Abstract
Lung cancer is a common cancer and the leading cause of cancer-related death worldwide. Aberrant activation of WNT signaling is implicated in lung carcinogenesis. EMX2, a human homologue of the Drosophila empty spiracles gene is a homeodomain-containing transcription factor. The function of EMX2 has been linked to the WNT signaling pathway during embryonic patterning in mice. However, little is known about the role of EMX2 in human tumorigenesis. In this study, we found that EMX2 was dramatically downregulated in lung cancer tissue samples and this downregulation was associated with methylation of the EMX2 promoter. Restoration of EMX2 expression in lung cancer cells lacking endogenous EMX2 expression suppressed cell proliferation and invasive phenotypes, inhibited canonical WNT signaling, and sensitized lung cancer cells to the treatment of the chemo cytotoxic drug cisplatin. On the other hand, knockdown of EMX2 expression in lung cancer cells expressing endogenous EMX2 promoted cell proliferation, invasive phenotypes and canonical WNT signaling. Taken together, our study suggests that EMX2 may have important roles as a novel suppressor in human lung cancer.
Keywords: EMX2, methylation, lung cancer, WNT signaling, tumor suppression
The American Cancer Society lists lung cancer as the leading cause of cancer death in the United States, exceeding all combined deaths from the next four most deadly cancers, that is breast, colon, prostate and pancreatic cancers (American Cancer Society, 2008). More than 80% of lung cancers are non-small cell lung cancer, which includes distinct histological subtypes: adenocarcinoma, squamous cell carcinoma and large cell carcinoma (Travis, 2002). The mainstays of conventional treatments have offered patients only a limited and generally short-term benefit. Overall, 5-year survival has remained at a dismal ~15% for over two decades (Travis, 2002; Jemal et al., 2008). The molecular carcinogenesis of lung cancer is characterized by multiple alterations of gene expression and function. These arise from a series of molecular and morphological events affecting oncogenes such as K-ras and EGFR (Fong et al., 2003) and tumor suppressor genes such as p53 and p16 (Zochbauer-Muller et al., 2000), all leading inexorably to abnormal changes in cell signaling transduction pathways.
The homeobox gene family encodes transcription factors that regulate morphogenesis and cell differentiation during embryogenesis by activating or repressing the expression of target genes (Boersma et al., 1999). In addition, several homeobox genes have recently been shown to be associated with cancers (Raman et al., 2000; Abate-Shen, 2002; Samuel and Naora, 2005; Yoshida et al., 2006). EMX2 is a human homologue of the Drosophila empty spiracles gene (ems), a homeodomain-containing transcription factor with important functions during early development. For example, mice harboring homozygous mutation of EMX2 (EMX2−/−) exhibit small cerebral hemispheres and olfactory bulbs (Dalton et al., 1989). EMX2 affects the proliferation of adult neural stem cells by regulating the frequency of symmetric divisions that generate two stem cells within the adult neural stem cell population, and overexpression of EMX2 decreases the frequency of symmetric divisions (Galli et al., 2002). EMX2 controls mammalian reproduction by adjusting endometrial cell proliferation without affecting differentiation (Taylor and Fei, 2005). Moreover, it has been reported that loss of EMX2 function leads to ectopic WNT1 expression in the developing mammalian telecephalon, resulting in cortical dysplasia (Ligon et al., 2003). The WNT pathway is well known to have important roles in (Klaus and Birchmeier, 2008), including that in lung cancer (Mazieres et al., 2004; You et al., 2004; He et al., 2005; Huang et al., 2008; Akiri et al., 2009). There have been only a limited number of recent studies suggesting possible involvement of EMX2 in human cancer. For example, EMX2 may be anti-proliferative in the endometrium, and its expression is decreased in endometrial tumors (Noonan et al., 2001, 2003). EMX2 also displays methylation but rarely in non-seminomas (Lind et al., 2006). The role of EMX2 in tumorigenesis, however, is still largely unknown. In this study, we seek to investigate the role of EMX2 in human lung cancer.
We first examined the mRNA levels of EMX2 in human lung cancer tissue samples and their matched adjacent normal tissues obtained from 64 patients with lung cancer. Upon comparison, 71.8% (46–64) lung cancer samples analyzed were found to have less EMX2 expression than their matched adjacent normal tissues (Figure 1a), and this downregulation was statistically significant (mean values of EMX2 expression measured by quantitative RT–PCR were 3.78 and 18.01 in cancer tissues and their matched adjacent normal tissues, respectively; P<0.001). Diminished/absent EMX2 expression was consistently associated with hypermethylation of the EMX2 promoter in these cases evaluated by qMSP (Figure 1b showed an example of 10 paired tissue samples). We also analyzed EMX2 expression and the EMX2 promoter methylation status in 12 lung cancer cell lines to verify these results. In all, 10 of the 12 lines examined were found to lack EMX2 expression (Figure 1c). Using qMSP, we found that the EMX2 promoter in the same 10 cells lines was also methylated (Figure 1d). Next, using a de-methylating reagent, 5-aza-2′-deoxycytidine (DAC), we restored EMX2 expression in cell lines with initially silenced EMX2 (Figure 1e). These data indicate that epigenetic modification may be one of the important mechanisms to silence EMX2 gene in lung cancer. Interestingly, methylation silencing was recently reported for several other homeobox gene family members in cancer. For example, HOXA5 was identified as a direct transcription activator of tumor suppressor p53 and HOXA5 silencing by hypermethylation consequently limited p53 expression in breast cancer (Raman et al., 2000). The HOXA9 promoter was found to be frequently methylated in non-seminomatous TGCT (Lind et al., 2006). Our data also revealed that in a few cases, promoter hypermethylation of EMX2 did not correlate with decreased EMX2 expression, suggesting that alternative mechanisms may account for EMX2 downregulation. Indeed, a report showed that EMX2 transcripts were reduced in a subset of endometrial cancers investigated with a 35% incidence of LOH for the 10q25.3–q26.1 region that included the EMX2 gene. Sequencing analysis uncovered multiple EMX2 variants, including somatic mutations and polymorphisms (Noonan et al., 2001).
Figure 1.
EMX2 expression was downregulated by methylation in lung cancer tissues and cell lines. Fresh samples (lung cancer tissue and its adjacent normal tissue) were collected from patients undergoing surgical resection with approval by the Committee on Human Research at the University of California, San Francisco (UCSF). Samples were promptly snap frozen in liquid nitrogen and stored at −170 °C before use. Total RNA was extracted using TRIzol LS (Invitrogen, Carlsbad, CA, USA). Human lung cancer cell lines were all purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in RPMI 1640 with 10% fetal bovine serum, penicillin (100 IU/ml)/streptomycin (100 μg/ml) at 37°C in a humidified 5% CO2 incubator. cDNA synthesis and Taqman PCR were performed as previously described (Raz et al., 2008). Hybridization probes and primers (Supplementary Information Table S1) were purchased from Applied Biosystems (ABI, Foster City, CA, USA). EMX2 expression of samples was calculated by using the 2−ddCt method (normalizing to their housekeeping gene GAPDH and then comparing to total RNA of adult normal lung tissue (BioChain, Hayward, CA, USA)). Quantitative methylation-specific PCR (qMSP) was performed as previously described (Fackler et al., 2004; Grote et al., 2005, 2006). Genomic DNA was extracted with Qiagen DNeasy kits (Qiagen, Valencia, CA, USA) and bisulfite modification of genomic DNA was performed using EZ DNA Methylation-Gold Kits (Zymo Research, Orange, CA, USA). Primers and probes (Supplementary Table S1) were designed using Primer Express and Methyl Primer Express Software v1.0 (ABI) and purchased from Operon (Huntsville, AL, USA). Relative EMX2 methylation levels were determined by using the 2−dCt method (normalizing to the housekeeping gene ACTB (Raz et al., 2008)) and then calculating the ratio (tumor/matched normal for tissues; cell line/an adult normal lung tissue (BioChain) for cell lines). Both quantitative RT–PCR and qMSP were done in triplicate using an ABI 7300 Real-time PCR System. (a) Quantitative RT–PCR of 64 tumors and their matched adjacent normal lung tissues. The y axis represents normalized relative EMX2 mRNA expression (arbitrary unit). (b) Quantitative RT–PCR (upper panel) and quantitative MSP (lower panel) of 10 representative tumors (black bars) compared with their matched adjacent normal lung tissues (gray bars). (c) Quantitative RT–PCR analysis and (d) Quantitative MSP analysis in lung cancer cell lines. An adult normal lung tissue was used as a control. Results are means±s.d. (error bars). (e) DAC treatment of lung cancer cell lines. Treatment of cells lines with 5μM DAC (Sigma, St Louis, MO, USA) was performed as previously described (Mazieres et al., 2004). Total RNA was isolated using Qiagen RNeasy kit 72 h after treatment, and EMX2 expression was examined by semiquantitative RT–PCR (primers (Supplementary Table S1) were purchased from Operon). GAPDH served as control for RNA quality and loading.
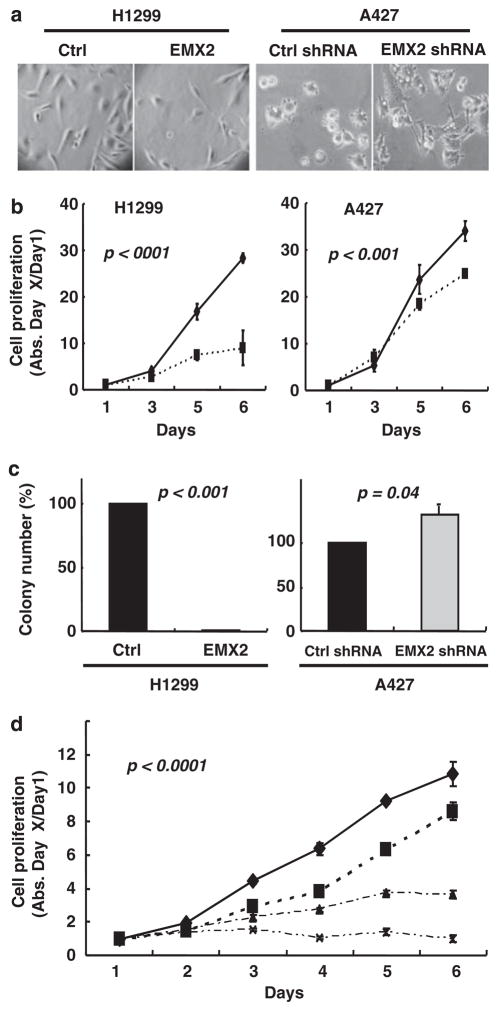
We next investigated the roles of EMX2 in the growth of lung cancer cells in which EMX2 was methylation silenced. One week after transfection and subsequent G418 selection, we found that EMX2 restoration in H1299 led to significant proliferative suppression (MTS: P<0.001; colony formation assays: P<0.001) without dramatic morphological changes (Figures 2a–c). In contrast, when endogenous EMX2 expression in A427 was silenced by anti-EMX2 shRNAs, cell proliferation was stimulated (MTS: P<0.001; colony formation: P=0.04) with dramatic morphological changes (cells were larger with more branches after EMX2 shRNA treatment) (Figures 2a–c). These results complement and support the data observed in H1299 cells. In addition, by using H1299 stably transfected with EMX2, we examined the chemo-synergistic effect between EMX2 and cisplatin, a widely used chemo drug in the clinic to treat lung cancer (Figure 2d). We observed that the suppressive potential of moderate doses of cisplatin was significantly enhanced by reexpressing EMX2 in H1299 cells (P<0.0001), indicating potential future therapeutic role for EMX2 in combination with current cytotoxic agents in lung cancer.
Figure 2.
EMX2 suppressed lung cancer cell proliferation and sensitized lung cancer cells to cisplatin. H1299 cells were transfected with pcDNA 3.1/EMX2 mammalian expression vector (subcloned from pCMV6-XL5/EMX2 vector (Origene, Rockville, MD, USA)). A427 cells were transfected with EMX2 shRNAs (5′-TCAAGCCATTTACCAGGCTTCGGAGGAAG-3′ and 5′-CGG TGGAGAATCGCCACCAAGCAGGCGAG-3′) and non-silencing shRNA (all in pRFP-C-RS vector, Origene). Transfection was done using Lipofectamine2000 (Invitrogen). Transfected cells were re-plated from six-well plates to 10 cm dishes for selection with G418 (500 μg/ml; Invitrogen). Stable transfectants were maintained in regular medium with G418 (300 μg/ml) before analyses. (a) Morphology under light microscope (×40). (b) MTS assay of H1299 cells stably transfected with EMX2 (solid diamonds) and empty pCDNA3.1 vector control (solid squares); and A427 cells stably transfected with EMX2 shRNA (solid squares) and non-silencing shRNA control (solid diamonds). Controls were set as 100%. Proliferation assay was performed by plating the stably transfected cells in 96-well plates at a density of 500–1000 cells/well in 100 μl of G418 culture medium. Medium was changed every day. Cell viability was evaluated in triplicate by CellTiter 96 AQueous (Promega, Madison, WI, USA). (c) Colony formation assay. In all, 500 individual stably transfected cells were seeded in 10 cm dishes and cultured for 10 days. Colonies were then fixed by 10% formalin, stained with 0.5% crystal violet and counted. (d) Synergistic effect between EMX2 and cisplatin in H1299. Diamonds, squares, triangles and crosses are treatments of control vector alone, control vector+cisplatin (0.3 ng/ml), EMX2 cDNA alone and EMX2 cDNA+cisplatin (0.3 ng/ml), respectively. All results are means±s.d. (error bars). Differences between groups were compared with a two-sided Student’s t-test. A P-value of ≤0.05 was considered to be significant.
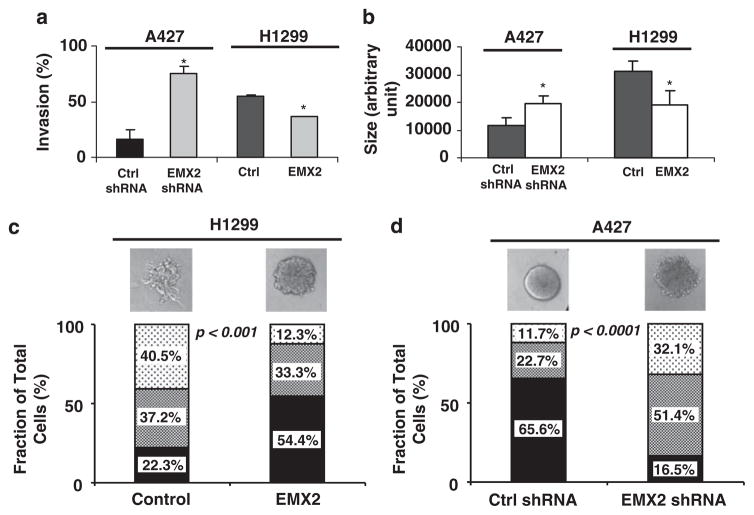
To further assess the function of EMX2 in lung cancer progression, we performed a cell invasion assay and found that the percent invasion of EMX2-stably transfected H1299 cells was significantly reduced (P<0.05) and that the percent invasion of EMX2 shRNA-stably transfected A427 cells was significantly increased (P<0.05) (Figure 3a). Consistently, using a 3D-spheroid model to mimic an in vivo microenvironment, we observed that EMX2-stably transfected H1299 formed smaller spheroids (P<0.05) and EMX2 shRNA-stably transfected A427 formed larger spheroids (P<0.05) (Figure 3b). More importantly, control (non-EMX2 expressing) H1299 cells formed 3D-spheroids with multiple invasive cellular branch-like structures indicating broken basal membrane. However, EMX2-stably transfected H1299 formed a less invasive rounder phenotype (P<0.001) (Figure 3c). In contrast, A427 cells treated with control shRNA formed more round/less invasive spheroids than those treated with EMX2 shRNA (P<0.001) (Figure 3d). Together, our results suggest that EMX2 may have a role as a novel suppressor of malignant lung cell progression or metastasis. To test this hypothesis and explore mechanisms involved in EMX2 function in lung cancer, we examined the canonical WNT signaling pathway known to be aberrantly activated in lung cancer and important for proliferation, survival and metastasis of lung cancer cells (Mazieres et al., 2004; You et al., 2004; He et al., 2005; Huang et al., 2008; Akiri et al., 2009; Nguyen et al., 2009). EMX2 cDNA and anti-EMX2 shRNA were stably transfected into both H1299 cells lacking EMX2 expression and into EMX2-expressing A427 cells, respectively. After stable transfectants were established, we used real-time PCR to examine EMX2 expression. H1703 cell line expressing endogenous EMX2 at similar levels to that of adult normal lung tissue (Figure 1c) was used as an EMX2 expression level control. We confirmed that EMX2 expression in EMX2-stably transfected H1299 was compatible with the physiological expression in normal lung, and determined that EMX2 levels in shRNA-stably transfected A427 were significantly downregulated (Figure 4a). Interestingly, we observed that EMX2 restoration in H1299 cells led to decreased WNT signaling-dependent transcription activity (Figure 4b). In contrast, shRNA silencing of EMX2 in A427 cells resulted in increased transcription activity of the canonical WNT signaling pathway (Figure 4c). In these stable lines, analysis of the canonical WNT downstream effector cytosolic β-catenin and its target Cyclin D1, corroborated the association between EMX2 and the canonical WNT pathway in lung cancer (Figure 4d). After treatment of H1299 cells with DAC, a de-methylation agent, we found that both cytosolic β-catenin and Cyclin D1 were downregulated, confirming our hypothesis (Figure 4e). After DAC treatment in A427 cells, we also observed some downregulation of these WNT downstream effectors (Figure 4e). Our interpretation is that DAC is not a specific de-methylation agent for a single gene such as EMX2. Thus, expression of other tumor suppressors that are known to be methylation silenced in lung cancer such as WNT pathway antagonists WIF-1 and SFRP (Mazieres et al., 2004; Fukui et al., 2005) should also be re-activated after the DAC treatment. This would contribute to the downregulation of those WNT downstream effectors. Moreover, our microarray analysis revealed that restoration of EMX2 expression in lung cancer cells downregulated metastasis genes such as S100P and S100A4, members of the EF-hand calcium-binding protein family (Figure 4f). These proteins have been reported to induce metastasis in rodent mammary model systems for breast cancer and are apparently associated with poor patient outcome in breast, colon, lung and esophageal carcinomas (Donato, 2001; Heizmann et al., 2002; Zimmer et al., 2003; Marenholz et al., 2004). It is possible that EMX2 regulates transcription of these metastasis genes in lung cancer by directly or indirectly downregulating canonical WNT signaling. This is supported by recent evidence that S100P and S100A4 may be direct downstream targets of canonical WNT signaling (Stein et al., 2006; Ganesan et al., 2008). Taken together, our results suggest that EMX2 may suppress lung cancer, either directly or indirectly, through regulation of the canonical WNT signaling activity. In addition, a preliminary analysis of co-expression of several WNT family genes along with EMX2 in lung cancer cell lines (Supplementary Figure S1) supported a possible association between reduced EMX2 expression and canonical WNT activation in lung cancer. It is possible that EMX2 functions as a transcriptional repressor in a similar manner in lung cancer as it does during embryonic development. However, other co-factors may have important roles in EMX2-related transcription regulation. The identification of direct target(s) of EMX2 and its interacting proteins is needed to further elucidate the function of EMX2 and its relationship with canonical WNT signaling in lung cancer.
Figure 3.
EMX2 suppressed invasive phenotypes of lung cancer cells. (a) Invasion assay using trans-well chamber with and without matrigel (BD BioCoat Matrigel Invasion Chamber, BD Biosciences, Lexington, KY, USA) was performed in triplicate for each stable transfectant according to the manufacturer’s protocol. Cells from five different fields of each insert membrane were counted under a light microscope (×40) and percent invasion was determined as follows: % invasion=(mean # of cells invading through matrigel insert membrane/mean # of cells migrating through control insert membrane without matrigel)× 100. (b, c) Analyses of 3D cultures of stably transfected cells. Eight-chambered culture slides (BD) were coated with 35 μl growth-factor reduced Cultrex Basement Membrane Extract (Trevigen, Gaithersburg, MD, USA) per well and left to solidify for 15 min. H1299 or A427 cells were treated with trypsin and resuspended in regular culture medium with serum. Cultrex was added to a total concentration of 2%, and 500 μl of the cell suspension was added to each chamber of the matrigel-coated slide. Medium was replaced every 2 days. After 1 week, 100+ acini were measured for size and graded for disruption. (b) Quantification of size of spheroids. (c) and (d) Phenotypes of spheroids (H1299 and A427) were categorized into three types (round (solid filling), asymmetric (dense dotted) and disrupted (sparse dotted)) and quantified. Representative phenotypes in each treatment were also shown. Symbol (*) in each graph represents statistical significance (P<0.05) by two-sided Student’s t-test.
Figure 4.
EMX2 suppressed canonical WNT signaling in lung cancer cells. (a) Quantitative RT–PCR of EMX2 expression in cell lines stably transfected with control or EMX2 expression vector (in H1299) and with non-silencing control or EMX2-specific shRNA (in A427). H1703 served as an EMX2 expression level control. (b, c) TOP/FOP luciferase assays (performed 24 h after transfection as previously described in Clement et al., 2008) in H1299 cells stably transfected with EMX2 cDNA and in A427 cells stably transfected with EMX2 shRNA, respectively. (d) Western blotting of key canonical WNT downstream effector (cytosolic β-catenin; antibody from BD Biosciences) and target protein (Cyclin D1; antibody from Cell Signaling Technology, Danvers, MA, USA). β-Actin (antibody from Sigma) was used as protein control. Cytosolic proteins were prepared by using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s protocol. (e) The effect of DAC on key canonical WNT downstream effectors. Semiquantitative RT–PCR was used to confirm reactivation of the EMX2 expression after treatment of cells lines with 10 μM DAC. DAC treatment and semiquantitative RT–PCR were performed as described in Figure 1e. GAPDH served as control for RNA quality and loading. Western blotting of cytosolic β-catenin and Cyclin D1 was performed as described in Figure 4d. β-Actin was used as protein control. (f) Microarray profiling of lung cancer cell line H1299 stably transfected with EMX2 and empty vector control. Partial heatmap plot of hierarchical clustering including S100A4 and S100P is shown. Total RNA quality was assessed using a Pico Chip on an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). RNA was amplified and labeled with Cy3-CTP or Cy5-CTP using the Agilent low RNA input fluorescent linear amplification kits following the manufacturer’s protocol. Labeled cRNA was assessed using the Nandrop ND-100, and equal amounts of Cy3- and Cy5-labeled target were hybridized to Agilent whole-human genome 44 K ink-jet arrays. Hybridization samples were randomized on the 3 × 44K format to correct any batch bias. Hybridizations were performed for 14 h according to the manufacturer’s protocol. Arrays were scanned using the Agilent microarray scanner and raw signal intensities were extracted with Feature Extraction v9.5 software (Agilent).
In conclusion, this is the first demonstration of the importance of EMX2 as a suppressor in lung carcinogenesis. Epigenetic silencing of the EMX2 expression may be important for aberrant activation of the canonical WNT signaling in lung cancer and consequent proliferation and metastasis of lung cancer cells.
Acknowledgments
This study was supported by grant from Joan’s Legacy: uniting against Lung Cancer Research Grant, NIH/NCI grant R01CA125030 and the Eileen D Ludwig endowed for Thoracic Oncology Research (to BH); the Bonnie J Addario Lung Cancer Foundation, the Kazan, McClain, Abrams, Fernandez, Lyons, Greenwood, Harley and Oberman Foundation and the Barbara Isackson Lung Cancer Research Fund (DJ); Swedish Cancer Institute (MJ); NIH/NCI grants R01CA130980, R01CA13256 and DOD BCRP Era of Hope Scholar Award (W81XWH-06-1-0416) (to LMC). Microarray profiling was done by the UCSF Shared Microarray Core Facilities. We thank M Roshni Ray for editing this paper.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;10:777–785. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- Akiri G, Cherian MM, Vijayakumar S, Liu G, Bafico A, Aaronson SA. Wnt pathway aberrations including autocrine Wnt activation occur at high frequency in human non-small-cell lung carcinoma. Oncogene. 2009;28:2163–2172. doi: 10.1038/onc.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Cancer Society. Cancer facts and figures. 2008. [Google Scholar]
- Boersma CJ, Bloemen M, Hendriks JM, van Berkel EA, Olijve W, van Zoelen EJ. Homeobox proteins as signal transduction intermediates in regulation of NCAM expression by recombinant human bone morphogenetic protein-2 in osteoblast-like cells. Mol Cell Biol Res Commun. 1999;2:117–124. doi: 10.1006/mcbr.1999.0115. [DOI] [PubMed] [Google Scholar]
- Clement G, Guilleret I, He B, Yagui-Beltrán A, Lin YC, You L, et al. Epigenetic alteration of theWnt inhibitory factor-1 promoter occurs early in the carcinogenesis of Barrett’s esophagus. Cancer Sci. 2008;99:46–53. doi: 10.1111/j.1349-7006.2007.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D, Chadwick R, McGinnis W. Expression and embryonic function of empty spiracles: a Drosophila homeo box gene with two patterning functions on the anterior-posterior axis of the embryo. Genes Dev. 1989;3:1940–1956. doi: 10.1101/gad.3.12a.1940. [DOI] [PubMed] [Google Scholar]
- Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–668. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- Fackler MJ, McVeigh M, Mehrotra J, Blum MA, Lange J, Lapides A, et al. Quantitative multiplex methylation-specific PCR assay for the detection of promoter hypermethylation in multiple genes in breast cancer. Cancer Res. 2004;64:4442–4452. doi: 10.1158/0008-5472.CAN-03-3341. [DOI] [PubMed] [Google Scholar]
- Fong KM, Sekido Y, Gazdar AF, Minna JD. Lung cancer. 9: molecular biology of lung cancer: clinical implications. Thorax. 2003;58:892–900. doi: 10.1136/thorax.58.10.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T, Kondo M, Ito G, Maeda O, Sato N, Yoshioka H, et al. Transcriptional silencing of secreted frizzled related protein 1 (SFRP 1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene. 2005;24:6323–6327. doi: 10.1038/sj.onc.1208777. [DOI] [PubMed] [Google Scholar]
- Galli R, Fiocco R, De Filippis L, Muzio L, Gritti A, Mercurio S, et al. Emx2 regulates the proliferation of stem cells of the adult mammalian central nervous system. Development. 2002;129:1633–1644. doi: 10.1242/dev.129.7.1633. [DOI] [PubMed] [Google Scholar]
- Ganesan K, Ivanova T, Wu Y, Rajasegaran V, Wu J, Lee MH, et al. Inhibition of gastric cancer invasion and metastasis by PLA2G2A, a novel beta-catenin/TCF target gene. Cancer Res. 2008;68:4277–4286. doi: 10.1158/0008-5472.CAN-07-6517. [DOI] [PubMed] [Google Scholar]
- Grote HJ, Schmiemann V, Geddert H, Bocking A, Kappes R, Gabbert HE, et al. Methylation of RAS association domain family protein 1a as a biomarker of lung cancer. Cancer. 2006;108:129–134. doi: 10.1002/cncr.21717. [DOI] [PubMed] [Google Scholar]
- Grote HJ, Schmiemann V, Geddert H, Rohr UP, Kappes R, Gabbert HE, et al. Aberrant promoter methylation of p16(INK4a), RARB2 and SEMA3B in bronchial aspirates from patients with suspected lung cancer. Int J Cancer. 2005;116:720–725. doi: 10.1002/ijc.21090. [DOI] [PubMed] [Google Scholar]
- He B, Lee AY, Dadfarmay S, You L, Xu Z, Reguart N, et al. SFRP4 is silenced by hypermethylation and induces apoptosis in β-catenin-deficient human mesothelioma cells. Cancer Res. 2005;65:743–748. [PubMed] [Google Scholar]
- Heizmann CW, Fritz G, Schafer BW. S100 proteins: structure, functions and pathology. Front Biosci. 2002;7:d1356–d1368. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- Huang CL, Liu D, Ishikawa S, Nakashima T, Nakashima N, Yokomise H, et al. Wnt1 overexpression promotes tumour progression in non-small cell lung cancer. Eur J Cancer. 2008;44:2680–2688. doi: 10.1016/j.ejca.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signaling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Echelard Y, Assimacopoulos S, Danielian PS, Kaing S, Grove EA, et al. Loss of Emx2 function leads to ectopic expression of Wnt1 in the developing telencephalon and cortical dysplasia. Development. 2003;130:2275–2287. doi: 10.1242/dev.00421. [DOI] [PubMed] [Google Scholar]
- Lind GE, Skotheim RI, Fraga MF, Abeler VM, Esteller M, Lothe RA. Novel epigenetically deregulated genes in testicular cancer include homeobox genes and SCGB3A1 (HIN-1) J Pathol. 2006;210:441–449. doi: 10.1002/path.2064. [DOI] [PubMed] [Google Scholar]
- Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature) Biochem Biophys Res Commun. 2004;322:1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- Mazieres J, He B, You L, Xu Z, Lee AY, Mikami I, et al. Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer Res. 2004;64:4717–4720. doi: 10.1158/0008-5472.CAN-04-1389. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan FC, Goodfellow PJ, Staloch LJ, Mutch DG, Simon TC. Antisense transcripts at the EMX2 locus in human and mouse. Genomics. 2003;81:58–66. doi: 10.1016/s0888-7543(02)00023-x. [DOI] [PubMed] [Google Scholar]
- Noonan FC, Mutch DG, Ann Mallon M, Goodfellow PJ. Characterization of the homeodomain gene EMX2: sequence conservation, expression analysis, and a search for mutations in endometrial cancers. Genomics. 2001;76:37–44. doi: 10.1006/geno.2001.6590. [DOI] [PubMed] [Google Scholar]
- Raman V, Tamori A, Vali M, Zeller K, Korz D, Sukumar S. HOXA5 regulates expression of the progesterone receptor. J Biol Chem. 2000;275:26551–26555. doi: 10.1074/jbc.C000324200. [DOI] [PubMed] [Google Scholar]
- Raz D, Ray MR, Kim J, He B, Taron M, Skrzypski M, et al. A multi-gene assay is prognostic of survival in patients with early-stage lung adenocarcinoma. Clin Cancer Res. 2008;14:5565–5570. doi: 10.1158/1078-0432.CCR-08-0544. [DOI] [PubMed] [Google Scholar]
- Samuel S, Naora H. Homeobox gene expression in cancer: insights from developmental regulation and deregulation. Eur J Cancer. 2005;16:2428–2437. doi: 10.1016/j.ejca.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Stein U, Arlt F, Walther W, Smith J, Waldman T, Harris ED, et al. The metastasis-associated gene S100A4 is a novel target of beta-catenin/T-cell factor signaling in colon cancer. Gastroenterology. 2006;131:1486–1500. doi: 10.1053/j.gastro.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Fei X. Emx2 regulates mammalian reproduction by altering endometrial cell proliferation. Mol Endocrinol. 2005;19:2839–2846. doi: 10.1210/me.2005-0130. [DOI] [PubMed] [Google Scholar]
- Travis WD. Pathology of lung cancer. Clin Chest Med. 2002;23:65–81. viii. doi: 10.1016/s0272-5231(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Broaddus R, Cheng W, Xie S, Naora H. Deregulation of the HOXA10 homeobox gene in endometrial carcinoma: role in epithelial-mesenchymal transition. Cancer Res. 2006;2:889–897. doi: 10.1158/0008-5472.CAN-05-2828. [DOI] [PubMed] [Google Scholar]
- You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, et al. A novel anti-human Wnt-2 monoclonal antibody induces programmed cell death in human non-small-cell lung cancer cells. Oncogene. 2004;23:6170–6174. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- Zimmer DB, Wright Sadosky P, Weber DJ. Molecular mechanisms of S100-target protein interactions. Microsc Res Tech. 2003;60:552–559. doi: 10.1002/jemt.10297. [DOI] [PubMed] [Google Scholar]
- Zochbauer-Muller S, Wistuba II, Minna JD, Gazdar AF. Fragile histidine triad (FHIT) gene abnormalities in lung cancer. Clin Lung Cancer. 2000;2:141–145. doi: 10.3816/clc.2000.n.027. [DOI] [PubMed] [Google Scholar]