Abstract
Given the high genetic heterogeneity of inherited retinal degenerations (IRDs), a wide applicable treatment would be desirable to halt/slow progressive photoreceptor (PR) cell loss in a mutation-independent manner. In addition to its erythropoietic activity, erythropoietin (EPO) presents neurotrophic characteristics. We have previously shown that adeno-associated viral (AAV) vector-mediated systemic EPO delivery protects from PR degeneration. However, this is associated with an undesired hematocrit increase that could contribute to PR protection. Non-erythropoietic EPO derivatives (EPO-D) are available which allow us to dissect erythropoiesis's role in PR preservation and may be more versatile and safe than EPO as anti-apoptotic agents. We delivered in animal models of light-induced or genetic retinal degeneration either intramuscularly or subretinally AAV vectors encoding EPO or one of the three selected EPO-D: the mutant S100E, the helix A- and B-derived EPO-mimetic peptides. We observed that (i) systemic expression of S100E induces a significantly lower hematocrit increase than EPO and provides similar protection from PR degeneration, and (ii) intraocular expression of EPO-D protects PR from degeneration in the absence of significant hematocrit increase. On the basis of this, we conclude that erythropoiesis is not required for EPO-mediated PR protection. However, the lower efficacy observed when EPO or S100E is expressed intraocularly rather than systemically suggests that hormone systemic effects contribute to PR protection. Unlike S100E, EPO-mimetic peptides preserve PR only when given locally, suggesting that different EPO-D have a different potency or mode of action. In conclusion, our data show that subretinal delivery of AAV vectors encoding EPO-D protects from light-induced and genetic PR degeneration.
INTRODUCTION
Inherited retinal degenerations (IRDs) are common untreatable conditions leading to blindness, which include retinitis pigmentosa (RP) (1), Leber congenital amaurosis (LCA) (2) and cone–rod dystrophies (3). IRDs are mostly inherited as Mendelian traits and characterized by high genetic heterogeneity. Mutations in 208 genes have been so far identified in patients with IRDs (RetNet: www.sph.uth.tmc.edu/retnet/). Independently of the primary causative gene, IRDs share a common degenerative process in which photoreceptor (PR: rods and cones) apoptosis represents the final outcome that leads to vision loss (4,5). However, the specific molecular mechanisms by which different genetic defects result in PR apoptosis are not thoroughly characterized. The delivery of genes or compounds that are able to either inhibit/slow PR apoptosis or sustain PR function and/or survival could be used as mutation-independent treatments for IRDs (6–10).
Erythropoietin (EPO), the cytokine that stimulates proliferation and differentiation of erythroid progenitor cells has many extra-erythropoietic functions, such as neuroprotection, anti-inflammation and regeneration (11), and it has been reported to protect neurons from cell death associated with acute or chronic injuries (12,13). Erythropoiesis is promoted by EPO binding to the homodimeric EPO receptor (EPOR2) (14), whereas non-erythropoietic functions have been shown to be mediated by EPO interaction with either EPOR2 (15) or other poorly characterized EPOR complexes (16–20). So far, the beta common receptor (βCR or CD131) has been reported to interact with EPOR (21–24) and to mediate EPO-protective functions in some tissues (25). However, the role of the βCR in the EPO-mediated protection is still controversial and low/no βCR expression has been reported in most neurons of the central nervous system (26,27), thus suggesting the existence of other yet unidentified tissue-protective receptor complexes. EPOR (28–31) and βCR (30) have been reported to be expressed in the retina where they may mediate EPO-protective functions. Notably, systemically delivered EPO, able to cross the blood–retina barrier (28,32), protects the retina from light damage or genetic degeneration (28,33), ischemic injury (29,34,35) and glaucoma (36); whereas intraocularly delivered EPO has been shown to protect retinal ganglion cells (RGC) from axotomy-induced degeneration (37) and experimental glaucoma (38) and to reduce neuronal and vascular cells apoptosis in models of retinal degeneration (39) and diabetes (40).
EPO delivery through viral vectors allows sustained and/or regulatable expression (41,42) that avoids repeated systemic or intraocular administrations that would be required to treat chronic and progressive diseases like IRDs. We have previously shown that systemic adeno-associated viral (AAV) vector-mediated delivery of rhesus EPO protects the retina of light-damaged rats and rds mice (33). However, systemic EPO delivery is accompanied by various side effects such as a significant hematocrit increase (12,33), increased thrombotic risk (43) and platelet hyper-reactivity (44,45). Recently, several non-erythropoietic EPO derivatives (EPO-D) have been engineered to retain tissue-protective functions while avoiding EPO-mediated erythropoiesis (46–49) as well as the other hormone side effects (45). Three of these derivatives appear particularly promising: the EPO mutant S100E (S100E) (47), the helix A- (49–52) and B-derived peptides (48,51,53) defined as EPO-mimetic peptides. The S100E protein shows drastically reduced affinity for the EPOR2 (47) and protects against experimental focal cerebral ischemia in vivo (54). The EPO-mimetic peptides derived from helices A and B protect from ischemic stroke, diabetes-induced retinal edema and peripheral nerve trauma (48,49,51). EPO-mimetic peptides do not stimulate erythropoiesis; however, they have been hypothesized to bind EPOR (48,49,51), thus corroborating the hypothesis that EPO has separate domains that mediate neurotrophic and/or erythropoietic functions (48).
In the present work, we sought to (i) establish whether delivery of non-erythropoietic EPO-D may represent a mutation-independent treatment for IRDs, safer than EPO since it lacks EPO's systemic side effects, and (ii) shed light on the contribution of the different EPO functions (erythropoietic versus non-erythropoietic) to retinal protection. To this end, we compared the efficacy of systemic versus subretinal (SR) AAV-mediated delivery of either EPO or the three selected non-erythropoietic EPO-D in the following models of PR degeneration. These include (i) the rat and murine models of light-induced retinal degeneration (55) and (ii) the rds (retinal degeneration slow) (56) and the Aipl1 (Aryl hydrocarbon receptor interacting protein like-1)−/− (57) mice, which recapitulate RP and LCA, respectively.
RESULTS
EPOR and βCR are expressed in the retina
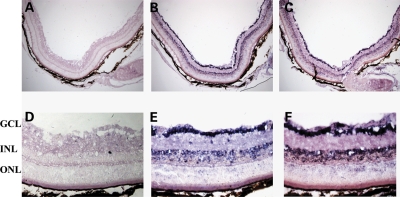
We initially set up to analyze the retinal expression profile of known EPORs to determine (i) whether a local effect can be exerted following SR AAV-mediated delivery of EPO and EPO-D, and (ii) which are EPO and EPO-D target cells in the retina. To date, EPO and EPO-D functions have been shown to be mediated by two distinct receptor complexes composed of EPOR and βCR (15,20,25), both reported to be expressed in the retina (28,30,31). We defined EPOR and βCR expression pattern by in situ hybridization analysis (ISH) on retinal sections from 4-week-old CBA mice. ISH showed that both EPOR and βCR are expressed in RGC, in the inner nuclear layer and to a lower extent in PR (Fig. 1). Notably, the major expression of EPORs in ganglion cells and interneurons (Fig. 1) is consistent with the evidence that most neurotrophic factors (such as the brain-derived neurotrophic factor, the ciliary neurotrophic factor, the basic fibroblast growth factor 2 and the glial cell-derived neurotrophic factor) promote PR survival acting through other retinal cell types and in particular through interneurons (58,59).
Figure 1.
EPOR and βCR expression pattern in the adult murine retina. In situ hybridization on retinal cross-sections from 1-month-old CBA mouse; upper panel: pictures at 10× magnification (A–C); lower panel: pictures at 40× magnification (D–F). EPOR and βCR sense control probes (A, D). EPOR antisense probe (B, E). βCR antisense probe (C, F). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. The blue/violet staining shows that the corresponding transcript is expressed in various retinal layers.
Assessment of hematocrit levels, functional and morphological PR preservation following EPO and EPO-D gene delivery in the rat and murine models of light-induced retinal degeneration
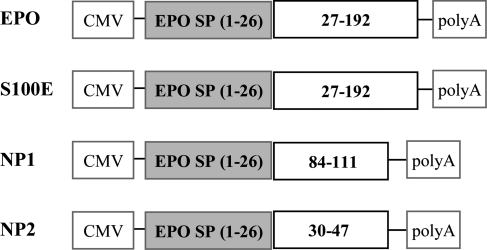
To perform gene transfer, we generated AAV1-based vectors (AAV2/1), which result in efficient outer retina transduction and intraocular secretion of therapeutic products (60–62), that encode EPO or the three selected EPO-D: the mutant S100E (S100E), the EPO-mimetic peptides derived from helix B (referred to as NP1, 28 mer) and helix A (referred to as NP2, 18 mer) (Fig. 2). The proper expression of EPO and EPO-D from our constructs was tested in vitro prior to the generation of AAV vectors and in vivo use (Supplementary Material, Fig. S1).
Figure 2.
Schematic representation of the expression cassettes containing EPO and EPO-D. Each expression construct has been cloned between the AAV2-inverted terminal repeats using the pAAV2.1 plasmid for AAV2/1 vector production. EPO, S100E, NP1 and NP2 CDSs are all of murine origin. The amino acidic region relative to the murine EPO protein is shown. CMV, cytomegalovirus promoter; EPO, erythropoietin; EPO SP, EPO signal peptide; polyA, polyadenilation signal sequence; S100E, EPO carrying the S to E mutation at amino acidic position 100 of the mature EPO (corresponding to amino acidic position 126 from the ATG start codon).
Light damage of albino rodents is a useful model of induced retinal degeneration to test the effect of trophic/antiapoptotic molecules (55). We injected 4-week-old albino Lewis rats either intramuscularly or subretinally with AAV2/1 vectors encoding EPO, S100E, and NP1 plus NP2 (NP1–NP2). Subretinal injections of AAV2/1 vectors encoding the enhanced green fluorescence protein (AAV2/1-EGFP) were used as controls of the SR treatments. Uninjected rats served as controls for the animals that received AAV systemically. Four weeks following vector delivery, we measured EPO and S100E protein levels by ELISA assay (Table 1) and found them to be significantly increased in the sera of rats injected systemically and in the anterior chamber fluid (ACF) of rats injected subretinally compared with controls (Table 1). In addition, detectable levels of both proteins were measured in the ACF of rats injected systemically, confirming EPO and S100E ability to cross the blood–retina barrier (Table 1). We could not use the ELISA to measure NP1 and NP2 levels as the commercially available anti-EPO antibodies do not bind these two short peptides. Lewis rats that were injected systemically with vectors encoding EPO showed a significant hematocrit increase compared with controls (Table 1). Interestingly, rats injected systemically with vectors encoding S100E showed a slight increase in hematocrit, indicating that the S100E derivative has significantly lower erythropoietic activity than EPO. A modest, albeit significant, hematocrit increase was also observed in rats injected subretinally with vectors encoding EPO, probably due to leakage of trace amounts of hormone from the eye into the circulation (Table 1). Subretinal delivery of vectors encoding S100E as well as systemic or SR administration of vectors encoding NP1–NP2 did not result in hematocrit variations (Table 1), indicating that the combined delivery of these two EPO-mimetic peptides is not erythropoietic in vivo.
Table 1.
Serum and intraocular EPO and S100E protein and hematocrit levels in the light-damaged rats and murine models of IRDs
|
Serum levels (pg/ml) |
ACF levels (pg/ml) |
Hct (%) |
|||||
|---|---|---|---|---|---|---|---|
| EPO | S100E | EPO | S100E | EPO | S100E | NP1–NP2 | |
| LD CTRL | U (20) | U (10) | 47.2 ± 1.3 (24) | ||||
| LD IM | 90 ± 16 (10) | 261 ± 24 (10) | 12 ± 6 (10) | 17 ± 2 (10) | 71.5 ± 5.7 (10)* | 52.3 ± 5.2 (10)* | 48.5 ± 5.6 (10) |
| LD SR | 4 ± 2 (4/10) | 6 ± 2 (3/10) | 2400 ± 750 (9) | 4550 ± 1370 (8) | 57.8 ± 7.9 (10)* | 49.8 ± 3.7 (10) | 45.4 ± 5.6 (10) |
| rds CTRL | 45 ± 5 (4) | U (24) | 50.7 ± 1.6 (28) | ||||
| rds IM | 492 ± 26 (3) | 507 ± 45 (11) | 19 ± 2 (3) | 22 ± 4 (3) | 88.8 ± 4.2 (3)* | 60 ± 8 (12)* | 44.4 ± 5.6 (4) |
| rds SR | 49 ± 26 (3) | 73 ± 15 (6) | 2375 ± 165 (3) | 2900 ± 1200 (6) | 52.5 ± 13 (5)* | 50.5 ± 2.8 (3) | 50 ± 2.3 (3) |
| Aipl1−/− CTRL | 15.3 ± 2.5 (7) | \ | 44.3 ± 3.3 (7) | ||||
| Aipl1−/− IM | 443 ± 59 (3) | 532 ± 79 (3) | \ | \ | 53.7 ± 4.4 (3)* | 47.7 ± 8 (3) | 42.1 ± 5.8 (4) |
| Aipl1−/− SR | 53 ± 20 (3) | 94 ± 30 (3) | \ | \ | 49.3 ± 6.8 (3)* | 44.5 ± 2.3 (3) | 43.7 ± 5 (4) |
LD, light-damaged Lewis rats; CTRL, uninjected animals; ACF, anterior chamber fluid; Hct, hematocrit; \ denotes not measured (due to the small eye size); U, levels below the ELISA sensitivity which is 0.65 pg/ml for murine EPO and 0.33 pg/ml for rat EPO; SR, subretinal injection; IM, intramuscular injection. NP1 and NP2 peptide levels cannot be measured by ELISA assay. Measurements were performed at P65 for LD rats, at P90 for rds mice and at P21 for Aipl1−/− mice. Values are means ± SE for the ELISA assay, means ± SD for hematocrits.
*Values significantly increased (P < 0.05) compared with controls.
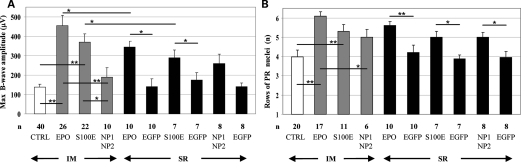
Electroretinographic analyses (ERG) were used to assess retinal function. The B-wave amplitude originates from bipolar cells that are post-synaptic to PR and reflects the global retinal function, whereas the A-wave represents PR function (63). Retinal function is not altered by intraocular EPO nor by EPO-D overexpression; ERG recorded 4 weeks following SR AAV injection and before light damage are similar to those of contralateral eyes injected with AAV2/1-EGFP (data not shown). Systemic delivery of vectors encoding either EPO or S100E but not of those encoding NP1 and NP2 in combination significantly preserved retinal function following light damage (Fig. 3A; Supplementary Material, Fig. S2). Systemic EPO overexpression, associated with a high hematocrit increase, resulted in B- and A-wave amplitudes similar to those obtained following systemic S100E overexpression (Fig. 3A; Supplementary Material, Fig. S2), which was associated with a minimal hematocrit variation (Table 1). Notably, a significant preservation of both B- and A-wave amplitudes was observed following SR injection of vectors encoding either EPO or S100E (but not NP1–NP2) when compared with EGFP-injected contralateral eyes (Fig. 3A; Supplementary Material, Fig. S2). Although this protection was lower than when systemic administration of vectors was used (Fig. 3A), the PR-protective effect observed following SR administration of S100E suggested that EPO-mediated neuroprotection can be exerted independently of erythropoiesis. However, since neuroprotection is more robust after systemic than intraocular EPO or S100E expression, we hypothesize that other systemic effects of the hormone may contribute to PR preservation (Fig. 3A).
Figure 3.
Retinal functional and morphological protection following AAV delivery in light-damaged albino Lewis rats. (A) ERG maximum B-wave amplitudes. The maximum B-wave amplitude from age-matched wild-type Lewis rats is 1291.3 ± 92 µV (n= 8). (B) The number of rows of PR nuclei in the ONL. The number of rows in age-matched wild-type Lewis rats is 11 ± 1.5 (n= 8). (A and B) Values are means ± SE. The number (n) of eyes in each group is depicted under the corresponding bar. CTRL, uninjected rats. Statistical significance has been calculated by ANOVA. *P ≤ 0.05. **P ≤ 0.001. In addition to those depicted in (A) and (B), significant differences (P < 0.05) were found between the following groups of rats: CTRL versus SR EPO; CTRL versus SR S100E; IM EPO versus SR EGFP; IM S100E versus SR EGFP; IM EPO versus SR S100E; IM EPO versus SR NP1–NP2.
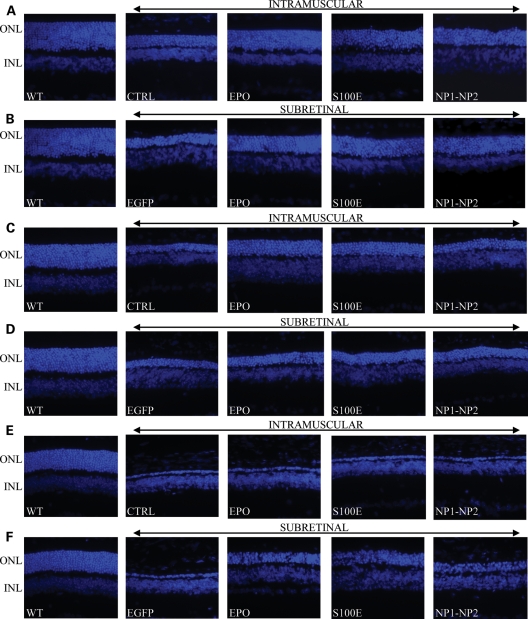
To further confirm the neuroprotection observed in the functional analysis, we evaluated PR survival counting the rows of nuclei remaining in the outer nuclear layer (ONL) of the retina in the various treated groups (Figs 3B and 4). Nine-week-old Lewis rats kept under physiological 12 h light–dark cycle present about 10 ± 2 rows of PR nuclei, whereas age-matched light-damaged rats progressively lose PR due to apoptosis. Treated rats showed significant morphological PR preservation, compared with controls (Figs 3B and 4). Consistent with the functional preservation, increased PR survival is associated with either a high or a low hematocrit increase (systemic administration of vectors encoding EPO, or S100E; SR administration of vectors encoding EPO). In addition, significant protection is obtained independently of increased erythropoiesis following SR delivery of vectors encoding either S100E or NP1–NP2 (Figs 3B and 4).
Figure 4.
Representative retinal histology in the various animal models following AAV delivery. (A, B) LD; (C, D) rds mice; (E, F) Aipl1−/− mice. LD, light damage; ONL, outer nuclear layer; INL, inner nuclear layer; WT, wild-type age-matched control animals corresponding to Lewis rats (A and B), CBA/JHsd (C and D) and C57BL/6 mice (E and F). CTRL, uninjected animals. Picture magnification is 40×.
Since different levels of PR protection from light damage by the same neurotrophic factors have been reported in albino rats versus mice (6), we tested the protective effect of AAV-mediated SR delivery of EPO and EPO-D in 4-week-old BALB/c mice subjected to light damage. Differently from what we observed in the rat model, significant protection of PR function from light damage is achieved following SR delivery of EPO but not EPO-D in the presence of increased hematocrits (Supplementary Material, Table S2). This could be explained by the lower levels of S100E than EPO achieved following SR AAV delivery in albino mice. Indeed, the EPO doses required to obtain neuroprotection are higher than those required for erythropoiesis (64).
Assessment of hematocrit levels and PR survival following EPO and EPO-D gene transfer to the retina of rds and Aipl1−/− mice
We then tested whether EPO and EPO-D gene delivery protected from IRD. The rds (also known as Prph2rd2/rd2) mouse is homozygous for a null mutation in the peripherin 2 gene, which encodes for a PR structural protein, that leads to failure of outer segment disc formation, negligible retinal function and progressive PR loss (65–67). The Aipl1 knock-out mouse (Aipl1−/−) exhibits absent retinal function and a fast degeneration of both rods and cones due to the destabilization of PR phosphodiesterases that are enzymes essential for PR survival and function (57,68,69). rds and Aipl1−/− mice were injected systemically or subretinally with AAV vectors during the first post-natal week and were analyzed at post-natal day (P) 90 and 21, respectively (as described in Materials and Methods) based on the different timing of PR loss in each model (57,65). EPO and S100E were detected in the sera and ocular fluids of rds at P90 and in the sera of Aipl1−/− at P21, confirming efficient AAV-mediated transduction in the various experimental groups and that both EPO and S100E cross the blood–retina barrier from the circulation (Table 1). ACF collection is not feasible in Aipl1−/− mice at P21 due to the small eye size. Hematocrits are significantly increased in mice injected with vectors encoding EPO (either systemically or subretinally) and in rds mice injected systemically with vectors encoding S100E (Table 1). The lower hematocrit levels observed following systemic S100E delivery compared with EPO confirms the low-erythropoietic activity of S100E in vivo (Table 1). The hematocrit increase we observed in animals receiving SR injections of vectors encoding EPO is due to EPO leakage into the circulation as confirmed by the EPO levels measured in the sera of a subset of mice (Table 1). No significant hematocrit increase was measured in mice injected either systemically or subretinally with AAV encoding EPO-mimetic peptides NP1 and NP2 or subretinally with vectors expressing S100E (Table 1).
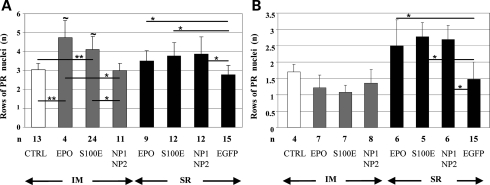
rds mice administered with AAV that encodes either EPO or its derivatives showed a significantly higher number of PR rows at P90 than controls, with the exception of those treated systemically with vectors encoding NP1 and NP2 (Figs 4 and 5A). As observed in the light-damage study, significant higher levels of PR survival were observed following systemic rather than intraocular delivery of either EPO or S100E in the presence of hematocrit increase, confirming that the systemic activities of the hormone in addition to its local functions provide the most robust retinal rescue (Figs 4 and 5A). In addition, we observed that EPO-D sustained PR survival in rds mice following intraocular delivery of vectors and that although at lower levels than after systemic administration, this effect is erythropoiesis-independent (Figs 4 and 5A).
Figure 5.
Retinal morphological protection following AAV delivery in rds and Aipl1−/− mice. The number of rows of PR nuclei in the ONL of rds (A) and Aipl1−/− (B) mice. Values are means ± SD. The number (n) of eyes (A and B) in each group is depicted under the corresponding bar. Statistical significance has been calculated by ANOVA. * and ∼ correspond to P ≤ 0.05; **P ≤ 0.001. ∼ refers to the comparison of the systemic versus the SR delivery of either EPO or S100E (A). In addition to those depicted in the figure, significant differences (P < 0.05) were found between the following groups of rds mice: CTRL versus SR EPO; CTRL versus SR S100E; CTRL versus SR NP1–NP2; IM EPO versus SR EGFP; IM S100E versus SR EGFP (A).
Finally, no amelioration of retinal morphology was observed in Aipl1−/− mice treated at P7–8 independently of the transgene used and of the AAV administration route (data not shown). Significant PR preservation was instead achieved treating 4-day-old Aipl1−/− mice subretinally but not systemically with AAV vectors encoding either EPO or EPO-D (Figs 4 and 5B). The discrete Aipl1−/− PR protection achieved when gene delivery is performed at P4 rather than at P7–8 suggests that the extremely fast and severe Aipl1−/− degenerative process requires the early- and fast-onset expression of neurotrophic genes which is presumably obtained more efficiently after retinal than muscular transduction. Indeed, the lower hematocrit increase measured in Aipl1−/− mice at P21 than in rds mice at P90 and in Lewis rats at P65 following intramuscular (IM) AAV2/1-CMV-EPO administration (Table 1) suggests that systemic EPO did not reach yet its plateau biological effect during the crucial phases of PR degeneration (i.e. around P12 in Aipl1−/− mice).
DISCUSSION
EPO is a well-known therapeutic protein widely used in the treatment of anemia (70,71). Over the past decade, several studies have shown that in addition to its role in the inhibition of erythrocyte apoptosis and stimulation of their differentiation, EPO is also a cytoprotective molecule and is produced in response to injuries or metabolic stress in several tissues, including the retina (28,34,72). In addition, EPO is able to protect retinal neurons following exogenous delivery (29,34–40). We have previously shown that systemic but not intraocular AAV-mediated rhesus EPO delivery provides functional and morphological PR protection from degeneration (33). Ours and other studies that used EPO and resulted in hematocrit increase (28) raised the important issue to determine whether EPO erythropoietic activity or EPO non-erythropoietic systemic effects (i.e. production of secondary systemic effects and/or intermediates acting at the retinal level) are required for retinal protection. The availability of EPO-D, which are EPO analogs, lacking erythropoietic activity, as well as of novel tools for improved retinal gene transfer, allowed us to address these important questions. Indeed, the PR protection observed after systemic delivery of the low-erythropoietic S100E in three different models of retinal degeneration suggests that EPO erythropoietic activity is not required to preserve PR from degeneration. This is further confirmed by the PR protection achieved following intraocular EPO-D delivery in the absence of any hematocrit increase. However, the highest levels of retinal protection were still observed following systemic delivery of EPO or S100E. Although the contribution of erythropoiesis to neuroprotection in the animals treated with vectors systemically cannot be completely ruled out based on a minimal hematocrit increase observed in the animals expressing S100E, the following mechanisms may be responsible for the superior effect of systemic versus intraocular vector administrations: (i) non-erythropoietic systemic functions of the hormones that contribute to retinal rescue enhancing the protective effect (i.e. activity on endothelial cells) (30,73,74); (ii) tissue-specific secondary modifications of the proteins following transduction of the muscle or retina. It is known that different EPO isoforms are produced by different tissues/cell types (75,76); thus, it is possible that different EPO isoforms have different biological activities (75,76); (iii) an inverse relation between the hormones efficacy and their concentrations with the highest efficacy obtained at the lowest intraocular concentrations. This has been previously observed in models of RGC and neuronal degeneration (17,77–79). Thus, although we show that the EPO and S100E doses we achieved using the constitutive cytomegalovirus (CMV) promoter are neuroprotective, the use of a regulatable gene expression system may be in the future required to define the hormone optimal therapeutic range.
Notably, we observe that EPO and EPO-D can provide PR protection following SR AAV-mediated delivery and that the protective effect mediated by EPO-D is achieved in the absence of any significant hematocrit increase, thus proving that a direct, local protective effect from light-induced degeneration is exerted. In addition, we observed that neither EPO nor EPO-D overexpression in the rat retina has detrimental effects on PR electrical activity. Interestingly, EPO and EPO-D neurotrophic effect seems less robust in albino mice (BALB/c) than in rats. This interspecies difference in PR protection from light damage has already been reported for several other neuroprotective molecules (6).
In the previous work, we did not achieve significant PR rescue following intraocular AAV-mediated delivery of EPO. Several crucial points could account for the intraocular protection we observe here: (i) the serotype: in this study, we switched from AAV2/2 to the more potent AAV2/1 which preferentially transduces the retinal pigment epithelium (RPE) and Muller cells (60–62) and may have provided a source of hormone more similar to the endogenous one, considering that in the retina EPO is mainly secreted by ganglion cells, interneurons and, in particular, Muller cells (29,31,60,80); (ii) differences in the transgene species used: in the previous study, we used the rhesus EPO protein that shares 80–82% of amino acidic identity with the murine and rat proteins, respectively, whereas the murine EPO used here shares 94% of amino acidic identity and a similar glycosylation profile to the rat protein (81). Thus, the amino acidic sequence together with secondary modifications of murine EPO could have contributed to maximize endogenous receptor binding and biological activity and in turn to increase the protective effect observed in this study following intraocular administration of vectors.
In addition to the light-induced model of PR degeneration, we tested AAV-mediated delivery of EPO and the selected derivatives in models of IRDs due to mutations in genes essential for PR structure and function. In these models, in the absence of gene replacement, preservation of the PR structure but not of cellular function is expected from a neurotrophic treatment as the one we tested here. We observed PR survival up to 3 months, the last time point of the analysis in rds mice following AAV-mediated systemic or intraocular delivery of either EPO or the selected EPO-D. Recently, systemic delivery of the R103E and S100E EPO-D has been reported to increase ONL thickness in rds mice (82); however, these EPO-D (82) were obtained by mutagenesis of EPO amino acidic residues numbered after the first methionine (82), whereas Leist et al. (47,83,84) and us mutagenized residues numbered after the first amino acid of the mature hormone that lacks the signal peptide. Indeed, the S100E generated by Sullivan et al. (82) is erythropoietic as EPO.
Importantly, we report for the first time increased PR survival in Aipl1−/− mice, achieved following early SR delivery of AAV-EPO, -S100E or -NP1–NP2. Interestingly and differently from what is observed in light-damaged rats and rds mice, no significant Aipl1−/− PR protection was observed following systemic administration of either vector. This could be explained by the later onset of AAV-mediated gene expression from the muscle than from the retina which reaches a plateau once Aipl1−/− PR degeneration is irreversibly triggered (60,85). However, the protection provided by SR injections supports further testing of EPO gene supply with Aipl1 gene replacement to investigate whether this results in stronger PR protection than that so far reported with gene replacement alone (86,87).
Unexpectedly, in all different animal models, we observed that the combined gene delivery of EPO-mimetic peptides (NP1–NP2) increases PR survival following SR but not IM delivery. One possible explanation for this is that NP1 and NP2 expression, cleavage and secretion from the retina differ from the muscle. However, this is unlikely since: (i) our data suggest that both muscular and retinal cells efficiently recognize and process the EPO signal peptide which is the one used in our NP1 and NP2 constructs; (ii) short peptides like somatostatin and other peptidic neurotransmitters are physiologically secreted by neurons (Neuropeptide Database: www.neuropeptides.nl). Alternatively, the lack of retinal protection following systemic administration of vectors expressing NP1–NP2 may be due to the peptides short plasma half-life (48,64). Similar to what is observed in the chronic, neurodegenerative mouse model of Huntington's disease (88), it is possible that retinal degeneration with ongoing continuous pro-apoptotic stimuli cannot be rescued using systemically administered compounds with such a short plasma half-life, despite their sustained AAV-mediated expression. Unfortunately, although we demonstrate the expression of NP1 and NP2 at the mRNA level, we could not test the peptides expression and secretion due to their small size and lack of appropriate antibodies, thus we cannot conclude whether the lack of efficacy of NP1 and NP2 expressed from the muscle is due to inappropriate expression/secretion or short plasma half-life.
In conclusion, our data show that EPO protection from PR degeneration, either induced or inherited, does not require hormone-induced erythropoiesis and can be exerted by non-erythropoietic EPO-D systemically or locally delivered to the retina by AAV. These may represent novel and safe therapeutic agents for common conditions characterized by retinal degeneration.
MATERIALS AND METHODS
Generation of the plasmids for AAV vector production
The pAAV2.1-CMV murine EPO (pAAV2.1-CMV-EPO) was obtained cloning the murine EPO coding sequence (CDS) into the pAAV2.1-CMV-EGFP3 plasmid (89) using the NotI and BamHI sites. The S to E amino acidic substitution generating the S100E protein was achieved by site-directed mutagenesis of the pAAV2.1-CMV-EPO plasmid using the Quick Change II XL Site-Directed Mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA). The sequence analysis of the pAAV2.1-CMV-S100E plasmid confirmed the AGT to GAA substitution at codon 126 of the murine EPO CDS which corresponds to the amino acidic position 100 of the mature EPO protein (S100E), as originally described (47,83,84). NP1 CDS was generated amplifying two overlapping DNA fragments from the pAAV2.1-CMV-EPO plasmid by polymerase chain reaction (PCR) using the following primers: NP1 (NotI-EPOFw 5′-AAGCGGCCGCCATGGGGGTGCCCGAAC-3′ and EPORev 5′-GGCCTTGCCAAACTTCTATGGCCTGTTC-3′, for one fragment, or NPFw 5′-GATTCCTCTGGGCCTCCCAGTCCTCTGTGAACAGGCCATAGAAGTTTGGCAAG-3′ and BamHI-NP1Rev, for the other fragment). The final full-length NP1 CDS was amplified using the following primers: NotI-EPO 5′-AAGCGGCCGCCATGGGGGTGCCCGAAC-3′ and BamHI-NP1rev 5′-AAGGATCCTCAGGAGGAATTGGCTAGCAGGGCCTG-3′. NP2 CDS has been generated by PCR amplification using the pAAV2.1-CMV-EPO as a template and the following primers: NP2 (NotI-EPOFw and BamHI-NP2rev 5′-AAGGATCCTCACTCCTTGGCCTCTAAGATGTACCTCTCCAGAACTCGACTGTCGCAGATGAGGCGACAGAGGACTGGGAGGCCCAG-3′). Each PCR product corresponding to either NP1 or NP2 CDS was cloned into pAAV2.1-CMV-EGFP3 using NotI and BamHI sites.
AAV vector production
AAV2/1 vectors encoding EPO, S100E, NP1, NP2 or EGFP were generated from the above-described pAAV2.1 plasmids. AAV2/1 vectors were produced by the TIGEM AAV vector core by triple transfection of 293 cells followed by two rounds of CsCl2 purification (90). For each viral preparation, physical titers [genome copies (GC)/ml] were determined by dot blot analysis (91) and by PCR quantification using TaqMan (Applied Biosystems, Monza, Italy) (92).
COS7 cell transfection and western blot analyses
COS7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and 2 mm l-glutamine (GIBCO, Invitrogen S.R.L., Milan, Italy) and plated in six-well plates at a density of 1 × 105 cell/well. Twenty-four hours later, the cells were transfected with 2 µg of pAAV2.1-CMV-EPO, pAAV2.1-CMV-S100E, pAAV2.1-CMV-NP1, pAAV2.1-CMV-NP2 or pAAV2.1-CMV-EGFP, using the PolyFect Transfection Reagent (Qiagen S.P.A., Milan, Italy). Thirty-six hours later, cells were incubated in serum-free DMEM. Medium and cells were harvested after 12 h for western blot or reverse transcriptase–PCR analysis. Cells were lysed in 50 mM Tris–HCl, pH 8, 150 mM NaCl, 1% NP40, 0.5% Na-deoxycholate, 1 mM EDTA, 0.1% sodium dodecyl sulfate (SDS), in the presence of protease inhibitors (Complete Protease inhibitor cocktail tablets, Roche, Milan, Italy) and 1 mm phenylmethylsulfonyl fluoride. Both cell lysates and media were separated by 12% SDS–polyacrylamide gel electrophoresis and immunoblotted using the anti-EPO antibody (EPO N-19 1:400, sc-1310; Santa Cruz Biotechnology Inc., Heidelberg, Germany).
RNA extraction, cDNA production and RT analyses
Total RNA was isolated from transfected COS7 cells using the RNeasy MiniKit (Qiagen). One microgram of RNA was submitted to DNase I digestion (RNase Free DNase set, Qiagen), then the cDNA was generated using the QuantiTect reverse transcription kit (Qiagen). PCR amplification was performed using 3 µl of cDNA and the following primers: Fw 5′-CTGCTCTCAGAAGCCATCCTGCAG-3′ and BgHpolyA-Rev 5′-GATGGCTGGCAACTAGAAGGCAC-3′, for the cDNA encoding NP1; Fw 5′-CATCTGCGACAGTCGAGTTCTGGAG-3′ and BgHpolyA-Rev, for the cDNA encoding NP2. The quality of each cDNA sample was checked amplifying the constitutively expressed β-actin with the following primers: Fw 5′-CAAGATCATTGCTCCTCCTGA-3′ and Rev 5′-CATCGTACTCCTGCTTGCTGA-3′.
Animal models and AAV2/1 vector administration
This study was carried out in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and the Italian Ministry of Health regulation for animal procedures.
Aipl1−/− mice (a kind gift from Dr Michael Dyer, St Jude Children's Research Hospital, Memphis, TN, USA) and rds mice (kindly provided by Prof. Robin Ali, University College London, UK) were bred and housed under physiological conditions: 12 h light–dark cycle (maximum light intensity <100 lux). Albino Lewis rats (LEW/Han™Hsd) and CBA/JHsd mice were purchased from Harlan Italy SRL (Udine, Italy), and C57BL/6 and BALB/c mice were purchased from Charles River Laboratories (Calco, Lecco, Italy).
Lewis rat and BALB/c injection
Before AAV vector injection, 4-week-old rats and mice were anesthetized with an intraperitoneal injection at 2 ml/100 g body weight of avertin (1.25% w/v of 2,2,2-tribromoethanol and 2.5% v/v of 2-methyl-2-butanol; Sigma-Aldrich, Milan, Italy) (93). For IM and SR injections, rats and mice received, respectively, 100 or 2 µl of AAV vector, corresponding to the following vector doses: AAV2/1-CMV-EPO (1 × 1010 GC IM; 1 × 109 GC SR), S100E (1 × 1011 GC IM; 1 × 109 GC SR), NP1 (1 × 1011 IM; 1 × 109 GC SR), and NP2 (1 × 1011 IM; 1 × 109 GC SR). NP1- and NP2-encoding vectors were injected in combination.
rds and Aipl1−/− mice injection
Before AAV vector administration, newborn rds (P7–8) and Aipl1−/− (P4 or P7-P8) mice were anesthetized by hypothermia; for IM and SR injections, mice received, respectively, 10 or 0.5 µl of AAV vector, corresponding to the following vector doses: AAV2/1-CMV-EPO (1 × 109 GC IM; 5 × 108 GC SR), S100E (1 × 1010 GC IM; 5 × 108 GC SR), NP1 (1 × 1010 IM; 5 × 108 GC SR), and NP2 (1 × 1010 IM; 5 × 108 GC SR). NP1- and NP2-encoding vectors were injected in combination. Early post-natal administration was chosen in order to provide the optimal therapeutic effect based on the disease progression and PR apoptotic peak corresponding to P16 in rds and to P12 in Aipl1−/− mice.
IM administration was performed injecting the vector preparation into three sites of the gastrocnemious using a Hamilton syringe (Microglass Heim SRL, Naples, Italy), whereas SR administration, consisting of eye exposure, conjunctival peritomy and subsequent vector delivery, was performed passing a 33 G needle (Microglass Heim SRL, Naples, Italy) through the sclera between the retina and the RPE, as described in detail elsewhere (94). AAV2/1-CMV-EGFP was injected subretinally in rats and mice as a negative control of the intraocular treatment. For the systemic treatment, we used animals either uninjected or injected with AAV2/1-CMV-EGFP. Since the results from both groups are similar, we show those from uninjected controls in Table 1, Figures 3–5 and Supplementary Material, Figure S2. The AAV2/1-CMV-EPO vector was administered intramuscularly to rats and mice at a dose 10-fold lower than that of vectors encoding EPO-D to avoid a fatal hematocrit increase observed when the full dose of 1 × 1011 GC of AAV2/1-CMV-EPO/animal was administered (P.C. and A.A., unpublished data).
Light damage in albino Lewis rats and BALB/c mice
The light-damage protocol was derived from that originally described by LaVail et al. (55) and subsequently modified (33,95). Briefly, before AAV injection and during the following 4 weeks (Lewis rats) or 2 weeks (BALB/c mice), animals were reared in physiological 12 h light–dark cycle. Rats (at P56) and mice (at P42) were housed separately in clear Plexiglas cages surrounded by eight 36 W white fluorescent bulbs (Osram Sylvania, Munich, Germany) and subjected to continuous light exposure for 48 or 96 h, respectively. During light damage, animals had free access to food and water. The average luminance measured in the cages was 2000 lux. Following light damage, animals were kept again under physiological 12 h light–dark cycle for 1 week and then submitted to ERG thus performed at P65 for rats and at P54 for mice.
Electrophysiological recordings
For ERG, Lewis rats and BALB/C mice were dark-adapted for 180 min, anesthetized with an intraperitoneal injection of avertin (1.25% w/v of 2,2,2-tribromoethanol and 2.5% v/v of 2-methyl-2-butanol; Sigma-Aldrich) at 2 ml/100 g of body weight, accommodated in a stereotaxic apparatus under dim red light, their pupils dilated with a drop of 1% tropicamide (Alcon Laboratories, Inc., Fort Worth, TX, USA) and the body temperature maintained at 37.5°C. Electroretinograms were evoked by 10 ms flashes of different light intensities ranging from 10−4 to 20 cd/m2/s generated through a Ganzfeld stimulator (CSO, Florence, Italy). To minimize the noise, three different responses evoked by light were averaged for each luminance step (the time interval between light stimuli was 4–5 min). The electrophysiological signals were recorded through gold plate electrodes inserted under the lower eyelids in contact with the cornea. Electrodes in each eye were referred to a needle electrode inserted subcutaneously at the level of the corresponding frontal region. The different electrodes were connected to a two-channel amplifier. Amplitudes of A- and B-waves were plotted as a function of increasing light intensities. After completion of responses obtained in dark-adapted conditions (scotopic), the recording session continued with the aim to dissect the cone pathway mediating the light response (photopic). To this end, the electroretinogram in response to light of 20 cd/m2 was recorded in the presence of a continuous background light (background light set at 50 cd/m2). For each group, the mean A- and B-wave amplitudes were plotted as a function of luminance (transfer curve) under scotopic and photopic conditions.
EPO and S100E quantification and hematocrit measurements
EPO and S100E levels (pg/ml) were measured in the serum and ACF of mice and rats using the Quantikine Mouse/Rat Erythropoietin ELISA (R&D Systems, Minneapolis, MN, USA). Rat and murine EPO proteins are similarly recognized by the ELISA as reported by the producer. We have indirectly tested whether the ELISA recognizes with similar affinity EPO and S100E by transfecting equal amounts of plasmids encoding the hormones in COS7 cells and measuring their levels in culture media. Similar amounts of EPO and S100E (4.5 and 4.7 ng/ml, respectively) per microgram of plasmid were indeed measured. Hematocrit levels were measured by microcapillary centrifugation of blood. ACF was collected from Lewis rats and rds mice using a Hamilton syringe equipped with a 33 G needle (Microglass Heim SRL, Napoli, Italy). Sample collection was performed at P65 for Lewis rats, P54 for BALB/C mice, P90 for rds mice and P21 for Aipl1−/− mice.
Histological analysis
Histological analyses were performed at P65 for Lewis rats, P90 for rds mice and at P21 for Aipl1−/− mice. Eyes were enucleated, oriented by cautery, fixed and embedded in tissue freezing medium (O.C.T. matrix, Kaltek, Padua, Italy) as described previously (95). Serial sections (10 μm thick) of eyes were cut along the horizontal meridian; sections were progressively distributed on slides so that each slide contained representative sections from the whole eye. Sections were stained with 4′,6-diamidino-2-phenylindole (Vectashield, Vector Lab, Inc., Peterborough, UK) or hematoxylin and eosin (Richard-Allen Scientific, Kalamazoo, MI, USA; Sigma-Aldrich) according to the standard procedures; PR counting and global retinal histology were analyzed by fluorescent or light microscopy, respectively. To quantify the number of PR nuclei in the ONL of the retina and assess PR protection, a minimum of three central sections through the optic nerve head (ONH) per slide were used. The number of PR nuclei was counted at 40× magnification in two different locations on each side of the ONH; the nasal and central on one side and the temporal and central on the other side. The nasal, temporal and central counts of three sections/eye were averaged. The counts from each group were then averaged and standard errors were calculated. P-values were calculated using ANOVA. Retinal pictures were captured using a Zeiss Axiocam (Carl Zeiss, Oberkochen, Germany).
In situ hybridization
RNA in situ experiments on eye sections from CBA mice were performed as described elsewhere (96). Digoxigenin-labeled antisense RNA probes for EPOR and βCR were obtained by PCR using oligonucleotides bearing RNA polymerase-binding sites. The RNA polymerases used are T3, T7 and SP6 (Roche Diagnostics, Milan, Italy). The pCMV-Sport6 vector (cDNA clone MGC: 54669, Invitrogen) or cDNA from the CBA adult retina were used as templates for EPOR and βCR RNA probes generation, respectively. The oligonucleotides used are the following: EPOR (T3-Fw: 5′-TAATGATTAACCCTCACTAAAGGGCACCGCCGGACTCTGCAG-3′; T7Rev: 5′-TAATGTAATACGACTCACTATAGGGCTAGGAGCAGGCCACATAGC-3′); βCR: (Sp6Fw: 5′-TGATTTAGGTGACACTATAGGCTACAAGAATAGACATGGTCC-3′, T3Rev: 5′-TGATTTAGGTGACACTATAGTGGATCATGGAGTGCCTGAC-3′). Retinal pictures were captured using a Leica DM5000B microscope and a Leica DFC 350FX camera (Leica Mycrosystems, Milan, Italy).
Statistical analysis
Data sets shown in Figures 3 and 5 and Supplementary Material, Figure S2, were analyzed by ANOVA to evaluate statistically significant differences. The Tukey multiple comparison procedure (post hoc) was used to make comparison among groups. Significance at P≤ 0.05 (*) or P≤ 0.001(**) is indicated in the corresponding figures. P-values depicted in Table 1 and Supplementary Material, Table S2, were calculated using Student's t-test.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the Foundation Fighting Blindness (grant no. TA-GT-0808-0459-TIGEM), the European Commission under the FP7 AAVEYE project (grant no. HEALTH-2007-B-223445); the FP7 TREATRUSH project (grant no. 242013); and the Telethon Foundation (grant TIGEM P21). Funding to pay the Open Access publication charges for this article was provided by Fondazione Telethon.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs Pietro Ghezzi, Graciana Diez-Roux and Luciana Borrelli for the critical reading of the manuscript. We thank the TIGEM AAV Vector Core for the production of the AAV vectors used in this study and the TIGEM Bioinformatics Core for the ANOVA analysis reported in this paper.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Hartong D.T., Berson E.L., Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 2.den Hollander A.I., Roepman R., Koenekoop R.K., Cremers F.P. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Hamel C.P. Cone rod dystrophies. Orphanet J. Rare Dis. 2007;2:7. doi: 10.1186/1750-1172-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marigo V. Programmed cell death in retinal degeneration: targeting apoptosis in photoreceptors as potential therapy for retinal degeneration. Cell Cycle. 2007;6:652–655. doi: 10.4161/cc.6.6.4029. [DOI] [PubMed] [Google Scholar]
- 5.Wright A.F., Chakarova C.F., Abd El-Aziz M.M., Bhattacharya S.S. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat. Rev. Genet. 2010;11:273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- 6.LaVail M.M., Yasumura D., Matthes M.T., Lau-Villacorta C., Unoki K., Sung C.H., Steinberg R.H. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest. Ophthalmol. Vis. Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- 7.Wenzel A., Grimm C., Samardzija M., Reme C.E. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Allocca M., Tessitore A., Cotugno G., Auricchio A. AAV-mediated gene transfer for retinal diseases. Expert Opin. Biol. Ther. 2006;6:1279–1294. doi: 10.1517/14712598.6.12.1279. [DOI] [PubMed] [Google Scholar]
- 9.Buch P.K., MacLaren R.E., Ali R.R. Neuroprotective gene therapy for the treatment of inherited retinal degeneration. Curr. Gene Ther. 2007;7:434–445. doi: 10.2174/156652307782793531. [DOI] [PubMed] [Google Scholar]
- 10.Colella P., Cotugno G., Auricchio A. Ocular gene therapy: current progress and future prospects. Trends Mol. Med. 2009;15:23–31. doi: 10.1016/j.molmed.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Maiese K., Chong Z.Z., Li F., Shang Y.C. Erythropoietin: elucidating new cellular targets that broaden therapeutic strategies. Prog. Neurobiol. 2008;85:194–213. doi: 10.1016/j.pneurobio.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brines M., Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat. Rev. Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- 13.Hasselblatt M., Ehrenreich H., Siren A.L. The brain erythropoietin system and its potential for therapeutic exploitation in brain disease. J. Neurosurg. Anesthesiol. 2006;18:132–138. doi: 10.1097/00008506-200604000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Youssoufian H., Longmore G., Neumann D., Yoshimura A., Lodish H.F. Structure, function, and activation of the erythropoietin receptor. Blood. 1993;81:2223–2236. [PubMed] [Google Scholar]
- 15.Um M., Gross A.W., Lodish H.F. A ‘classical' homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell. Signal. 2007;19:634–645. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Masuda S., Nagao M., Takahata K., Konishi Y., Gallyas F., Jr., Tabira T., Sasaki R. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J. Biol. Chem. 1993;268:11208–11216. [PubMed] [Google Scholar]
- 17.Sakanaka M., Wen T.C., Matsuda S., Masuda S., Morishita E., Nagao M., Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl Acad. Sci. USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siren A.L., Knerlich F., Poser W., Gleiter C.H., Bruck W., Ehrenreich H. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. 2001;101:271–276. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z.Y., Asavaritikrai P., Prchal J.T., Noguchi C.T. Endogenous erythropoietin signaling is required for normal neural progenitor cell proliferation. J. Biol. Chem. 2007;282:25875–25883. doi: 10.1074/jbc.M701988200. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez P.E., Fares R.P., Risso J.J., Bonnet C., Bouvard S., Le-Cavorsin M., Georges B., Moulin C., Belmeguenai A., Bodennec J., et al. Optimal neuroprotection by erythropoietin requires elevated expression of its receptor in neurons. Proc. Natl Acad. Sci. USA. 2009;106:9848–9853. doi: 10.1073/pnas.0901840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanazono Y., Sasaki K., Nitta H., Yazaki Y., Hirai H. Erythropoietin induces tyrosine phosphorylation of the beta chain of the GM-CSF receptor. Biochem. Biophys. Res. Commun. 1995;208:1060–1066. doi: 10.1006/bbrc.1995.1442. [DOI] [PubMed] [Google Scholar]
- 22.Jubinsky P.T., Krijanovski O.I., Nathan D.G., Tavernier J., Sieff C.A. The beta chain of the interleukin-3 receptor functionally associates with the erythropoietin receptor. Blood. 1997;90:1867–1873. [PubMed] [Google Scholar]
- 23.Blake T.J., Jenkins B.J., D'Andrea R.J., Gonda T.J. Functional cross-talk between cytokine receptors revealed by activating mutations in the extracellular domain of the beta-subunit of the GM-CSF receptor. J. Leukoc. Biol. 2002;72:1246–1255. [PubMed] [Google Scholar]
- 24.Su K.H., Shyue S.K., Kou Y.R., Ching L.C., Chiang A.N., Yu Y.B., Chen C.Y., Pan C.C., Lee T.S. Beta common receptor integrates the erythropoietin signaling in activation of endothelial nitric oxide synthase. J. Cell. Physiol. 2011 doi: 10.1002/jcp.22678. [DOI] [PubMed] [Google Scholar]
- 25.Brines M., Grasso G., Fiordaliso F., Sfacteria A., Ghezzi P., Fratelli M., Latini R., Xie Q.W., Smart J., Su-Rick C.J., et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc. Natl Acad. Sci. USA. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadam J., Navarro F., Sanchez P., Moulin C., Georges B., Laglaine A., Pequignot J.M., Morales A., Ryvlin P., Bezin L. Neuroprotective effects of erythropoietin in the rat hippocampus after pilocarpine-induced status epilepticus. Neurobiol. Dis. 2007;25:412–426. doi: 10.1016/j.nbd.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez P.E., Navarro F.P., Fares R.P., Nadam J., Georges B., Moulin C., Le Cavorsin M., Bonnet C., Ryvlin P., Belmeguenai A., et al. Erythropoietin receptor expression is concordant with erythropoietin but not with common beta chain expression in the rat brain throughout the life span. J. Comp. Neurol. 2009;514:403–414. doi: 10.1002/cne.22020. [DOI] [PubMed] [Google Scholar]
- 28.Grimm C., Wenzel A., Groszer M., Mayser H., Seeliger M., Samardzija M., Bauer C., Gassmann M., Reme C.E. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat. Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- 29.Junk A.K., Mammis A., Savitz S.I., Singh M., Roth S., Malhotra S., Rosenbaum P.S., Cerami A., Brines M., Rosenbaum D.M. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA. 2002;99:10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J., Connor K.M., Aderman C.M., Smith L.E. Erythropoietin deficiency decreases vascular stability in mice. J. Clin. Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munro K., Rees S., O'Dowd R., Tolcos M. Developmental profile of erythropoietin and its receptor in guinea-pig retina. Cell Tissue Res. 2009;336:21–29. doi: 10.1007/s00441-009-0754-5. [DOI] [PubMed] [Google Scholar]
- 32.Brines M.L., Ghezzi P., Keenan S., Agnello D., de Lanerolle N.C., Cerami C., Itri L.M., Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc. Natl Acad. Sci. USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rex T.S., Allocca M., Domenici L., Surace E.M., Maguire A.M., Lyubarsky A., Cellerino A., Bennett J., Auricchio A. Systemic but not intraocular Epo gene transfer protects the retina from light-and genetic-induced degeneration. Mol. Ther. 2004;10:855–861. doi: 10.1016/j.ymthe.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 34.Grimm C., Hermann D.M., Bogdanova A., Hotop S., Kilic U., Wenzel A., Kilic E., Gassmann M. Neuroprotection by hypoxic preconditioning: HIF-1 and erythropoietin protect from retinal degeneration. Semin. Cell Dev. Biol. 2005;16:531–538. doi: 10.1016/j.semcdb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Grimm C., Wenzel A., Acar N., Keller S., Seeliger M., Gassmann M. Hypoxic preconditioning and erythropoietin protect retinal neurons from degeneration. Adv. Exp. Med. Biol. 2006;588:119–131. doi: 10.1007/978-0-387-34817-9_11. [DOI] [PubMed] [Google Scholar]
- 36.Zhong L., Bradley J., Schubert W., Ahmed E., Adamis A.P., Shima D.T., Robinson G.S., Ng Y.S. Erythropoietin promotes survival of retinal ganglion cells in DBA/2J glaucoma mice. Invest. Ophthalmol. Vis. Sci. 2007;48:1212–1218. doi: 10.1167/iovs.06-0757. [DOI] [PubMed] [Google Scholar]
- 37.King C.E., Rodger J., Bartlett C., Esmaili T., Dunlop S.A., Beazley L.D. Erythropoietin is both neuroprotective and neuroregenerative following optic nerve transection. Exp. Neurol. 2007;205:48–55. doi: 10.1016/j.expneurol.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Tsai J.C., Wu L., Worgul B., Forbes M., Cao J. Intravitreal administration of erythropoietin and preservation of retinal ganglion cells in an experimental rat model of glaucoma. Curr. Eye Res. 2005;30:1025–1031. doi: 10.1080/02713680500320729. [DOI] [PubMed] [Google Scholar]
- 39.Rex T.S., Wong Y., Kodali K., Merry S. Neuroprotection of photoreceptors by direct delivery of erythropoietin to the retina of the retinal degeneration slow mouse. Exp. Eye Res. 2009;89:735–740. doi: 10.1016/j.exer.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J., Wu Y., Jin Y., Ji F., Sinclair S.H., Luo Y., Xu G., Lu L., Dai W., Yanoff M., et al. Intravitreal injection of erythropoietin protects both retinal vascular and neuronal cells in early diabetes. Invest. Ophthalmol. Vis. Sci. 2008;49:732–742. doi: 10.1167/iovs.07-0721. [DOI] [PubMed] [Google Scholar]
- 41.Auricchio A., Rivera V.M., Clackson T., O'Connor E.E., Maguire A.M., Tolentino M.J., Bennett J., Wilson J.M. Pharmacological regulation of protein expression from adeno-associated viral vectors in the eye. Mol. Ther. 2002;6:238–242. doi: 10.1006/mthe.2002.0660. [DOI] [PubMed] [Google Scholar]
- 42.Lebherz C., Auricchio A., Maguire A.M., Rivera V.M., Tang W., Grant R.L., Clackson T., Bennett J., Wilson J.M. Long-term inducible gene expression in the eye via adeno-associated virus gene transfer in nonhuman primates. Hum. Gene Ther. 2005;16:178–186. doi: 10.1089/hum.2005.16.178. [DOI] [PubMed] [Google Scholar]
- 43.Rosenzweig M.Q., Bender C.M., Lucke J.P., Yasko J.M., Brufsky A.M. The decision to prematurely terminate a trial of R-HuEPO due to thrombotic events. J. Pain Symptom Manage. 2004;27:185–190. doi: 10.1016/j.jpainsymman.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 44.Stohlawetz P.J., Dzirlo L., Hergovich N., Lackner E., Mensik C., Eichler H.G., Kabrna E., Geissler K., Jilma B. Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood. 2000;95:2983–2989. [PubMed] [Google Scholar]
- 45.Kirkeby A., Torup L., Bochsen L., Kjalke M., Abel K., Theilgaard-Monch K., Johansson P.I., Bjorn S.E., Gerwien J., Leist M. High-dose erythropoietin alters platelet reactivity and bleeding time in rodents in contrast to the neuroprotective variant carbamyl-erythropoietin (CEPO) Thromb. Haemost. 2008;99:720–728. doi: 10.1160/TH07-03-0208. [DOI] [PubMed] [Google Scholar]
- 46.Campana W.M., Misasi R., O'Brien J.S. Identification of a neurotrophic sequence in erythropoietin. Int. J. Mol. Med. 1998;1:235–241. doi: 10.3892/ijmm.1.1.235. [DOI] [PubMed] [Google Scholar]
- 47.Leist M., Ghezzi P., Grasso G., Bianchi R., Villa P., Fratelli M., Savino C., Bianchi M., Nielsen J., Gerwien J., et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 48.Brines M., Patel N.S., Villa P., Brines C., Mennini T., De Paola M., Erbayraktar Z., Erbayraktar S., Sepodes B., Thiemermann C., et al. Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc. Natl Acad. Sci. USA. 2008;105:10925–10930. doi: 10.1073/pnas.0805594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brines M., Cerami A., Coleman T. Tissue Protective Peptides and Uses Thereof. Warren Pharmaceuticals, Inc; 2009. US Patent Application 2009/0221482. Available at http://www.wipo.int/pctdb/en/wo.jsp?KEY=07%2F019545&IA=US2006031061&DISPLAY=DESC; and http://www.freepatentsonline.com/y2009/0221482.html. [Google Scholar]
- 50.Elliott S., Lorenzini T., Chang D., Barzilay J., Delorme E. Mapping of the active site of recombinant human erythropoietin. Blood. 1997;89:493–502. [PubMed] [Google Scholar]
- 51.Dumont F., Bischoff P. Non-erythropoietic tissue-protective peptides derived from erythropoietin: WO2009094172. Expert Opin. Ther. Pat. 2010;20:715–723. doi: 10.1517/13543771003627464. [DOI] [PubMed] [Google Scholar]
- 52.Cerami A., Brines M. Tissue Protective Peptides and Peptides Analogs for Preventing and Treating Diseases and Disorders Associated with Tissue Damage. Araim Pharmaceuticals, Inc; 2009. US Patent Application WO/2009/094172. Available at http://www.wipo.int/pctdb/en/wo.jsp?WO=2009094172. [Google Scholar]
- 53.Syed R.S., Reid S.W., Li C., Cheetham J.C., Aoki K.H., Liu B., Zhan H., Osslund T.D., Chirino A.J., Zhang J., et al. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998;395:511–516. doi: 10.1038/26773. [DOI] [PubMed] [Google Scholar]
- 54.Villa P., van Beek J., Larsen A.K., Gerwien J., Christensen S., Cerami A., Brines M., Leist M., Ghezzi P., Torup L. Reduced functional deficits, neuroinflammation, and secondary tissue damage after treatment of stroke by nonerythropoietic erythropoietin derivatives. J. Cereb. Blood Flow Metab. 2007;27:552–563. doi: 10.1038/sj.jcbfm.9600370. [DOI] [PubMed] [Google Scholar]
- 55.LaVail M.M., Unoki K., Yasumura D., Matthes M.T., Yancopoulos G.D., Steinberg R.H. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc. Natl Acad. Sci. USA. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang B., Hawes N.L., Hurd R.E., Davisson M.T., Nusinowitz S., Heckenlively J.R. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 57.Dyer M.A., Donovan S.L., Zhang J., Gray J., Ortiz A., Tenney R., Kong J., Allikmets R., Sohocki M.M. Retinal degeneration in Aipl1-deficient mice: a new genetic model of Leber congenital amaurosis. Brain Res. Mol. Brain Res. 2004;132:208–220. doi: 10.1016/j.molbrainres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 58.Wahlin K.J., Campochiaro P.A., Zack D.J., Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Invest. Ophthalmol. Vis. Sci. 2000;41:927–936. [PubMed] [Google Scholar]
- 59.Hauck S.M., Kinkl N., Deeg C.A., Swiatek-de Lange M., Schoffmann S., Ueffing M. GDNF family ligands trigger indirect neuroprotective signaling in retinal glial cells. Mol. Cell. Biol. 2006;26:2746–2757. doi: 10.1128/MCB.26.7.2746-2757.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Auricchio A., Kobinger G., Anand V., Hildinger M., O'Connor E., Maguire A.M., Wilson J.M., Bennett J. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum. Mol. Genet. 2001;10:3075–3081. doi: 10.1093/hmg/10.26.3075. [DOI] [PubMed] [Google Scholar]
- 61.Yang G.S., Schmidt M., Yan Z., Lindbloom J.D., Harding T.C., Donahue B.A., Engelhardt J.F., Kotin R., Davidson B.L. Virus-mediated transduction of murine retina with adeno-associated virus: effects of viral capsid and genome size. J. Virol. 2002;76:7651–7660. doi: 10.1128/JVI.76.15.7651-7660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabinowitz J.E., Rolling F., Li C., Conrath H., Xiao W., Xiao X., Samulski R.J. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weymouth A.E., Vingrys A.J. Rodent electroretinography: methods for extraction and interpretation of rod and cone responses. Prog. Retin. Eye Res. 2008;27:1–44. doi: 10.1016/j.preteyeres.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 64.Brines M., Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J. Intern. Med. 2008;264:405–432. doi: 10.1111/j.1365-2796.2008.02024.x. [DOI] [PubMed] [Google Scholar]
- 65.Sanyal S., Jansen H.G. Absence of receptor outer segments in the retina of rds mutant mice. Neurosci. Lett. 1981;21:23–26. doi: 10.1016/0304-3940(81)90051-3. [DOI] [PubMed] [Google Scholar]
- 66.Reuter J.H., Sanyal S. Development and degeneration of retina in rds mutant mice: the electroretinogram. Neurosci. Lett. 1984;48:231–237. doi: 10.1016/0304-3940(84)90024-7. [DOI] [PubMed] [Google Scholar]
- 67.Chang G.Q., Hao Y., Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron. 1993;11:595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- 68.Kirschman L.T., Kolandaivelu S., Frederick J.M., Dang L., Goldberg A.F., Baehr W., Ramamurthy V. The Leber congenital amaurosis protein, AIPL1, is needed for the viability and functioning of cone photoreceptor cells. Hum. Mol. Genet. 2010;19:1076–1087. doi: 10.1093/hmg/ddp571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramamurthy V., Niemi G.A., Reh T.A., Hurley J.B. Leber congenital amaurosis linked to AIPL1: a mouse model reveals destabilization of cGMP phosphodiesterase. Proc. Natl Acad. Sci. USA. 2004;101:13897–13902. doi: 10.1073/pnas.0404197101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eschbach J.W. The future of r-HuEPO. Nephrol. Dial. Transplant. 1995;10(Suppl. 2):96–109. doi: 10.1093/ndt/10.supp2.96. [DOI] [PubMed] [Google Scholar]
- 71.Testa U. Erythropoietic stimulating agents. Expert Opin. Emerg. Drugs. 2010;15:119–138. doi: 10.1517/14728210903499273. [DOI] [PubMed] [Google Scholar]
- 72.Chung H., Lee H., Lamoke F., Hrushesky W.J., Wood P.A., Jahng W.J. Neuroprotective role of erythropoietin by antiapoptosis in the retina. J. Neurosci. Res. 2009;87:2365–2374. doi: 10.1002/jnr.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang L., Zhang Z.G., Zhang R.L., Gregg S.R., Hozeska-Solgot A., LeTourneau Y., Wang Y., Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J. Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chong Z.Z., Shang Y.C., Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr. Neurovasc. Res. 2007;4:194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stieger K., Le Meur G., Lasne F., Weber M., Deschamps J.Y., Nivard D., Mendes-Madeira A., Provost N., Martin L., Moullier P., et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol. Ther. 2006;13:967–975. doi: 10.1016/j.ymthe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Toledo J.R., Sanchez O., Segui R.M., Garcia G., Montanez M., Zamora P.A., Rodriguez M.P., Cremata J.A. High expression level of recombinant human erythropoietin in the milk of non-transgenic goats. J. Biotechnol. 2006;123:225–235. doi: 10.1016/j.jbiotec.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 77.Weishaupt J.H., Rohde G., Polking E., Siren A.L., Ehrenreich H., Bahr M. Effect of erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest. Ophthalmol. Vis. Sci. 2004;45:1514–1522. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- 78.Keswani S.C., Leitz G.J., Hoke A. Erythropoietin is neuroprotective in models of HIV sensory neuropathy. Neurosci. Lett. 2004;371:102–105. doi: 10.1016/j.neulet.2004.08.080. [DOI] [PubMed] [Google Scholar]
- 79.Toth C., Martinez J.A., Liu W.Q., Diggle J., Guo G.F., Ramji N., Mi R., Hoke A., Zochodne D.W. Local erythropoietin signaling enhances regeneration in peripheral axons. Neuroscience. 2008;154:767–783. doi: 10.1016/j.neuroscience.2008.03.052. [DOI] [PubMed] [Google Scholar]
- 80.Grimm C., Wenzel A., Stanescu D., Samardzija M., Hotop S., Groszer M., Naash M., Gassmann M., Reme C. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J. Neurosci. 2004;24:5651–5658. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wen D., Boissel J.P., Tracy T.E., Gruninger R.H., Mulcahy L.S., Czelusniak J., Goodman M., Bunn H.F. Erythropoietin structure-function relationships: high degree of sequence homology among mammals. Blood. 1993;82:1507–1516. [PubMed] [Google Scholar]
- 82.Sullivan T., Kodali K., Rex T.S. Systemic gene delivery protects the photoreceptors in the retinal degeneration slow mouse. Neurochem. Res. 2011;36:613–618. doi: 10.1007/s11064-010-0272-6. [DOI] [PubMed] [Google Scholar]
- 83.Chern Y.J., Chung T.W., Sytkowski A.J. Structural role of amino acids 99–110 in recombinant human erythropoietin. Eur. J. Biochem. 1991;202:225–229. doi: 10.1111/j.1432-1033.1991.tb16366.x. [DOI] [PubMed] [Google Scholar]
- 84.Grodberg J., Davis K.L., Sytkowski A.J. Functional and structural role of arginine 103 in human erythropoietin. Arch. Biochem. Biophys. 1996;333:427–431. doi: 10.1006/abbi.1996.0411. [DOI] [PubMed] [Google Scholar]
- 85.Vincent-Lacaze N., Snyder R.O., Gluzman R., Bohl D., Lagarde C., Danos O. Structure of adeno-associated virus vector DNA following transduction of the skeletal muscle. J. Virol. 1999;73:1949–1955. doi: 10.1128/jvi.73.3.1949-1955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan M.H., Smith A.J., Pawlyk B., Xu X., Liu X., Bainbridge J.B., Basche M., McIntosh J., Tran H.V., Nathwani A., et al. Gene therapy for retinitis pigmentosa and Leber congenital amaurosis caused by defects in AIPL1: effective rescue of mouse models of partial and complete Aipl1 deficiency using AAV2/2 and AAV2/8 vectors. Hum. Mol. Genet. 2009;18:2099–2114. doi: 10.1093/hmg/ddp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun X., Pawlyk B., Xu X., Liu X., Bulgakov O.V., Adamian M., Sandberg M.A., Khani S.C., Tan M.H., Smith A.J., et al. Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Ther. 2010;17:117–131. doi: 10.1038/gt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 88.Gil J.M., Leist M., Popovic N., Brundin P., Petersen A. Asialoerythropoietin is not effective in the R6/2 line of Huntington's disease mice. BMC Neurosci. 2004;5:17. doi: 10.1186/1471-2202-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Auricchio A., O'Connor E., Hildinger M., Wilson J.M. A single-step affinity column for purification of serotype-5 based adeno-associated viral vectors. Mol. Ther. 2001;4:372–374. doi: 10.1006/mthe.2001.0462. [DOI] [PubMed] [Google Scholar]
- 90.Auricchio A., Hildinger M., O'Connor E., Gao G.P., Wilson J.M. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum. Gene Ther. 2001;12:71–76. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]
- 91.Drittanti L., Rivet C., Manceau P., Danos O., Vega M. High throughput production, screening and analysis of adeno-associated viral vectors. Gene Ther. 2000;7:924–929. doi: 10.1038/sj.gt.3301191. [DOI] [PubMed] [Google Scholar]
- 92.Gao G., Qu G., Burnham M.S., Huang J., Chirmule N., Joshi B., Yu Q.C., Marsh J.A., Conceicao C.M., Wilson J.M. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum. Gene Ther. 2000;11:2079–2091. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
- 93.Papaioannou V.E., Fox J.G. Efficacy of tribromoethanol anesthesia in mice. Lab. Anim. Sci. 1993;43:189–192. [PubMed] [Google Scholar]
- 94.Liang F.Q., Anand V., Maguire A.M., Bennett J. Intraocular Delivery of Recombinant Virus. Totowa, NJ: Humana Press Inc.; 2000. [DOI] [PubMed] [Google Scholar]
- 95.Allocca M., Di Vicino U., Petrillo M., Carlomagno F., Domenici L., Auricchio A. Constitutive and AP20187-induced Ret activation in photoreceptors does not protect from light-induced damage. Invest. Ophthalmol. Vis. Sci. 2007;48:5199–5206. doi: 10.1167/iovs.07-0140. [DOI] [PubMed] [Google Scholar]
- 96.Armentano M., Chou S.J., Tomassy G.S., Leingartner A., O'Leary D.D., Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat. Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.