Abstract
Short-term facilitation and depression coexist at many CNS synapses. Facilitation, however, has not been fully characterized at hair cell synapses. Using paired recordings and membrane capacitance measurements we find that paired-pulse plasticity at an adult frog auditory hair cell synapse depends on pulse duration and interpulse intervals. For short 20 ms depolarizing pulses, and interpulse intervals between 15 and 50 ms, facilitation occurred when hair cells were held at −90 mV. However, hair cells held at −60 mV displayed only paired-pulse depression. Facilitation was dependent on residual free Ca2+ levels because it was greatly reduced by the Ca2+ buffers EGTA and BAPTA. Furthermore, low external Ca2+ augmented facilitation, whereas depression was augmented by high external Ca2+, consistent with depletion of a small pool of fast releasing synaptic vesicles. Recovery from depression had a double-exponential time course with a fast component that may reflect the rapid replenishment of a depleted vesicle pool. We suggest that hair cells held at more depolarized in vivo-like resting membrane potentials have a tonic influx of Ca2+; they are thus in a dynamic state of continuous vesicle release, pool depletion and replenishment. Further Ca2+ influx during paired-pulse stimuli then leads to depression. However, at membrane potentials of −90 mV, ongoing release and pool depletion are minimized, so facilitation is revealed at time intervals when rapid vesicle pool replenishment occurs. Finally, we propose that vesicle pool replenishment kinetics is not rate limited by vesicle endocytosis, which is too slow to influence the rapid pool replenishment process.
Introduction
Auditory hair cell synapses have some distinctive features that set them apart from conventional synapses. Instead of being triggered by all-or-none action potential spikes, glutamate is continuously released with remarkable temporal precision by graded membrane potential changes in hair cells. The amplitude, duration, and frequency of sound are thus encoded by the patterns of glutamate-evoked spikes in the afferent fiber. Hair cells also have unique electron-dense disc-like structures, called synaptic ribbons, where exocytosis occurs preferentially (Zenisek et al., 2003). Although the functions of synaptic ribbons at hair cells are not completely understood, they may promote a highly synchronous form of multivesicular release (Glowatzki and Fuchs, 2002).
Short-term plasticity of hair cell synapses can be critical for auditory function. Ongoing facilitation may enhance the accuracy of signal onset with minimum delay, while short-term depression may help to match the stimuli strength for limited dynamic ranges of spiking, a process known as adaptation (Chimento and Schreiner, 1991; Spassova et al., 2004). The mechanisms that underlie paired-pulse facilitation and depression vary at different synapses, and the observed behavior is a mix of the offsetting strengths of these influences. In general, at synapses with a high probability of release, depression dominates, while synapses with a low release probability tend to show facilitation (Thomson, 2000). It has been widely accepted that residual calcium from previous stimuli causes activity-dependent facilitation (Katz and Miledi, 1968), although another possible mechanism is saturation of local calcium buffers during the stimulus in a pair of stimuli (Klingauf and Neher, 1997). In contrast, depletion of a readily releasable pool of vesicles can cause synaptic depression (von Gersdorff and Matthews, 1997). In addition, postsynaptic receptors can be desensitized or saturated by repetitive exposure to neurotransmitter release (Trussell et al., 1993).
Previous studies of short-term plasticity using ribbon-type synapses generally showed only short-term depression, though experimental conditions such as intensity of stimulus, concentrations of external calcium, and internal calcium buffer varied greatly (Edmonds et al., 2004; Rabl et al., 2006). The recovery rate of depression was generally fast with short and/or weak stimulus, and it was slower as the stimulus became longer and/or stronger. However, facilitation has not been so evident at ribbon-type synapses perhaps because it is masked by the strong synaptic depression.
Here we use the amphibian papilla of adult bullfrogs (Rana catesbeiana) to study short-term plasticity at mature hair cell synapses (Keen and Hudspeth, 2006; Li et al., 2009). At this preparation both the presynaptic hair cells and connecting postsynaptic afferent fibers are exposed and simultaneous patch-clamp recordings are possible. Our results show that hair cell synapses can show both paired-pulse depression and facilitation depending on the durations of the depolarizing pulse and/or interpulse intervals and on the hair cell holding potential. Furthermore, we propose that the phenomena of recovery from forward masking observed with in vivo fast-adapting frog afferent fiber recordings can be explained by the hair cell's recovery rate from paired-pulse depression (Megela and Capranica, 1982; Smotherman and Narins, 2000).
Materials and Methods
Hair cell preparation.
Following an Oregon Health & Science University (Institutional Animal Care and Use Committee)-approved animal care protocol, amphibian papillae were carefully dissected from adult female or male bullfrogs (R. catesbeiana) that had been sedated in ice water bath, double-pithed, and decapitated. Amphibian papillae were placed in a recording chamber with oxygenated artificial perilymph containing (in mm): 95 NaCl, 2 KCl, 2 CaCl2, 1 MgCl2, 25 NaHCO3, 3 glucose, 1 creatine, 1 Na-pyruvate, pH adjusted to 7.30 with NaOH, and continuously bubbled with 95% O2 and 5% CO2 (230 mOsm). Semi-intact preparations of hair cells and their connecting afferent fibers were obtained as described by Keen and Hudspeth (2006) and Li et al. (2009). During the recordings, the preparation was perfused continuously (2–3 ml/min) with oxygenated artificial perilymph.
Electrophysiology.
For whole-cell recordings, patch pipettes of borosilicate glass were pulled to resistances of 5–6 MΩ for hair cells and 7–10 MΩ for afferent fibers. Unless described otherwise, pipettes were filled with the internal solution containing (in mm): 77 Cs-gluconate, 20 CsCl, 1 MgCl2, 10 tetraethylammonium chloride, 10 HEPES, 2 EGTA, 3 Mg-ATP, 1 Na-GTP, and 5 Na2-phosphocreatine (adjusted to pH 7.3 with CsOH). Perforated patch recordings were made with nystatin (250 μg/ml, 0.4% DMSO). Patch-clamp recordings were performed with a double EPC-9/2 (HEKA Elektronik) patch-clamp amplifier and Pulse software (HEKA) at room temperature. Hair cells and connecting afferent fibers were viewed with differential interference contrast microscopy through a 60× water-immersion objective lens (Olympus) and CCD camera (XC-75; Sony).
Hair cells were held at a resting membrane potential of either −60 or −90 mV and afferent fibers were held at −90 mV. Membrane potentials were corrected for a liquid junction potential of 10 mV. The averaged uncompensated series resistances (Rs) in whole-cell recordings were 12.6 ± 0.3 MΩ for 30 hair-cell recordings and 29.5 ± 1.3 MΩ for 25 afferent-fiber recordings. Rs was compensated in some afferent fiber recordings by up to 60% (n = 10), however we did not use electronic series resistance compensation for most of the recordings because this added noise to the recordings and often resulted in the loss of the fiber recording. Therefore, an off-line Rs compensation procedure was performed for peak EPSC values following the methods outlined by Traynelis (1998) and Leão et al. (2005). Each peak current was scaled by a factor, K, that corrects for the loss of driving force due to series resistance errors. The factor K = (Vc − Vrev)/(Vc − I · Rs − Vrev), where Vc is the command potential, Vrev is the calculated reversal potential (Vrev = 0 for AMPA receptor-mediated EPSCs), and I is the current at any point in time and Rs is the uncompensated series resistance. As expected (Leão et al., 2005), the average recovery time constants of peak EPSCs from synaptic depression tended to be slightly slower after this off-line Rs compensation procedure, but this difference was not statistically significant (data not shown). The average EPSC recovery time course data shown here were thus corrected for any artificial speeding-up of the time constants due to series resistance errors. The current signal was low-pass filtered at 2 kHz and sampled at 10 μs intervals.
Capacitance measurements.
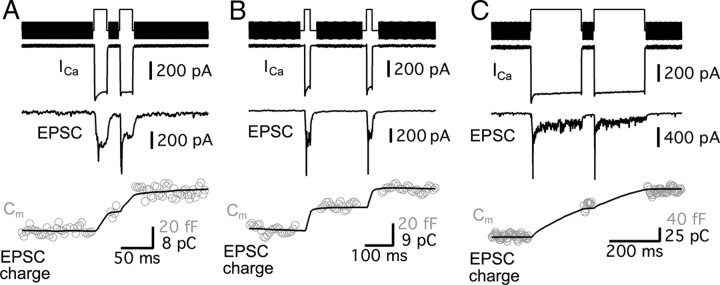
The measurements of the whole-cell membrane capacitance (Cm) from hair cells were performed under voltage-clamp with the “Sine + DC” method (Lindau and Neher, 1988; Gillis, 2000) using a double EPC-9/2 (HEKA Elektronik) patch-clamp amplifier and Pulse software (HEKA). Patch pipettes were coated with dental wax to minimize their stray capacitance and to achieve better C-fast compensation. Sine waves (50 mV peak-to-peak, 1 kHz) were superposed on the holding potential, −90 mV, and the resulting current response was used to calculate Cm via a Pulse software emulator of a lock-in amplifier (Gillis, 2000). The increase of Cm (ΔCm), evoked by membrane depolarization, was measured as ΔCm = Cm (response) − Cm (baseline) and was used as a measure of synaptic vesicle exocytosis from hair cells. An average of Cm (response) and Cm (baseline) was obtained by averaging capacitance data points before and after the depolarizing pulse, and the amount of points used depended on the interpulse interval for paired pulses.
Data analysis.
Data analysis was performed with Igor Pro software (Wavemetrics) and Prism (GraphPad Software). Statistical significance was assessed with paired and unpaired Student's t tests with p < 0.05 considered significant. Data are expressed as mean ± SEM.
Results
Short-term plasticity at hair cell synapses
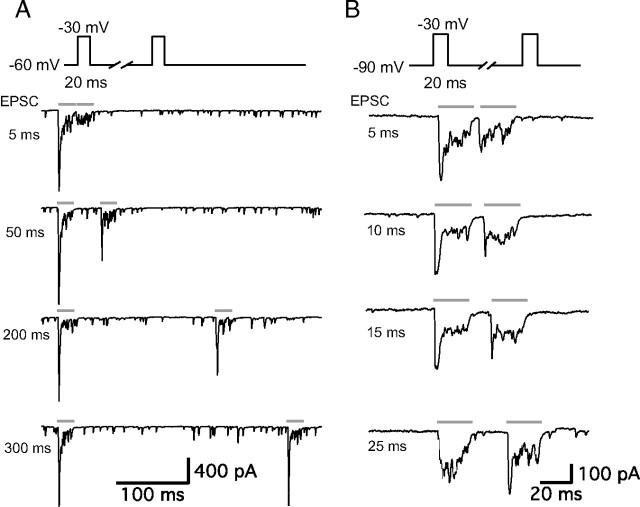
We studied short-term plasticity using a pair of pulses in adult auditory hair cell synapses from the bullfrog amphibian papilla. Hair cells were stimulated by a pair of 20 ms depolarizing pulses from a holding potential of −60 to −30 mV with various interpulse intervals (from 3 to 500 ms) and EPSCs were recorded from the connected postsynaptic afferent fibers (Fig. 1). EPSCs during the depolarizing pulse consisted of a fast, transient component and a smaller sustained component. As the interpulse interval was made shorter, the peak amplitude of the second EPSC became smaller (Fig. 1A). With these stimulating conditions and protocols, hair cell synapses displayed severe paired-pulse depression.
Figure 1.
Paired-pulse depression and facilitation of afferent fiber EPSCs. Paired recordings of EPSCs from an afferent fiber and a connected hair cell that was depolarized by a pair of 20-ms-long pulses (gray bars) from holding potentials of −60 mV (A) or from −90 mV (B) to −30 mV with various interpulse intervals. A, The stimulation protocol (top trace) and EPSCs with 5, 50, 200, and 300 ms interpulse intervals. The hair cell had a resting potential of −60 mV. Note the severe synaptic depression of the second EPSC for a 5 ms interpulse interval and the gradual recovery of the peak EPSC. B, The stimulation protocol (top trace) and EPSCs with 5, 10, 15, and 25 ms interpulse intervals. The hair cell had a resting potential of −90 mV. Note the faster recovery time course from depression of the EPSCs and the facilitation of the second peak EPSC at the 25 ms interpulse interval. Spontaneous EPSCs were also less frequent at a hair cell holding potential of −90 mV than at −60 mV.
Although −60 mV is closer to the physiological in vivo resting membrane potential of auditory hair cells (Crawford and Fettiplace, 1980; Pitchford and Ashmore, 1987), at this membrane potential significant Ca2+ influx and glutamate release occur (Li et al., 2009). To uncover the underlying mechanisms of short-term plasticity in the absence of this continuous Ca2+ influx, we also used −90 mV as a holding potential for the presynaptic hair cells. When we stimulated hair cells, using a pair of 20 ms pulses from −90 to −30 mV, with various interpulse intervals (from 3 to 500 ms), hair cell synapses showed both paired-pulse depression and facilitation depending on the interpulse interval (Fig. 1B). At short interpulse intervals, paired-pulse depression dominated and EPSCs evoked by the second pulse usually showed only a small fast peak, whereas for interpulse intervals from 15 to 50 ms facilitation was observed (Fig. 1B). Note also that for hair cells held at −90 mV the recovery time from paired-pulse depression was greatly accelerated (Fig. 1B), a finding that may help to explain frequency selectivity of exocytosis in frog saccular hair cells, where a short hyperpolarizing gap in a depolarizing step enhances the overall amount of exocytosis (Rutherford and Roberts, 2006). Full recovery of the EPSC peak was evident at interpulse intervals of <20 ms for hair cells held at −90 mV, whereas recovery was not complete for hair cells held at −60 mV even after interpulse intervals of 200 ms (Fig. 1A,B).
We next calculated the paired-pulse ratio (EPSC2/EPSC1) from the peak amplitude of EPSCs. For hair cells held at −60 mV, at a 3 ms interpulse interval the averaged paired-pulse EPSC peak ratio was 0.23 ± 0.09 (n = 9), whereas for hair cells held at −90 mV, this averaged paired-pulse EPSC peak ratio was 0.45 ± 0.06 (n = 7) (Fig. 2A,B). EPSC peaks thus undergo more severe depression at very short interpulse intervals when hair cells are held at −60 mV. As the interpulse interval became longer, the paired-pulse ratio recovered and for a certain range of interpulse intervals, such as 20 or 50 ms, the peak amplitude of the second EPSC became even bigger than the first EPSC peak amplitude for hair cells held at −90 mV (Fig. 2B). With a 20 ms interpulse interval, the averaged paired-pulse ratio was 1.26 ± 0.05 (n = 8), and with a 50 ms interpulse interval, the averaged paired-pulse ratio was 1.37 ± 0.06 (n = 8) (Fig. 2B). Paired-pulse facilitation dwindled as the interpulse interval became even longer and the averaged paired-pulse ratio eventually approached 1 (Fig. 2B). The recovery of peak EPSC paired-pulse depression for hair cells held at −60 mV could be fit with a double exponential function with fast (τf = 15.0 ms; 64%) and slow (τs = 581 ms; 36%) time constants, which produces a median time constant τmedian = 213 ms (Fig. 2A). For hair cells held at −90 mV, the averaged paired-pulse ratio with interpulse interval from 3 ms (paired-pulse ratio 0.45 ± 0.06) to 50 ms (paired-pulse ratio 1.37 ± 0.06) increased as a single exponential function with 10.9 ms time constant (Fig. 2B).
Figure 2.
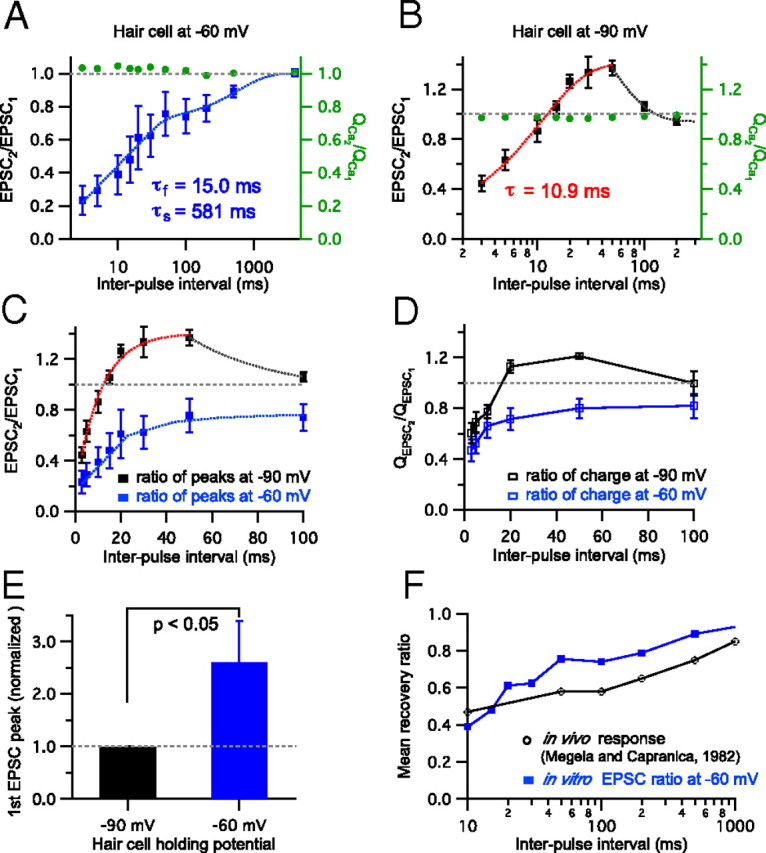
Recovery from paired-pulse depression and facilitation. EPSCs were recorded from the afferent fiber while a connected hair cell was depolarized by a pair of 20 ms long pulses with various interpulse intervals. The Ca2+ influx charge ratio mediated by hair-cell open Ca2+ channels during the pulse was calculated by integrating the Ca2+ currents. A, The paired-pulse peak EPSC ratio with hair cells held at −60 mV and interpulse intervals varied from 3 ms to 4 s. Data were plotted with a logarithmic scale on interpulse interval (n = 4–9 paired recordings per data point). The EPSC paired-pulse ratio recovered exponentially with fast (τf = 15.0 ms; 64%) and slow (τs = 581 ms) time constants (the blue dashed curve shows the exponential fits). The gray dashed line indicates that the ratio is 1 (EPSC2 = EPSC1). The ratio of Ca2+ charge (QCa2/QCa1) was ∼1 (0.99–1.05; green, right axis). B, The EPSC peak ratios with hair cells held at −90 mV (n = 4–8). The red dashed line shows a single exponential fit. From 3 ms to 50 ms intervals, the paired-pulse ratio increased exponentially (τ = 10.9 ms). QCa2/QCa1 was relatively constant (0.96–0.99; green, right axis). C, The relationship between EPSC peak ratios and interpulse intervals (3–100 ms) when hair cells were held at −90 mV (n = 4–8) and at −60 mV (n = 5–9). D, The relationship between EPSC charge ratios and interpulse intervals (3–100 ms) when hair cells were held at −90 mV (n = 6) and at −60 mV (n = 4–6). E, The peak amplitudes of the first EPSCs for hair cells held at −60 mV were normalized to the first EPSCs at −90 mV. For seven paired recordings, the normalized peak of the first EPSC at −60 mV increased significantly (2.6 ± 0.8; n = 7). F, Paired-pulse depression of hair cells held at −60 mV (blue filled square; same data as in A) shows recovery rates similar to that of in vivo fast-adapting afferent fiber spikes recovering from a pure tone sound stimulus in R. pipiens frogs (black open square; modified with permission from Megela and Capranica, 1982).
We also checked whether Ca2+ influx through the L-type calcium channels on hair cells contributed to paired-pulse depression and facilitation. We calculated Ca2+ charge (QCa) by integrating Ca2+ currents evoked by a pair of pulses. Both at −60 mV and at−90 mV, the ratio of second and first QCa (QCa2/QCa1) was consistently very close to 1 regardless of paired-pulse depression or facilitation (Fig. 2A,B). This indicates that Ca2+ current inactivation or facilitation does not contribute significantly to paired-pulse plasticity at hair cell synapses.
We next compared the paired-pulse ratios for hair cells held at −60 and −90 mV (Fig. 2C). When we held hair cells at −90 mV, hair cell synapses showed first paired-pulse depression for very short intervals, a brief overshoot period of paired-pulse facilitation, and eventually a recovery back to 1 within ∼100 ms. In contrast, paired-pulse depression generally dominated for all ranges of the interpulse intervals when hair cells were held at −60 mV (Fig. 2A,C). Furthermore, hair cells held at −60 mV showed a significantly stronger initial paired-pulse depression for short intervals, such as 3 or 5 ms, than for hair cells held at −90 mV, and the recovery rate of paired-pulse depression was faster at −90 mV than at −60 mV (Fig. 2C). When we also calculated the paired-pulse ratio from EPSC charge transfers, the paired-pulse ratios for hair cells held at −60 and −90 mV showed results similar to those obtained from EPSC peaks (Fig. 2D). Although the paired-pulse depression of EPSC charges for short intervals was less severe in comparison with that of EPSC peaks, this small difference in paired-pulse ratios from EPSC peaks and EPSC charges was not statistically significant for the individual interpulse intervals for hair cells held at −60 mV and at −90 mV. We also compared the peak amplitudes of the first EPSCs for hair cells held at −60 and −90 mV (Fig. 2E). For seven paired recordings, we recorded EPSCs from the same synapse while holding the hair cell at −90 or −60 mV. When the hair cell was held at −60 mV, the averaged amplitude of the first EPSC (1562 ± 841 pA) was larger than the first EPSC amplitude of the hair cell held at −90 mV (524 ± 292 pA; n = 7). Including other paired recordings in which EPSCs were recorded separately while holding the hair cells at −90 or −60 mV, the first EPSC peak amplitude at −60 mV (2493 ± 626 pA; n = 12) was also significantly larger than the first peak EPSC at −90 mV (613 ± 206 pA; n = 20). However, the total charge transfers of the first EPSC for hair cells held at −60 and −90 mV were not significantly different for 20 ms pulses. Remarkably, the average amplitude of the peak Ca2+ current was also not significantly different at the two holding potentials for the same hair cell: −347 ± 44 pA for −60 mV and −366 ± 49 pA for −90 mV (p > 0.2; paired t test; n = 8).
We next compared the recovery of peak EPSC paired-pulse depression for hair cells held at −60 mV (Fig. 2A) with a previous in vivo study using Rana pipiens frogs (Megela and Capranica, 1982). In these in vivo studies a sequence of two tones, 10 dB above threshold and at the fiber's best frequency, were given at variable intervals (the first and second tone durations were 750 and 200 ms, respectively). The recovery from response decrement was then measured. Surprisingly, the in vivo recovery of auditory nerve fibers originating from the amphibian papilla resembled closely our recovery curve of paired-pulse depression while hair cells were held at −60 mV (Fig. 2F). Importantly, the in vivo data shown here were obtained from fast-adapting fibers that have a characteristic (best) frequency of ∼350–550 Hz (Megela and Capranica, 1982; Megela, 1984), which is also the resonant frequency of our bullfrog hair cells (409.0 ± 2.2 Hz; n = 20; data not shown). Based on the correlation of Figure 2F, we propose that paired-pulse depression observed in our in vitro study may be physiologically relevant to in vivo auditory nerve recovery from adaptation (Spassova et al., 2004).
As mentioned above, changes in hair cell Ca2+ influx do not seem to significantly contribute to paired-pulse EPSC depression or facilitation (Fig. 2A,B). Though the ratios of the Ca2+ charges were very close to 1 at the two different holding potentials, the ratio of Ca2+ charges at −60 mV was slightly above 1, whereas it was slightly below 1 at −90 mV (Fig. 2A,B). The kinetics of the Ca2+ currents at different holding potentials is shown in Figure 3. Interestingly, there was a small initial outward component in the first Ca2+ current at −60 mV (Fig. 3A,B1). This was not present when hair cells were held at −90 mV (Fig. 3B2). The Ca2+ currents of Figure 3B1 resemble those observed in retinal bipolar cells and may reflect a pH-mediated inhibition of Ca2+ current due to the exocytosis of protons (Palmer et al., 2003), or this may be related with modulation via activation of protein kinase C (Abreu et al., 2008). It is also possible that heterogeneous types of Ca2+ channels exist, as in frog saccular hair cells (Su et al., 1995; Rodriguez-Contreras and Yamoah, 2001). The transient outward component causes the ratio of Ca2+ charges to be slightly above 1 at the −60 mV holding potential. By contrast, at −90 mV, the first Ca2+ current shows a slightly bigger peak than the second Ca2+ current for short interpulse intervals (Fig. 3B2). This probably reflects Ca2+-dependent Ca2+ current inactivation (von Gersdorff and Matthews, 1996; Schnee and Ricci, 2003), and the fastest component of inactivation may reflect near-channel Ca2+ nanodomains that can rapidly reach high Ca2+ levels (Grant and Fuchs, 2008). However, as the interpulse intervals get longer, the first and the second Ca2+ currents become identical.
Figure 3.
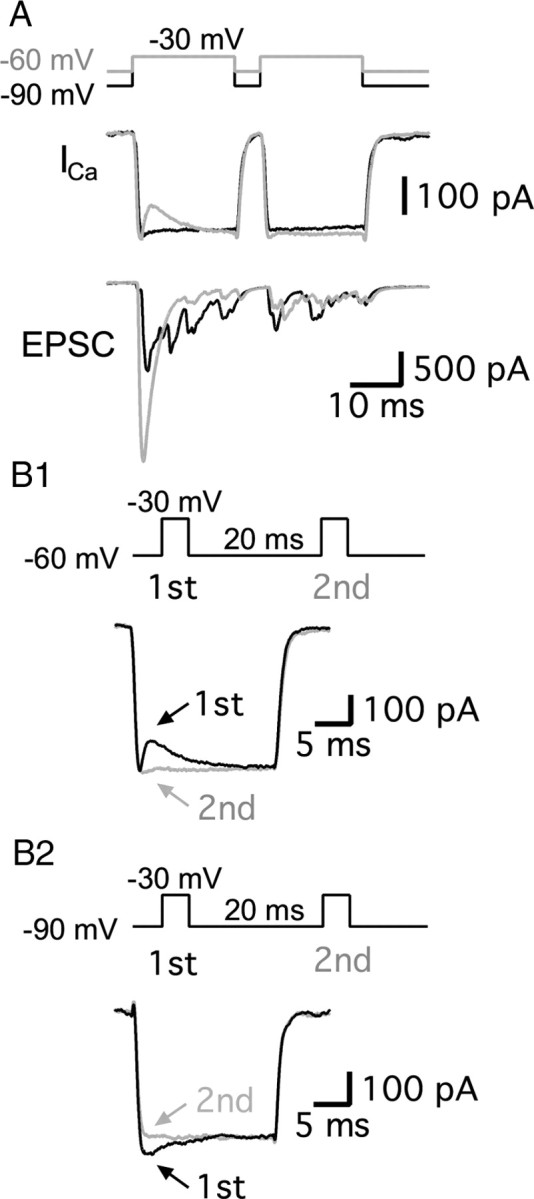
A comparison of Ca2+ currents from hair cells held at −60 and −90 mV. A, Hair cells were depolarized from −60 mV (gray) or −90 mV (black) to −30 mV for 20 ms with 5 ms interpulse intervals in the same paired recording. Ca2+ currents (ICa) showed similar peaks at both holding potentials, but the first ICa at −60 mV showed a transient outward component (or Ca2+ current inhibition). Note the larger first pulse EPSC amplitude when hair cells are held at −60 mV and the strong paired-pulse depression (gray trace). B1, B2, Ca2+ currents were evoked by a pair of 20 ms pulses with a 20 ms interpulse interval from holding potentials of −60 mV (B1) or −90 mV (B2) to −30 mV at the same hair cell. The first Ca2+ currents (black) and the second Ca2+ currents (gray) were superimposed. At −60 mV, the first Ca2+ current showed a transient outward component (B1). At −90 mV, the first Ca2+ current showed a slightly larger peak (B2).
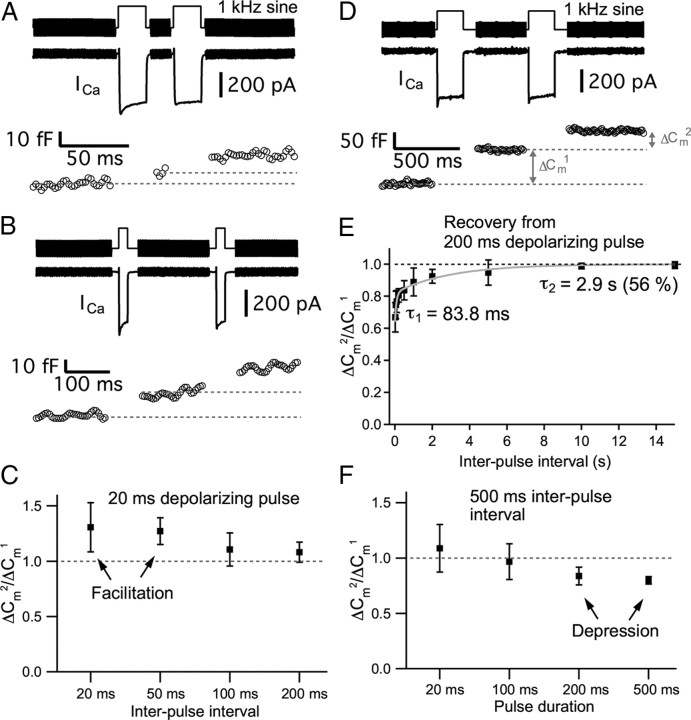
AMPA receptor-mediated EPSCs can undergo saturation and/or desensitization during a prolonged and strong presynaptic depolarization. So, in addition to EPSC recordings, we also monitored exocytosis directly from hair cells using Cm measurements. The electrical capacitance of a cell is proportional to the cell surface area and vesicle fusion into the plasma membrane increases the cell surface area (Lindau and Neher, 1988). Presynaptic Ca2+ currents from hair cells were recorded and Cm measurements used a 1 kHz sine wave superimposed on the holding potential of −90 mV. This hyperpolarized membrane potential guaranteed that Ca2+ current was not activated during the sine wave stimulus used to measure Cm. The change in membrane capacitance (capacitance jump; ΔCm) was used as a measure of exocytosis. We depolarized hair cells from −90 to −30 mV for 20 ms with 20, 50, 100, or 200 ms interpulse intervals (Fig. 4A–C). The averaged paired-pulse ratio of ΔCm (ΔCm2/ΔCm1) evoked by a pair of 20 ms pulses with a 20 ms interpulse interval was 1.30 ± 0.22 (n = 14; Fig. 4A,C). With a 50 ms interval, ΔCm2/ΔCm1 was 1.27 ± 0.12 (n = 15; Fig. 4C). As the results of EPSCs showed (Fig. 2), hair cells with these protocols of stimulation (a pair of 20 ms pulse with 20 or 50 ms interpulse interval) showed paired-pulse facilitation (Fig. 4A,C). With a 100 ms interval, ΔCm2/ΔCm1 was 1.11 ± 0.15 (n = 12) and with a 200 ms interval, ΔCm2/ΔCm1 was 1.08 ± 0.09 (n = 9; Fig. 4B). As the interpulse interval became longer, ΔCm2/ΔCm1 approached a value of 1 (Fig. 4C), which was also consistent with the results obtained from EPSCs (Fig. 2).
Figure 4.
Paired-pulse depression and facilitation measured with Cm changes from hair cells. Hair cells were depolarized from −90 to −30 mV. A, ICa and ΔCm evoked by a pair of 20 ms pulses with 20 ms interpulse interval shows paired-pulse facilitation (ΔCm2/ΔCm1 > 1). B, ICa and ΔCm evoked by a pair of 20 ms pulses with 200 ms interpulse interval. ΔCm2/ΔCm1 was close to 1. C, The averaged ratio of ΔCm (ΔCm2/ΔCm1) evoked by a pair of 20 ms voltage steps from −90 to −30 mV with interpulse interval of 20, 50, 100, and 200 ms. D, ICa and ΔCm evoked by a pair of 200 ms pulses with 500 ms interpulse interval. E, Recovery from depression elicited by a pair of 200 ms pulses. The averaged ΔCm2/ΔCm1 was measured with various interpulse intervals (20 ms, 50 ms, 100 ms, 200 ms, 500 ms, 1 s, 2 s, 5 s, 10 s, and 15 s; n = 4–17). Paired-pulse depression recovered with a two exponential time course with time constants of 83.8 ms (τ1) and 2.9 s (τ2). F, ΔCm evoked by a pair of voltage steps from −90 to −30 mV for various durations (20, 100, 200, and 500 ms) with 500 ms interpulse interval. The dashed line indicates that ΔCm2/ΔCm1 is 1.
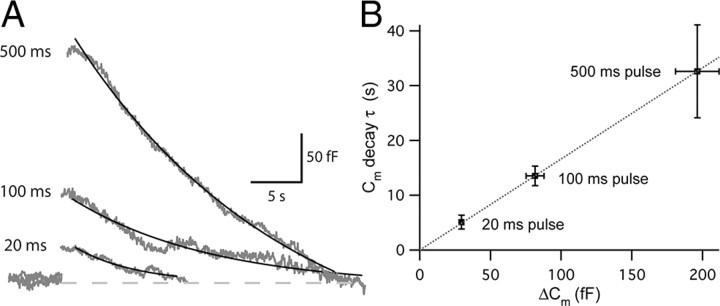
To completely deplete the fast component of release (Li et al., 2009), we next used a longer duration of depolarization to stimulate the hair cells (Fig. 4D,F). When hair cells were depolarized from −90 to −30 mV for 200 ms, we also measured how fast the paired-pulse depression recovered. The averaged ΔCm2/ΔCm1 was measured with various interpulse intervals (20 ms, 50 ms, 100 ms, 200 ms, 500 ms, 1 s, 2 s, 5 s, 10 s, and 15 s). Paired-pulse depression recovered as a two exponential function with a fast time constant of 83.8 ms (τ1; 44%) and a slow time constant 2.9 s (τ2; 56%) (n = 4–17; Fig. 4E). Comparing with the recovery rate using a shorter pulse (20 ms) from EPSC data (Fig. 2), the recovery rate of paired-pulse depression with a longer pulse (200 ms) was dramatically slower. When hair cells were depolarized from −90 to −30 mV for 20, 100, 200, and 500 ms with a constant interpulse interval of 500 ms, they generally showed paired-pulse depression (Fig. 4F). With a pair of 20 ms pulses, ΔCm2/ΔCm1 was 1.09 ± 0.22 (n = 8), and with a pair of 100 ms pulses, ΔCm2/ΔCm1 was 0.97 ± 0.16 (n = 11). With a pair of 200 ms pulses, ΔCm2/ΔCm1 was 0.84 ± 0.06 (n = 17), and with a pair of 500 ms pulses, ΔCm2/ΔCm1 was 0.80 ± 0.03 (n = 28).
From the results of both EPSCs and ΔCm, we have shown that hair cell synapses display both paired-pulse depression and facilitation depending on the duration of stimulation and interpulse interval (Figs. 2, 4). This is quite different from other ribbon-type synapses such as those found in goldfish retinal bipolar cell terminals, which show only depression for pulses of variable durations (Mennerick and Matthews, 1996; Burrone and Lagnado, 2000; Palmer et al., 2003; Coggins and Zenisek, 2009).
Ca2+ dependence of short-term plasticity at hair cell synapses
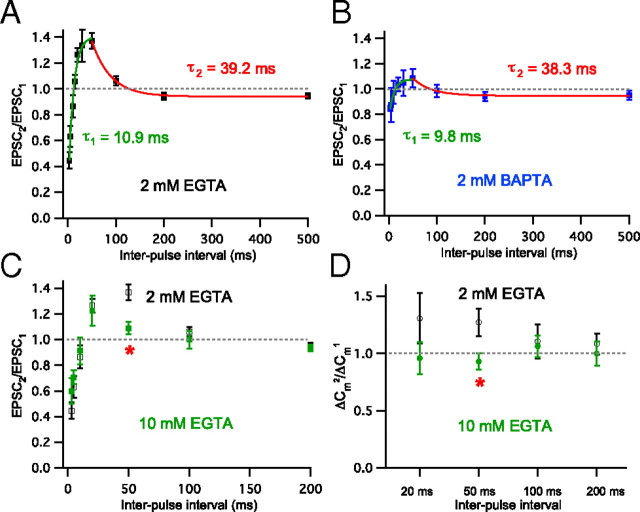
To study whether paired-pulse facilitation and depression at hair cell synapses are dependent on Ca2+, we held presynaptic hair cells at −90 mV and measured paired-pulse ratios of EPSCs using different conditions of presynaptic Ca2+ buffer in the internal solution (Fig. 5A–C). As in Figure 2, EPSCs were recorded from afferent fibers while connected hair cells were depolarized by a pair of 20 ms voltage steps from −90 to −30 mV with various interpulse intervals (3–500 ms) and the averaged ratios of EPSC peak amplitudes (EPSC2/EPSC1) were fitted exponentially (Fig. 5). As a control condition, 2 mm EGTA, EPSC2/EPSC1 with interpulse intervals from 3 to 50 ms increased double exponentially (τ1 = 10.9 ms) (green in Fig. 5A, same data as in Fig. 2B) and EPSC2/EPSC1 with interpulse intervals from 50 to 500 ms decreased as a single exponential with time constant, τ2 = 39.2 ms (n = 6–9; Fig. 5A, red). When the presynaptic calcium buffer in the pipette internal solution was changed to 2 mm BAPTA (n = 4–8), EPSC2/EPSC1 ratios with 3, 20, and 50 ms interpulse intervals were significantly different from those of 2 mm EGTA (p < 0.05) (Fig. 5B). The time constant (τ) of single exponential fitting between 3 and 50 ms interpulse intervals in 2 mm BAPTA (τ1 = 9.8 ms; green) was similar to the time constant of 2 mm EGTA (10.9 ms in Fig. 5A). EPSC2/EPSC1 with interpulse intervals from 50 to 500 ms decreased single exponentially with a time constant, 38.3 ms (τ2) (Fig. 5B). This was also very similar to time constant in 2 mm EGTA (39.2 ms in Fig. 5A). These results indicate that paired-pulse facilitation and depression at hair cell synapses are dependent on Ca2+. Although the concentration of Ca2+ buffer was the same, BAPTA can bind with free Ca2+ ions 150-fold faster than EGTA and this difference may affect the concentration of residual Ca2+ and paired-pulse facilitation at this synapse. The effect of 2 mm BAPTA on paired-pulse depression with very short interpulse interval (3 ms) may result from the decrease in the first pulse response. Indeed, the first pulse ΔCm decreased significantly in 2 mm BAPTA (12.3 ± 1.4 fF; n = 10) compared with 2 mm EGTA (18.2 ± 1.9 fF; n = 24).
Figure 5.
Ca2+ buffer dependence of paired-pulse ratios. EPSCs were elicited with a pair of 20 ms pulses from −90 to −30 mV (A–C). In A and B, EPSCs were recorded from afferent fibers while the hair cells were depolarized by a pair of 20 ms voltage steps from −90 to −30 mV with various interpulse intervals (3–500 ms). The averaged paired-pulse ratios of EPSC peak amplitudes (EPSC2/EPSC1) were fit by exponentials (green and red lines). In D, paired-pulse ratio was calculated from ΔCm evoked by a pair of 20 ms voltage steps from −90 to −30 mV with interpulse interval of 20, 50, 100, and 200 ms. A, The averaged EPSC2/EPSC1 with 2 mm EGTA as an internal calcium buffer in hair cells (black; n = 6–9). EPSC2/EPSC1 with interpulse intervals from 3 to 50 ms (3, 5, 10, 15, 20, 30, and 50 ms) increased exponentially (τ1 = 10.9 ms, green). The red line shows a single exponential fit with a time constant of 39.2 ms. B, The averaged EPSC2/EPSC1 with 2 mm BAPTA as an internal calcium buffer in hair cells (blue; n = 4–8). EPSC2/EPSC1 values with 3, 20, and 50 ms interpulse intervals were significantly different from those of 2 mm EGTA (p < 0.05). The time constant of a single exponential fit between the 3 ms and 50 ms intervals was 9.8 ms (τ1; green) and the exponential time constant between 50 ms and 500 ms intervals was 38.3 ms (τ2; red). C, The averaged paired-pulse ratio of EPSCs (3, 5, 10, 20, 50, 100, and 200 ms interpulse intervals) with 10 mm EGTA internal calcium buffer in the hair cells (green squares; n = 4–9). The averaged paired-pulse ratio with 10 mm EGTA was significantly decreased only for the 50 ms interval compared with 2 mm EGTA (red asterisk, p < 0.05). D, With 10 mm EGTA (green filled circle), ΔCm2/ΔCm1 was not significantly different from 2 mm EGTA (black open square, same data as in with Fig. 4C) except for the data point with 50 ms interval (red asterisk; p < 0.05).
We next tested a higher concentration (10 mm) of EGTA as a presynaptic Ca2+ buffer (Fig. 5C,D). The averaged EPSC2/EPSC1 with 50 ms interpulse interval was significantly decreased from that of 2 mm EGTA (n = 4–9; p < 0.05). In addition, we also tested the effect of 10 mm EGTA using the ΔCm measurements. Using 2 mm EGTA as our control Ca2+ buffer, a ΔCm was evoked by a pair of 20 ms voltage steps from −90 to −30 mV with interpulse intervals of 20, 50, 100, and 200 ms with 2 mm external Ca2+ (black in Fig. 5D, same data as in Fig. 4C). When we increased the internal Ca2+ buffer concentration from 2 mm EGTA to 10 mm EGTA, this facilitation was blocked significantly with 50 ms interpulse interval (p < 0.05; Fig. 5D, green). This also indicates that paired-pulse facilitation is Ca2+ dependent. Higher concentrations of EGTA (10 mm) may block facilitation at a certain range of interpulse intervals by removing more of free Ca2+ and leaving less residual Ca2+. Importantly, and in contrast with 2 mm BAPTA, 10 mm EGTA (18.1 ± 2.0 fF; n = 16) did not change the first pulse ΔCm in comparison with 2 mm EGTA (18.2 ± 1.9 fF; n = 24) and it did not change paired-pulse depression with very short interpulse intervals (Fig. 5C).
Manipulating the release probability by changing the extracellular Ca2+ concentration can alter the form of short-term plasticity that dominates at synapses (Thomson, 2000; Johnson et al., 2005). To investigate the role of Ca2+ in short-term plasticity further, we altered the Ca2+ concentration in the external bath solution during the recordings (Fig. 6). When we increased Ca2+ concentration to 5 mm, paired-pulse facilitation in control, 2 mm Ca2+ in the bath (Fig. 6A, filled squares), changed into paired-pulse depression (Fig. 6A, open circles). With all interpulse intervals that we tested with 5 mm external Ca2+ in the bath, the paired-pulse ratio decreased significantly (n = 7–9). These results show that high external Ca2+ concentrations promote paired-pulse synaptic depression (Johnson et al., 2005; Frank et al., 2010). This may result from the increase in ΔCm evoked by the first pulse with 5 mm external Ca2+ in the bath. Compared with 2 mm Ca2+ (18.2 ± 1.9 fF; n = 24), the first pulse ΔCm significantly increased in 5 mm Ca2+ (29.3 ± 2.0 fF; n = 15; p < 0.001). In contrast, paired-pulse facilitation became stronger by decreasing the concentration of external Ca2+ to 1 mm (Fig. 6B, open triangles). With a 50 ms interpulse interval, the paired-pulse ratio significantly increased (n = 5–9; p < 0.001). In the case of 20 ms interpulse intervals, it showed the tendency of increased facilitation, but this was not statistically significant because of biological variability. In contrast to the 5 mm external Ca2+ condition, 1 mm external Ca2+ in the bath significantly decreased the first pulse ΔCm (5.9 ± 1.4 fF; n = 9; p < 0.001) from that with 2 mm Ca2+.
Figure 6.
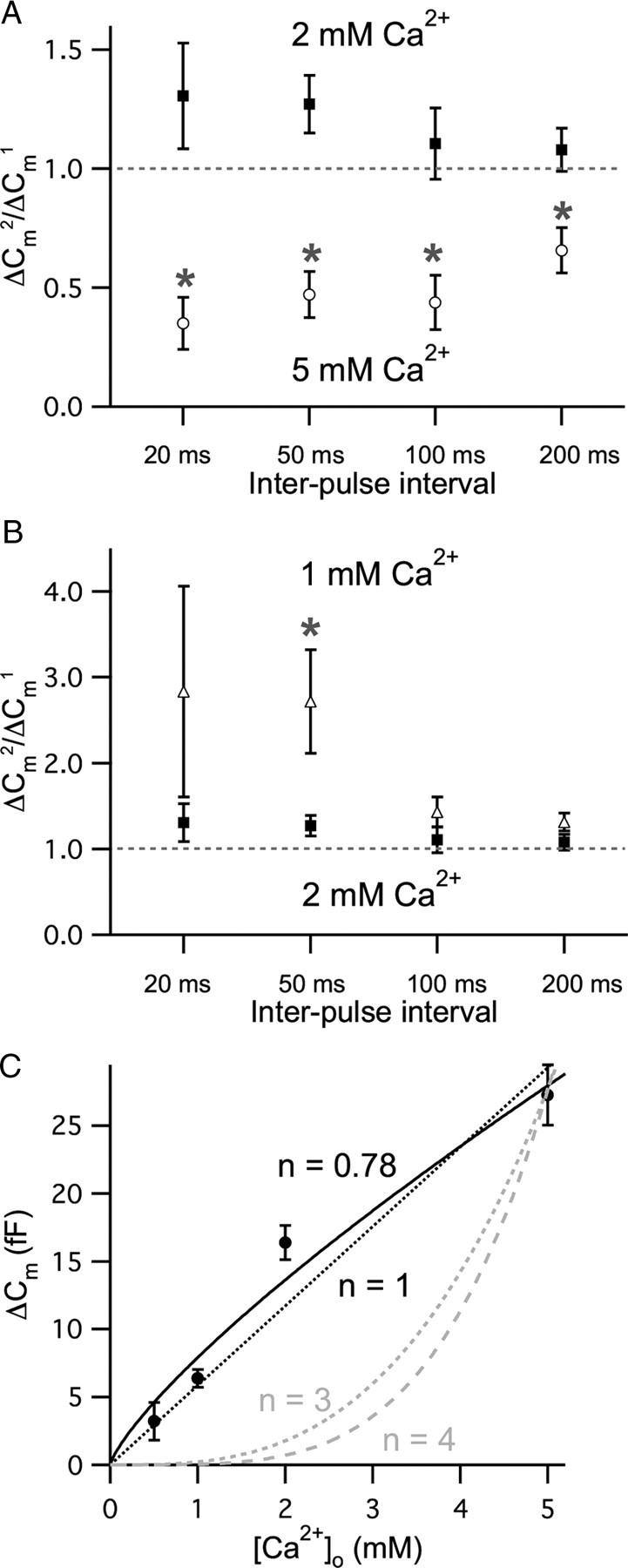
Dependence of paired-pulse ratios on external Ca2+ concentration. ΔCm evoked by a pair of 20 ms voltage steps from −90 to −30 mV with interpulse intervals of 20, 50, 100, and 200 ms with 2 mm EGTA as an internal Ca2+ buffer. A, When the external Ca2+ concentration was increased from 2 mm (filled squares) into 5 mm (open circles), the averaged paired-pulse ratios of ΔCm with interpulse intervals ranging from 20 to 200 ms were significantly decreased (n = 7–9; asterisk; p < 0.001). B, When the external Ca2+ concentration decreased to 1 mm (open triangles), the averaged paired-pulse ratios of ΔCm with interpulse intervals ranging from 20 to 200 ms were increased (n = 5–9) comparing with data in 2 mm external Ca2+ (filled squares). The ratio especially with 50 ms interval was significantly increased (asterisk; p < 0.001). C, The relationship between ΔCm and external Ca2+ concentration ([Ca2+]o) for a 20 ms long-depolarizing pulse (n = 11–17). The lines are fits using the equation ΔCm = A · [Ca2+]n. The best fit to the data is shown by the black solid line (n = 0.78). The gray dashed lines (n = 3 or n = 4) demonstrate the third and the fourth power relationships between [Ca2+]o and ΔCm, respectively. The black dashed line is a linear fit (R2 = 0.95).
We next measured ΔCm elicited by 20 ms pulses while changing external Ca2+ concentrations ([Ca2+]o) over the range of 0.5 to 5 mm to study the quantitative relationship between ΔCm and external Ca2+ concentration (n = 11–17). When we fitted the data using the equation ΔCm = A · [Ca2+]n, it showed a slightly sublinear relationship (n = 0.78; Fig. 6C, black solid line). The data could also be approximated by a linear fit (R2 = 0.95; Fig. 6C, black dashed line). Note that unlike mouse retinal bipolar cells, exocytosis does not saturate from 2 mm to 5 mm [Ca2+]o suggesting a wide dynamic range of release for hair cells (Jarsky et al., 2010). This slightly sublinear Ca2+ dependence of exocytosis is similar to that in previous studies using mature mammalian inner hair cells (Johnson et al., 2008), or using Ca2+ current peak amplitudes and EPSC charge transfers with our bullfrog hair cell preparation (Keen and Hudspeth, 2006).
The underlying mechanisms for depression
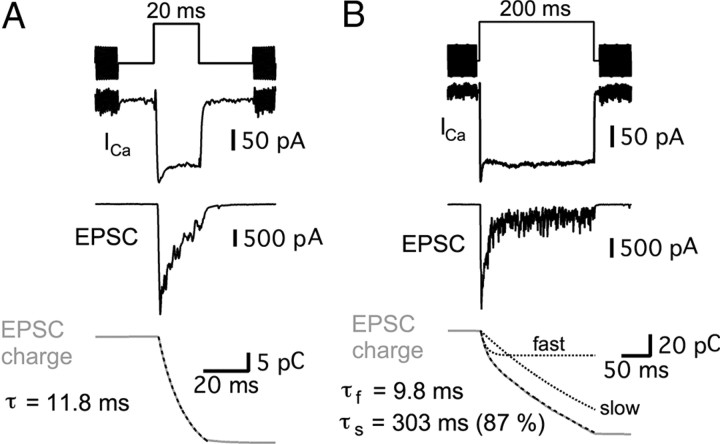
The relationship between paired-pulse EPSC ratio and interpulse intervals (Fig. 2) suggests that there is a very small and fast component of release, which can be recovered quickly (Moser and Beutner, 2000; Spassova et al., 2004; Rutherford and Roberts, 2006; Johnson et al., 2008; Bartoletti et al., 2010). Previous studies have shown that depletion of the vesicles within the readily releasable pool can cause synaptic depression (Betz, 1970; Glavinovic and Narahashi, 1988; von Gersdorff and Matthews, 1997; Heidelberger et al., 2005). Therefore, we investigated the kinetic components of evoked EPSCs during the hair cell depolarization. We depolarized hair cells from −90 to −30 mV for 20 and 200 ms and recorded the evoked EPSCs from the afferent fibers (Fig. 7A,B). The transferred EPSC charges were calculated by integrating the EPSCs. For a 20 ms pulse, the EPSC charge had a single time constant (τ = 13.3 ± 1.6 ms) in five synapses, whereas in four other synapses it showed very fast (τf = 3.6 ± 1.5 ms) and slow (τs = 15.9 ± 2.6 ms; 78% of total charge) components. For a 200 ms pulse, the EPSC change was fit with a double exponential equation with fast (τf = 12.2 ± 1.1 ms) and slow (τs = 599 ± 179 ms; 81% of total charge) components (n = 4; Fig. 7). Depletion of the fast component of transmitter release (τf ≈ 12 ms) thus likely results in our observed paired-pulse depression at hair cell synapses.
Figure 7.
Prolonged hair cell depolarizations evoke EPSCs with two kinetic components. Paired recordings of hair-cell Ca2+ currents (ICa) and EPSCs from the connected afferent fiber. A, B, Examples of EPSCs evoked with 20 ms (A) and 200 ms (B) step depolarizations from a holding potential of −90 to −30 mV at the same hair cell to afferent fiber synapse. Black dashed lines are exponential fits of calculated EPSC charges (gray). With a 20 ms pulse, the exponential time constant (τ) was 11.8 ms. The EPSC charge transfer (gray) evoked by a 200 ms stimulus shows fast and slow components. The time constant of the fast component (τf) was 9.8 ms and the time constant of the slow component (τs) was 303 ms (87% of the total amplitude). The dashed black lines show the fast and slow components of the double-exponential fit to the data.
It is possible that desensitization or saturation of postsynaptic AMPA receptors contribute to paired-pulse depression. To test this possibility, we simultaneously recorded ΔCm from the presynaptic hair cell and EPSCs from the connected afferent fiber. We compared ΔCm and the transferred EPSC charges during that time (Fig. 8). A pair of short pulses with a short interval (a pair of 20 ms depolarizations with 20 ms interpulse interval; Fig. 8A), a pair of short pulses with a long interval (a pair of 20 ms depolarizations with 200 ms interpulse interval; Fig. 8B) and a pair of long pulses with a short interval (a pair of 200 ms depolarizations with 50 ms interpulse interval; Fig. 8C) were tested. In all cases, the ΔCm and EPSC charge transfers were correlated very well. This is consistent with our previous studies with single depolarizing pulses of variable durations (Li et al., 2009) and suggests that desensitization and/or saturation of AMPA receptors may not contribute significantly to short-term plasticity. Though blockers of AMPA receptor desensitization affected EPSC responses in young rat auditory nerve fibers (Goutman and Glowatzki, 2007), studies at other ribbon-type synapses also do not show evidence for AMPA receptor desensitization (Singer and Diamond, 2006; Wölfel et al., 2007; Pang et al., 2008).
Figure 8.
Paired recordings and simultaneous ΔCm measurements. A–C, EPSCs mediated by AMPA receptors are not significantly desensitized or saturated during exocytosis with pairs of 20 ms (A, B) or 200 ms (C) depolarizing pulses from a holding potential of −90 to −30 mV in hair cell synapses. Presynaptic ICa, Cm (gray open circle, bottom), and afferent fiber EPSC were simultaneously recorded while a voltage-clamped hair cell was depolarized by a pair of 20 ms pulses with 20 ms interpulse interval (A), by a pair of 20 ms pulses with 200 ms interpulse interval (B), or by a pair of 200 ms pulses with 50 ms interpulse interval (C). The intermediate changes of EPSC charge transfer (black lines) are well correlated with the increases of ΔCm (gray open circles) after the traces are normalized to the peak EPSC and ΔCm changes.
To investigate whether AMPA receptor desensitization contributes to paired-pulse depression for very short interpulse intervals, we compared the ratio of ΔCm evoked by a 40 ms single pulse to ΔCm evoked by a 20 ms pulse from the same hair cells. We chose a 40 ms pulse (equivalent to a 20 ms pulse + 0 ms interval + 20 ms pulse), because this should show maximum EPSC desensitization, if it is present. For 5 cells, we calculated the paired-pulse ratio that was derived from ΔCm using 40 and 20 ms pulses [(ΔCm of 40 ms pulse − ΔCm of 20 ms pulse)/ΔCm of 20 ms pulse]. This ratio for “0 ms” interpulse intervals was 0.62 ± 0.07, which is very close to the paired-pulse ratio for EPSC charges for 3 ms interpulse intervals at −90 mV hair cell potentials (0.61 ± 0.08; Fig. 2D). Together, these results strongly suggest that short-term depression at hair cell synapses results from presynaptic mechanisms within hair cells.
Exocytosis and endocytosis coupling
What are the mechanisms that control vesicle pool replenishment and recovery from paired-pulse depression? It is possible that vesicles undergo endocytosis quickly after being released and thus constitute a main source for this replenishment. Since the decay of Cm after exocytosis can be interpreted as due to endocytosis, we monitored Cm for prolonged durations to measure the rate of endocytosis. Unfortunately, we could not observe significant endocytosis with 20–500 ms long-depolarizing pulses under regular whole-cell recordings in hair cells. This suggests that our capacitance jump measurements reflect solely exocytosis without being contaminated by endocytic activity. However, previous recordings obtained with the nystatin-perforated patch method showed clear endocytosis (Parsons et al., 1994; Moser and Beutner, 2000). This suggests that small mobile intracellular molecules, that are required for endocytosis, may be dialyzed during the whole-cell recording (Hull and von Gersdorff, 2004). Alternatively, it is possible that a component of our internal solution in regular whole-cell recordings may inhibit endocytosis. Previous studies of endocytosis at hair cells have reported a Cm decay time constant τ of ∼7.5 s (Moser and Beutner, 2000; Schnee et al., 2005; Rutherford and Roberts, 2006; Dulon et al., 2009). Hair cells were depolarized from −90 to −30 mV for 20, 100, and 500 ms under the nystatin-perforated patch-clamp mode of recording. Cm increased after exocytosis and returned back to the baseline exponentially (Fig. 9A). As the duration of stimulation got longer, the rate of endocytosis became slower. The decay τ of Cm was 5.1 ± 1.3 s for a 20 ms pulse (n = 9), 13.5 ± 1.8 s for a 100 ms pulse (n = 9), and 32.6 ± 8.5 s for a 500 ms pulse (n = 6). The relationship between endocytic decay τ and ΔCm was linear (ΔCm = 0.17 · τ, R2 = 0.99997; Fig. 9B). Smaller amounts of exocytosis thus have a faster rate of endocytosis, perhaps because endocytic proteins are more abundant per fused vesicle (Renden and von Gersdorff, 2007; Smith et al., 2008). Although asynchronous release after a depolarizing pulse was usually very rare for hair cells held at −90 mV, it is possible that some asynchronous release from hair cells may interfere with the rate of endocytosis measured by the decay of Cm. However, given the 10 ms to 1 s time scale for recovery from depression (Figs. 1, 2), the endocytosis rate is relatively slow and unlikely to be rate limiting for the recovery from depression. Moreover, we could observe endocytosis routinely only in the perforated patch mode of recording. It is thus likely that vesicles recruited from reserve pools may be used preferentially to replenish the fast component of transmitter release rather than vesicles that have been freshly endocytosed. A fast clearance rate of fused vesicular membrane after exocytosis at synaptic ribbons, which is not dependent on fast endocytosis, is also implied by these results and the rapid recovery from short-term depression (Neher and Sakaba, 2008).
Figure 9.
The coupling of exocytosis to endocytosis at hair cell synapses. The kinetics of endocytosis was measured using the nystatin-perforated patch mode of recording. A, Cm traces with 20, 100, and 500 ms, long step depolarizations from −90 to −30 mV (gray). The decay of Cm was fit with a single exponential function (black). B, The relationship between the ΔCm and the Cm decay time constant (τ) shows a linear relationship (R2 = 0.99997). The rate of endocytosis is thus faster when the amount of exocytosis is smaller.
Discussion
Our observations at hair cell synapses suggest that short-term facilitation was dependent on residual presynaptic Ca2+ levels, whereas depression resulted from the depletion of a small pool of readily releasable vesicles. An extremely rapid vesicle replenishment process allows the depleted synapse to recover quickly from depression. Finally, we suggest the recovery draws mainly from preformed vesicles mobilized from a reserve pool near the synaptic ribbon, rather than from the rapid reuse of vesicles freshly formed via the retrieval of vesicular membrane (endocytosis).
Comparisons with other ribbon-type synapses
Previous studies using other ribbon synapses, such as goldfish and rat retinal bipolar cells, photoreceptors of salamanders, frog saccular hair cells and cochlear inner hair cells of the mouse showed depression only (Mennerick and Matthews, 1996; Moser and Beutner, 2000; Palmer et al., 2003; Edmonds et al., 2004; Spassova et al., 2004; Singer and Diamond, 2006; Babai et al., 2010). However, we emphasize that previous studies used a variety of experimental protocol parameters such as the duration and amplitude of depolarization, the duration of interpulse intervals, external Ca2+ concentration, internal Ca2+ buffer, and holding potentials. So it is difficult to directly compare them with our study. Nevertheless, retinal bipolar cell terminals exhibit strong paired-pulse depression and a relatively slow double-exponential recovery from depression (τf = 1.03 s and τs = 11.8 s) (Palmer et al., 2003; see also Mennerick and Matthews, 1996; Wan et al., 2008; Coggins and Zenisek, 2009). Rod bipolar cells in the rat retina also exhibited paired-pulse depression with a slow recovery (τ = 4 s) (Singer and Diamond, 2006). However, acutely isolated frog saccular hair cells recover quickly from paired-pulse depression with a τ = 29 ms (Edmonds et al., 2004). Chick and cat afferent fibers also recover quickly from sound-evoked adaptation [chick: τ = 70 ms (Spassova et al., 2004); cat: τf = 22 ms and τs = 184 ms (Chimento and Schreiner, 1991)]. In immature mouse inner hair cells, depletion of the readily releasable pool also had a rapid and biphasic recovery (τf = 140 ms and τs = 3 s) (Moser and Beutner, 2000). Compared with these previous studies, our recovery rate from paired-pulse depression was relatively fast (Fig. 2A,B), although depression elicited by a longer pulse showed slower recovery (Fig. 4E). We conclude that hair cell ribbon synapses recover more quickly from depression than retinal ribbon synapses. Perhaps hair-cell synaptic ribbons, and their surrounding vesicle mobilization machinery, are tailored for fast replenishment of depleted vesicle pools (Schnee et al., 2005). The fast time course of recovery from depression in hair cell synapses may thus reflect an acute requirement for speed in the processing of ongoing acoustic signals.
Short-term plasticity and the resting membrane potential of hair cells
When hair cells were held at −60 mV, a more physiological condition (Crawford and Fettiplace, 1980; Pitchford and Ashmore, 1987), hair cell synapses showed only depression. In contrast, the synapse showed both paired-pulse depression and facilitation at the holding potential of −90 mV. Holding hair cells at different membrane potentials provided us the experimental conditions to dissect the mechanisms underlying both dominant plasticity (paired-pulse depression at −60 mV) and masked plasticity (paired-pulse facilitation at −90 mV), instead of observing only one type of behavior, or a competition of both. One explanation of these differing results can lie in the resting Ca2+ concentration of neurons at different holding potentials (Kobayashi and Tachibana, 1995). At −60 mV, the Ca2+ current in hair cells is already slightly activated (Edmonds et al., 2004; Li et al., 2009), and the concentration of resting Ca2+ is probably higher than at −90 mV. This can affect many Ca2+-dependent processes, such as vesicle priming for fusion, facilitation of release, and vesicle recruitment rates (Awatramani et al., 2005). Changes in intracellular Ca2+ (or stimulation frequency) can also modify the size of the readily releasable pool (Voets, 2000; Ruiz et al., 2011). In general, at synapses with a high probability of release, depression dominates, while synapses with a low release probability tend to show facilitation. Given the larger average EPSC amplitude of hair cells held at −60 mV compared with those held at −90 mV, it seems likely that release probability was larger for hair cells held at −60 mV, so their synapses exhibited mostly paired-pulse depression.
A relatively slow decay of residual Ca2+ is thought to cause facilitation at conventional synapses. Accordingly, we found that higher concentrations of a slow Ca2+ buffer (EGTA), or dialysis of a fast Ca2+ buffer (BAPTA), blocked facilitation in hair cells (Fig. 5). Moreover, a higher concentration of external Ca2+ evoked strong paired-pulse depression and increased Ca2+ influx, consistent with an increase in release probability (Fig. 6). In contrast, decreasing the concentration of external Ca2+ resulted in smaller amplitude Ca2+ currents and clear paired-pulse facilitation.
Ca2+-dependent release and vesicle recruitment
While many conventional synapses have shown a third to fourth order power-law dependence of release on Ca2+, hair cell ribbon synapses show a linear or sublinear Ca2+ dependence of neurotransmitter release (Johnson et al., 2005; Keen and Hudspeth, 2006; Goutman and Glowatzki, 2007). Moreover, there is a marked developmental change in the Ca2+ dependence of exocytosis (Johnson et al., 2005). Our Ca2+ dependence of exocytosis (n = 0.78; Fig. 6C) is in good agreement with previous studies from mature hair cells (Keen and Hudspeth, 2006; Johnson et al., 2008). Recently, otoferlin has been shown to be a Ca2+ sensor for exocytosis at mouse cochlea hair cells (Roux et al., 2006), although it is likely to also be involved in a Ca-dependent vesicle recruitment process (Pangrsic et al., 2010). The existence of unique Ca2+ sensors in hair cells may produce the near-linear Ca2+ dependence of transmitter release (Safieddine and Wenthold, 1999; Dulon et al., 2009; Beurg et al., 2010; Johnson et al., 2010).
Continuous release and endocytosis at hair cell synapses
Our results show that hair cells can release a small pool of vesicles at a fast rate (τfast ≈ 12 ms) and replenish this pool at comparably fast rates [τf = 15 ms (64%) with τmedian = 213 ms at −60 mV]. How can the fastest component of release be replenished so quickly? Moreover, hair cell synapses can keep releasing vesicles at high rates during prolonged stimulation (Figs. 7B, 8C). What are the mechanisms that sustain vesicle mobilization for continuous release? We suggest here that high internal Ca2+ levels may facilitate vesicle mobilization. In addition, to avoid premature vesicle pool depletion and maintain a proper cell size, a fast rate of endocytosis seems necessary for hair cells. However, the fastest rate of endocytosis we observed was relatively slow (τ = 5.1 s with 20 ms depolarizing pulses; Fig. 9), and may be too slow to participate significantly in the recovery from depression. In mouse hair cells, high internal Ca2+ concentrations (> 15 μm) promoted a faster component of endocytosis (τ = 300 ms) (Beutner et al., 2001), although we observed here that prolonged Ca2+ influx produced slower rates of endocytosis (Fig. 9). Nevertheless, it is expected that endocytosed vesicles will eventually join the releasable vesicle pool. Indeed, previous studies have demonstrated that vesicles labeled with horseradish peroxidase during endocytosis attached to the synaptic bodies of hair cells after a few minutes (Siegel and Brownell, 1986). Endocytosis will thus aid vesicle recycling during repetitive or prolonged stimulation on a long-time basis, but it probably does not play an important role for the fast recovery from paired-pulse depression.
Adaptation and recovery from synaptic depression
Synaptic vesicles exist in distinct pools that show different fusion competency as demonstrated by multiple kinetic components of exocytosis (Neher and Sakaba, 2008). The charge transfer of EPSCs from afferent fibers showed that there were two distinct kinetic components: a fast rising, transient component (τfast ≈ 12 ms), and a slow, sustained component that did not saturate (Fig. 7B). Mouse cochlear inner hair cells also have a fast component of exocytosis, the readily releasable pool, that is depleted with a time constant τ = 10 ms (Moser and Beutner, 2000). Previous studies have suggested that the depletion of this fast-releasing vesicle pool causes auditory adaptation and the replenishment of this exhausted vesicle pool mediates recovery from adaptation (Furukawa et al., 1978; Westerman and Smith, 1984; Moser and Beutner, 2000; Spassova et al., 2004).
Hair cells in frog amphibian papilla are tonotopically arranged and the average characteristic (or resonant) frequency of our hair cells was ∼400 Hz. Our bullfrog hair cells are thus in the mid-frequency portion of the amphibian papilla, which shows fast adaptation of afferent fiber spikes in response to a pure tone (Feng et al., 1975; Megela, 1984; Lewis, 1986; Christensen-Dalsgaard and Jørgensen, 1996). Considering the similar time course of the in vivo recovery rate from forward masking and the in vitro recovery rate from paired pulse depression (Fig. 2F), we propose that the rapid recovery from spike adaptation at the auditory nerve may be mediated by the fast recovery of the hair cell ribbon synapse from synaptic depression.
Footnotes
This research was funded by Grant RO1 DC04274 from the National Institute on Deafness and Other Communication Disorders (NIDCD) to H.v.G., a Deafness Research Foundation grant and a Tartar fellowship to S.C., and a K99 research award from the NIDCD to G.-L.L.
References
- Abreu BJ, Guimarães M, Uliana LC, Vigh J, von Gersdorff H, Prado MA, Guatimosim C. Protein kinase C modulates synaptic vesicle acidification in a ribbon type nerve terminal in the retina. Neurochem Int. 2008;53:155–164. doi: 10.1016/j.neuint.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Babai N, Bartoletti TM, Thoreson WB. Calcium regulates vesicle recruitment at the cone ribbon synapse. J Neurosci. 2010;30:15866–15877. doi: 10.1523/JNEUROSCI.2891-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoletti TM, Babai N, Thoreson WB. Vesicle pool size at the salamander cone ribbon synapse. J Neurophysiol. 2010;103:419–423. doi: 10.1152/jn.00718.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz WJ. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970;206:629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Michalski N, Safieddine S, Bouleau Y, Schneggenburger R, Chapman ER, Petit C, Dulon D. Control of exocytosis by synaptotagmin and otoferlin in auditory hair cells. J Neurosci. 2010;30:13281–13290. doi: 10.1523/JNEUROSCI.2528-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Burrone J, Lagnado L. Synaptic depression and the kinetics of exocytosis in retinal bipolar cells. J Neurosci. 2000;20:568–578. doi: 10.1523/JNEUROSCI.20-02-00568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimento TC, Schreiner CE. Adaptation and recovery from adaptation in single fiber responses of the cat auditory nerve. J Acoust Soc Am. 1991;90:263–273. doi: 10.1121/1.401296. [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J, Jørgensen MB. One-tone suppression in the frog auditory nerve. J Acoust Soc Am. 1996;100:451–457. doi: 10.1121/1.415872. [DOI] [PubMed] [Google Scholar]
- Coggins M, Zenisek D. Evidence that exocytosis is driven by calcium entry through multiple calcium channels in goldfish retinal bipolar cells. J Neurophysiol. 2009;101:2601–2619. doi: 10.1152/jn.90881.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R. The frequency selectivity of auditory nerve fibers and hair cells in the cochlea of the turtle. J Physiol. 1980;306:79–125. doi: 10.1113/jphysiol.1980.sp013387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon D, Safieddine S, Jones SM, Petit C. Otoferlin is critical for a highly sensitive and linear calcium-dependent exocytosis at vestibular hair cell ribbon synapses. J Neurosci. 2009;29:10474–10487. doi: 10.1523/JNEUROSCI.1009-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds BW, Gregory FD, Schweizer FE. Evidence that fast exocytosis can be predominantly mediated by vesicles not docked at active zones in frog saccular hair cells. J Physiol. 2004;560:439–450. doi: 10.1113/jphysiol.2004.066035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng AS, Narins PM, Capranica RR. Three populations of primary auditory fibers in the bullfrog (Rana catesbeiana): their peripheral origins and frequency sensitivities. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1975;100:221–229. [Google Scholar]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangrŝič T, Khimich D, Fetjova A, Gundelfinger ED, Liberman MC, Harke B, Bryan KE, Lee A, Egner A, Riedel D, Moser T. Bassoon and the synaptic ribbon organize Ca2+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Hayashida Y, Matsuura S. Quantal analysis of the size of excitatory post-synaptic potentials at synapses between hair cells and afferent nerve fibres in goldfish. J Physiol. 1978;276:211–226. doi: 10.1113/jphysiol.1978.sp012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis KD. Admittance-based measurement of membrane capacitance using the EPC-9 patch-clamp amplifier. Pflugers Arch. 2000;439:655–664. doi: 10.1007/s004249900173. [DOI] [PubMed] [Google Scholar]
- Glavinovic MI, Narahashi T. Depression, recovery and facilitation of neuromuscular transmission during prolonged tetanic stimulation. Neuroscience. 1988;25:271–281. doi: 10.1016/0306-4522(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA. Transmitter release at the hair cell ribbon synapse. Nat Neurosci. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Goutman JD, Glowatzki E. Time course and calcium dependence of transmitter release at a single ribbon synapse. Proc Natl Acad Sci U S A. 2007;104:16341–16346. doi: 10.1073/pnas.0705756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant L, Fuchs P. Calcium- and calmodulin-dependent inactivation of calcium channels in inner hair cells of the rat cochlea. J Neurophysiol. 2008;99:2183–2193. doi: 10.1152/jn.01174.2007. [DOI] [PubMed] [Google Scholar]
- Heidelberger R, Thoreson WB, Witkovsky P. Synaptic transmission at retinal synapses. Prog Retin Eye Res. 2005;24:682–720. doi: 10.1016/j.preteyeres.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, von Gersdorff H. Fast endocytosis is inhibited by GABA-mediated chloride influx at a presynaptic terminal. Neuron. 2004;44:469–482. doi: 10.1016/j.neuron.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J Neurosci. 2010;30:11885–11895. doi: 10.1523/JNEUROSCI.1415-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Marcotti W, Kros CJ. Increase in efficiency and reduction in Ca2+ dependence of exocytosis during development of mouse inner hair cells. J Physiol. 2005;563:177–191. doi: 10.1113/jphysiol.2004.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Forge A, Knipper M, Münkner S, Marcotti W. Tonotopic variation in the calcium dependence of neurotransmitter release and vesicle pool replenishment at mammalian auditory ribbon synapses. J Neurosci. 2008;28:7670–7678. doi: 10.1523/JNEUROSCI.0785-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Franz C, Kuhn S, Furness DN, Rüttiger L, Münkner S, Rivolta MN, Seward EP, Herschman HR, Engel J, Knipper M, Marcotti W. Synaptotagmin IV determines the linear Ca2+ dependence of vesicle fusion at auditory ribbon synapses. Nat Neurosci. 2010;13:45–52. doi: 10.1038/nn.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen EC, Hudspeth AJ. Transfer characteristics of the hair cell's afferent synapse. Proc Natl Acad Sci U S A. 2006;103:5537–5542. doi: 10.1073/pnas.0601103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J, Neher E. Modeling buffered Ca2+ diffusion near the membrane: implications for secretion in neuroendocrine cells. Biophys J. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Tachibana M. Ca2+ regulation in the presynaptic terminals of goldfish retinal bipolar cells. J Physiol. 1995;483:79–94. doi: 10.1113/jphysiol.1995.sp020569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão RM, Kushmerick C, Pinaud R, Renden R, Li GL, Taschenberger H, Spirou G, Levinson SR, von Gersdorff H. Presynaptic Na+ channels: locus, development, and recovery from inactivation at a high-fidelity synapse. J Neurosci. 2005;25:3724–3738. doi: 10.1523/JNEUROSCI.3983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ER. Adaptation, suppression and tuning in amphibian acoustical fibers. In: Moore BCJ, Patterson RD, editors. Auditory frequency selectivity. New York: Plenum; 1986. pp. 129–136. [Google Scholar]
- Li GL, Keen E, Andor-Ardó D, Hudspeth AJ, von Gersdorff H. The unitary event underlying multiquantal EPSCs at a hair cell's ribbon synapse. J Neurosci. 2009;29:7558–7568. doi: 10.1523/JNEUROSCI.0514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflügers Arch. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Megela AL. Diversity of adaptation patterns in responses of eighth nerve fibers in the bullfrog, Rana catesbeiana. J Acoust Soc Am. 1984;75:1155–1162. doi: 10.1121/1.390764. [DOI] [PubMed] [Google Scholar]
- Megela AL, Capranica RR. Differential patterns of physiological masking in the anuran auditory nerve. J Acoust Soc Am. 1982;71:641–645. doi: 10.1121/1.387538. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996;17:1241–1249. doi: 10.1016/s0896-6273(00)80254-8. [DOI] [PubMed] [Google Scholar]
- Moser T, Beutner D. Kinetics of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse of the mouse. Proc Natl Acad Sci U S A. 2000;97:883–888. doi: 10.1073/pnas.97.2.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Hull C, Vigh J, von Gersdorff H. Synaptic cleft acidification and modulation of short-term depression by exocytosed protons in retinal bipolar cells. J Neurosci. 2003;23:11332–11341. doi: 10.1523/JNEUROSCI.23-36-11332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Barrow A, Jacoby RA, Wu SM. How do tonic glutamatergic synapses evade receptor desensitization? J Physiol. 2008;586:2889–2902. doi: 10.1113/jphysiol.2008.151050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangrsic T, Lasarow L, Reuter K, Takago H, Schwander M, Riedel D, Frank T, Tarantino LM, Bailey JS, Strenzke N, Brose N, Müller U, Reisinger E, Moser T. Hearing requires otoferlin-dependent efficient replenishment of synaptic vesicles in hair cells. Nat Neurosci. 2010;13:869–876. doi: 10.1038/nn.2578. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Pitchford S, Ashmore JF. An electrical resonance in hair cells of the amphibian papilla of the frog Rana temporaria. Hear Res. 1987;27:75–83. doi: 10.1016/0378-5955(87)90027-x. [DOI] [PubMed] [Google Scholar]
- Rabl K, Cadetti L, Thoreson WB. Paired-pulse depression at photoreceptor synapses. J Neurosci. 2006;26:2555–2563. doi: 10.1523/JNEUROSCI.3667-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renden R, von Gersdorff H. Synaptic vesicle endocytosis at a CNS nerve terminal: faster kinetics at physiological temperatures and increased endocytotic capacity during maturation. J Neurophysiol. 2007;98:3349–3359. doi: 10.1152/jn.00898.2007. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, Yamoah EN. Direct measurement of single-channel Ca2+ currents in bullfrog hair cells reveals two distinct channel subtypes. J Physiol. 2001;534:669–689. doi: 10.1111/j.1469-7793.2001.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Ruiz R, Cano R, Casañas JJ, Gaffield MA, Betz WJ, Tabares L. Active zones and the readily releasable pool of synaptic vesicles at the neuromuscular junction of the mouse. J Neurosci. 2011;31:2000–2008. doi: 10.1523/JNEUROSCI.4663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford MA, Roberts WM. Frequency selectivity of synaptic exocytosis in frog saccular hair cells. Proc Natl Acad Sci U S A. 2006;103:2898–2903. doi: 10.1073/pnas.0511005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieddine S, Wenthold RJ. SNARE complex at the ribbon synapses of cochlear hair cells: analysis of synaptic vesicle- and synaptic membrane-associated proteins. Eur J Neurosci. 1999;11:803–812. doi: 10.1046/j.1460-9568.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- Schnee ME, Ricci AJ. Biophysical and pharmacological characterization of voltage-gated calcium currents in turtle auditory hair cells. J Physiol. 2003;549:697–717. doi: 10.1113/jphysiol.2002.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee ME, Lawton DM, Furness DN, Benke TA, Ricci AJ. Auditory hair cell-afferent fiber synapses are specialized to operate at their best frequencies. Neuron. 2005;47:243–254. doi: 10.1016/j.neuron.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Brownell WE. Synaptic and Golgi membrane recycling in cochlear hair cells. J Neurocytol. 1986;15:311–328. doi: 10.1007/BF01611434. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol. 2006;95:3191–3198. doi: 10.1152/jn.01309.2005. [DOI] [PubMed] [Google Scholar]
- Smith SM, Renden R, von Gersdorff H. Synaptic vesicle endocytosis: fast and slow modes of membrane retrieval. Trends Neurosci. 2008;31:559–568. doi: 10.1016/j.tins.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman MS, Narins PM. Hair cells, hearing and hopping: a field2 guide to hair cell physiology in the frog. J Exp Biol. 2000;203:2237–2246. doi: 10.1242/jeb.203.15.2237. [DOI] [PubMed] [Google Scholar]
- Spassova MA, Avissar M, Furman AC, Crumling MA, Saunders JC, Parsons TD. Evidence that rapid vesicle replenishment of the synaptic ribbon mediates recovery from short-term adaptation at the hair cell afferent synapse. J Assoc Res Otolaryngol. 2004;5:376–390. doi: 10.1007/s10162-004-5003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZL, Jiang SC, Gu R, Yang WP. Two types of calcium channels in bullfrog saccular hair cells. Hear Res. 1995;87:62–68. doi: 10.1016/0378-5955(95)00079-j. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Facilitation, augmentation and potentiation at central synapses. Trends Neurosci. 2000;23:305–312. doi: 10.1016/s0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- Traynelis SF. Software-based correction of single compartment series resistance errors. J Neurosci Methods. 1998;86:25–34. doi: 10.1016/s0165-0270(98)00140-x. [DOI] [PubMed] [Google Scholar]
- Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- Voets T. Dissection of three Ca2+-dependent steps leading to secretion in chromaffin cells from mouse adrenal slices. Neuron. 2000;28:537–545. doi: 10.1016/s0896-6273(00)00131-8. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Calcium-dependent inactivation of calcium current in synaptic terminals of retinal bipolar neurons. J Neurosci. 1996;16:115–122. doi: 10.1523/JNEUROSCI.16-01-00115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Depletion and replenishment of vesicle pools at a ribbon-type synaptic terminal. J Neurosci. 1997;17:1919–1927. doi: 10.1523/JNEUROSCI.17-06-01919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan QF, Vila A, Zhou ZY, Heidelberger R. Synaptic vesicle dynamics in mouse rod bipolar cells. Vis Neurosci. 2008;25:523–533. doi: 10.1017/S0952523808080711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman LA, Smith RL. Rapid and short-term adaptation in auditory nerve responses. Hear Res. 1984;15:249–260. doi: 10.1016/0378-5955(84)90032-7. [DOI] [PubMed] [Google Scholar]
- Wölfel M, Lou X, Schneggenburger R. A mechanism intrinsic to the vesicle fusion machinery determines fast and slow transmitter release at a large CNS synapse. J Neurosci. 2007;27:3198–3210. doi: 10.1523/JNEUROSCI.4471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003;23:2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]