Abstract
Recognition of the strength of nonhuman primate models in investigating metabolic disorders has resulted in an expanded need for in vivo research techniques. We studied adipose metabolism in 10 baboons (13.0 ± 4.2 years old, 29.5 ± 5.5 kg). Part 1 evaluated the effect of different sedatives on the rate of appearance of plasma free fatty acids (RaFFA), assessed using 13C4-labeled palmitate infusion (7 µmol/kg/min). Animals, were studied with no sedation, with complete isoflurane sedation, and with minimal midazolam infusion (0.04 mg/kg/h), with the last scheme allowing for the most consistent values and animals that were visually more calm. In Part 2, RaFFA and RaGlycerol (D5-glycerol, 5 mg/kg lean body mass/h) were measured. From midnight to 0300, flux fell and came to a steady state between 0500 and 0700 h (RaFFA, 39.4 ± 29.8 μmol/kg fat mass/min; and RaGlycerol, 26.9 ± 7.3 μmol/kg/min). The RaFFA-to-RaGlycerol ratio was 1.5 ± 0.8 (49% reesterification). The decline in turnover throughout the night reflects natural circadian processes and was mirrored by reductions in FFA and glycerol to 0.62 and ± 0.14 and 0.16 and ± 0.03 mmol/l, respectively. The concurrent changes in both FFA and glycerol kinetics indicate physiologic validity of the method. These techniques will support needed research to determine mechanisms by which treatments act upon the adipocyte in vivo.
Keywords: adipose tissue, fatty acid metabolism, glycolipids, lipids, lipolysis, lipoprotein kinetics, transport, triglycerides
Nonhuman primates, particularly Old World monkeys, have proven to be valuable models for the study of a variety of complex diseases and physiological processes expressing a pattern of susceptibility and complications similar to that seen in humans (1). The close evolutionary relationship between humans and nonhuman primates suggests that they share many of the specific genetic mechanisms involved in determining differential susceptibility to disease (1). Not only do nonhuman primates offer a large, long-lived animal for the study of chronic diseases associated with metabolic dysregulation (e.g., refs. 4 and 5), they provide a model that is physiologically and genetically very similar to that of humans. The primary energy reserve in mammals is stored as adipose triacylglycerols (TG), and the release of that energy through the process of lipolysis is a metabolic event of great importance to the maintenance of health. Lipolysis is found to be dysregulated in disease (2, 3), and when the kinetics of both FFA and glycerol are measured in plasma, the ratio of these turnovers has been used as a means to estimate intra-adipocyte reesterification (4–6). To our knowledge, such methodology has not been developed for use in the baboon, and if available, these techniques would allow the strengths of this animal model in genetic studies to be extended to translational research in the area of obesity and metabolic disease. Accordingly, the objective of the present work was to optimize human (7) and rodent (8) protocols to establish clinical procedures for the measurement of blood fatty acid and glycerol fluxes in vivo in Papio hamadryas monkeys. Using an established tether system (9), we modified a meal-feeding paradigm and refined protocols for animal acclimation to reduce stress and maintain the animals’ body weights in the clinic. In addition, we present data showing the effects of different management techniques (anxiolytic treatment, sedation) and how numerous variables can be measured using the minimum amount of blood, and we document for the first time turnovers of FFA and glycerol as they change throughout the night. As in humans, these fluxes in primates do not appear to come to steady state reflecting fasting until 5–7 AM (10). The availability of these methods will support needed research to determine how adipose insulin resistance predisposes to the development of chronic diseases, using the overweight baboon as a model. Such studies will allow testing of in vivo mechanisms by which weight loss and pharmacologic treatments aid in the treatment of metabolic disorders (11).
MATERIALS AND METHODS
The baboons studied herein were selected from a population at the Southwest National Primate Research Center (SNPRC) under study at the Texas Biomedical Research Institute (San Antonio, TX). The protocol was approved by the Texas Biomedical Research Institute Animal Care and Use Committee (protocol 1203PC 0). Animals were baboons (Papio hamadryas sp.) >6 years of age (i.e., fully sexually mature), housed individually during the duration of these studies in cages with access to a light cycle from the room lights in the clinic, set to be on every day from 0600 to 1800 h. Using an established tether catheter system (9), this project was conducted in two parts: in Part 1, the 2 females and 1 male baboon were selected by using body weight criteria deemed normal for adults (females, 14–20 kg; males, 25–30 kg; unpublished SNPRC observations). As shown in Table 1, we evaluated the effect of different anxiolytic doses of the benzodiazepine agonist midazolam on the rate of appearance of plasma free fatty acids (RaFFA) in the 3 animals. Each animal was tested twice with an identical infusion of [1,2,3,4-13C4]hexadecanoate administered at 7 µg/kg/min, complexed to human albumin in a ratio of 2:1. The use of sedatives was varied between the two tests. The first animal (ID 11597) was studied once without the administration of the anxiolytic compound and once with midazolam administration beginning at 2245 h with a 0.025 mg/kg bolus given over a 2 min period, followed by a continuous infusion of 0.010 mg/kg/h. Isotope infusion and blood drawing occurred through the tether catheters. The second animal (ID 11953) was studied under conditions of two doses of midazolam; dose 1 was a 0.025 mg/kg bolus begun at 2245 h and given over a period of 2 min, followed by a continuous infusion of 0.020 mg/kg/h; and dose 2 was a bolus of 0.025 mg/kg, begun earlier, at 1900 h, followed by a higher continuous infusion of 0.040 mg/kg/h. The third animal (ID 11044) was studied under the conditions of higher anxiolytic dose of midazolam beginning at 1900 h and then with complete isoflurane sedation given as an inhalant (1.5% v/v gas). The isoflurane was tested because previous studies in dogs have shown isoflurane to reduce fatty acid release (12). The repeat studies in animals were conducted on average 19.3 ± 11.6 days apart to allow for decay of the isotopes.
TABLE 1.
Summary of method development experiments
| Baboon ID | Experimental protocol | Recorded behavioral data and metabolic results |
|---|---|---|
| 11597A (male) | No treatment | Behavior agitated (grunting, stamping, etc.). FFA concentration and RaFFA unstable |
| 11597Ba | Midazolam; 0.025 mg/kg bolus and Continuous infusion of 0.010 mg/kg/h starting at 22:45 | No aggressive behaviors; FFA Concentration unstable; RaFFA stable |
| 11953A (female) | Midazolam; 0.025 mg/kg bolus and continuous infusion of 0.020 mg/kg/h starting at 22:45 | Animal moving around in cage but quiet. FFA concentration and RaFFA unstable |
| 11953Ba | Midazolam; 0.025 mg/kg bolus and continuous infusion of 0.040 mg/kg/h starting at 19:00 | Animal quiet and sleeping. FFA concentration more stable throughout the night. |
| 11044A (female) | Complete isoflurane sedation | Animal completely sedated.FFA concentration and RaFFA unstable |
| 11044Ba | Midazolam; 0.025 mg/kg bolus and continuous infusion of 0.040 mg/kg/h starting at 19:00 | Animal quiet and sleeping. FFA concentration and RaFFA stable |
Data from these experiments were combined with those from Part 2 and used for correlational analysis (see supplementary Fig. III).
In Part 2 of the project, as a result of the data generated in Part 1, we chose the higher dose of midazolam beginning at 1900 h and expanded data collection to include measurement of plasma glycerol turnover (RaGlycerol), as RaFFA and RaGlycerol are both indicators of adipose lipolysis. The 7 males studied in Part 2 were chosen to represent a range of body weight (Table 2) so that the group would contain animals with large variations in adiposity and insulin sensitivity, as previously demonstrated by our group (13). The baboons were admitted to the clinic and allowed to acclimate. During this time, each animal was observed for aggressive-submissive behaviors, daily food consumption was monitored, and the animal was acclimatized to the tether jacket system (9). When acclimation was achieved, the animal underwent ketamine sedation, and baseline assessments were made as follows: body composition was measured by dual-energy X-ray absorptiometry scan (DEXA; Lunar Prodigy whole-body scanner; GE Medical Systems, Madison, WI); and measurements of body weight, waist circumference, and body surface area and blood sampling were made for clinical biochemistry analyses. For DEXA, animals were placed in the supine position on the bed, and the extremities were positioned within the scanning region. Scans were analyzed using Encore2007 software (version 11.40.004; GE Healthcare, Madison, WI). A euglycemic, hyperinsulinemic clamp study was performed the same day, using previously published methods (14). Following these assessments, the animal underwent surgical tether implantation, as described in detail previously (9). Catheter implants in the femoral artery and vein for tether were prepared as follows: heparin (low-molecular-weight)-coated polyurethane (7 Fr) catheters were placed in the femoral artery and vein by laparotomy. An incision was made over the femoral artery and vein just below the inguinal lymph node, and the femoral artery/vein and the profundus artery/vein were exposed by blunt dissection. Through a small incision in the profundus artery/vein, a catheter was introduced. The catheter was advanced through the profundus artery/vein into the femoral artery/vein. Both catheters were routed subcutaneously to the midscapular region of the back where they exited the skin into a molded plastic box (backpack) attached to the tether jacket. At the end of the study, the tether catheters were removed by another surgery. During the 24 h occurring after catheter placement, the animal was carefully monitored, and after the animal recuperated over a period of 10 days, the stable isotope study was performed. Because the isotope infusion studies were planned to be performed at night, the veterinary technicians assigned to the project were exposed to the animals on a daily basis during the 3 week acclimation period, including entering the room at night to have the animals become accustomed to them doing so. Also, throughout the acclimation period and during and in between the catheter placement and isotope infusion, food intake was measured daily. Body weight was monitored weekly, and food intake was adjusted downward for increases in body weight. If body weight began to fall, additional enrichments (fruits, vegetables) were added. Changes in an animal's behavior (e.g., depressive posture, interactions with handlers) were monitored by technical staff.
TABLE 2.
Characteristics of baboons
| Parameter | Unit | Mean ± SD | Range |
|---|---|---|---|
| Age | year | 13.0 ± 4.2 | 7.4–18.1 |
| Body weight | kg | 29.5 ± 5.5 | 16.2–35.9 |
| Body fat mass | kg | 2.4 ± 1.5 | 0.6–5.3 |
| Body fat mass | % | 8.5 ± 5.7 | 3.9–21.8 |
| Lean mass | kg | 25.9 ± 5.1 | 15.3–32.5 |
| Lean mass | % | 89.0 ± 5.1 | 78.2–94.9 |
| Waist circumference | cm | 56.0 ± 6.6 | 42.0–64.5 |
| Body surface area | m2 | 1.21 ± 0.05 | 0.81–1.38 |
| Total cholesterola | mmol/l | 2.62 ± 0.69 | 1.81–3.49 |
| Plasma TG | mmol/l | 0.45 ± 0.21 | 0.21–0.69 |
| Glucose | mmol/l | 4.88 ± 0.52 | 4.22–5.74 |
| Insulina | μU/ml | 7.7 ± 6.8 | 2.8–19.3 |
| Glucose disposal ratea | mg/kg/min | 7.2 ± 0.6 | 4.8–10.0 |
| HOMA IRab | —- | 1.71 ± 1.60 | 0.33–4.49 |
n = 10, except where noted: 8 male and 2 female baboons. All values are from fasted animals (under no drug treatments), except the glucose disposal rate, derived from the clamp procedure.
n = 7.
Homeostasis model of insulin resistance, calculated as [glucose (mg/dl) × insulin (μU/ml)]/405.
Dietary intake and isotope study
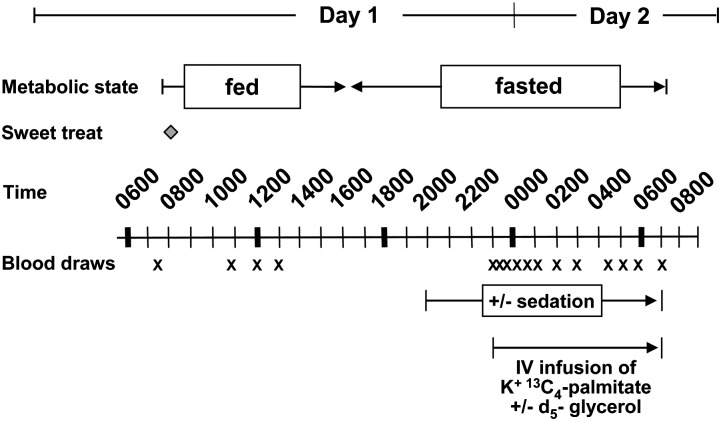
For all animals, food was made available from 0800 through 1600 h each day. This protocol was followed to ensure that during the fed-state measurements (made in the morning), all animals would be actively eating and that during the fasted-state measurements (through the night between 2300 and 0700 h), animals would be approaching a standardized fasted state. The baboons were acclimatized to this feeding regimen during the preparatory phases of this work (i.e., during the 3 week duration of clinic and tether jacket acclimation and surgery recovery). The quantity of food offered to each baboon daily was based on the estimated metabolizable energy requirements for adult captive baboons. Specifically, the animals were fed a commercial diet targeted to meet an expected energy requirement to sustain constant body weight (BW) of 40–51 kcal/BW (in kg) (167–213 kJ) (15). The average consumption of dietary feed totaled 1,590 calories (6.65 kJ per day as shown in supplementary Fig. I). The diets consisted of standard chow (Monkey Diet 15%, constant nutrition, 5LE0; Purina), which contained carbohydrate, 57.7% (g/100 g weight); and protein, 15.3%, fat (ether extract) 4.7%. Total saturated fatty acids were 1.2% (% weight); monounsaturated fatty acids were 1.3%; C18:2 was 1.9%; C18:3 was 0.2%; ω-3-fatty acid was 0.2%; and cholesterol was 49 mg/kg diet. Water was provided to the animals ad libitum, and fresh fruits and vegetables were given for enrichment. Along with their food each morning, all animals received a single peanut butter-based sweet treat to test this mixture as a vehicle for drug delivery. The sweet treat contained 44.9% peanut butter, 24.7% graham cracker, 20.9% powdered sugar, and 9.7% butter by weight. Each treat bolus weighed 15 g and contained 82.3 kcal (344 kJ) (55.6% of energy from fat, 36.1% from carbohydrate, and 8.3% from protein). The fatty acid composition of the TG in the treat was C16:0, 22.0% (weight %); C18:0, 8.9%; C18:1, 45.4%; C16:2, 22.0%; C18:2, 0.5%; and 1.2% other fatty acids. The addition of this treat raised the dietary fat intake from 12.7% to 15.2% total dietary energy as fat. The stable isotope infusion protocol was modified from human protocols used previously (16, 17), while taking into account the different metabolic body size of the baboon (18). Infusions were performed through the femoral vein, and blood was drawn from the arterial catheter. As shown in Fig. 1, on day 1 of the isotope study, fasting blood was drawn at 0750 h and then at 0800 h, and the animal was presented with food and the treat. Fed-state blood was drawn at 1100, 1200, and 1300 h. At 1600 h, the food was removed as per daily protocol. At 1900 h, the bolus of midazolam (0.025 mg/kg) was administered, followed by the iv drip (0.040 mg/kg/h) to induce anti-anxiolytic effects in the animal. This treatment was continued until the end of the isotope study on day 2 at 0700 h. To further reduce stress, fasting metabolism in the baboons was assessed at night, and the animals rested or slept when the measurements were made. At 2300 h on day 1, an infusion of isotopes began that contained K+[1,2,3,4-13C4]hexadecanoate administered as described in Part 1, and d5-glycerol (5 mg/kg lean body mass/h). Nightly blood samples were drawn at 2300, 2320, 2340, 2400, 0030, 0100, 0130, 0200, 0300, 0430, 0600, and 0700 h. Sterile, pyrogen-free isotopes were purchased from Isotec (Miamisburg, OH) and from Cambridge Isotope Laboratory (Andover, MA) and were prepared using sterile techniques. Isotopic purity was greater than 98% for all the tracers used.
Fig. 1.
Metabolic infusion protocol. Animals were acclimated to food availability between 0800 and 1600 h. Blood was drawn through the tether system catheter periodically during the day and night. Stable isotope infusions were performed starting at 2300 h and continued until 0700 h. Isotope infusion and blood drawing occurred through the tether catheter. For details of feeding and animal management protocols, see Materials and Methods.
Analysis of blood metabolites
All blood samples were 5 ml volume and were drawn into chilled tubes containing EDTA; plasma was separated immediately by centrifugation (3,000 rpm; 1,500 g, 10 min) at 4°C. The samples were kept on ice while benzamidine, gentamicin sulfate, chloramphenicol, phenylmethylsulfonyl fluoride, and Trolox® were added as preservatives (19). TG-rich lipoproteins (TRL) with a d of <1.006 g/l were isolated from plasma by ultracentrifugation as described previously (20). The top 2 ml containing TRL was removed by tube slicing, and the infranatant was used to isolate FFA and glycerol for analysis by GC-MS (16). On a separate day from all other tests, blood samples for serum cortisol concentration determination were drawn at 0700, 1030, 1400, and 1630 h, and concentrations were measured by immunoassay (Immulite1000 cortisol; Siemens, Inc.). For measurement of plasma metabolite concentrations, enzymatic kits were used for plasma TG and TRL-TG (product nos. 461-09092, 461-08992; Wako, Richmond, VA), glucose (product no. 439-90901; Wako), and FFA (product nos. 999-34691 and 991-34891;Wako); insulin was measured by ELISA ( product no. EZHI-14L; Millipore), and TRL-apoB100 was measured by using a human total apoB100 kit (Kamiya Biomedical Co., Seattle, WA). TRL-apoB48 concentration was measured using a human apoB48 ELISA kit (Shibayagi, Inc.). Sufficient TRL samples were available in 8 of 10 animals for the measurement of these apolipoproteins, and in 1 animal, the concentration of apoB48 was 10-fold that of the other animals. Since a cross-reacting protein was suspected, this animal's values were excluded from the analysis. Results of these assays were all read with a Power wave XS microtiter plate reader (Biotek, Inc).
Analysis of fatty acids and glycerol
The fatty acid composition of plasma FFA was analyzed on an HP 6890 series gas chromatograph (GC) using an SP 2560 fused-silica capillary column (100 m × 0.25 mm inner diameter, 0.020 µm film; Supelco Inc., Bellefonte, PA) and equipped with a flame ionization detector (Hewlett-Packard, Palo Alto, CA). The temperature was programmed at 140°C for 5 min, followed by an increase of 4°C/min to reach a final temperature of 240°C and then held for 50 min. The injector was operated in the split-less mode and was kept at 260°C. Individual fatty acids were identified by retention time in comparison to known standards. For fatty acid isotopomer analysis by GC-MS, we used a 6890N gas chromatograph coupled to a 5975 mass selective detector (Agilent Technologies, Palo Alto, CA). The GC-MS was equipped with an Agilent DB-225 fused silica capillary column (30 m × 0.25 mm inside diameter, 0.25 µm film) with helium as a carrier gas flowing at the rate of 1.5 ml/min. The initial temperature was 75°C, which then rose to 220°C at a rate of 40°C/min, followed by a rise to 230°C at a rate of 5°C/min. The final temperature was held constant for a total run time of 14.75 min. The injector was operated in the split-less mode and kept at 230°C. The GC-MS method used selected ion monitoring for m/z of 270 and 274. Palmitate methyl ester enrichments were calculated using five-point standard curves for M4 analysis, and the average steady-state M4% enrichment was 3.00% ± 1.37%. For plasma glycerol enrichments, a DB-17 ms, 30 m column was used (0.25 mm inner diameter, 0.25 μm phase thickness) with a split-less injection and GC conditions as follows: 70°C for 1 min, then temperature was ramped at 20°C/min to 220°C, held for 2 min, ramped at 30°C/min to 280°C, and held for 2 min. The total run time was 14.5 min (21). The injector temperature was 250°C; transfer line, 280°C; source, 230°C; quadrupole, 150°C; with helium as the carrier gas flowing at a constant rate of 1 ml/min. Isotopomers of the propionic ester of glycerol (m/z 171, 172, 173, 175, and 176) were assessed in the EI mode as described by Sunehag (21). The fragment assessed for the derivatized D5-glycerol had a m/z of 173, and the 175/176 ions were derived from the internal standard that was added (13C3, d8-glycerol). Comparable ion peak areas between the standard curve and biological samples were achieved by either dilution or concentration of the sample. The average glycerol steady-state enrichment was 8.95% ± 3.95%.
Calculations and statistical analysis
The fatty acid infusate composition and enrichments were analyzed by GC and GC-MS, and the calculations of the RaFFA were adjusted for the amount of unlabeled fatty acids present in the FFA pool that were derived from less than 100% purity of the isotope and fatty acids present on the albumin used in the infusion. RaGlycerol was calculated as described by Wolfe (7). The final RaFFA and RaGlycerol values presented Table 3 , represent the average of a single baboon's steady-state values collected between 0500 and 0700 h. Then, this number for each animal was averaged to obtain the group mean presented in the table. To calculate the theoretical adipose fatty acid intracellular reesterification rate for each animal, the ratio of RaFFA to RaGlycerol was calculated and then this value was subtracted from 3.0 (the theoretical maximal ratio of release rates of FFA and glycerol from adipose TG) to get the difference. This difference was then divided by 3.0 to estimate the percentage of fatty acids released from intracellular lipolysis that did not appear in the plasma compartment. Calculations were carried out using Excel (version 2007 software; Microsoft, Seattle, WA).
RESULTS
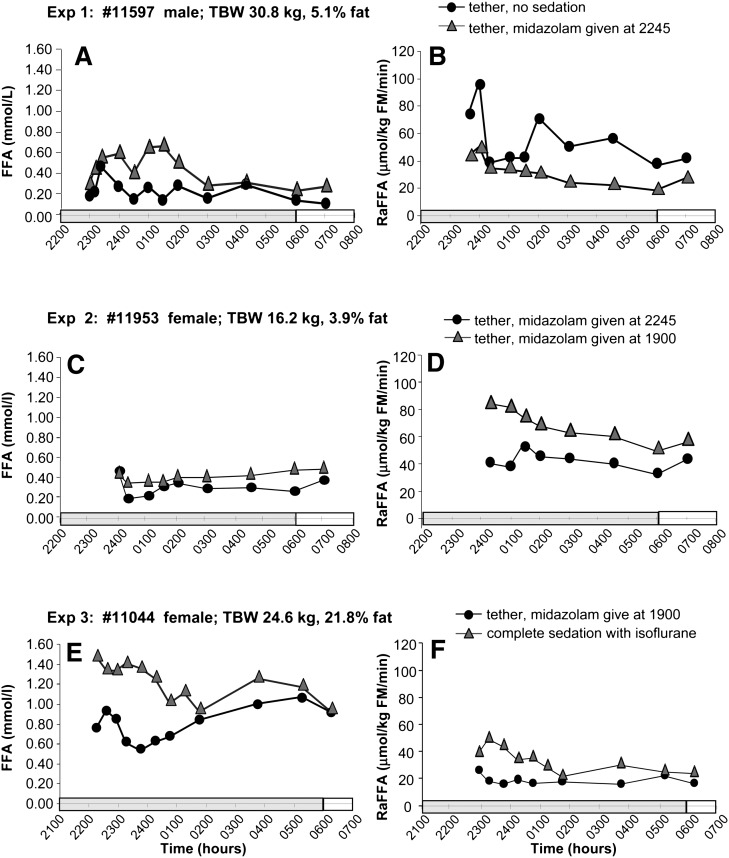
The clinical and laboratory values displayed in Table 2 reflect characteristics of adult baboons. Concentrations of total cholesterol, TG, and insulin were normal. Glucose disposal rates averaged 7.2 ± 0.6 mg/kg/min, which fell within the upper (insulin-sensitive) third and fourth quartiles, as defined by Chavez et al., who investigated insulin sensitivity in a group of baboons from the same colony (13). As described in Materials and Methods, Part 1 of the study tested various modes of animal management (with or without midazolam treatment vs. complete/no sedation) to determine their effect on the FFA concentration and RaFFA and the summary of these findings is shown in Table 1 (also see Table 2) The first animal studied underwent isotope infusions in the absence and presence of midazolam. With no midazolam, the animal's behavior was agitated, and the FFA concentration (Fig. 2A) and RaFFA (Fig. 2B) were variable. Treatment with midazolam, beginning at 2245 h, did not reduce the variability of the FFA concentrations, but turnover rates were steadier throughout the night. The animal was visibly quieter. Given these results, we also wondered whether administering the midazolam at 2245 h had allowed sufficient time for the drug to take effect before the measurements were made. Thus, in experiment 2, compared with midazolam begun at 2245 h, beginning the drug earlier in the evening at 1900 h resulted in an animal that was visibly calmer throughout the night and FFA concentrations that were less variable (Fig. 2C). RaFFA values were higher but approached similar values between 0600 and 0700 h in both experiments. In experiment 3, higher concentrations (Fig. 2E) and turnovers of FFA (Fig. 2F) were observed under isoflurane sedation than those with midazolam. We learned from this work that the use of midazolam gave similar steady-state concentrations and turnovers between 0600 and 0700 h and, importantly, resulted in calmer behavior in the animals. As a result of these studies, we chose a protocol using a midazolam bolus, 0.025 mg/kg, which was started at 1900 h, followed by a continuous infusion of 0.040 mg/kg/h.
Fig. 2.
Effects with or without midazolam treatment and complete/no sedation. Data are presented for 3 animals with nighttime plasma FFA concentrations in the graphs on the left (A, C, E) and FFA turnover rates in the graphs on the right (B, D, F). See Table 1 for details of interpretation.
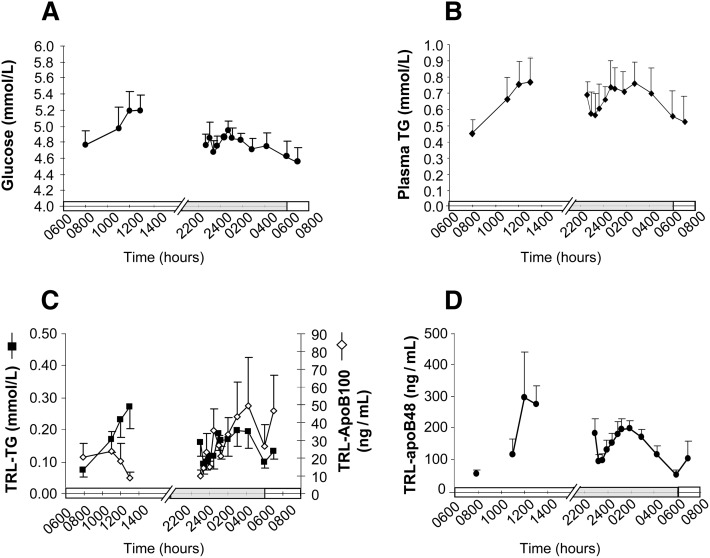
In Part 2 of this research, the animals were acclimated to food availability between 0800 and 1600 h to aid in the measurement of both fed and fasting metabolism. This protocol for monitoring and managing food intake resulted in tight control of body weights (supplementary Fig. I). From the date of sham tether placement through surgery and recovery after tether placement to the date of the metabolic infusion, the animals did not lose weight and only gained 0.33 ± 0.59 kg. Figure 3 shows the blood metabolite concentrations during the isotope infusion tests. The expected temporal patterns of metabolites were observed; e.g., after initiation of food consumption, the time-to-peak of glucose concentration was 4 h, and glucose concentrations remained relatively steady throughout the night and fell off after 0400 h (Fig. 3A). Concentrations of total plasma TG (Fig. 3B) and that in the TG-rich lipoproteins (chylomicrons and VLDL [Fig. 3C]), along with the apolipoprotein-B48 concentrations in these triacylglycerol-rich lipoproteins (TRL, Fig. 3D) rose steadily during the first 5 h of food intake. TRL-apoB100 concentration fell between 0800 and 1300 h (Fig. 3C). Throughout the day, TRL-TG represented 17%–35% of plasma TG, a ratio lower than that, 37%–67%, observed in humans (E. Parks, unpublished observations). During the night, concentrations of plasma TG, TRL-TG, TRL-apoB100, and TRL–B48 rose to peak between 0200 and 0400 h and then returned to baseline at 0600 h. The TRL-apoB48 concentrations rose and fell in a pattern similar to that of TRL-TG and apoB100 concentrations, suggesting that the elevation in TRL-TG observed between 0200 and 0400 h was partially due to the presence of chylomicrons in plasma (Fig. 3C). Serum cortisol concentrations were measured at four time points on a single day (see supplementary Fig. II). The values were within normal ranges and revealed typical circadian levels that fall throughout the day (22). These data suggest that the use of an acclimation phase successfully adapted the animals for study.
Fig. 3.
Concentrations of plasma glucose, total plasma-TG, TRL-TG, TRL-apoB100, and TRL-apoB48 during the 24h study. Data represent the means ± SEM for animals in Part 2 (n = 7): (A) glucose, (B) plasma-TG, (C) TRL-apoB100, and TRL-TG, and (D) TRL-apoB48. X-axis bars denote the light cycle (0600 to 1800 h) and the dark cycle (1800 to 0600 h) in the animal room.
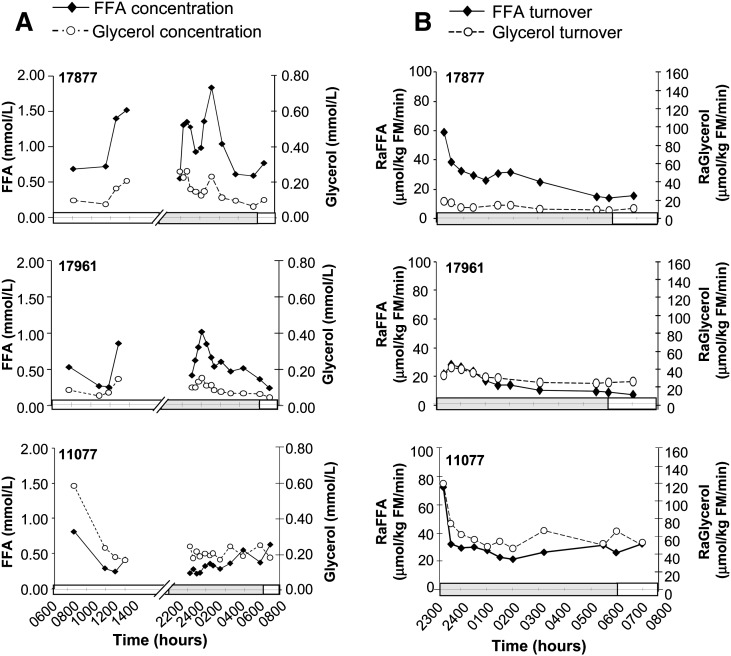
Day-long changes in plasma FFA and glycerol concentrations (Fig. 4A) demonstrated a postprandial pattern similar to that of the TG concentrations, rising as the animal began to eat. However, nighttime values, between 2300 and 0400 h, rose to levels found earlier in the day (1300 h). The nighttime peak occurred between 0100 and 0200 h, and then values fell toward morning. Among animals, FFA and glycerol concentrations were variable, yet, within a given animal, the changes in concentrations of FFA and glycerol mirrored one another. Shown in Fig. 4B are the turnover data for FFA and glycerol concentrations from 2300 to 0700 h (i.e., during the time of the isotopic infusions). The calculated values of appearance start higher for the first 2 h of the night because the FFA and glycerol pools have not sufficiently turned over to reach steady state. After 0100 h in all animals, the turnover rates of both metabolites approached a steady state, which was fully achieved at 0600 h. Again, the shapes of the FFA and glycerol turnover curves paralleled one another. The mean RaFFA in the baboons at steady state (Table 3) was 39.4 ± 29.8 μmol/kg fat mass (FM)/min, and RaGlycerol was 26.9 ± 7.3 μmol/kg FM/min. The ratios of FFA-to-glycerol turnover rates averaged 1.5 ± 0.8, and thus, it was estimated that 49.3% ± 25.1% of fatty acids were reesterified before leaving the adipose tissue. Baseline correlations between FFA turnover rates and body fat composition were explored to allow comparison to data available in humans. Negative correlations were found between the RaFFA and measures of body fat (see supplementary Fig. III), either per kilogram of fat mass (r = 0.699, P = 0.024, a relationship that may also be curvilinear), or waist circumference (r = 0.658, P = 0.039). In other words, those animals with the highest quantities of body fat had the lowest free fatty acid appearance rates in plasma.
Fig. 4.
Plasma concentrations and turnover rates of FFA and glycerol. Representative data from plasma concentrations of FFA and glycerol (A) and turnover rates (B) in three animals that had infusions of stable isotopes of fatty acid and glycerol from 2300 to 0700 h. The individual animal's identification number is in the upper left corner of each graph.
TABLE 3.
Metabolic parameters measured during isotope infusion studies
| Parameter | Unit | Mean ± SD | Range |
|---|---|---|---|
| Plasma FFA concentrationa | mmol/l | 0.553 ± 0.138 | 0.215–1.224 |
| Plasma glycerol concentrationb | mmol/l | 0.157 ± 0.032 | 0.093–0.222 |
| RaFFAa | μmol/kg FM/min | 39.4 ± 29.8 | 9.2–74.3 |
| RaGlycerolb | μmol/kg FM/min | 26.9 ± 7.3 | 9.7–53.9 |
| RaFFA:RaGlycerol ratiob | — | 1.5 ± 0.8 | 0.4–2.4 |
| FFA reesterification rateb | % | 49.3 ± 25.1 | 19.5–88.0 |
Values are means ± SD and represent averages of steady-state values between 0500 and 0700 h. See Materials and Methods for details of reesterification calculation.
n = 7.
n = 5.
DISCUSSION
The objective of the present work was to modify and optimize human (7) and rodent (8) protocols to establish clinical procedures for the measurement of blood fatty acid and glycerol fluxes in vivo in a nonhuman primate model. In order to obtain baseline data to assess the animals’ recoveries after implantation of the tether catheter, we first carefully monitored the food intake and body weight of animals as they were housed separately in cages in the clinical unit. Food was routinely delivered once per day in the morning. We found the natural timing of food intake to vary widely among the animals throughout the day. As is well appreciated by veterinary staff, most baboons would consume the daily ration of food during the daylight hours, but some animals would eat all of it beginning immediately when it was presented, while others would spread out their intake throughout the 24 h period. Acclimating the animals to a regimen of food availability between 0800 and 1800 h allowed the animals to adjust to consumption of all their food during this period, and, within an hour of the food's arrival in the morning, all animals were seen to be actively eating. We have used a similar protocol in mice previously (23), and the results are that blood draws could be timed specifically to test postprandial metabolism in the morning and that studies performed at night would assess the animal's metabolism as it transitioned to the fully fasted state the next morning (i.e., avoiding active nighttime eating). In human studies of fatty acid turnover, research subjects are frequently asked to fast for long periods of time so that metabolism can be measured in a steady state (16, 24). To achieve this same end in the baboon, we did not feel we could restrict food for a lengthy period because it would result in a situation disadvantageous for both the animal (under stress) and the research (erratic and various metabolic states among animals). The blood draws planned at night necessitated a technician entering the room containing the cage, and the use of midazolam is a limitation of this study, in that it may have had some unforeseen physiologic effects. However, the low-dose infusion of the drug midazolam, with its anxiolytic effects, allowed us to perform the overnight isotope infusions with less recorded stress to the animal and steady levels of metabolites. From these blood samples, lipoprotein particles were isolated, and the plasma FFA and glycerol molecules were extracted from the infranatant. This methodology maximized the number of metabolites that could be analyzed using the minimum amount of blood drawn at each time point. Thus, the final protocol developed during the method development phase demonstrated maintenance of food intake and body weight, limited the stress on the animal, and allowed sufficient blood sampling to achieve measurements of glucose, insulin, cortisol, plasma-TG, TRL-TG, apolipoproteins, FFA, and glycerol concentrations and the turnover of these latter metabolites as well.
The long-term energy reserve in mammals is stored as adipose TG, and the release of that energy through the process of lipolysis is a metabolic event of great importance to the maintenance of health. Lipolysis is found to be dysregulated in disease (2, 3). When both FFA and glycerol turnovers are measured, the ratio of plasma FFA to glycerol turnovers has been used as a means to estimate intra-adipocyte reesterification (4–6). There are clear limitations to assumptions at the basis of these calculations, including the potential for adipocyte glyceroneogenesis (25), the fact that fatty acids and glycerol from dietary TG breakdown contribute to the plasma FFA or glycerol pools (26), and lack of information on the activities of intracellular acyltransferases which may allow some diacylglycerol or monoacylglycerol species to reform TG (intracellular recycling) such that total lipolytic rate is not measureable, based on observation of turnover of plasma pools (27). Nonetheless, in vivo calculations of lipolysis and reesterification have provided important data to expand the understanding of mechanisms of many diseases, including that of body mass wasting (28), the impact of smoking on adipose metabolism (29), and the effects of acute exercise on energy balance (30), to name a few. Recently, the advantages of studying nonhuman primates to understand metabolic diseases have also been recognized (1, 13, 14). Given the intense focus on the role of adipose tissue-mediated insulin resistance in the development of obesity and type 2 diabetes (31), methodologies to quantitate fatty acid flux in vivo have become key tools in the study of adipose metabolism. Using the present methodology, we have established baseline values for a number of important variables in the baboon study that correspond well with those in the human literature.
The first novel observation here relates to changes in plasma FFA and glycerol concentrations after a meal. Changes of both metabolites result from a balance of suppression of adipose lipolysis by insulin and spillover of chylomicron fatty acids and glycerol liberated from lipoprotein-TG by the action of lipoprotein lipase (32–34). In the present study, during the postprandial period, 4/5 animals exhibited elevations in fatty acid and glycerol concentrations (net spillover) similar to that observed by us previously (35). However, one animal (11077) had reduced FFA concentrations postprandially (Fig. 4A). It is of note that this animal had the lowest fasting glucose concentrations and had the greatest lean mass of the group. In a pilot study in human subjects, we have recently shown that greater lean mass predicts lower appearance of dietary fatty acids in the plasma FFA pool after a meal (36). If this is the case in mammals generally, then clearance of dietary fatty acids to muscle (less spillover), along with clearance to adipose (37) in the postprandial state, may be an important mechanism to avoid ectopic deposition of lipid in other tissues. The present methodology would support testing of such a hypothesis.
Second, this study documented the fact that the turnover of FFA and glycerol do not come to steady state until morning, a fact also recently recognized in humans (10). In the present protocol, which provided ad libitum food intake during 8 daylight h and no food availability after 1600 h, the baboon's fatty acid and glycerol concentrations peaked in the middle of the night and then slowly approached a fasted value between 0500 and 0600 h. The appearance rates in plasma also became stable at this time, allowing for accurate kinetic appearance and disappearance rates to be calculated. Our third finding, a RaFFA of 39.4 ± 29.8 μmol/kg FM/min, is a value slightly faster than that observed in healthy, lean humans of ∼28 µmol/kg FM/min (4, 16). RaGlycerol in the baboons was 26.9 ± 7.3 μmol/kg FM/min, which appears significantly higher than that reported for humans, averaging 7.3 μmol/kg FM/min, with a range of 6.2–21.0 μmol/kg FM/min (4, 5, 38). We present individual data for animals because, to our knowledge, studies of this kind have not been attempted before in the baboon. The RaFFA turnover data demonstrate peaks and valleys during the early morning hours, and we see similar nocturnal variability in human (E. J. Parks, unpublished observations). These data alone might suggest that variances could be due to technical factors; however, the level of enrichments achieved during the isotope infusions were well within the range for accurate detection. Furthermore, the patterns of RaGlycerol data mirrored the RaFFA well, and the curves of FFA and glycerol concentration also varied in parallel. These observations support the concept that our findings represent true physiologic flux. This concept is also strengthened by the fact that the measurements of the metabolites were made independently, using different plasma extraction procedures, derivatization reagents, and separate GC-MS analysis protocols. Thus, the increasing and decreasing fluxes observed here are likely due to circadian rhythm in the baboon. The precise metabolic control systems that resulted in a rise in TRL-TG, apoB, FFA, and glycerol between midnight and 0500 h will be important to determine in future studies.
Last, the ratio of FFA to glycerol turnover rates at steady state was 1.5 ± 08, and thus, it was estimated that 49% of fatty acids were reesterified before leaving the adipose tissue. These data are slightly higher than the average taken from human literature, 34% (4, 5, 39), but fall within the range reported in these studies (17%–56%). We also found that animals with the greatest FM demonstrated the lowest fatty acid release rates, as also observed in humans (40). These data add support for the use of this animal model to investigate metabolic dysregulation. In summary, we present a detailed protocol for the measurement of adipose substrate flux in the baboon, and to our knowledge, these are the first data to document these fluxes in this animal, demonstrating nocturnal patterns of metabolism. These methods will support needed research to determine how adipose insulin resistance predisposes to the development of chronic diseases using the overweight baboon as a model. Such studies will allow testing of the in vivo mechanisms by which weight loss and pharmacologic treatments can aid in the treatment of metabolic disorders (11).
Supplementary Material
Acknowledgments
The authors thank the veterinarian technicians and the staff at the Southwestern National Primate Center for their excellent care of the animals and flexibility during the development of these procedures. In addition, we are grateful to Michael Adams at UTSW and Vicki Mattern at SFBR for expert technical assistance, to Dr. Agneta Sunehag at Baylor College of Medicine for advice on the mass spectrometry measurement of glycerol, and to Dr. Tony Comuzzie at SFBR for invaluable advice and guidance.
Footnotes
Abbreviations:
- BW
- body weight
- DEXA
- dual-energy X-ray absorptiometry scan
- FM
- fat mass
- RaFFA
- rate of appearance of plasma FFA
- RaGlycerol
- rate of appearance of plasma glycerol
- TBW
- total body weight
- TG
- triacylglycerol
- TRL
- triacylglycerol-rich lipoprotein
This study was funded by an unrestricted grant from Sanofi-Aventis, and the investigation was conducted in facilities constructed with support from the Research Facilities Improvement Program grant C06 RR (no. 014578, 013556, 015456, and 017515) from the National Center for Research Resources, National Institutes of Health, and with support from National Institutes of Health Grants PO1 HL028972 and P51 RR013986. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Comuzzie A. G., Cole S. A., Martin L., Carey K. D., Mahaney M. C., Blangero J., VandeBerg J. L. 2003. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes. Res. 11: 75–80. [DOI] [PubMed] [Google Scholar]
- 2.Unger R. H., Clark G. O., Scherer P. E., Orci L. 2010. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta. 1801: 209–214. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo R. A. 2010. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 53: 1270–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen M. D. 1999. Regional glycerol and free fatty acid metabolism before and after meal ingestion. Am. J. Physiol. 39: E863–E869. [DOI] [PubMed] [Google Scholar]
- 5.Klein S., Young V. R., Blackburn G. L., Bistran B. R., Wolfe R. R. 1986. Palmitate and glycerol kinetics during brief starvation in normal weight young adult and elderly subjects. J. Clin. Invest. 78: 928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W., Basinger A., Neese R. A., Christiansen M., Hellerstein M. K. 2000. Effects of nicotinic acid on fatty acid kinetics, fuel selection, and pathways of glucose production in women. Am. J. Physiol. Endocrinol. Metab. 279: E50–E59. [DOI] [PubMed] [Google Scholar]
- 7.Wolfe R. R. 1992. Radioactive and Stable Isotope Tracers in Biomedicine. Wiley and Sons, New York. [Google Scholar]
- 8.Grefhorst A., Parks E. J. 2009. Reduced insulin-mediated inhibition of VLDL secretion upon pharmacological activation of the liver X receptor in mice. J. Lipid Res. 50: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coelho A. M. J., Carey K. D. 1990. A social tethering system for nonhuman primates used in laboratory research. Lab. Anim. Sci. 40: 388–394. [PubMed] [Google Scholar]
- 10.Miles J. M., Wooldridge D., Grellner W. J., Windsor S., Isley W. L., Klein S., Harris W. S. 2003. Nocturnal and postprandial free fatty acids kinetics in normal and type 2 diabetes subjects. Diabetes. 52: 675–681. [DOI] [PubMed] [Google Scholar]
- 11.Langin D. 2006. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol. Res. 53: 482–491. [DOI] [PubMed] [Google Scholar]
- 12.Horber F. F., Krayer S., Miles J. M., Cryer P., Rehder K., Haymond M. W. 1990. Isoflurane and whole body leucine, glucose, and fatty acid metabolism in dogs. Anesthesiology. 73: 82–92. [DOI] [PubMed] [Google Scholar]
- 13.Chavez A. O., Gastaldelli A., Guardado-Mendoza R., Lopez-Alvarenga J. C., Leland M. M., Tejero M. E., Sorice G., Casiraghi F., Davalli A., Bastarrachea R. A., et al. 2009. Predictive models of insulin resistance derived from simple morphometric and biochemical indices related to obesity and the metabolic syndrome in baboons. Cardiovasc. Diabetol. 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez A. O., Lopez-Alvarenga J. C., Tejero M. E., Triplitt C., Bastarrachea R. A., Sriwijitkamol A., Tantiwong P., Voruganti V. S., Musi N., Comuzzie A. G., et al. 2008. Physiologic and molecular determinants of insulin action in the baboon. Diabetes. 57: 899–908. [DOI] [PubMed] [Google Scholar]
- 15.Nutrient Requirements of Nonhuman Primates. 2003. 2nd edition Ad Hoc Committee on Nonhuman Primate Nutrition, National Research Council of the National Academy of Science. National Academies Press, Washington, DC. [Google Scholar]
- 16.Parks E. J., Krauss R. M., Christiansen M. P., Neese R. A., Hellerstein M. K. 1999. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production and clearance. J. Clin. Invest. 104: 1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timlin M. T., Barrows B. R., Parks E. J. 2005. Increased dietary substrate delivery alters hepatic fatty acid recycling in healthy men. Diabetes. 54: 2694–2701. [DOI] [PubMed] [Google Scholar]
- 18.Kleiber M. 1987. The Fire of Life: An Introduction to Animal Energetics. Robert E. Krieger Publishing Co, Malabar, FL. [Google Scholar]
- 19.Kotite L., Bergeron N., Havel R. J. 1995. Quantification of apolipoproteins B-100, B-48, and E in human triglyceride-rich lipoproteins. J. Lipid Res. 36: 890–900. [PubMed] [Google Scholar]
- 20.Havel R. J., Eder H. A., Bragdon J. H. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunehag A., Treuth M. S., Toffolo G., Butte N. F., Cobelli C., Bier D. M., Haymond M. W. 2001. Glucose production, gluconeogenesis, and insulin sensitivity in children and adolescents: An evaluation of their reproducibility. Pediatr. Res. 50: 115–123. [DOI] [PubMed] [Google Scholar]
- 22.Van Cauter E. 2001. Endocrine rhythms In Principles and Practice of Endocrinology and Metabolism. Becker K. L., editor Lippincott Willams & Wilkins, Philadelphia: 56–64. [Google Scholar]
- 23.Parks E. J., Schneider T. L., Baar R. A. 2005. Meal-feeding studies in mice: effects of different diets on blood lipids and energy expenditure. Comp. Med. 55: 13. [PubMed] [Google Scholar]
- 24.Patterson B. W., Mittendorfer B., Elias N., Satyanarayana R., Klein S. 2002. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J. Lipid Res. 43: 223–233. [PubMed] [Google Scholar]
- 25.Reshef L., Hanson R. W., Ballard F. J. 1970. A possible physiological role for glyceroneogenesis in rat adipose tissue. J. Biol. Chem. 245: 5979–5984. [PubMed] [Google Scholar]
- 26.Coppack S. W., Fisher R. M., Gibbons G. F., Humphreys S. M., McDonough M. J., Potts J. L., Frayn K. N. 1990. Postprandial substrate deposition in human forearm and adipose tissues in vivo. Clin. Sci. 79: 339–348. [DOI] [PubMed] [Google Scholar]
- 27.Kim J., Saidel G. M., Kalhan S. C. 2008. A computational model of adipose tissue metabolism: evidence for intracellular compartmentation and differential activation of lipases. J. Theor. Biol. 251: 523–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strawford A., Hoh R., Neese R. A., Parks E. J., Turner S., Hellerstein M. K. 1999. A placebo-controlled trial of the effects of combining megestrol-acetate with testosterone replacement therapy in AIDS-wasting syndrome. J. Am. Med. Assoc. 281: 1282–1290. [Google Scholar]
- 29.Hellerstein M. K., Benowitz N. L., Neese R. A., Hoh R., Jacob P., Fong I., Hsieh J., Faix D. 1994. Effects of cigarette smoking and its cessation on lipid metabolism and energy expenditure in heavy smokers. J. Clin. Invest. 93: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfe R. R., Klein S., Carraro F., Weber J. 1990. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am. Physiol. Soc. 258: E382–E389. [DOI] [PubMed] [Google Scholar]
- 31.Cusi K. 2010. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr. Diab. Rep. 10: 306–315. [DOI] [PubMed] [Google Scholar]
- 32.Miles J. M., Park Y. S., Walewicz D., Russell-Lopez C., Windsor S., Isley W. L., Coppack S. W., Harris W. S. 2004. Systemic and forearm triglyceride metabolism: fate of lipoprotein lipase-generated glycerol and free fatty acids. Diabetes. 53: 521–527. [DOI] [PubMed] [Google Scholar]
- 33.Barrows B. R., Parks E. J. 2006. Contributions of different fatty acid sources to VLDL-triacylglycerol in the fasted and fed-states. J. Clin. Endocrinol. Metab. 91: 1446–1452. [DOI] [PubMed] [Google Scholar]
- 34.Barrows B. R., Timlin M. T., Parks E. J. 2005. Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylglycerol under two different feeding regimens. Diabetes. 54: 2668–2673. [DOI] [PubMed] [Google Scholar]
- 35.Donnelly K. L., Smith C. I., Schwarzenberg S. J., Jesserun J., Parks E. J. 2005. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 115: 1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belzer L. M., Parks E. J. 2010. Metabolic parameters impacting diet-derived non-esterified fatty acids. Triglyceride and Triglyceride-Rich Particles in Health and Disease. Keystone Symposium; Big Sky, MT. [Google Scholar]
- 37.Coppack S. W., Evans R. D., Fisher R. M., Frayn K. N. 1992. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism. 41: 264–272. [DOI] [PubMed] [Google Scholar]
- 38.Siler S. Q., Neese R. A., Parks E. J., Hellerstein M. K. 1998. VLDL-triglyceride production after alcohol ingestion, studied using [2-13C1] glycerol. J. Lipid Res. 39: 2319–2328. [PubMed] [Google Scholar]
- 39.Beylot M., Martin C., Beaufrere B., Riou J. P., Mornex R. 1987. Determination of steady state and nonsteady-state glycerol kinetics in humans using deuterium-labeled tracer. J. Lipid Res. 28: 4. [PubMed] [Google Scholar]
- 40.McQuaid S. E., Hodson L., Neville M. J., Dennis A. L., Cheeseman J., Humphreys S. M., Ruge T., Gilbert M., Fielding B. A., Frayn K. N., et al. 2011. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes. 60: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.