hand, the IRE1–XBP1 pathway also demonstrates high protein specificity. Ryoo et al. (42) found that polyglutamine expansions (Huntingtin-128Q and Ataxin3-78Q) and mutant Tau (TauR406W) expressed in the fly eye did not induce XBP1 splicing, whereas a mutant version of rhodopsin-1 (Rh-1), a membrane photosensitive protein associated with human retinal degeneration, did. The available evidence suggests that XBP1 may be more sensitive to proteins produced in the ER (Aβ, Rh-1) that, upon misfolding, have direct access to the ER stress sensors. In contrast, Tau and expanded polyglutamines aggregate primarily in the cytoplasm and nucleus and, thus, may be less efficient activators of ER stress. However, several polyglutamines and mutant SOD1 induce ER stress in mouse models, suggesting that secondary cellular mechanisms such as reduced proteasome activity, altered ERAD and retrotranslocation of misfolded substrates to the ER, may play a role in UPR activation. In this context, it is important to note that different sources of Aβ are efficient inducers of ER stress. In Drosophila, rapid misfolding of Aβ following synthesis in the ER can lead to direct activation of ER stress sensors. However, PC12 cells are treated with exogenous Aβ oligomers that have no direct access to the ER. Several potential mechanisms may explain extracellular Aβ neurotoxicity and induction of ER stress (43). (i) Aβ oligomers could enter the cell by endocytosis, reaching the ER by retrotranslocation. (ii) Aβ oligomers could accumulate in the cytosol, disrupt cellular homeostasis and, indirectly, induce ER stress. (iii) Aβ oligomers could traffic passively through ruptured membranes or through pores constituted of donut-shaped Aβ assemblies in the cell membrane and in the ER. (iv) Finally, ER stress could be the consequence of aberrant cellular signaling induced by the interaction of Aβ oligomers with membrane receptors. Although these mechanisms are possible contributors to Aβ neuropathology, there is little experimental support for their in vivo relevance.
In this report, we describe for the first time the neuroprotective activity of XBP1s in two models of AD. Based on its predicted protective function, we tested and demonstrated the ability of Drosophila XBP1 and mXBP1s to rescue Aβ neurotoxicity in the fly eye. These flies exhibited vastly improved eye organization, and this protection extended for 20 days in adult flies. Also, PC12 cells overexpressing mXBP1s were fully protected at lethal doses of Aβ oligomers (18 µg/ml) and partially protected at higher concentrations of Aβ. We also found that endogenous XBP1 plays a critical role in preventing Aβ neurotoxicity, as shown by the exacerbated cell death induced by Aβ in both models when XBP1 was eliminated, suggesting a physiological role in AD pathogenesis. This protective activity of the XBP1 pathway has been reported in other models of protein misfolding. In a cellular model of prion disease, XBP1s overexpression prevented prion protein (PrP) misfolding, whereas a dominant negative form of XBP1 favored PrP misfolding (44). Also, in a fly model of retinitis pigmentosa expressing mutant Rh-1, co-expression of ERAD components (downstream of XBP1s), including HRD-1, reduced the accumulation of Rh-1 and prevented retinal degeneration (45). Similarly, silencing of HRD-1 in SH-SY5Y cells led to increased APP processing and accumulation of Aβ, whereas cells overexpressing XBP1s showed reduced APP and Aβ (46). So far, the only evidence for the protective activity of XBP1s in mice comes from a non-transgenic mouse model of PD. In mice treated with the neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), adenoviral expression of XBP1s significantly prevented dopaminergic neuron loss (40). To test the neuroprotective activity of XBP1 in prion diseases, Hetz and Glimcher (39) engineered mice lacking XBP1 in the brain and inoculated them with prions. Unexpectedly, the animals showed normal prion replication and neuropathology, suggesting that the other UPR pathways could compensate for the absence of XBP1. The authors used the same rationale to test the role of XBP1 in ALS models, predicting that the lack of XBP1 would increase ALS pathology. However, the lack of XBP1 rescued ALS pathology and was associated with lower SOD1 aggregation in the spinal cord and increased autophagy, uncovering a new function for XBP1 (47). Overall, these results suggest that XBP1s has a significant protective activity in several disease models, although elimination of XBP1 uncovered the redundant activities of other UPR pathways.
We also propose here a new mechanistic connection between XBP1s and a pathway known to mediate Aβ neurotoxicity: XBP1s may reduce ER Ca2+ release by transcriptional regulation of a ryanodine Ca2+ channel (RyR3). For more than two decades, elevated intracellular Ca2+ has been proposed as a key neurotoxic pathway in AD—the ‘calcium dysregulation' hypothesis (27). Both intracellular Ca2+ stores and extracellular Ca2+ traffic are elevated in AD (5,6). However, elevated levels of RyRs in AD patients and AD models suggest that the increased release of ER Ca2+ stores through these channels may play a critical role in AD pathogenesis (8–12). Transgenic mice expressing mutant forms of APP, PS1 or triple transgenics also expressing mutant Tau show specific upregulation of RyR3, the major RyR isoform in the brain (9,10,12). In contrast, the levels of the other main ER Ca2+ transporters, including RyR1 and RyR2, the inositol-3 phosphate receptors (IP3R1–3) and the SERCA1–3 pumps, seem normal (11,12,48). Thus, elevated levels of RyR3 explain the increased Ca2+ release observed in several models of AD, which, in turn, stimulates the activity of IP3Rs by Ca2+-mediated Ca2+ release (CICR) mechanisms (5,9,11). The combined effect of overexpressed RyR3 and activated IP3Rs results in a persistent disruption of Ca2+ homeostasis, which may lead to the activation of cell death pathways (5,6). In this work, we show that PC12 cells start to release intracellular Ca2+ only a few minutes after exposure to Aβ oligomers, but cells that overexpress XBP1s release less Ca2+ and survive longer. This Ca2+-protective activity correlates with the reduced levels of RyR3 mRNA, suggesting that XBP1s prevents the release of Ca2+ through downregulation of RyR3. However, the reduced expression of RyR3 did not prevent Ca2+ release from the ER under high concentration of Aβ, suggesting that other Ca2+ transporters may be activated in response to Aβ. But the low levels of RyR3 allow these cells to recover after several minutes by avoiding further Ca2+ release, promoting their extended survival. It is still unclear whether the regulation of RyR3 by XBP1 is direct or indirect. Human and murine RyR3 contain multiple XBP1s-binding sites (ER stress elements) arguing for a direct repression by XBP1s (Supplemental Material, Fig. S5). Even though XBP1s is best known as a transcriptional activator, several expression-profiling experiments have identified numerous genes downregulated by XBP1s (28,49). This dual activity might be possible if XBP1s formed complexes with different partners that modulate its transcriptional activity. Additionally, transcriptional repression may be indirectly mediated by XBP1s target genes. This indirect repression mechanism has been demonstrated for Mist1, an XBP1s target that prevents the differentiation of myotubules through the repression of the muscle differentiation factor, MyoD (50). Having the ability to activate and repress gene expression provides XBP1s with an expanded repertoire of regulatory activities during cellular differentiation and ER stress response. Whatever the mechanism mediating the negative regulation of RyR3 by XBP1s, this activity was confirmed in flies, where the levels of Rya-r were also reduced. Moreover, reduced levels of Rya-r protect against Aβ neurotoxicity in flies, demonstrating the functional relevance of decreased Ca2+ release from the ER. Interestingly, a recent report identified loss-of-function alleles of Rya-r as suppressors of the deleterious effects of mutant constructs of PS1 and APP, further supporting the link between familial AD genes and Ca2+ dyshomeostasis (51). Overall, these results agree with previous results showing that the regulation of Ca2+ traffic prevents Aβ-induced cell death (52,53). Furthermore, one of the most effective current AD therapies includes memantine, which blocks the activity of the N-methyl-d-aspartate Ca2+ channel, suggesting that Ca2+ regulation at the cell membrane and the ER are viable therapeutic targets in AD and, possibly, other neurodegenerative conditions.
MATERIALS AND METHODS
Generation of transgenic flies
We created transgenic flies expressing two copies of human amyloid-β 1–42 peptide (2xAβ) to imitate the duplication of the APP gene associated with familial AD in humans (23). For this, a 161 nt fragment containing the Aβ sequence was amplified from pCMV-Aβ (a gift from C. Soto, UTMB) using primers B42Pst-F (CTGCAGATGCAGAATTCCGACAT) and B42Xho-R (GGCTTCCTCGAGTCGACTATC). The underlined sequence indicates engineered restriction sites. This fragment was digested with PstI, blunted and further digested with XhoI. To ensure that Aβ is secreted in flies, we amplified a 232 nt fragment containing the signal peptide from the argos gene using genomic DNA flanked by the restriction sites EcoRI and SfoI (AosRI-F:TCGAATTCAATTACAAAATTCATTG and AosSfoI-R: GGCGCCGCCGACAGCAACGG). Finally, the EcoRI–SfoI and the blunted PstI–XhoI fragments were mixed in a three-part ligation with pUAST (54) digested with EcoRI and XhoI. Then, we engineered a construct carrying two tandem copies of the Aβ expression cassette. For this, we released pUAS:SP-Aβ with BamHI and isolated a 1666 nt fragment carrying the UAS-binding sites, the minimal hsp70 promoter, the Argos-Aβ fusion and the polyA signal. This fragment was sucbloned into a partially BamHI-digested pUAS:SP-Aβ to generate pUAS:SP-Aβ (2X). In the final construct, each copy of SP-Aβ has its own UAS regulatory sequence for transcriptional control by the yeast Gal4 activator, and its own polyA for RNA stability and processing. These flies were briefly described before (22). The cDNA encoding mXBP1s [a gift from Hetz (49)] was released from pcDNA and subcloned into the NotI site of pUAST. The resulting Aβ2X and mXBP1s constructs were injected into yw embryos by standard procedures (55) and several independent transgenic lines were obtained for each.
Drosophila genetics
The reporter strains UAS–LacZ and UAS–CD8–GFP, the eye (gmr-Gal4), Kenyon cell (OK107-Gal4), pan-neural (Elav-Gal4) and ubiquitous (da-Gal4) driver strains, and the Rya-r16 internal deletion were obtained from the Bloomington Drosophila Stock Center (flystocks.bio.indiana.edu). The insertion expressing XBP1 (XBP1d08698) was obtained from the Exelixis collection at Harvard (drosophila.med.harvard.edu) and the UAS-XBP1–RNAi strain was obtained from the Vienna Drosophila RNAi Center (stockcenter.vdrc.at/control/main). The XBP1–GFP sensor was obtained from Yanicostas and colleagues (24). The strain SM5-Gal80 was derived by P-element mobilization of the Gal80 insertion. For transgene expression in the eye, homozygous females gmr-Gal4 UAS–Aβ were crossed with males carrying the desired constructs and the progeny was maintained at 27.5°C. For Aβ expression in brain neurons, homozygous females OK107-Gal4 were mated with males bearing UAS–Aβ constructs with or without the XBP1 constructs, and the progeny was maintained at 27°C.
Histology and immunofluorescence
For histological analysis of retinas, epon-embedded heads of 1- or 20-day-old flies were sectioned at 1 µm and stained with toluidine-blue as described before (56). Sections were documented in a Nikon 80i microscope with a Zeiss Axiocam digital camera and AxioVision software. Fresh eyes were photographed under a dissecting microscope using a Nikon Coolpix digital camera. For scanning electron microscopy (SEM), flies of the appropriate genotypes were collected at day 1, dehydrated in ethanol, dried in hexamethyldisilazane (Electron Microscope Sciences) and coated for observation in a Jeol 1500 SEM. Eye phenotypes were arbitrarily divided into five categories for quantification as follows: normal (Cat. 1): perfect eye morphology; mild (Cat. 2): slightly disrupted eye morphology; moderate (Cat. 3): moderately disrupted eyes, glassy (bright) center; strong (Cat. 4): highly disrupted eye morphology, glassy with necrotic spots; small (Cat. 5): small, depigmented and severely disorganized eyes. Progeny from three to six independent vials was assigned to each category for statistical analysis (t-test) and represented graphically in Microsoft Excel.
Flies expressing Aβ in the Kenyon cells alone (OK107-Gal4; UAS–Aβ) or with mXBP1s (OK107-Gal4; UAS–Aβ/mXBP1s) were collected at day 1 post-eclosion. Eye imaginal discs from third instar larvae were collected from flies carrying the XBP1–GFP sensor alone (gmr-Gal4; XBP1–GFP) or combined with Aβ (gmr-Gal4 Aβ; XBP1–GFP). Whole-mount brains and eye imaginal discs were processed following published protocols (56). Brains expressing Aβ were first stained with thioflavine-S (0.125%) for 20 min and then incubated with 4G8 anti-Aβ (Millipore) and Cy3 secondary antibody (Sigma). The neural maker anti-Elav was obtained from the Developmental Studies Hybridoma Bank. Brains and eye discs were imaged by confocal microscopy with a Zeiss Axiovert 40 microscope and LSM 510 software. All images were processed in Adobe Photoshop for preparation of final figures. Quantification of immunofluorescence signal was done in Photoshop, followed by statistical analysis (t-test) and plotting in Microsoft Excel.
Viability assay
Females UAS–Aβ/SM5, Gal80; da-Gal4 were crossed with males bearing a control construct (UAS–LacZ) or the Rya-r16 deletion and reared at 25°C. Progeny carrying Aβ (experimental) or SM5, Gal80 (control) chromosomes were identified and viability was calculated as the percentage of Aβ flies with respect to the control genotypes as follows: viability = observed (# Aβ flies)/expected (# SM5, Gal80 flies) × 100. Six independent vials for Aβ/Rya-r (n= 6) and three for Aβ (n= 3) were analyzed, with each vial producing at least 50 flies.
RT–PCR and analysis of XBP1 splicing
Two hundred micrograms of total RNA isolated from cultured cells and fly tissues (RNAqueous, Ambion) were subjected to RT (reverse transcription)–PCR using One Step RT-PCR (Qiagen). For detection of Drosophila XBP1s, we amplified a fragment of 148 bp using primers dXBP1s550F 5′-ACCAACCTTGGATCTGCCG-3′ and dXBP1s697R 5′-CGCCAAGCATGTCTTGTAGA-3′, where the 3′ end of the reverse primer straddles the ER-sensitive intron. To eliminate any unspecific amplification of XBP1u, we subjected the PCR products to PstI digestion, which only cleaves the intron included in the XBP1u isoform, leaving the XBP1s isoform intact. A 482 bp fragment from Actin5C was also amplified in these reactions as internal control using the primers Act5C3F 5′-CTGGACTTCGAGCAGGAGAT-3′ and Act5C2R 5′-GGTGGCTTGGATGCTTAGAA-3′. For amplification of both isoforms of rat XBP1 (PC12 cells), we used primers Rxbp3F 5-GAAACTGAAAAACAGAGTAGCAGC-3′ and Rxbp3R 5′-GCTTCCAGCTTGGCTGATG-3′. After RT–PCR, half of the reaction was digested with PstI to detect the XBP1s isoform. To detect specific mXBP1s transcripts in PC12 cells, we used primers 5′-ATCCTGACGAGGTTCCAG-3′ and 5′-CCAGAATGCCCAAAAGGATA-3′. To analyze rat RyR3 transcripts, we amplified two different regions of 331 nt (Rryr3-1F: 5′-GAGCTGCTCATCACCAACAA-3′ and Rryr3-1R: 5′-AGGTCTTGTGGGTCACCTTG-3′) and 466 nt (Rryr3-2F: 5′-CGGAACACCACAGCCTTATT-3′ and Rryr3-2R: 5′-TCAAGATGCAGTGCAAGACC-3′). In all RT–PCRs from PC12 RNA, a 823 bp actin fragment was amplified as internal control using primers against cross-reacting human actin: hActin1F 5′-CCACACCTTCTACAATGAGC-3′ and hActin1R 5′-ACTCCTGCTTGCTGATCCAC-3′.
Quantitative RT–PCR
Total RNA was isolated from fly heads (Trizol, Invitrogen), DNA traces were eliminated with Turbo DNAse (Ambion), and cDNAs were prepared with One Step RT-PCR (Qiagen). To quantify total XBP1 transcripts in Drosophila, we generated primers dXBP1t238F 5′-TGGGAGGAGAAAGTGCAAAG-3′ and dXBP1t362R 5′-TCCGTTCTGTCTGTCAGCTC-3′ that amplify a fragment of 125 bp in the 5′ common exon. As internal control, we also used the primers for Actin5C: Act5C922F 5′-CCAGGTATCGCTGACCGTAT-3′ and Act5C1069R 5′-ACATCTGCTGGAAGGTGGAC-3′ that amplify a fragment of 147 bp. For qPCR with the SYBR Green PCR Master Mix and the 7500 FAST Real-Time PCR System (Applied Biosystems), we used 10 ng of purified RNA. Cycling conditions were set at 95°C for 10 min and 40 cycles of 95°C for 15 s and 55°C for 1 min. The levels of RNA were calculated using the 7500 2.0.1 software (Applied Biosystems), which relies on the comparative Ct method of quantification. Total XBP1 mRNA levels were normalized to Actin5C.
For Drosophila Rya-r transcripts, Taqman real-time RT–PCR assays were performed with the following primers and probe: Ryar44F-11423F (5′-AGGAAGCGTGCCGTAATCG-3′), Ryar44F-11530R (5′-CTGCAGCCACTGCTCGTAGTAC-3′) and Ryar44F-11469T (5′-6FAM-CTCTACCCAGACATCG-3′). Real-time qPCR reactions were done in an ABI PRISM 7700 (Applied Biosystems). The relative amounts of mRNAs were calculated by amplifying RNA pol II mRNA duplicate reactions.
Preparation of Aβ oligomers
Soluble oligomers were prepared as shown by Kayed et al. (26) by dissolving 0.3 mg Aβ (previously re-solubilized in formic acid) in 200 µl of hexafluoroisopropanol (HFIP) for 20 min at room temperature. Two hundred microliters of this Aβ solution were added to 1000 µl DD H2O in a siliconized Eppendorf tube for evaporation of the HFIP. The samples were then stirred at 500 rpm using a Teflon coated micro stir bar for 8 h at 22°C for formation of oligomers. Before using Aβ oligomers for cytotoxicity assays, the samples were sonicated to break incipient fibers.
Cell culture, transfection and siRNA
Rat pheochromocytoma PC12 cells were cultured in Dubelcco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). To promote their differentiation to neural fate, they were incubated for 48 h in 10 µm retinoic acid and 1% FBS in 96-well plates at 7 × 104 cells/well. Cells were transfected with pCMV-mXBP1s (Lipofectamine 2000, Invitrogen) and supplemented with Geneticin-4G8 (0.5 mg/ml) to select independent clones. For Aβ cytotoxicity assays, differentiated cells were challenged with Aβ at concentrations 2.25–36 µg/ml. Cell viability was measured 6–8 h later using an indirect quantitative assay for adenosine triphosphate levels (CellTiter, Promega). Caspase-3/7 activation was measured at different time points using Apo-ONE® Homogeneous Caspase-3/7 Assay (Promega). All these experiments were carried out in triplicate and repeated at least twice. For tunicamycin and staurosporine toxicity, these drugs were added to differentiated PC12 cells and viability was determined after incubating for 6 h. Cells were stained against anti-Tubulin (Sigma) and anti-activated-Caspase3 (Cell Signaling Technology) antibodies and DAPI following standard protocols and then they were photographed in a Nikon 80i microscope by epifluorescence.
For siRNA, Silencer® pre-designed siRNA molecules against human XBP1s were obtained from Ambion (ID #130473, #13974 and #13975). Two different siRNAs were used individually for human XBP1 to ensure reproducibility of results. We included a ‘scrambled' siRNA as control for unspecific toxicity. For silencing experiments, cells were seeded in 96-well plates and transfected with 10pmol of siRNA. siRNAs were transfected using Lipofectamine 2000 following the manufacturer's recommendations.
Intracellular free calcium
The intracellular free calcium level ([Ca2+]i) in individual cells was determined by fluorescence imaging of the Ca2+-indicator dye Fura-2 (Invitrogen). In brief, cells were incubated for 1 h in the presence of 5 µm fura-2 and then exposed to low (9 µg/ml) and high (18 µg/ml) concentrations of Aβ oligomers. Cells were analyzed using a Nikon Eclipse TE200 microscope with a Roper CoolSnap HQ digital camera, and Universal Imaging Metamoprh and MetaFluor Software. The average level of [Ca2+]i in 20 cells/microscope fields was quantified for each condition. Five micromolar of ionomycin was used for complete [Ca2+]i release at the end of the experiment. Data were analyzed using a two-way ANalysis Of VAriance (ANOVA) including only the points after the treatment was initiated.
Tissue homogenates and western blot
Ten fly heads from each relevant genotype were used to prepare homogenates. Fly heads or PC12 cells were homogenized in 25 µl of radio immunoprecipitation assay buffer containing Complete Protease Inhibitors (Roche). For western blots, an equal volume of Tricine-SDS loading buffer was added to each sample, followed by boiling at 95°C for 5 min. Protein extracts were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) in 4–12 % Bis–Tris gels (Invitrogen) under reducing conditions and electroblotted into nitrocellulose membranes. Membranes were blocked in TBS containing 5% non-fat milk, and probed against Aβ (6E10, Signet), β-Tubulin (Sigma), Drosophila BiP/HSC3 (a gift from H. Steller, Rockefeller University) and ATF6 and XBP1 (Santa Cruz) antibodies. To improve reactivity of 6E10 antibody, membranes were boiled for 5 min in TBS. Immunoreactive bands were visualized by enhanced chemiluminescence (ECL, Amersham). Quantification of relative expression was done from three independent experiments using a loading control for normalization, and statistical differences were determined by t-test in Excel.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by a Jeanne B. Kempner postdoctoral fellowship (UTMB) to S.C.-T. and the NIH grant DP2 OD002721-01 to P.F.-F.
Supplementary Material
ACKNOWLEDGEMENTS
We thank J. Wen and Violet Ham for assistance with the eye sections, L. Vergara for assistance with calcium imaging, R. Kayed for the conformational antibodies and assistance in preparing oligomers, C. Hetz for the mXBP1s cDNA, C. Soto for helpful discussions, the Developmental Studies Hybridoma Bank for antibodies and the Bloomington, Vienna and Harvard Stock centers for fly strains.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Crews L., Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer's disease. Hum. Mol. Genet. 2010;19:R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haass C., Selkoe D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J. The amyloid hypothesis for Alzheimer's disease: a critical reappraisal. J. Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 4.Oddo S., Caccamo A., Kitazawa M., Tseng B.P., LaFerla F.M. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol. Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Bezprozvanny I., Mattson M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green K.N., LaFerla F.M. Linking calcium to Abeta and Alzheimer's disease. Neuron. 2008;59:190–194. doi: 10.1016/j.neuron.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Guo Q., Fu W., Sopher B.L., Miller M.W., Ware C.B., Martin G.M., Mattson M.P. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat. Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 8.Kelliher M., Fastbom J., Cowburn R.F., Bonkale W., Ohm T.G., Ravid R., Sorrentino V., O'Neill C. Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer's disease neurofibrillary and beta-amyloid pathologies. Neuroscience. 1999;92:499–513. doi: 10.1016/s0306-4522(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 9.Chan S.L., Mayne M., Holden C.P., Geiger J.D., Mattson M.P. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J. Biol. Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 10.Smith I.F., Hitt B., Green K.N., Oddo S., LaFerla F.M. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer's disease. J. Neurochem. 2005;94:1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 11.Stutzmann G.E., Smith I., Caccamo A., Oddo S., Laferla F.M., Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J. Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Supnet C., Grant J., Kong H., Westaway D., Mayne M. Amyloid-beta-(1–42) increases ryanodine receptor-3 expression and function in neurons of TgCRND8 mice. J. Biol. Chem. 2006;281:38440–38447. doi: 10.1074/jbc.M606736200. [DOI] [PubMed] [Google Scholar]
- 13.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 14.Lee A.H., Iwakoshi N.N., Anderson K.C., Glimcher L.H. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl Acad. Sci. USA. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 16.Chang R.C., Wong A.K., Ng H.K., Hugon J. Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer's disease. Neuroreport. 2002;13:2429–2432. doi: 10.1097/00001756-200212200-00011. [DOI] [PubMed] [Google Scholar]
- 17.Hoozemans J.J., Veerhuis R., Van Haastert E.S., Rozemuller J.M., Baas F., Eikelenboom P., Scheper W. The unfolded protein response is activated in Alzheimer's disease. Acta Neuropathol. (Berl.) 2005;110:165–172. doi: 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- 18.Kakimura J., Kitamura Y., Takata K., Tsuchiya D., Taniguchi T., Gebicke-Haerter P.J., Smith M.A., Perry G., Shimohama S. Possible involvement of ER chaperone Grp78 on reduced formation of amyloid-beta deposits. Ann. N. Y. Acad. Sci. 2002;977:327–332. doi: 10.1111/j.1749-6632.2002.tb04834.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.H., Won S.M., Suh J., Son S.J., Moon G.J., Park U.J., Gwag B.J. Induction of the unfolded protein response and cell death pathway in Alzheimer's disease, but not in aged Tg2576 mice. Exp. Mol. Med. 2010;42:386–394. doi: 10.3858/emm.2010.42.5.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katayama T., Imaizumi K., Sato N., Miyoshi K., Kudo T., Hitomi J., Morihara T., Yoneda T., Gomi F., Mori Y., et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat. Cell Biol. 1999;1:479–485. doi: 10.1038/70265. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.Y., Hwang D.Y., Kim Y.K., Lee J.W., Shin I.C., Oh K.W., Lee M.K., Lim J.S., Yoon D.Y., Hwang S.J., et al. PS2 mutation increases neuronal cell vulnerability to neurotoxicants through activation of caspase-3 by enhancing of ryanodine receptor-mediated calcium release. FASEB J. 2006;20:151–153. doi: 10.1096/fj.05-4017fje;1. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Funez P., Salinas J., Nino-Rosales L., Rincon-Limas D.E. In: New Trends in Alzheimer and Parkinson Disorders: ADPD 2007. Hanin I., Windisch M., Poewe W., Fisher A., editors. Salzburg, Austria: Medimont; 2007. pp. 37–41. [Google Scholar]
- 23.Rovelet-Lecrux A., Hannequin D., Raux G., Le Meur N., Laquerriere A., Vital A., Dumanchin C., Feuillette S., Brice A., Vercelletto M., et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat. Genet. 2006;38:24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- 24.Souid S., Lepesant J.A., Yanicostas C. The xbp-1 gene is essential for development in Drosophila. Dev. Genes Evol. 2007;217:159–167. doi: 10.1007/s00427-006-0124-1. [DOI] [PubMed] [Google Scholar]
- 25.Kayed R., Head E., Sarsoza F., Saing T., Cotman C.W., Necula M., Margol L., Wu J., Breydo L., Thompson J.L., et al. Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers. Mol. Neurodegener. 2007;2:18. doi: 10.1186/1750-1326-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayed R., Head E., Thompson J.L., McIntire T.M., Milton S.C., Cotman C.W., Glabe C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 27.Khachaturian Z.S. Calcium, membranes, aging, and Alzheimer's disease. Introduction and overview. Ann. N. Y. Acad. Sci. 1989;568:1–4. doi: 10.1111/j.1749-6632.1989.tb12485.x. [DOI] [PubMed] [Google Scholar]
- 28.Kakiuchi C., Ishiwata M., Hayashi A., Kato T. XBP1 induces WFS1 through an endoplasmic reticulum stress response element-like motif in SH-SY5Y cells. J. Neurochem. 2006;97:545–555. doi: 10.1111/j.1471-4159.2006.03772.x. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan K.M., Scott K., Zuker C.S., Rubin G.M. The ryanodine receptor is essential for larval development in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 2000;97:5942–5947. doi: 10.1073/pnas.110145997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 31.Lin J.H., Walter P., Yen T.S. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holtz W.A., O'Malley K.L. Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J. Biol. Chem. 2003;278:19367–19377. doi: 10.1074/jbc.M211821200. [DOI] [PubMed] [Google Scholar]
- 33.Kikuchi H., Almer G., Yamashita S., Guegan C., Nagai M., Xu Z., Sosunov A.A., McKhann G.M., 2nd, Przedborski S. Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model. Proc. Natl Acad. Sci. USA. 2006;103:6025–6030. doi: 10.1073/pnas.0509227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobisawa S., Hozumi Y., Arawaka S., Koyama S., Wada M., Nagai M., Aoki M., Itoyama Y., Goto K., Kato T. Mutant SOD1 linked to familial amyotrophic lateral sclerosis, but not wild-type SOD1, induces ER stress in COS7 cells and transgenic mice. Biochem. Biophys. Res. Commun. 2003;303:496–503. doi: 10.1016/s0006-291x(03)00353-x. [DOI] [PubMed] [Google Scholar]
- 35.Hetz C., Russelakis-Carneiro M., Maundrell K., Castilla J., Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Unterberger U., Hoftberger R., Gelpi E., Flicker H., Budka H., Voigtlander T. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J. Neuropathol. Exp. Neurol. 2006;65:348–357. doi: 10.1097/01.jnen.0000218445.30535.6f. [DOI] [PubMed] [Google Scholar]
- 37.Hetz C., Glimcher L.H. Fine-tuning of the unfolded protein response: assembling the IRE1alpha interactome. Mol. Cell. 2009;35:551–561. doi: 10.1016/j.molcel.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atkin J.D., Farg M.A., Turner B.J., Tomas D., Lysaght J.A., Nunan J., Rembach A., Nagley P., Beart P.M., Cheema S.S., et al. Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. J. Biol. Chem. 2006;281:30152–30165. doi: 10.1074/jbc.M603393200. [DOI] [PubMed] [Google Scholar]
- 39.Hetz C., Lee A.H., Gonzalez-Romero D., Thielen P., Castilla J., Soto C., Glimcher L.H. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc. Natl Acad. Sci. USA. 2008;105:757–762. doi: 10.1073/pnas.0711094105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sado M., Yamasaki Y., Iwanaga T., Onaka Y., Ibuki T., Nishihara S., Mizuguchi H., Momota H., Kishibuchi R., Hashimoto T., et al. Protective effect against Parkinson's disease-related insults through the activation of XBP1. Brain Res. 2009;1257:16–24. doi: 10.1016/j.brainres.2008.11.104. [DOI] [PubMed] [Google Scholar]
- 41.Ikezoe K., Nakamori M., Furuya H., Arahata H., Kanemoto S., Kimura T., Imaizumi K., Takahashi M.P., Sakoda S., Fujii N., et al. Endoplasmic reticulum stress in myotonic dystrophy type 1 muscle. Acta Neuropathol. 2007;114:527–535. doi: 10.1007/s00401-007-0267-9. [DOI] [PubMed] [Google Scholar]
- 42.Ryoo H.D., Domingos P.M., Kang M.J., Steller H. Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J. 2007;26:242–252. doi: 10.1038/sj.emboj.7601477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S.J., Desplats P., Sigurdson C., Tsigelny I., Masliah E. Cell-to-cell transmission of non-prion protein aggregates. Nat. Rev. Neurol. 2010;6:702–706. doi: 10.1038/nrneurol.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hetz C., Castilla J., Soto C. Perturbation of endoplasmic reticulum homeostasis facilitates prion replication. J. Biol. Chem. 2007;282:12725–12733. doi: 10.1074/jbc.M611909200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang M.J., Ryoo H.D. Suppression of retinal degeneration in Drosophila by stimulation of ER-associated degradation. Proc. Natl Acad. Sci. USA. 2009;106:17043–17048. doi: 10.1073/pnas.0905566106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaneko M., Koike H., Saito R., Kitamura Y., Okuma Y., Nomura Y. Loss of HRD1-mediated protein degradation causes amyloid precursor protein accumulation and amyloid-beta generation. J. Neurosci. 2010;30:3924–3932. doi: 10.1523/JNEUROSCI.2422-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hetz C., Thielen P., Matus S., Nassif M., Court F., Kiffin R., Martinez G., Cuervo A.M., Brown R.H., Glimcher L.H. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stutzmann G.E., Caccamo A., LaFerla F.M., Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer's-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J. Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee A.H., Iwakoshi N.N., Glimcher L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acosta-Alvear D., Zhou Y., Blais A., Tsikitis M., Lents N.H., Arias C., Lennon C.J., Kluger Y., Dynlacht B.D. XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell. 2007;27:53–66. doi: 10.1016/j.molcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 51.van de Hoef D.L., Hughes J., Livne-Bar I., Garza D., Konsolaki M., Boulianne G.L. Identifying genes that interact with Drosophila presenilin and amyloid precursor protein. Genesis. 2009;47:246–260. doi: 10.1002/dvg.20485. [DOI] [PubMed] [Google Scholar]
- 52.Suen K.C., Lin K.F., Elyaman W., So K.F., Chang R.C., Hugon J. Reduction of calcium release from the endoplasmic reticulum could only provide partial neuroprotection against beta-amyloid peptide toxicity. J. Neurochem. 2003;87:1413–1426. doi: 10.1111/j.1471-4159.2003.02259.x. [DOI] [PubMed] [Google Scholar]
- 53.Ferreiro E., Oliveira C.R., Pereira C. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1,4,5-triphosphate receptors in the neurotoxic effects induced by the amyloid-beta peptide. J. Neurosci. Res. 2004;76:872–880. doi: 10.1002/jnr.20135. [DOI] [PubMed] [Google Scholar]
- 54.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 55.Rubin G.M., Spradling A.C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez-Funez P., Nino-Rosales M.L., de Gouyon B., She W.C., Luchak J.M., Martinez P., Turiegano E., Benito J., Capovilla M., Skinner P.J., et al. Identification of genes that modify ataxin-1-induced neurodegeneration. Nature. 2000;408:101–106. doi: 10.1038/35040584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.