Abstract
To determine if the dose of peptide administered or the plasma level was more important, doses of 0.15, 0.45, 4.5, or 45 mg/kg/day of the peptide D-4F were administered orally or subcutaneously (SQ) to apoliptotein (apo)E null mice. Plasma levels of peptide were ∼1,000-fold higher when administered SQ compared with orally. Regardless of the route of administration, doses of 4.5 and 45 mg/kg significantly reduced plasma serum amyloid A (SAA) levels and the HDL inflammatory index (P < 0.0001); doses of 0.15 or 0.45 mg/kg did not. A dose of 45 mg/kg/day administered to apoE null mice on a Western diet reduced aortic atherosclerosis by ∼50% (P < 0.0009) whether administered orally or SQ and also significantly reduced plasma levels of SAA (P < 0.002) and lysophosphatidic acid (P < 0.0009). Remarkably, for each dose administered, the concentration and amount of peptide in the feces was similar regardless of whether the peptide was administered orally or SQ. We conclude: i) the dose of 4F administered and not the plasma level achieved determines efficacy; ii) the intestine may be a major site of action for the peptide regardless of the route of administration.
Keywords: atherosclerosis, apolipoprotein A-I, apolipoprotein A-I mimetic peptides, lipoproteins
The peptide known as 4F contains 18 amino acids and has no sequence homology to apolipoprotein (apo)A-I but forms an amphipathic α-helix that allows the peptide to mimic many of the properties of apoA-I (1). When this peptide was synthesized from all D-amino acids (D-4F) and administered orally, it was found to inhibit lesion formation in mouse models of atherosclerosis (2).
Bloedon et al. (3) reported that single oral doses of 4.3 and 7.14 mg/kg of the apoA-I mimetic peptide D-4F significantly improved the HDL-inflammatory index (HII) of patients with coronary heart disease (CHD) or equivalents compared with patients that received placebo but doses of 0.43 and 1.43 mg/kg did not. The maximal concentration (Cmax) plasma levels of peptide after administration of 4.3 mg/kg or 7.14 mg/kg were 8.1 ± 6 or 16 ± 7 ng/ml, respectively (3). Based on three lines of evidence [ i) the peptide plasma levels in the study by Bloedon et al. (3), which were associated with a significant improvement in HII; ii) the peptide plasma levels achieved in animal studies demonstrating efficacy (4) (e.g., an oral dose of D-4F of 25 mg/kg produced a Cmax of 322 ng/ml in apoE null mice and significantly improved HII, paraoxonase activity, and HDL-mediated cholesterol efflux); and iii) D-4F added at a concentration of 250 ng/ml to the plasma of patients with CHD significantly improved HII (1)], Watson et al. (5) designed studies of 4F synthesized from all L-amino acids (L-4F) in patients with CHD to achieve pretargeted peptide plasma levels.
Peptides are expensive to produce compared with small organic molecules. In developing drugs for large numbers of patients, the cost of producing the drug is an important factor. Because peptides are expensive to produce, using the smallest effective dose limits the cost and facilitates use of the therapy in large patient populations. Watson et al. (5) administered the 4F peptide by intravenous (IV) injection or subcutaneous (SQ) injection to maximize plasma levels and minimize the dose. In the studies of Watson et al. (5), the Cmax achieved for a dose of 0.43 mg/kg was ∼3,000 ng/ml for IV administration and ∼400 ng/ml for SQ administration. Despite achieving these plasma levels of intact peptide, there was no significant improvement in HII values compared with placebo (5).
The doses used in the studies of Watson et al. (5) were much lower than the doses that were used in the studies of Bloedon et al. (3). The doses that were used by Bloedon et al. (3) significantly improved HII compared with placebo, whereas those used by Watson et al. (5) did not. Van Lenten et al. (6) demonstrated that the efficacy of D-4F and L-4F was equal when the peptides were administered by injection, suggesting that the difference in outcome between these two studies (3, 5) was not due to the different preparations of the peptide (D-4F vs. L-4F). Therefore, we hypothesized that the dose of these peptides may be more important than plasma levels. The data reported here strongly support this hypothesis and have significant implications for the future design of clinical trials of apoA-I mimetic peptides. Additionally, the data presented here strongly suggest that the intestine maybe a major site of action for the peptide regardless of the route of administration.
MATERIALS and METHODS
Peptides
The peptide 4F (Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2) was synthesized from all D- or all L-amino acids (D-4F and L-4F, respectively) by solid phase synthesis as described (4). A control peptide, scrambled D-4F (Sc-D-4F), with the same D-amino acids as in D-4F but in a sequence that does not promote amphipathic α-helix formation (Ac-D-W-F-A-K-D-Y-F-K-K-A-F-V-E-E-F-A-K-NH2) was also synthesized by solid phase synthesis as described previously (4). For the synthesis of 15N, 13C-4F, 15N-13C-Trp (Cambridge Isotopes, Andover, MA) was treated with fluorenylmethyloxycarbonyl (FMOC)-chloride and the resulting FMOC-15N-13C-Trp was used instead of FMOC-Trp during the solid phase synthesis of 4F. The rest of the steps were as described previously (4).
Other materials
Niclosamide was purchased from Sigma-Aldrich (St. Louis, MO; Catalogue Number N3510), and when administered, was given orally at a dose of 2 mg per mouse per day in the food. All other materials were purchased from sources previously described (7).
Mice
ApoE null mice originally purchased from the Jackson Laboratories on a C57BL/6J background were obtained from the breeding colony of the Department of Laboratory and Animal Medicine at the David Geffen School of Medicine at UCLA. The mice were maintained on a chow diet (Ralston Purina). In some experiments, the mice were switched to a Western diet (Teklad, Harlan, catalog # TD88137). The mice were kept in metabolic cages for experiments to determine peptide concentration in feces and urine. Peptides were administered to the mice by daily SQ injection on the back or were administered orally by providing the peptides in the drinking water or by incorporating the peptides into the diet. For SQ administration, the peptides were uniformly dissolved in normal saline, pH 7.4, using a glass-glass homogenizer. The solutions with lower concentrations, i.e., 3 μg per ml and 9 μg per ml, were prepared in the mouse room immediately before injection. For SQ administration, each mouse received a daily injection of 1 ml containing the peptide in normal saline, pH 7.4.
The following protocol was used to conveniently and accurately provide peptide in the Western diet. One hundred and fifty milliliters of water were added to 250 g of powdered Western diet and a highly uniform mixture was generated using a small industrial-type mixer. The desired quantity of the peptide (D-4F or Sc-D-4F) was first mixed using a laboratory mortar into 5–10 g of powdered regular rodent chow (Ralston Purina) to yield a highly uniform mixture followed by gradual addition and mixing with powdered Western diet. This uniform mixture containing the peptides was then carefully and gradually added and mixed in an industrial-type mixer with the 250 g of powdered Western diet to which 150 ml of water had been added and mixed as described above. Mixing was continued at high speed for 1 min at which time the mixer was stopped and the material was mixed in the opposite direction using a spatula to ensure a highly uniform distribution of the peptide. This process was continued for a total of 5–10 cycles. The resulting mixture containing the peptide was spread and flattened uniformly on a sheet of aluminum foil in a tray to achieve a height of ∼1 cm. The mixture was then cut into blocks of ∼4 g each using a rotary knife. These blocks were stored at −20°C. Each evening, four blocks of the frozen diet containing the peptides were provided to each cage containing four mice. The mice ate all of the diet by morning. All experiments were performed using protocols approved by the Animal Research Committee at UCLA.
Peptide levels in plasma, liver, feces, and urine
Plasma, liver, feces, and urine peptide levels were determined by LC-ESI-MS/MS using an internal standard of 15N13C labeled 4F peptide as described previously with a lower limit of quantification of 1 ng/ml (3).
Plasma extraction.
Twenty microliters of plasma were placed in an Eppendorf tube. A stock solution of 1 mg/ml of 15N13C-4F peptide was diluted 1:50 in 50:50:0.1 acetonitrile-H2O-formic acid. Twenty microliters of this solution were added to the Eppendorf tube containing the plasma sample. Two hundred microliters of methanol and 100 μl of chloroform were added and the contents were mixed for 1–2 min by vortexing; 200 μl of water were added and the content was mixed for 1–2 min by vortexing followed by centrifugation at 10,000 rpm in a microfuge. The aqueous phase was removed and kept frozen until use. The sample was lyophilized on a Speed Vac and brought to 5% phase B (5% acetonitrile, 0.1% formic acid) prior to analysis by LC-ESI-MS/MS.
Liver extraction.
As described below, the livers were flushed to remove blood and snap-frozen in liquid nitrogen. Subsequently, the livers were thawed and minced and samples were weighed to determine wet weight. Water was added to the samples (5:1; volume/weight); they were homogenized, extracted, and analyzed as described above for plasma.
Feces extraction.
Fecal material was collected from the metabolic cages and weighed. Pyrogen free water (Baxter) was added to the fecal samples (5:1; volume/weight); the samples were placed on ice and allowed to hydrate for 5 min. Subsequently the samples were ground in an IKA tissue homogenizer for 2 min. Using a cut-off blue tip on a P1000 Eppendorf pipette, 50 μl were transferred into an Eppendorf tube. The volume was brought to 100 μl using HPLC water (Fisher HPLC-grade water, submicron filtered) and the sample was then extracted and analyzed as described above for plasma samples.
Urine extraction.
Urine was collected from the metabolic cages and was extracted and analyzed as described above for plasma.
Accuracy and precision.
A plasma sample from an apoE null mouse that had not received peptide was spiked with D-4F to give a plasma concentration of 1,000, 200, 40, 10, or 1 ng/ml. The plasma was extracted and the D-4F concentration was determined in quadruplicate. The values obtained are shown in Table 1.
TABLE 1.
Accuracy and precision of D-4F plasma measurements
| Peptide Added (ng/ml) | 1,000 | 200 | 40 | 10 | 1 |
|---|---|---|---|---|---|
| Values obtained from quadruplicates (ng/ml) | 925 | 206 | 41.8 | 9.31 | 1.32 |
| 965 | 192 | 34.5 | 8.46 | 1.17 | |
| 961 | 187 | 35.4 | 7.24 | 0.931 | |
| 1090 | 185 | 35.0 | 6.75 | 1.03 | |
| Mean ± SD | 985.3 ± 72 | 192.5 ± 9.47 | 36.68 ± 3.43 | 7.94 ± 1.16 | 1.11 ± 0.169 |
| 95% CI | (870.5–1100) | (177.4–207.6) | (31.21–42.14) | (6.09–9.79) | (0.84–1.38) |
| CV% | 7.3% | 4.9% | 9.4% | 14.6% | 15.2% |
A plasma sample from an apoE null mouse that had not received peptide was spiked with D-4F to give a plasma concentration of 1,000, 200, 40, 10, or 1 ng/ml. The plasma was extracted and the D-4F concentration was determined in quadruplicate as described in Materials and Methods.
Removal of hepatic blood for determination of liver peptide levels
The livers were washed free of blood using the following procedure. The vena cava was nicked with fine scissors to start blood flow. To perfuse the liver, a 21-gauge needle was inserted into the portal vein. The needle was connected by tubing to a bag containing the perfusion solution. The perfusing solution contained 0.9% saline, Baxter USP for injection, adjusted to 2 mM EDTA (Sigma), and 20 μM BHT (Sigma). The bag was placed at a height of 30 cm above the liver and the perfusion was continued for a minimum of 10 min using a minimum of 20 ml delivered through a Baxter Continu-Flo solution set. Remaining blood and saline continued to flow out of the hole made in the vena cava. This fluid was removed by vacuum. The perfusion was continued until the liver appeared white or tan. If needed, gentle massaging of the tissues or repositioning of the needle was used to facilitate perfusion. Once the perfusion was completed, the needle was removed and the liver was harvested and snap-frozen in liquid nitrogen after wrapping it in labeled foil.
SAA plasma levels
Serum amyloid A (SAA) plasma levels were determined by ELISA (Invitrogen, catalog # KMA0011) according to the manufacturer's instructions.
HII
The HII was determined as previously described (6).
Aortic atherosclerosis
The percent of the aorta with atherosclerosis was determined by en face analysis as described previously (8).
Other methods
Lysophosphatidic acid (LPA) was determined by LC-MS/MS as previously described (9). Lipid and lipoprotein and protein determinations were determined by methods described previously (6). Cholesterol was measured enzymatically (cholesterol esterase, cholesterol oxidase, peroxidase, hydroxybenzoic acid, 4-aminoantipyrine) using a 96-well microplate-based absorbance method (end point mode and at primary wavelength of 500 nm and secondary wavelength of 600 nm) according to the manufacturer's instructions. The components used consisted of Cholesterol Reagent (Thermo Trace/DMA reagent #TR13303, Fisher # NC9767174, Fisher Diagnostics, Fremont, CA), Cholesterol Standard 300 mg/dl (Fisher #NC9343697, Thermo Scientific) or Pointe Scientific General Chemistry Standards (200 mg/dl, Mfr. # C7509-STD, Fisher Scientific #: 23-666-198), Internal Controls (high, medium, and low controls), Verichem Labs Matrix Plus Cholesterol Reference Kit (Controls A, B, and E), List No. 9550 (Verichem Labs, Providence, RI), for monitoring accuracy and precision of lipid assays and for calibration or calibration verification of serum cholesterol test systems, and a 96-well plate reader (Versamax Tunable microplate reader, Molecular Devices, Sunnyvale, CA). The linearity of the assay was optimal between 0 and 20 mmol/L (0 to 774 mg per dl), and the sensitivity was at 60 delta mA per mmol/L. The coefficient of variation for within runs was 2.56% and 4.12% for between runs. The cholesterol concentration was calculated by delta Abs/min of the unknown divided by delta Abs/min of calibrator multiplied by the calibrator value. When needed, samples were diluted using normal saline to fall within the linear range of the standard curve.
Statistical analyses
Statistical analyses were performed by ANOVA or unpaired two-tail t-test using GraphPad Prism version 5.03 (GraphPad Software, San Diego, CA).
RESULTS
Comparing the efficacy of L-4F administered orally with niclosamide to L-4F administered SQ without niclosamide
Lewis et al. (10) previously reported that SAA levels correlate well with lesion area in mice. As shown in supplementary Fig. I, administration of L-4F at a dose of 10 mg/kg/day without pravastatin was equally effective in reducing SAA levels in old apoE null mice whether administered orally with niclosamide (which protects the peptide from degradation in the intestine; see Ref. 7) or administered SQ at the same dose without niclosamide. We did not measure plasma levels in this first pilot experiment, but the results suggested to us that efficacy was not likely determined by plasma levels because plasma levels of L-4F after administration of this dose of L-4F with niclosamide previously only produced a Cmax of ∼150 ng/ml (7). From unpublished preclinical studies, we would expect that this dose of L-4F administered by SQ injection would have produced a Cmax 10–100 times higher. We also recently reported that oral administration of L-4F in mouse chow without niclosamide but at a high dose of L-4F (100 mg/kg/day) decreased plasma levels of LPA and significantly reduced tumor burden in a mouse model of ovarian cancer (9). We did not measure plasma levels of L-4F in those studies (9), but based on prior work (7), we would have expected only low levels of intact peptide in the plasma. Thus, we were encouraged by the pilot experiment shown in supplementary Fig. I and by our recently published experiment with oral L-4F without niclosamide (9) to proceed with studies directly comparing dose versus plasma levels as determinants of efficacy.
D-4F plasma levels after oral or SQ administration
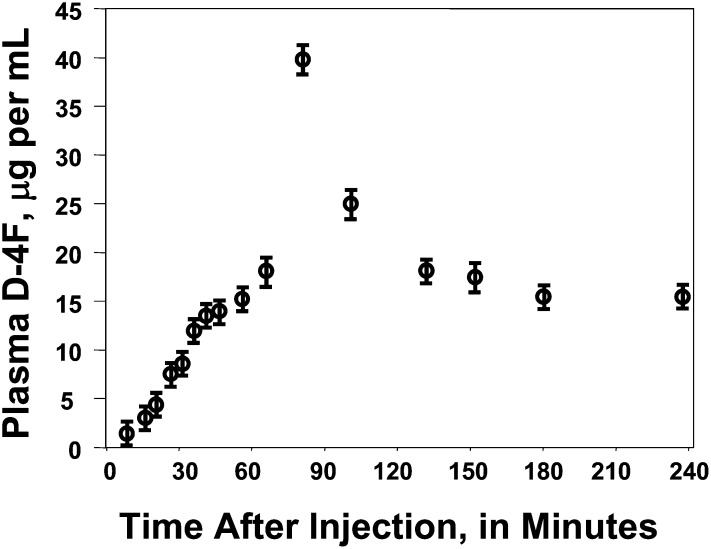
To avoid the use of niclosamide and to allow testing of peptide administered either orally or SQ at equal doses across a wide range of dosing, we chose to use D-4F for the remainder of the experiments reported here. We began by determining plasma levels of D-4F as a function of time after administration of equal doses given orally or by SQ injection. As shown in Fig. 1, the peak plasma level of D-4F after SQ injection of 900 μg/mouse (45 mg/kg) was achieved 60–90 min after injection giving a Cmax of ∼40 μg/ml of intact D-4F as determined by LC-ESI-MS/MS. In these SQ experiments, the plasma level of D-4F did not fall below ∼15 μg/ml during the 150 min following Cmax.
Fig. 1.
Plasma levels of D-4F after subcutaneous (SQ) injection. Female apoE null mice 6–7 months of age (n = 48) were placed on a Western diet for 2 weeks and then fasted overnight in individual cages. In the morning, water was removed for 3 h and each mouse was administered 900 μg of D-4F (45 mg/kg) by SQ injection on the back. The mice were returned to their cages after injection and allowed to drink up to 3 ml of water. The mice were bled at the time points shown on the X-axis and the plasma concentration of intact D-4F peptide was determined by LC-ESI-MS/MS as described in Materials and Methods. The data shown are mean ± SD for three mice that were used for each time point.
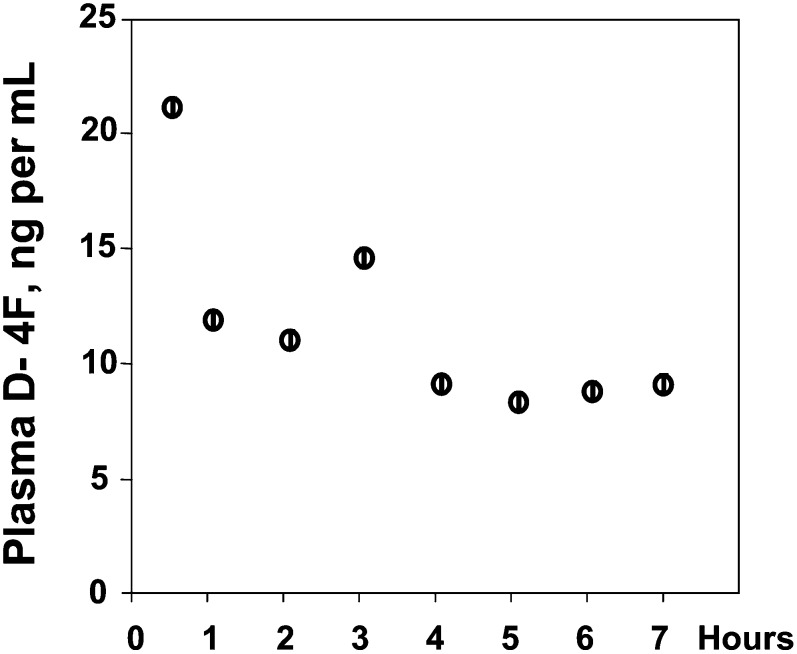
As shown in Fig. 2, when the peptide was administered in the drinking water at a concentration of 300 μg/ml and the mice were allowed to drink 3 ml to give the same dose of D-4F orally, the Cmax was only ∼20 ng/ml. Comparing Figs. 1 and 2 demonstrates that in addition to the differences in Cmax, there was a difference in the pattern of plasma D-4F levels. SQ administration produced a peak between 1 and 2 h after injection with a Cmax of 40 ± 1 μg/ml and after this peak, the lowest value recorded was 15 ± 1 μg/ml, which was at 240 min after injection. When the peptide was administered in the drinking water, a Cmax of 22 ± 1 ng/ml was obtained at the first time point tested, which was at 30 min after allowing the mice to drink; subsequent values varied between a high of 14 ± 1 ng/ml and a low of 8 ± 1 ng/ml.
Fig. 2.
Plasma levels of D-4F after administration of the peptide in the drinking water. Female apoE null mice 6–7 months of age (n = 24) were placed on a Western diet for 2 weeks and then fasted overnight in individual cages. In the morning, water was removed for 3 h after which drinking water containing 300 μg/ml of D-4F was provided and the mice were allowed to drink 3 ml of water over a period of 3 h and the mice were not injected with D-4F. The mice were bled at the time points shown on the X-axis. The data shown are mean ± SD for three mice that were used for each time point.
Comparing the efficacy of administering D-4F orally versus SQ as determined by the reduction in SAA levels
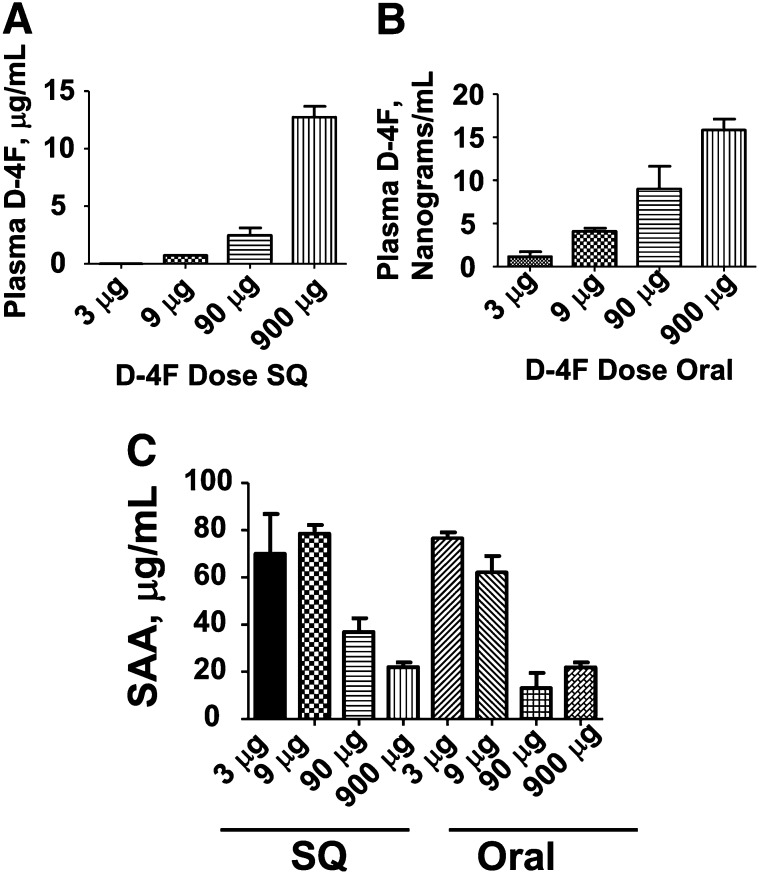
As shown in Fig. 3, administering D-4F by SQ injection in apoE null mice produced plasma levels 100- to 1,000-fold higher compared with the same dose administered orally in the drinking water. As also shown in Fig. 3, despite the marked differences in plasma levels of D-4F, the reduction in SAA levels was similar between mice that received D-4F orally and those receiving the same dose by daily SQ injection.
Fig. 3.
Administering D-4F by SQ injection in apoE null mice produced plasma levels 100- to 1,000-fold higher compared with the same dose administered orally in the drinking water, but the reduction in SAA levels was similar. Female apoE null mice 6–7 months of age (n = 12 per group) were fed a Western diet and were administered D-4F in the drinking water (B) to provide each mouse a daily dose of peptide of 3, 9, 90, or 900 μg per day of D-4F (Oral) or the mice received drinking water without peptide but received the same dose of D-4F by daily SQ injection on the back (A). In the morning of the 8th day of treatment after an overnight fast, water was removed for 3 h and then replaced with water that did or did not contain D-4F at the concentration each mouse had been receiving. The mice that received water without D-4F were injected SQ on the back with the dose of D-4F that they had been receiving and 2 h later were bled. The mice that received drinking water with D-4F were allowed to drink for 3 h and were then bled. The plasma from four mice in each group was pooled and plasma levels of D-4F were determined as described in Materials and Methods. The data shown are mean ± SD and are representative of two of two separate experiments. C: The plasma of the mice described in panels A and B was analyzed for SAA levels as described in Materials and Methods. The data shown are mean ± SD and are representative of two of two separate experiments.
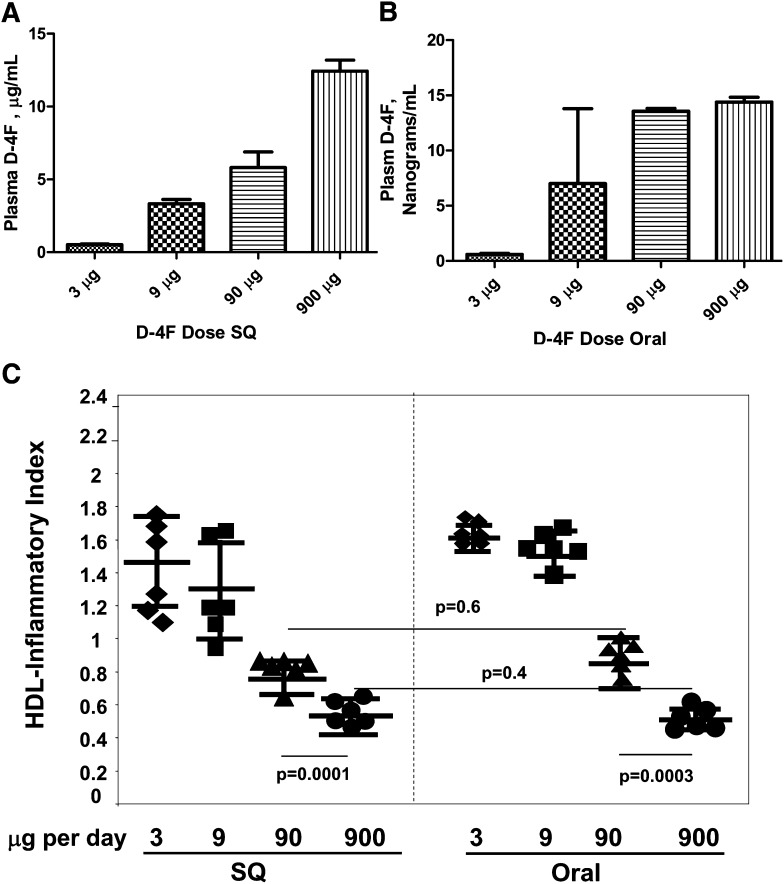
As shown in Fig. 3A, administering 9 μg per mouse per day (0.45 mg/kg/day) by daily SQ injection produced plasma levels of D-4F of 727 ± 6 ng/ml. As shown in Fig. 3C, this dose was not effective in improving SAA levels.
As shown in Fig. 3B, administering 90 μg per mouse per day (4.5 mg/kg/day) in the drinking water only produced plasma levels of D-4F of 9.0 ± 3 ng/ml, but as shown in Fig. 3C, this dose was effective in improving SAA levels (P = 0.003). In this experiment, administering 90 μg per mouse per day (4.5 mg/kg/day) in the drinking water was modestly but significantly (P = 0.02) more effective in lowering SAA levels compared with the same dose of D-4F administered SQ.
Administering 900 μg per mouse per day (45 mg/kg/day) of D-4F in the drinking water (Fig. 3B) produced plasma D-4F levels of 16 ± 1 ng/ml compared with plasma D-4F levels of 12.7 ± 0.9 μg/ml after administering the same dose by daily SQ injections (Fig. 3A). Despite a nearly 1,000-fold difference in plasma levels of D-4F, the lowering of SAA was similar whether the peptide was administered orally or by SQ injection (Fig. 3C).
In the experiments described in Fig. 3, the Western diet was only fed for 8 days and plasma from four mice in each group was pooled to give three pools for analysis in each experiment. The next experiment was designed to i) test a longer period of oxidative stress by increasing the time of the Western diet to six weeks, ii) analyze the plasma of individual mice, and iii) compare D-4F treatment to treatment with scrambled D-4F (Sc-D-4F), which contains the same D-amino acids as in D-4F but arranged in a sequence that prevents formation of an amphipathic α-helix. There was considerable variation in D-4F plasma levels among individual mice (supplementary Fig. II). Table 2 summarizes these data. As shown in Table 2, at the lower doses of D-4F, the plasma levels of peptide were often below the lower limit of quantification, which was 1 ng/ml of peptide.
TABLE 2.
The minimum value, maximum value, median, and mean ± SD for plasma D-4F levels are shown for the mice described in Fig. 4
| D-4F Dose μg/mouse/day | Route of Administration | Minimum Value | Maximum Value | Median | Mean ± SD |
|---|---|---|---|---|---|
| 3 | Oral | BLLOQ | 65 ng/ml | BLLOQ | 17 ± 4 ng/ml |
| 3 | SQ | BLLOQ | 660 ng/ml | 56 ng/ml | 150 ± 196 ng/ml |
| 9 | Oral | BLLOQ | BLLOQ | BLLOQ | BLLOQ |
| 9 | SQ | 317 ng/ml | 625 ng/ml | 410 ng/ml | 442 ± 96 ng/ml |
| 90 | Oral | BLLOQ | 7 ng/ml | 1 ng/ml | 2 ± 2 ng/ml |
| 90 | SQ | 2.3 μg/ml | 5.7 μg/ml | 4.0 μg/ml | 3.8 ± 1 μg/ml |
| 900 | Oral | 2 ng/ml | 94 ng/ml | 27 ng/ml | 35 ± 28 ng/ml |
| 900 | SQ | 23 μg/ml | 61 μg/ml | 39 μg/ml | 39 ± 11 μg/ml |
BLLOQ, below lower limit of quantification.
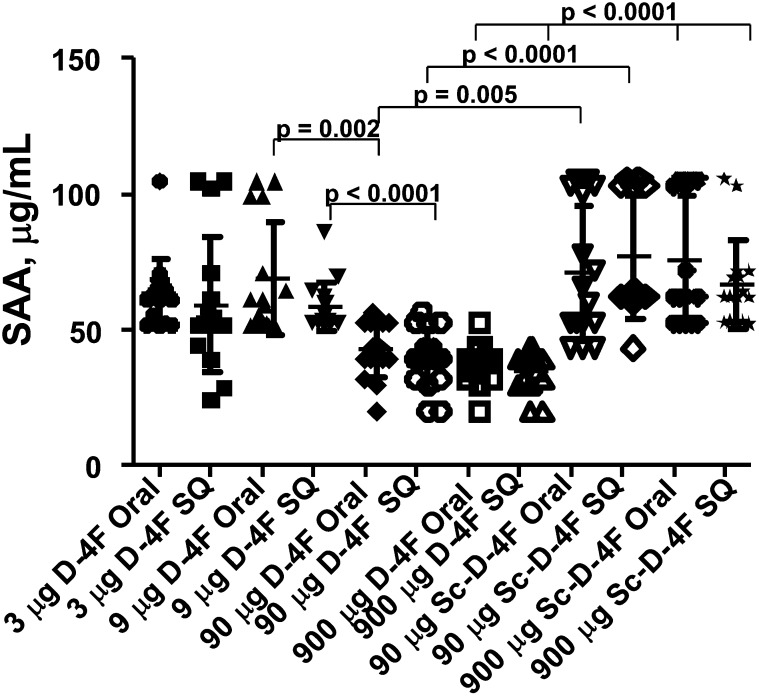
A dose of 9 μg per mouse per day of D-4F administered SQ produced D-4F plasma levels of 442 ± 96 ng/ml of peptide and this dose was not effective in reducing SAA levels (Fig. 4). In contrast, a dose of 90 μg per mouse per day of D-4F administered in the drinking water produced D-4F plasma levels of only 2 ± 2 ng/ml but significantly reduced SAA levels (P < 0.002) (Fig. 4). The plasma level of D-4F after administration of 90 μg per mouse per day of D-4F by SQ administration was 3.8 ± 1 μg/ml (Table 2) but was no more effective in reducing SAA levels than was the same dose administered orally (Fig. 4), which produced plasma levels of peptide ∼1,000 times lower (supplementary Fig. II). Thus, neither plasma peptide levels nor the route of administration determined the reduction in SAA levels but the dose administered did.
Fig. 4.
The route of administration does not determine SAA levels but the dose administered does. Female apoE null mice (n = 16 per group) 6–7 months of age were fed a Western diet and were administered drinking water with or without D-4F at the following doses: 3, 9, 90, or 900 μg per day per mouse. Some of the mice received scrambled D-4F (Sc-D-4F) in the drinking water instead of D-4F. The doses of Sc-D-4F provided to these mice were 90 or 900 μg per mouse per day of Sc-D-4F. Mice that did not receive D-4F in their drinking water received D-4F SQ at doses of 3, 9, 90, or 900 μg per mouse per day. Some of the mice received Sc-D-4F SQ instead of D-4F at doses of 90 or 900 μg per mouse per day of Sc-D-4F. After 6 weeks, in the morning after an overnight fast, water was removed for 3 h and then replaced with water that did or did not contain D-4F at the concentration each mouse had been receiving. The mice that received water without D-4F were injected SQ on the back with the dose of D-4F that they had been receiving and 2 h later were bled. The mice that received drinking water with D-4F were allowed to drink for 3 h and were then bled. Plasma SAA levels were determined as described in Materials and Methods. The symbols represent individual values for each mouse that received D-4F; the longer horizontal line represents the mean and the shorter horizontal lines define 1 SD above and below the mean.
Peptide treatment did not significantly alter plasma total cholesterol levels and there was a trend toward slightly higher HDL-cholesterol levels in mice that received 90 or 900 μg per mouse per day of D-4F by either route of administration (data not shown).
Comparing the efficacy of administering D-4F orally versus SQ as determined by HII
The experiments shown in Fig. 5 confirm with a different measure (HII) that plasma levels of peptide do not predict efficacy. The Y-axis in Fig. 5A is given in micrograms/ml and the Y-axis of Fig. 5B is given in nanograms/ml. Despite the ∼1000-fold difference in plasma peptide levels, the values for HII were similar at the same dose whether administered SQ or orally (Fig. 5C).
Fig. 5.
Plasma levels of peptide did not predict improvement in the HDL-inflammatory index (HII). Eight groups of female apo E null mice 7–8 months of age (n = 23 per group) were fed a Western diet and treated daily with 3, 9, 90, or 900 μg of D-4F administered in the drinking water daily (Oral) or SQ daily for 2 weeks at which time they were bled. The plasma levels of D-4F after SQ administration (A) and after oral administration (B) was determined as described in Materials and Methods. The data shown are the mean ± SD. For determination of HII, plasma from three to four mice was pooled to yield six pools of plasma for each treatment group and HDL from each pool was isolated by FPLC and the HII was determined as described in Materials and Methods (C). In C, the symbols represent the values for each of the six pools for each treatment group; the longer horizontal line represents the mean and the shorter horizontal lines define 1 SD above and below the mean.
Comparing the efficacy of administering D-4F orally versus SQ as determined by the reduction in aortic atherosclerosis and the reduction in SAA and LPA levels
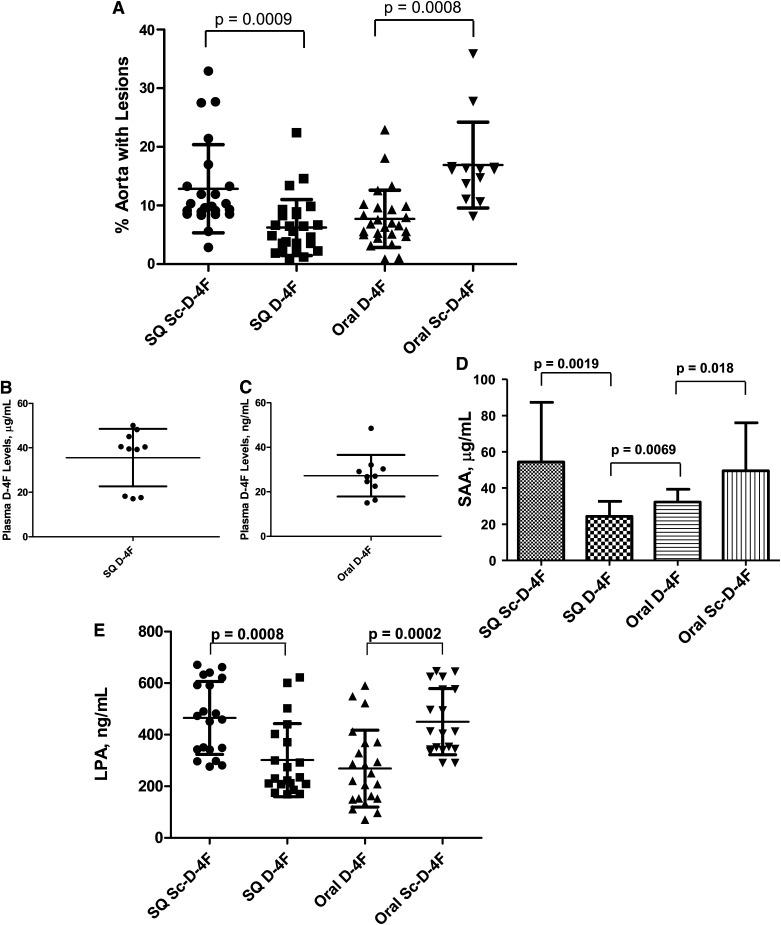
We tested the ability of D-4F at a dose of 45 mg/kg to alter aortic atherosclerosis and plasma SAA and LPA levels in apoE null mice on a Western diet when the peptide was administered SQ daily or was incorporated into the diet. There was a highly significant ∼50% reduction in aortic atherosclerosis (Fig. 6A), which was not significantly different whether the peptide was administered SQ daily or was incorporated into the diet despite the dramatic difference in plasma D-4F levels (Fig. 6B, C). The percent aorta with lesions for mice that received SQ D-4F was 6.2 ± 4.8% (mean ± SD) compared with 7.7 ± 4.9% for mice that received oral D-4F (Fig. 6A). The percent aorta with lesions for mice that received SQ Sc-D-4F was 12.9 ± 7.5% compared with 16.9 ± 7.3% for mice that received oral Sc-D-4F and this difference was not statistically significant (Fig. 6A). The plasma D-4F levels in the mice receiving SQ D-4F were 36 ± 13 μg/ml (Fig. 6B) compared with 27 ± 9 ng/ml for mice receiving the same dose of D-4F in the diet (Fig. 6C).
Fig. 6.
Administering D-4F SQ daily or administering the same dose incorporated into the diet reduced aortic atherosclerosis to the same degree. A: Female apoE null mice 8–9 months of age were fed a Western diet. The mice received 900 μg per mouse per day of D-4F or Sc-D-4F SQ daily or received the same daily dose orally by incorporating the peptide in the diet. After 8 weeks on the Western diet, mice from each group were euthanized daily; by the 9th week, all mice had been euthanized. The percent of aorta containing lesions was determined by en face analysis as described in Materials and Methods. The symbols represent individual values for each mouse; the longer horizontal line represents the mean and the shorter horizontal lines define 1 SD above and below the mean. In the evening 2 days before euthanasia, food was removed from the mice described in A and the mice were fasted overnight. B: The next morning (1 day prior to euthanasia), the mice receiving peptide SQ were injected with D-4F or Sc-D-4F at a dose of 900 μg per mouse. Two hours later, these mice were bled and D-4F levels were determined by LC-ESI-MS/MS in 10 randomly chosen plasma samples. C: For the mice receiving peptides in the diet, on the morning prior to euthanasia, after the overnight fast, the mice were given frozen blocks of diet prepared as described in Materials and Methods, each containing 900 μg of D-4F or Sc-D-4F. The mice were allowed to eat all of the diet presented to them over a period of 3–4 h at which time they were bled and D-4F levels were determined by LC-ESI-MS/MS in 10 randomly chosen plasma samples. The symbols represent individual values for each mouse; the longer horizontal line represents the mean and the shorter horizontal lines define 1 SD above and below the mean. D: On the day of euthanasia, after an overnight fast, a terminal bleed was performed in the mice described in A and SAA levels were determined as described in Materials and Methods. The data shown are mean ± SD. E: Lysophosphatidic acid (18:1) (LPA) levels were determined as described in Materials and Methods on the plasma taken from the mice described in D. The symbols represent individual values for each mouse; the longer horizontal line represents the mean and the shorter horizontal lines define 1 SD above and below the mean.
SAA levels were significantly decreased whether D-4F was administered SQ or was administered in the diet. In this experiment, in contrast to the case in Fig. 5, SAA levels were slightly but significantly higher after oral administration of D-4F compared with SQ administration (24 ± 8 μg/ml compared with 32 ± 7 μg/ml for SQ vs. oral administration, respectively) (Fig. 6D). Treatment with either oral or SQ D-4F also significantly reduced LPA levels to the same degree (Fig. 6E).
There was no significant difference in plasma total cholesterol as a function of treatment (918 ± 202, 870 ± 157, 888 ± 176, and 929 ± 136 mg/dl for SQ Sc-D-4F, SQ D-4F, oral D-4F, and oral Sc-D-4F, respectively). The mice that received D-4F by either route had a small but significant increase in HDL-cholesterol levels (30 ± 7 mg/dl for SQ D-4F and 31 ± 10 mg/dl for oral D-4F) compared with mice that received SQ Sc-D-4F (25 ± 7 mg/dl) (P = 0.0131 for SQ D-4F vs. SQ Sc-D-4F) or oral Sc-D-4F (21 ± 5 mg/dl) (P = 0.0006 for oral D-4F vs. oral Sc-D-4F).
If the concentration of peptide in the plasma does not determine efficacy, where does the peptide act?
Using a variety of measures of efficacy (plasma SAA levels, HII, aortic lesion area, plasma LPA levels), it is clear that peptide plasma concentrations do not determine efficacy, so where does the peptide act? It has been postulated that the 4F peptide acts by binding bioactive lipids with very high affinity (11). The data presented here show that efficacy is dose related but independent of plasma concentrations. Is there a compartment where peptide concentrations are equal whether the peptide was administered orally or SQ, suggesting that this compartment may be a major site of peptide action?
In searching for a compartment outside of the plasma compartment where there might be equal concentrations of peptide, we administered D-4F SQ and determined plasma and hepatic peptide levels as a function of time. Similar to the data in Fig. 1, the plasma Cmax was achieved ∼2 h after SQ administration (data not shown). In contrast, the hepatic Cmax was achieved 16 h after SQ administration (supplementary Fig. III). After oral administration of D-4F, hepatic D-4F levels were very low and below the detection limit and after a dose of 900 μg, reached a Cmax of ∼30 ng/g liver wet weight (supplementary Fig. IV A) compared with ∼100 μg/g liver wet weight after SQ administration (supplementary Fig. IV B).
Next, we examined the content of the peptide in feces and urine. Surprisingly, despite the enormous difference in D-4F plasma levels when the peptide was administered orally versus SQ, peptide concentrations in the feces and the total peptide excreted in the feces were similar at each dose regardless of whether the peptide was administered orally or SQ (Table 3). Only about 5% of the amount of peptide contained in the feces was found in the urine (data not shown).
TABLE 3.
Plasma D-4F levels were dramatically different after oral or subcutaneous (SQ) administration but the concentration of D-4F in the feces and the total amount of D-4F excreted in the feces were similar regardless of whether the peptide was administered SQ or orally
| D-4F Dose μg/mouse/day | Route of Administration | Plasma D-4F Levels | D-4F in Feces μg/gram feces | μg D-4F in Feces per 24 Hours per Mouse | % Dose Excreted |
|---|---|---|---|---|---|
| 3 | Oral | 3.7 ± 2 ng/ml | 0.14 ± 0.01 | 0.075 ± 0.019 | 2.5 ± 0.6 |
| 3 | SQ | 0.59 ± 0.1 μg/ml | 0.27 ± 0.12 | 0.093 ± 0.06 | 3.1 ± 2 |
| 9 | Oral | 8.8 ± 8 ng/ml | 5.5 ± 2.9 | 0.71 ± 0.79 | 7.9 ± 8.8 |
| 9 | SQ | 0.99 ± 05 μg/ml | 7.8 ± 2.8 | 0.94 ± 0.48 | 10 ± 5 |
| 90 | Oral | 13 ± 4 ng/ml | 24.3 ± 9.6 | 3.5 ± 1.5 | 3.9 ± 1.7 |
| 90 | SQ | 13.3 ± 0.6 μg/ml | 20.0 ± 6.6 | 3.5 ± 1.5 | 3.9 ± 1.7 |
| 900 | Oral | 46 ± 1 ng/ml | 252 ± 14 | 47 ± 8.3 | 5.2 ± 0.9 |
| 900 | SQ | 63.3 ± 4 μg/ml | 315 ± 128 | 52 ± 36 | 5.8 ± 4.0 |
ApoE null female mice, 6–8 months of age were placed on a Western diet. After 2 weeks, the Western diet was continued and the mice were transferred to metabolic cages (n = 8–10 mice for each dose group and for each route of administration) and administered D-4F SQ or by addition to the drinking water (Oral) to give a dose of 3, 9, 90, or 900 μg of peptide per day. Feces and urine were discarded from the first 24 h of treatment. Feces and urine were collected from the second 24 h of treatment and analyzed for D-4F concentration and content as described in Materials and Methods. Immediately following the second 24 h period in the metabolic cages, the mice receiving the peptide in drinking water were deprived of water for 3 h and then allowed access to water containing peptide. The mice were allowed to drink the water containing their daily dose of peptide over a period of 3 h and were bled. Similarly, immediately following the second 24 h period in the metabolic cages, the SQ groups were injected with their daily dose of peptide and bled 2–3 h later. Plasma D-4F levels were determined as described in Materials and Methods. The values shown for plasma D-4F levels are the mean ± SD of three plasma samples randomly selected from each dose and each route of administration. The values shown for the fecal analyses are the mean ± SD.
DISCUSSION
The failure of Watson et al. (5) to see efficacy in vivo despite achieving plasma peptide levels that were orders of magnitude higher than those achieved by Bloedon et al. (3) prompted the studies reported here. Bloedon et al. (3) saw a significant improvement compared with placebo in HII after administering oral doses of D-4F of 4.3 mg/kg or 7.14 mg/kg, which yielded plasma D-4F levels of 8.1 ± 6 or 16 ± 7 ng/ml, respectively. Watson et al. (5) achieved plasma peptide levels for L-4F of 3,255 ± 640 ng/ml after administering the peptide IV at a dose of 0.43 mg/kg but failed to see significant improvement in HII compared with placebo. Van Lenten et al. (6) have shown that when administered by injection, there is no difference in efficacy between L-4F and D-4F. The data presented here conclusively demonstrate that in apoE null mice, the efficacy of the 4F peptide as determined by a number of measures (SAA levels, HII, lesion area, LPA levels) is not determined by plasma levels but is dose dependent.
Given the similar efficacy at similar doses regardless of the route of administration or the plasma level achieved, we thought that it was likely that in some compartment, at equal doses of peptide, there must be equal peptide concentrations to account for the similar efficacy. The data in Table 3 show that peptide concentration in the feces and the amount of peptide excreted in the feces was similar at equal doses regardless of whether the peptide was administered orally or SQ. These results strongly suggest that the intestine may be a major site of action for the peptide regardless of whether the peptide is administered orally or SQ. Because we did not measure peptide levels in intestinal tissue, we cannot rule out the possibility that the peptide acted in the liver when administered SQ but acted in the intestine when administered orally. We think such a scenario is unlikely because of the similar concentration of peptide in the feces after oral or SQ administration coupled with the similar dose response after oral or SQ administration.
Pappenheimer et al. (12) reported that lipophilic peptides that are undegradable by mammalian enzymes combine with bile salts to form hydrophilic complexes that are secreted rapidly at high concentration in bile. They reported that “At physiological concentrations of bile salts (5–40 mM) and nanomolar concentrations of peptide the binding is so complete that these undegradable peptides are rapidly cleared from liver to duodenal fluid in association with the bile salts. After reaching the ileum the bile salts are reabsorbed to blood, leaving the original lipophilic peptides to be excreted in the feces…” (12, p. 292). Future studies will be needed to determine if the mechanism suggested by Pappenheimer et al. (12) accounts for the findings in our studies.
The appearance of the peptide in the plasma after oral administration was much more variable than after SQ administration. The slope of a line describing the increase in peptide plasma levels after increasing doses administered SQ would be much greater compared with the case for increasing doses administered orally. The modest increase and variability in peptide plasma levels after oral administration is not unexpected because the absorption from the intestine of peptides synthesized from all D-amino acids appears to be by solvent drag in fluid absorbed through tight junctions and intercellular channels (12). As a result, the plasma concentrations of peptide after SQ administration are less variable and 100- to 1,000-fold greater than after oral administration of the same dose.
After SQ administration, the time course of peptide appearing in the plasma (Fig. 1 and data not shown) was much faster than was the time course for the appearance of the peptide in the liver (supplementary Fig. III). The cause for this difference in plasma and hepatic concentrations cannot be known from these studies. Another apoA-I mimetic peptide (18A) was cleared from rat plasma at similar rates whether synthesized from all L-amino acids or from all D-amino acids (13). After 3 days, ∼55% of the 125I-label was found in the liver whether the peptide was synthesized from L- or D-amino acids (13). However, after 3 days, most of the peptide synthesized from all L-amino acids was degraded and most of the released label was incorporated into the thyroid or excreted into the urine. This was not the case with the peptide synthesized from all D-amino acids. Our studies only measure intact peptide because of the detection system (LC-ESI-MS/MS). The time course for the appearance of the peptide in liver (supplementary Fig. III) compared with the time course in plasma (Fig. 1 and data not shown) suggests that intact peptide left the plasma compartment rather rapidly, entered into the tissues, and later was returned to the liver, presumably for secretion into the bile. Future studies will be required to determine the exact time course and mechanism by which peptide is transported from plasma to tissues, and after a delay, from the tissues to the liver. The time course and mechanism for secretion into bile and intestine will also need to be determined in future studies.
Similar to the findings of Garber et al. (13), we found that very little intact peptide was secreted into the urine (only about 5% of that excreted in the feces). The concentration of peptide in the feces and the amount of peptide excreted into the feces was similar at each dose regardless of the route of administration. After a 900 μg dose of peptide (45 mg/kg), ∼50 μg of D-4F were excreted per mouse per day in the feces whether the peptide was administered orally or SQ (Table 3). It may seem surprising that approximately the same concentration and amount of administered peptide were found in the feces after SQ administration or oral administration. Also, the apparent low recovery in the feces (2.5 ± 0.6% to 10 ± 5% of the administered dose) after oral administration may seem surprising for a D-peptide that is resistant to degradation by most mammalian enzymes. However, it should be noted that although peptides synthesized from all D-amino acids are resistant to degradation by most mammalian enzymes, this is not the case for bacterial enzymes (14). Indeed, bacteria use D-amino acids for a number of important metabolic purposes (15, 16).
If, after injection, the peptide is nearly quantitatively excreted into the intestine in the duodenum with the bile, as suggested by the work of Pappenheimer et al. (12), the peptide would enter the intestine at a site that would expose it to the vast majority of the intestinal flora as it progressed through the intestine and was excreted in the feces. Conversely, because only a tiny fraction of the intestinal flora is present prior to the duodenum, peptide entering orally would essentially be exposed to the same bacterial load as would be the case for peptide entering the duodenum through the bile. Thus, assuming that i) equal amounts of peptide reached the duodenum regardless of the route of administration and ii) most of the peptide entering the duodenum was degraded or altered by the intestinal flora as it passed through to the feces, it would not be surprising if the concentration and amount of peptide in the feces was the same whether the peptide was administered orally or SQ.
The mechanism of action of the 4F peptides appears to be largely due to their remarkable ability to bind oxidized lipids compared with apoA-I (11). Administration of L-4F by SQ injection at a dose of 10 mg/kg/day or L-4F administered in mouse food at a dose of 100 mg/kg/day significantly reduced plasma levels of the tumor promoting lipid LPA and significantly decreased tumor burden in a mouse model of ovarian cancer (9). Administration of D-4F to diabetic apoE null mice at a dose of ∼50 mg/kg/day in the drinking water significantly reduced atherosclerotic lesions and also decreased hepatic concentrations of arachidonic acid, prostaglandin E2, prostaglandin D2, 15-hydroxyeicosatetraenoic acid, 12-hydroxyeicosatetraenoic acid, and 13-hydroxyoctadecanoic acid without significantly altering plasma glucose, insulin, total cholesterol, HDL-cholesterol, or triglyceride levels (17). As shown in Fig. 6E in this article, oral and SQ administration was equally effective in reducing plasma LPA levels despite the dramatic differences in plasma peptide levels (Fig. 6B, C).
Wool et al. (18) recently reported that administering L-4F at a dose of ∼1.19 mg/kg by intraperitoneal injection every other day to apoE null mice on a chow diet starting at age 10 weeks and continuing for 4 weeks dramatically reduced atherosclerosis in two arterial sites. However, the same protocol, when started at age 20 weeks and continued for 8 weeks, was not effective. Wool et al. concluded that the peptide “…inhibited early atherogenesis but was ineffective against more mature lesions” (18). The experiments in Fig. 6 were carried out with apoE null mice that were older than the 20-week-old mice used by Wool et al. (18); the mice in Fig. 6 were 8–9 months of age at the start of the experiment. The period of treatment for the mice in was approximately the same as used by Wool et al. (8–9 weeks of treatment). The mice in the experiments described in Fig. 6 were under much more oxidative stress (i.e., Western diet vs. chow diet). Plasma cholesterol levels were much higher in the mice in Fig. 6 compared with the mice in Wool et al. (800–900 mg/dl vs. 300–500 mg/dl). Although there was no significant reduction in lesions in the studies of Wool et al. in the apoE null mice on a chow diet started on peptide treatment at 20 weeks of age, there was a significant ∼50% reduction in lesions in our studies (Fig. 6A). One obvious difference between the experiments in Fig. 6 and those reported by Wool et al. was that the dose of peptide used in Fig. 6 was 45 mg/kg/day or nearly 80-fold greater than that used by Wool et al. (18) when taking into consideration daily versus every-other-day dosing.
The studies reported here demonstrate: i) in apo E null mice on a Western diet, plasma and hepatic levels of the 4F peptide do not determine efficacy in vivo, ii) the concentration and amount of peptide excreted into the feces is similar for each dose administered regardless of whether the peptide is administered orally or SQ, and iii) similar efficacy is achieved at similar doses whether the peptide is administered orally or SQ. Taken together, these data strongly suggest that the intestine maybe a major site of action for the peptide.
One limitation of the studies reported here is that the fecal measurements of peptide only accounted for a minority of the peptide administered. Although this was likely due to the action of intestinal bacteria, future studies are needed to determine if this is indeed the case. If this is the case, which intestinal bacteria are involved and where are they located in the intestine? Where in the intestine does the peptide work? Does the peptide act on intestinal tissue, or on intestinal contents? Does the peptide work on the bile in the duodenum, or does the peptide act on intestinal cells, or does it work on the contents in the duodenum, jejunum, ileum, or colon? We measured plasma, hepatic, and fecal levels of peptide, but what was the tissue content of the peptide at each level of the intestine? What in the intestine does the peptide bind? Is the peptide binding oxidized lipid as might be expected or is the peptide binding an entirely different class of substances? Is the content of oxidized lipids or LPA changed in intestinal cells after peptide treatment? Is the material bound by the peptide in the intestine excreted with the peptide in the feces or does the binding target the material for destruction by intestinal bacteria? Is the interaction of the peptide with intestinal bacteria a requirement for bioactivity and what is the nature of that interaction? How does the peptide's action on substances in intestinal cells or on intestinal contents rapidly lead to changes in plasma constituents such as SAA or LPA levels or HII? These are important questions for future studies.
We previously reported that oral administration of D-4F at a dose of 12.5 mg/kg/day improved reverse cholesterol transport from macrophages in apoE null mice (4). Future studies will be needed to determine if the findings described here regarding the importance of dose independent of plasma peptide levels and the likely role of the intestine will also apply to other apoA-I mimetic peptides, particularly those which are thought to be more active on reverse cholesterol transport (19–21).
The results of the studies reported here and those recently reported by Watson et al. (5) strongly suggest that much higher doses of 4F and perhaps other apoA-I mimetic peptides will be needed for treating humans than was thought to be the case based on a strategy of targeting plasma peptide levels. These studies also suggest that there is likely to be no advantage to administering the 4F peptide SQ compared with administering it orally.
Supplementary Material
Footnotes
Abbreviations:
- apo
- apolipoprotein
- CHD
- coronary heart disease
- Cmax
- maximal concentration
- FMOC
- fluorenylmethyloxycarbonyl
- HII
- HDL-inflammatory index
- IV
- intravenous
- LPA
- lysophosphatidic acid
- SAA
- serum amyloid A
- SQ
- subcutaneous
- 4F
- (Ac-D-W-F-K-A-F-Y-D-K-V-A-E-K-F-K-E-A-F-NH2)
- D-4F
- 4F synthesized from all D-amino acids
- L-4F
- 4F synthesized from all L-amino acids
- Sc-D-4F
- (Ac-D-W-F-A-K-D-Y-F-K-K-A-F-V-E-E-F-A-K-NH2) a control scrambled peptide containing the same D-amino acids as in D-4F but in a sequence that does not promote α-helical formation
This work was supported in part by US Public Health Service Grants HL-30568, HL-34343 and the Laubisch, Castera, and M.K. Grey Funds at UCLA. M.N., S.T.R., G.M.A., and A.M.F. are principals in Bruin Pharma and A.M.F. is an officer in Bruin Pharma.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of four figures.
REFERENCES
- 1.Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Yu N., Ansell B. J., Datta G., Garber D. W., et al. 2005. Apolipoprotein A-I mimetic peptides. Arterioscler. Thromb. Vasc. Biol. 25: 1325–1331. [DOI] [PubMed] [Google Scholar]
- 2.Navab M., Anantharamaiah G. M., Hama S., Garber D. W., Chaddha M., Hough G., Lallone R., Fogelman A. M. 2002. Oral administration of an apoA-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 105: 290–292. [DOI] [PubMed] [Google Scholar]
- 3.Bloedon L. T., Dunbar R., Duffy D., Pinell-Salles P., Norris R., DeGroot B. J., Movva R., Navab M., Fogelman A. M., Rader D. J. 2008. Safety, pharmacokinetics and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 49: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navab M., Anantharamaiah G. M., Reddy S. T., Hama S., Hough G., Grijalva V. R., Wagner A. C., Frank J. S., Datta G., Garber D., et al. 2004. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation. 109: 3215–3220. [DOI] [PubMed] [Google Scholar]
- 5.Watson C. E., Weissbach N., Kjems L., Ayalasomayajula S., Zhang Y., Chang I., Navab M., Hama S., Hough G., Reddy S. T., et al. 2011. Treatment of patients with cardiovascular disease with L-4F, an apoA-1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 52: 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Lenten B. J., Wagner A. C., Navab M., Anantharamaiah G. M., Hama S., Reddy S. T., Fogelman A. M. 2007. Lipoprotein inflammatory properties and serum amyloid A levels but not cholesterol levels predict lesion area in cholesterol-fed rabbits. J. Lipid Res. 48: 2344–2353. [DOI] [PubMed] [Google Scholar]
- 7.Navab M., Ruchala P., Waring A. J., Lehrer R. I., Hama S., Hough G., Palgunachari M. N., Anantharamaiah G. M., Fogelman A. M. 2009. A novel method for oral delivery of apolipoprotein mimetic peptides synthesized from all L-amino acids. J. Lipid Res. 50: 1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navab M., Anantharamaiah G. M., Hama S., Hough G., Reddy S. T., Frank J. S., Garber D. W., Handattu S., Fogelman A. M. 2005. D-4F and statins synergize to render HDL anti-inflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 25: 1426–1432. [DOI] [PubMed] [Google Scholar]
- 9.Su F., Kozak K. R., Imaizumi S., Gao F., Amneus M. W., Grijalva V., Ng C., Wagner A., Hough G., Farias-Eisner G., et al. 2010. Apolipoprotein A-I (apoA-I) and apoA-I mimetic peptides inhibit tumor development in a mouse model of ovarian cancer. Proc. Natl. Acad. Sci. USA. 107: 19997–20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis K. E., Kirk E. A., McDonald T. O., Wang S., Wight T. N., O'Brien K. D., Chait A. 2004. Increase in serum amyloid A evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 110: 540–545. [DOI] [PubMed] [Google Scholar]
- 11.Van Lenten B. J., Wagner A. C., Jung C. L., Waring A. J., Lehrer R. I., Watson A. D., Hama S., Navab M., Anantharamaiah G. M., Fogelman A. M. 2008. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J. Lipid Res. 49: 2302–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappenheimer J. R., Karnovsky M. L., Maggio J. E. 1997. Absorption and excretion of undegradable peptides: role of lipid solubility and net charge. J. Pharmacol. Exp. Ther. 280: 292–300. [PubMed] [Google Scholar]
- 13.Garber D. W., Venkatachalapathi Y. V., Gupta K. B., Ibdah J., Phillips M. C., Hazelrig J. B., Segrest J. P., Anantharamaiah G. M. 1992. Turnover of synthetic class A amphipathic peptide analogues of exchangeable apolipoproteins in rats. Correlation with physical properties. Arterioscler. Thromb. Vasc. Biol. 12: 886–894. [DOI] [PubMed] [Google Scholar]
- 14.Korza H. J., Bochtler M. 2005. Pseudomoas aeruginaosa LD-carboxypeptidase, a serine peptidase with a Ser-His-Glu triad and a nucleophilic elbow. J. Biol. Chem. 280: 40802–40812. [DOI] [PubMed] [Google Scholar]
- 15.Lam H., Oh D-C., Cava F., Takacs C. N., Clardy J., de Pedro M. A., Waldor M. K. 2009. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science. 325: 1552–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolodkin-Gal I., Romero D., Cao S., Clardy J., Kolter R., Losick R. 2010. D-amino acids trigger biofilm disassembly. Science. 328: 627–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgantini C., Imaizumi S., Grijalva V., Navab M., Fogelman A. M., Reddy S. T. 2010. ApoA-I mimetic peptides prevent atherosclerosis development and reduce plaque inflammation in a mouse model of diabetes. Diabetes. 59: 3223–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wool G. D., Cabana V. G., Lukens J., Shaw P. X., Binder C. J., Witztum J. L., Reardon C. A., Getz G. S. 2011. 4F peptide reduces nascent atherosclerosis and induces natural antibody production in apolipoprotein E-null mice. FASEB J. 25: 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wool G. D., Reardon C. A., Getz G. S. 2008. Apolipoprotein-I mimetic peptide helix number and helix linker influence potentially anti-atherogenic properties. J. Lipid Res. 49: 1268–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bielicki J. K., Zhang H., Cortez Y., Zheng Y., Nrayanaswami V., Patel A., Johansson J., Azhar S. 2010. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis. J. Lipid Res. 51: 1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Souza W., Stonik J. A., Murphy A., Demosky S. J., Sethi A. A., Moore X. L., Chin-Dusting J., Remaley A. T., Sviridov D. 2010. Structure/function relationships of apolipoprotein a-I mimetic peptides: implications for antiatherogenic activities of high-density lipoprotein. Circ. Res. 107: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.