Abstract
Members of the rhizobia are distinguished for their ability to establish a nitrogen-fixing symbiosis with leguminous plants. While many details of this relationship remain a mystery, much effort has gone into elucidating the mechanisms governing bacterium-host recognition and the events leading to symbiosis. Several signal molecules, including plant-produced flavonoids and bacterially produced nodulation factors and exopolysaccharides, are known to function in the molecular conversation between the host and the symbiont. Work by several laboratories has shown that an additional mode of regulation, quorum sensing, intercedes in the signal exchange process and perhaps plays a major role in preparing and coordinating the nitrogen-fixing rhizobia during the establishment of the symbiosis. Rhizobium leguminosarum, for example, carries a multitiered quorum-sensing system that represents one of the most complex regulatory networks identified for this form of gene regulation. This review focuses on the recent stream of information regarding quorum sensing in the nitrogen-fixing rhizobia. Seminal work on the quorum-sensing systems of R. leguminosarum bv. viciae, R. etli, Rhizobium sp. strain NGR234, Sinorhizobium meliloti, and Bradyrhizobium japonicum is presented and discussed. The latest work shows that quorum sensing can be linked to various symbiotic phenomena including nodulation efficiency, symbiosome development, exopolysaccharide production, and nitrogen fixation, all of which are important for the establishment of a successful symbiosis. Many questions remain to be answered, but the knowledge obtained so far provides a firm foundation for future studies on the role of quorum-sensing mediated gene regulation in host-bacterium interactions.
INTRODUCTION
Bacterial populations coordinately regulate gene expression by producing diffusible signal molecules. These signals, known as autoinducers, accumulate extracellularly and interact specifically with a receptor protein to affect changes not related to their own metabolism. Production of autoinducers typically occurs at specific stages of growth or in response to changes in the environment and induces a concerted response once a critical concentration has been reached. These diffusible signals frequently act to induce gene expression in response to bacterial cell density in a process often referred to as quorum sensing (8, 59, 60, 69, 120, 166, 178, 182, 185). Alternatively, autoinducer secretion and response may confer on the bacterium the ability to determine whether secreted molecules move away from the cell. This process, termed diffusion sensing by Rosemary Redfield, could allow the cells to regulate the secretion of effectors, such as degradative enzymes, antibiotics, surfactants, and siderophores, to minimize losses to extracellular diffusion (137). The best characterized quorum-sensing mechanism is found in gram-negative organisms and involves the use of acylated homoserine lactones (AHLs) as signal molecules (8, 59, 60, 63, 69, 120, 145, 166, 178, 182, 185).
Recent publications have shown that quorum sensing plays a major role in preparing and perhaps coordinating the symbiotic nitrogen-fixing rhizobia during the establishment of their interactions with the host plant.
HOW BACTERIA TALK TO EACH OTHER
AHL-Mediated Cell-Cell Communication
Historically, it was thought that bacteria were solitary individuals, each growing independently of the population. However, in 1970 Nealson et al. (114) discovered that bacteria can sense and respond to the rest of the population. This phenomenon is called quorum sensing and is defined as the cell density-dependent regulation of gene expression (for reviews, see reference 51, 59, 60, 120, and 181). One of the best-studied examples of quorum sensing is in Photobacterium fischeri (formerly Vibrio fischeri), a marine bacterium that is a symbiont of several marine fish and squids (Fig. 1) (142, 173). In this model organism, the basal-level synthesis of autoinducers occurs at low cell densities, like those found in seawater. The autoinducers, which belong to the AHL family of signal molecules, are thought to pass through the cell membrane by diffusion (86). As the cell density increases during the symbiotic association with the animal host, autoinducers accumulate in and around the cells (86).
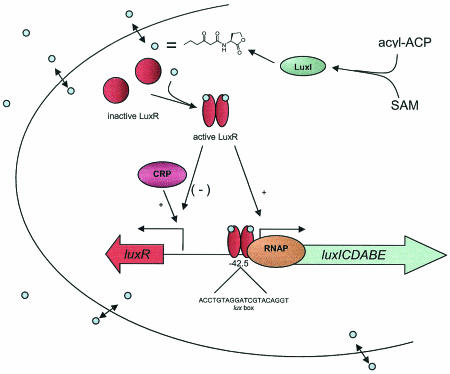
FIG. 1.
Quorum-sensing model in P. fischeri. At low cell densities, transcription of the luxICDABE operon occurs at basal levels. LuxI encodes the AHL synthase, which synthesizes 3-oxo-C6-HSL from acyl-ACP and SAM substrates. High cell densities lead to the accumulation of AHLs, which bind and activate the LuxR transcriptional activator. LuxR binds to an inverted repeat referred to as the lux box, which is centered at −42.5 from the transcriptional start site, and makes contact with the RNA polymerase to stimulate the expression of the luxICDABE genes. Expression of the luxR gene is regulated by several factors such as heat shock, catabolite repression, and even LuxR itself (only at very high cell densities).
When a threshold level of AHLs (about 10 nM) is reached, the LuxR regulator is activated by binding the AHL (73, 86). LuxR, a transcriptional activator, then induces expression of the lux operon (Fig. 1). The lux operon contains luxI (the AHL synthase) along with the genes necessary for luminescence (48, 49, 164). Activation of the lux operon leads to a rapid rise in the levels of autoinducer and creates a positive-feedback loop, which is followed by the onset of luminescence. LuxR is regulated at the transcriptional level by cyclic AMP receptor protein (41) and presumably at the posttranscriptional level by GroEL (37). At low AHL levels, LuxR activates its expression, while at high AHL levels, the active LuxR represses itself (152, 153).
Conjugal Transfer of the A. tumefaciens Ti Plasmid
Agrobacterium tumefaciens has become one of the paradigms for quorum sensing, especially as it relates to soil organisms. This plant pathogen induces crown gall tumors on susceptible plant hosts (181, 190). This is mediated by the transfer of oncogenic DNA fragments from its Ti plasmid directly into the nuclei of the host plant cell (63, 181). These DNA fragments encode the production and secretion of opines by the plant, which are then utilized by A. tumefaciens as nutrients. In addition, the Ti plasmids mediate their own conjugal transfer between associated agrobacteria (51, 128). This process is regulated in part by quorum sensing. The Ti plasmid carries the key quorum-sensing regulators: traI (a luxI homolog), traR (a luxR homolog), and traM (an antiactivator of TraR) (58, 62, 83, 84). TraI synthesizes an AHL, 3-oxo-C8-homoserine lactone (3-oxo-C8-HSL), which binds and activates TraR (135). TraR then goes on to activate its targets: traAFB, traCDG, and traI-trb (58, 62, 83, 130, 188). When the level of TraR is low, TraM binds to it and forms an inactive complex, thereby preventing plasmid transfer until the cell density is high (85, 104, 132, 165). The Ti plasmid copy number is also influenced by the quorum-sensing system. Transcription of the Ti repABC operon is regulated by TraR in combination with 3-oxo-C8-HSL. This activation leads to an elevated Ti plasmid copy number and enhanced tumorigenesis (94, 118, 119). In addition to TraM, the tra genes are under another level of regulation. Opines secreted by crown gall tumors bind a receptor protein, AccR or OccR, depending on the strain (14, 72). The opine-receptor protein combination initiates the transcription of traR by either induction (OccR) or derepression (AccR) (131). Therefore, conjugal transfer of the Ti plasmid does not occur in the absence of opines. Thus, while the quorum-sensing network is similar to that of the P. fischeri model, an additional regulatory network, required for proper induction of conjugal plasmid transfer, is superimposed on the tra system.
AHL-Mediated Cell-Cell Communication Plays a Role in Symbiosis and Pathogenesis in Some Organisms
In the organisms characterized so far, the quorum-sensing mechanisms are similar to those in the P. fischeri paradigm but the activated target genes are diverse. Examples of genes regulated by quorum sensing include the lux (luminescence) genes in P. fischeri, the tra (Ti plasmid transfer) genes in A. tumefaciens, exoenzymes and virulence factors in Pseudomonas aeruginosa and Erwinia carotovora, swarming motility in Serratia liquefaciens, antibiotics and violacein pigment in Chromobacterium violaceum, and exopolysaccharide production in Pantoea stewartii (43, 63, 145, 166). All of these organisms have one or more LuxR and LuxI homologues. In addition to sharing similar quorum-sensing mechanisms, most of the organisms establish symbiotic or pathogenic relationships with eukaryotic hosts. Therefore, it is not surprising that symbiosis, pathogenesis, and quorum sensing are intertwined in a complex story of gene regulation. Furthermore, the possibility has been raised that in natural habitats, different bacterial species communicate with one another to coordinate their behavior (7, 8). An example of this is the bacterial community that naturally colonizes the roots of tomato plants (156). It has been suggested that the AHLs act as signals for coordination of the functions of the different populations within this rhizosphere community. Further evidence for interspecies signaling through AHLs was shown by McKenney et al. (110), when they demonstrated that AHLs in spent culture supernatants from P. aeruginosa enhanced virulence factor production in Burkholderia cepacia.
MOLECULAR MECHANISMS
AHL Family of Autoinducers
AHL characteristics.
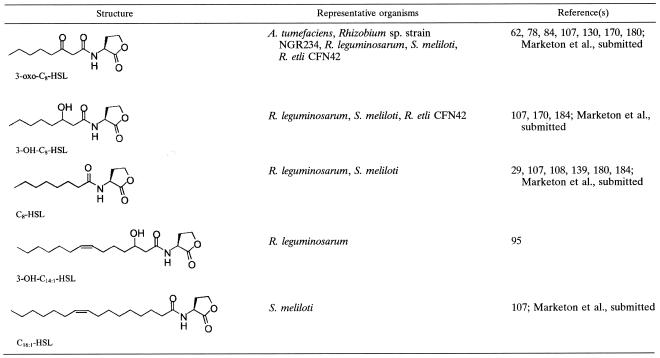
AHLs consist of an HSL head group attached to a variable acyl side chain (Table 1). The amphipathy of the AHL molecule seems to be a balance between the hydrophobic side chain and the hydrophilic HSL ring. These characteristics presumably allow the AHLs to traverse the phospholipid bilayer of the cell membrane and to navigate the aqueous intracellular and extracellular environments (60). The acyl chain varies in length, from 4 to 18 carbons in those AHLs identified so far (71, 77, 108, 134, 150). Variability also exists in the third carbon position of the acyl chain, where there can be a hydrogen, hydroxyl, or oxo substitution (see Table 1). A few AHLs that have unsaturated acyl chains have also been identified (71, 134, 150). The overall length of the side chain and the chemical modification at the third carbon position provide the specificity to quorum-sensing signals. To add complexity, most organisms produce more than one type of AHL and different organisms can produce the same AHL (77). Therefore, there is some overlap in the production and recognition of AHLs by different organisms.
TABLE 1.
Characteristics of AHLs
AHL Synthases
LuxI-type synthases.
All LuxI-type proteins identified to date resemble the LuxI of P. fischeri and catalyze the ligation of S-adenosylmethionine (SAM) with an acylated acyl carrier protein (acyl-ACP), which form the HSL and acyl chain components, respectively, of the resulting AHL (60, 111, 147). The catalytic model proposed by Parsek, Greenberg, and coworkers suggests a nucleophilic attack on the C-1 position of the acyl-ACP molecule by the amino nitrogen of SAM, resulting in an amide linkage. This is followed by the lactonization of SAM together with amide bond formation, which results in ring formation and release of the AHL (42, 74, 122). LuxI homologues are about 200 amino acids in length, and disruption of certain conserved residues in the amino-terminal half of the protein leads to a reduction or loss of synthase activity. It has been suggested that conserved amino acids in the carboxy terminus may be necessary for acyl-ACP selection (76, 121). The recently determined crystal structure of EsaI, the LuxI homologue from P. stewartii, shows remarkable similarity to N-acetyltransferases, and its core catalytic fold has features essential for phosphopantetheine binding (175, 176). The structural analysis suggests that the N acylation of SAM is likely to include abstraction of an amine proton by a catalytic base. In addition, variable residues in the C-terminal half of the protein and the nature of the amino acid at position 140 constitute the basis for the acyl chain specificity (175, 176).
LuxM/AinS-type synthases.
The products of the luxM gene from V. harveyi and the ainS gene from P. fischeri synthesize AHLs despite their lack of similarity to LuxI (10, 64). These AHL synthases direct the synthesis of 3-OH-C4-HSL and C8-HSL, respectively. These two proteins share a strong conserved region, and null mutations in either of these genes abolish synthesis of their respective AHLs. In vitro studies revealed that AinS catalyzes C8-HSL synthesis from SAM and either octanoyl-ACP or octanoyl coenzyme A (octanoyl-CoA) conjugates (75). This is in contrast to LuxI-type proteins, which use acyl-ACP as their primary source of fatty acyl chains.
HdtS-type synthases.
More recently, a third AHL synthase, HdtS, has been detected in Pseudomonas fluorescens F113 (87). When expressed in Escherichia coli, hdtS directs the production of three AHLs (3-OH-C14:1-HSL, C10-HSL, and C6-HSL). The HdtS protein sequence does not show homology to either the LuxI or the LuxM families of AHL synthases (87). Instead, it seems to be related to the lysophosphatidic acid acyl transferase family, which is responsible for the transfer of an acyl chain from either acyl-ACP or acyl-CoA to lysophosphatidic acid, resulting in the production of phosphatidic acid. It has been suggested that HdtS could transfer acyl chains from acyl-ACP or acyl-CoA to SAM to generate AHLs (87).
AI-2 Family of Autoinducers
LuxS-type synthases.
A second autoinducer response system, independent of the AHL/LuxR-type system, controls the density-dependent expression of the luciferase (lux) operon in V. harveyi (7-9, 11). This system synthesizes and responds to a signal termed autoinducer-2 (AI-2), as opposed to the classical AHL (AI-1) based system. This second autoinducer is a furanosyl borate diester (25). AI-2 is also produced from SAM and requires three enzymatic steps (148). The use of SAM as a methyl donor produces S-adenosylhomocysteine (SAH). Hydrolysis of SAH results in adenine and S-ribosylhomocysteine (SRH), and LuxS catalyzes the conversion of SRH to 4,5-dihydroxy-2,3-pentanedione (DPD) and homocysteine. DPD undergoes additional rearrangements, resulting in two fused five-member rings with a boron atom bridging the diester (25). Synthesis of AI-2 is dependent on the luxS gene (162), which has no homology to the luxI, luxM/ainS, or hdtS families. Highly conserved luxS homologues have been identified in both gram-negative and gram-positive bacterial species. Despite this, it is not clear that all bacteria that produce AI-2 use it as a signaling molecule. Some evidence suggests that AI-2 may be a metabolic waste product that could under some circumstances play a role in signaling (162, 163). So far, no luxS homologues have been found in the α-proteobacteria.
Quorum-Sensing Regulators
LuxR-type regulators.
LuxR-type proteins share two regions of sequence conservation, an AHL binding domain and a DNA binding motif (154, 155). The current model suggests that LuxR acts as a dimeric protein (135, 192). LuxR regulators have an amino-terminal domain that binds AHLs and mediates protein oligomerization (28). The cytoplasmic carboxy-terminal domain includes a helix-turn-helix DNA binding region that is thought to be involved in transcriptional regulation (27, 28). Specific interactions between the cognate AHLs and purified LuxR homologues have been demonstrated by various laboratories (135, 177, 191). Studies of A. tumefaciens have suggested that TraR, on binding the AHL signal, undergoes a conformational change, dimerizes, and activates transcription (192). Transcriptional activation by LuxR-type proteins requires cis-acting DNA elements, normally referred to as lux-type boxes (34, 70). The typical lux-type box is an 18- to 22-bp inverted-repeat sequence centered at about −40 from the transcriptional start site (44). Although many target genes of the LuxR-type regulators contain lux-type boxes within their promoters, there are reports of targets lacking a discernible lux box (58, 61). Once bound, LuxR facilitates the binding of RNA polymerase to the target promoter (157-159), leading to activation of transcription. Although most instances show AHL-bound LuxR-type proteins to function as transcriptional activators, a few examples of these proteins have been reported to function as repressors (2, 13, 15).
A new and exciting development in the study of quorum sensing is in the area of structural biology. Recently the TraR protein was crystallized in the presence of 3-oxo-C8-HSL and a self-complementary oligonucleotide containing the canonical tra box sequence (171, 189). The crystal unit contains two TraR dimers, each binding two molecules of 3-oxo-C8-HSL and one duplex DNA fragment. The N-terminal domain of each TraR monomer binds the AHL molecule, while the C-terminal domain contains a four-helix bundle that binds to half a tra box (171, 189). These two domains are joined by a 12-amino acid linker. The AHL binding domains seem to provide a significant dimerization interface, while the DNA binding domains add additional dimer interactions (189). Interestingly, the AHL molecule is fully embedded within the protein and shows no significant contact with solvent (171, 189). Earlier studies from the laboratory of Steve Winans suggested that TraR may require the AHL molecule as a scaffold that helps the protein to acquire a protease-resistant tertiary structure (191, 192). The recent structural studies seem to confirm the key role of the AHL in the correct folding of the nascent TraR protein (171, 189). Another interesting finding of the structural work is the overall asymmetry of the dimer complex. The TraR dimer displays a twofold symmetry axis in each domain, but the two axes of symmetry are at a 90° angle (171, 189).
The authors of one of these studies (189) propose that the autoinducer indirectly affects gene activation by increasing the stability of TraR and the formation of active dimers. The dimers are then predisposed to recognize specific TraR binding sites and activate transcription (189).
AHL Accumulation and Transport
AHL concentrations are thought to rise mainly as a result of the increase in population density (143). Although AHLs can clearly accumulate as a result of a simple increase in bacterial numbers, other factors could also influence the environmental concentration of these signal molecules. Bacterial aggregation, biofilm formation, and physical confinement could play roles in increasing the local concentration of AHLs (137). Other conditions such as high pH or enzymatic degradation could effectively decrease the concentration of signal molecules available for LuxR-type protein activation.
In the mid-1980s it was shown by Kaplan and Greenberg that radiolabeled 3-oxo-C6-HSL from P. fischeri freely diffuses in and out of both P. fischeri and E. coli cells (86). Their studies showed that the distribution of 3-oxo-C6-HSL was dictated largely by its concentration gradient across the bacterial membrane. AHLs from other bacteria were also thought to cross the bacterial envelope by diffusion, since exogenous AHLs activated LuxR-type proteins. However, with the identification of AHLs with long acyl chains, the idea that all AHLs are freely diffusible has come into question. Of particular significance is the observation by Iglewski and colleagues that the 3-oxo-C12-HSL produced by P. aeruginosa does not rely solely on diffusion to exit the cells. In a series of elegant experiments, they showed that 3-oxo-C12-HSL was subject to active export by a specific efflux pump encoded by the mexAB-oprM operon (124), a member of a large family of antibiotic transporters. In the absence of this pump, cellular levels of 3-oxo-C12-HSL were much higher than external levels. Therefore, while the long-chain AHL was able to diffuse across the cell membranes, it was not very efficient in doing so and was likely to partition to the lipid bilayer (124). This is in contrast to the C4-HSL also produced by P. aeruginosa, which freely diffuses into and out of the cells (124).
Although active transport of an AHL has been demonstrated for 3-oxo-C12-HSL only in P. aeruginosa, increasing numbers of organisms have been shown to produce long-chain AHLs (108, 146). It is not yet known if pumps similar to the MexAB-type pump are involved in AHL transport in those organisms. However, the efflux pump encoded by the mexAB-oprM operon belongs to the resistance/nodulation/cell division family, which includes a number of other multidrug resistance proteins (AcrB and AcrF from E. coli and MtrD from Neisseria gonorrhoeae) (116, 123). Interestingly, this family also includes the NolGHI system from S. meliloti, which may be involved in the export of nodulation signals (144).
ENVIRONMENTAL CONSIDERATIONS AND THE ECOLOGY OF QUORUM SENSING
Soil Relationships
A wide variety of soil- and plant-associated bacteria produce AHLs (24). Recent work has suggested that AHL production is more common in plant-associated than in soil-borne pseudomonads (46). In a survey study conducted in Stephen Farrand's laboratory, the pattern of AHLs produced by numerous plant-associated bacteria was analyzed by reverse-phase C18 thin-layer chromatography (24). Although this technique cannot identify the exact AHL structures, it is interesting that several different species appeared to have one or more AHLs in common. For instance, Erwinia carotovora pv. atroseptica, several xanthomonads, A. tumefaciens, and several rhizobia all have an AHL with a mobility identical to 3-oxo-C8-HSL (24). In addition, most rhizobia had an AHL that was strongly nonpolar and stayed at the thin-layer chromatography plate origin. It has been suggested that in the soil, various chemical signals serve as a bacterial Esperanto, helping microorganisms interact with or avoid each other in their quest to interrelate with their plant hosts. Elegant studies by Bassler and coworkers (7) suggested that quorum sensing modulates both intra- and interspecies cell-cell communication. It is interesting to speculate that evolution might have allowed the development of this type of chemical communication to ultimately increase cell survivability by coordinating interactions among potential bacterial competitors and their plant hosts.
AHL Mimics
A large number of bacteria associated with eukaryotes are known to regulate important phenotypes like motility, virulence, exoenzyme production, exopolysaccharide production, and antibiotic production by quorum sensing (63, 77). As stated above, a variety of plant-associated bacteria produce AHL quorum-sensing signals (24). Therefore, the potential exists for the eukaryotic hosts to disrupt this regulatory system by producing homologs (AHL mimics) to the quorum-sensing signals and thus protect themselves from pathogens by modifying bacterial behavior.
Pisum sativum (pea) and other higher plants produce AHL-mimicking compounds that interfere with the quorum-sensing-regulated behavior of several reporter strains (167). Another example of a plant signal affecting quorum sensing in an associated bacterium is that of the Australian red alga Delisea pulchra, which interferes with the swarming motility of Serratia liquefaciens. D. pulchra produces halogenated furanones that are structurally similar to the AHL signals produced by S. liquefaciens. The furanones successfully inhibit swarming motility in S. liquefaciens (65). It was recently demonstrated that the halogenated furanones modulate LuxR activity through accelerated degradation of the transcriptional activator rather than by blocking or displacing the binding of the AHL signal (105).
There could be more such mechanisms that are utilized by eukaryotic hosts to interfere with the quorum-sensing behavior of associated bacteria. It would be very interesting and rewarding to investigate such systems, since they could prove to be a potential solution for the manipulation of pathogenic and symbiotic bacteria.
AHL Degradation
Another potential way to interfere with quorum sensing is through the degradation or inactivation of the AHL signal molecules. A strain of Variovorax paradoxus was isolated from soil based on its ability to utilize AHLs as the sole source of energy and nitrogen, an activity that could disrupt the signaling process of other bacteria sharing the same environment (88). More recently, Zhang and colleagues isolated a Bacillus sp. capable of inactivating AHLs. They cloned from this species a gene encoding an AHL-lactonase capable of inactivating AHLs by hydrolyzing the lactone bond (39). Furthermore, transgenic plants expressing the AHL-lactonase activity were found to be more resistant to E. carotovora, a plant pathogen that requires AHLs for expression of the genes necessary for pathogenicity (38).
BACTERIUM-PLANT SYMBIOTIC INTERACTIONS
The symbiotic relationships formed between the nitrogen-fixing rhizobia and their legume hosts are the result of an intricate signaling network between the host and symbiont. As yet, many aspects of the signal exchange are still a mystery; however, quorum sensing has been implicated as a key player in the symbiotic process (29, 30, 98, 106). The fact that quorum sensing regulates aspects of symbiosis is not surprising. The process of symbiosis (discussed below) leads to the concentration of bacteria in and around the plant's roots and nodules. This rise in rhizobial cell density, as determined by quorum sensing, is therefore an important component of the signaling process.
Nodulation by Rhizobia
The nodulation of leguminous plants by rhizobia is a complex and fascinating developmental phenomenon that requires a series of biochemical interactions between the bacterium and its host (18, 55, 101, 103, 140, 172) (Fig. 2). In the course of this association, the bacteria undergo chemotaxis toward the plant roots and alter the growth of the epidermal hairs on the surface of the roots such that they curl. Subsequently, the bacteria induce cell division in the normally quiescent cells of the inner cortex of the root, which leads to the establishment of a nodule meristem. The bacteria trapped in the curled root hair induce the formation of an infection thread, a tube of plant origin, which penetrates the outer plant cells while the bacteria proliferate inside. As the nodule develops, infection threads ramify and penetrate individual target cells (79). The bacteria are surrounded by a membrane of plant origin and then released into the cytoplasm of these cells. Once released, the bacteria differentiate into morphologically altered forms termed bacteroids and begin to synthesize nitrogenase and the other proteins required for nitrogen fixation. The plant cells also differentiate and express a number of nodule-specific proteins termed nodulins, such as leghemoglobin. The symbiotic interaction results in the reduction of atmospheric dinitrogen to ammonia by the bacteroids, which is then utilized by the host plant.
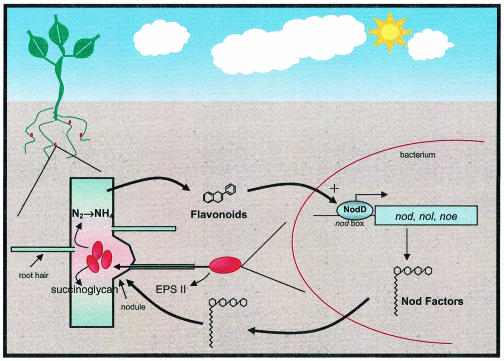
FIG. 2.
Rhizobium-legume symbiosis model. The process of nodule invasion begins with the production, by the plant roots, of phenolic signals called flavonoids. Flavonoids serve as inducers for the NodD transcriptional activator, which then binds to conserved promoter elements, referred to as nod boxes, upstream of the nodulation genes (nod, noe, and nol). Expression of the nodulation genes results in the production of Nod factor molecules composed of a backbone of N-acetylglucosamine residues (two to five), a fatty acyl moiety with variable length and degrees of saturation, and various decorations on the backbone, all of which are species specific. Bacterial Nod factors then act on the plant roots to induce nodule formation and root hair curling. The bacteria are then able to invade the root hairs; this process requires the production of certain symbiotically active exopolysaccharides, which also vary depending on the species. Once inside, the bacteria undergo a differentiation process and begin expressing nitrogenase and other genes necessary for nitrogen fixation.
Nodulation (Nod) Factors
Most of the research on rhizobia has focused on the nod genes (23, 100, 102), mutants of which do not induce root hair curling or nodule formation (101). Control of nod gene expression in rhizobia varies from strain to strain but is usually mediated by NodD, a transcriptional regulator that belongs to the LysR family. NodD binds to a conserved 47-bp region (the nod box) found upstream of the nodulation genes (nod, nol, and noe) (126). The presence of plant-produced flavonoids is generally necessary, but not essential, for the expression of the nod genes. Direct binding of flavonoids to NodD remains to be shown, but they clearly activate nod gene expression and subsequent Nod factor production (54, 112, 126, 127). The Nod factors produced by all rhizobia have the same generic lipochitooligosaccharide structure: generally, three, four, or five β-1,4-linked N-acetyl glucosamines, with the terminal nonreducing sugar N acylated by a fatty acid usually of 16 or 18 carbon atoms (35).
Biosynthesis of the Nod factor requires the nodABC genes. The NodC protein is a β-glucosaminyl transferase that links the UDP-N-acetyl glucosamine monomers. NodB removes an acetyl group from the terminal residue of the chitin-like backbone, while NodA catalyzes the transfer of a fatty acyl chain onto the resulting free amino group by using acyl-ACP from fatty acid biosynthesis (80). Many of the other nodulation genes determine the nature of the substitutions at the terminal residues and the structure of the acyl chain, both important features that play a role in determining host specificity (3, 32, 33, 45, 55, 151). The structure of the acyl moiety attached to lipochitooligosaccharide can contain one of a broad variety of acyl groups that also occur commonly as moieties of the phospholipids (for summaries, see references 21, 32, 33, 55, 92, and 138). Evidence suggests that the ratios of the common types of Nod factor acyl substituents reflect the composition of the fatty acyl pool that is present as components of the phospholipids.
Treatment of alfalfa seedlings with concentrations of purified Nod factor as low as 10−12 M produces an ion flow across the plant plasma membrane. This results in a depolarization of the membrane, causing periodic oscillations in intracellular calcium concentrations (calcium spiking) (for a review, see reference 40). This is followed by root hair deformation and the development of empty nodules (169) reminiscent of those elicited by exopolysaccharide-deficient mutants of Sinorhizobium meliloti (see below). The identity of the Nod factor receptor(s) in legumes is unknown, but biochemical and genetic approaches have led to the characterization of various putative high-affinity binding sites. One interesting candidate is NFBS2, located in the plasma membrane of alfalfa cells. NFBS2 binds S. meliloti Nod factors with high affinity but does not select for the presence of sulfate on the reducing sugar. Sulfated S. meliloti Nod factors are required for optimal nodulation of alfalfa (17, 31, 115). A second candidate, Db-LNP, is an unusual Nod factor binding lectin with apyrase activity. It was shown that ATPase activity of Db-LNP increases on Nod factor binding to the lectin domain of the protein (50).
Another interesting approach to the study of Nod factor signaling is the analysis of nodulation-deficient plant mutants (160). Various plant hosts blocked in the early steps of symbiosis have been identified, and positional cloning of the plant symbiotic genes has been initiated (47, 149, 174). Analysis of these plant mutants promises to be a very active area of study in the near future.
Requirement for Exopolysaccharides in Nodule Invasion
Work from Graham Walker's laboratory and others has helped to focus attention on the importance of rhizobial exopolysaccharides in nodulation. Most rhizobia produce a variety of polysaccharides (12, 22, 89, 129), and it was hypothesized that they might play roles in bacterium-plant interactions, such as being at least partially responsible for the host specificity of various Rhizobium species. Arguably, the best-characterized symbiotically important exopolysaccharides can be found in S. meliloti. S. meliloti is capable of synthesizing two different exopolysaccharides, succinoglycan and EPS II. The synthesis of at least one of these exopolysaccharides by S. meliloti Rm1021 is absolutely required for the development of normal nitrogen-fixing nodules (32, 33, 67, 68, 138). Mutants that are unable to synthesize either exopolysaccharide form empty nodules that lack bacteria and bacteroids (53, 90, 91, 186) and are similar to the empty nodules elicited by treatment of alfalfa roots with purified Nod factor (169). Root hair curling is delayed, and normal infection threads are not seen in the curled root hairs; infection threads are detected on sectioning, but these abort within the peripheral cells of the developing nodule (26, 91, 186). The empty nodules elicited by mutants unable to make either exopolysaccharide appear to be arrested at an intermediate state of nodule development and express only 2 of the 17 nodule-specific plant proteins (nodulins) that are synthesized in nodules containing wild-type bacteria (186).
Importance of Rhizobial Plasmids in Symbiosis
In different Rhizobium species, most nodulation, nitrogen fixation, and exopolysaccharide biosynthesis genes are present on one or more megaplasmids known as symbiotic (Sym) plasmids (4, 16, 82, 179). In contrast, Bradyrhizobium, Azorhizobium, and Mesorhizobium species carry most of the symbiotic information in clusters or islands found on the chromosome. Recent evidence suggests that the symbiosis island from Mesorhizobium loti can be transferred horizontally by conjugation from a nitrogen-fixing-proficient strain to a nonsymbiotic mesorhizobium (161). Several laboratories have demonstrated that these Sym plasmids can be cured under the proper permissive conditions without affecting growth and reproduction but that they are essential for effective symbiosis (117). In addition to the Sym plasmids, rhizobia can harbor various cryptic plasmids, most of them of unknown function.
In S. meliloti, for example, there are two classes of plasmids, megaplasmids and pRme plasmids (4, 5). S. meliloti typically contains two megaplasmids of approximately 1.4 and 1.7 Mb, termed pSymA and pSymB, respectively (6). pSymA carries genes for nitrogen fixation (nif) and for nodulation (nod), while the genes involved in the production of the symbiotically essential exopolysaccharides (exo and exp), thiamine (thi) biosynthesis, and dicarboxylic acid transport (dct) are found on pSymB (6). pSymA and pSymB are transmissible and stably maintained in new hosts, such as A. tumefaciens (4, 82). On the other hand, the pRme plasmids, whose number varies widely among strains, do not seem to be essential for effective nodulation and nitrogen fixation (4, 82). It is unclear what functions are carried on these plasmids, but many of them contain regions with extensive homology to the conjugal plasmid transfer genes from the Ti plasmids of A. tumefaciens (82). Most of these plasmids appear to be self-transmissible and could be involved not only in their own transfer but also in mediation of the low-frequency transfer of the symbiotic megaplasmids (4, 82).
A similar story can be found in Rhizobium sp. strain NGR234, R. etli, and R. leguminosarum. The broad-host-range strain NGR234 carries a symbiotic plasmid (pNGR234 a) with genes homologous to the plasmid replication (rep) and conjugal transfer (tra) genes of Agrobacterium Ti plasmids (56, 57). Recent work by Fuqua and colleagues showed that these genes are indeed involved in low-frequency plasmid transfer and that at least some of these tra genes are regulated by quorum sensing (78; also see below). R. etli also contains a large symbiotic plasmid (pSym) in addition to one or more “cryptic” plasmids, most of which remain uncharacterized (19, 136). Some of these plasmids are self-transmissible at a high frequency. pSym of R. etli CFN42 is also transmissible, but this event is dependent on the presence of the self-transmissible plasmids (20). In R. leguminosarum, most of the symbiotic genes are also located in a symbiotic plasmid designated pRL1JI. Recent work by Downie and colleagues has identified a cluster of genes on pRL1JI with homology to the trb operon and the tra regulators of A. tumefaciens, and they appear to be involved in transfer of this symbiotic plasmid (180; also see below).
QUORUM SENSING IN SELECTED RHIZOBIA
In addition to the well-characterized signal molecules (flavonoids, Nod factors, and exopolysaccharides) that are involved in the nodulation process, AHLs produced by bacterial quorum sensing can now be included in the list of symbiotic signals. As discussed below, quorum sensing has recently been linked to various phenomena including nodulation efficiency, symbiosome development, exopolysaccharide production, and nitrogen fixation, all of which are important for the establishment of a successful symbiosis.
R. leguminosarum bv. viciae
Of the nitrogen-fixing rhizobia, quorum sensing is best characterized in R. leguminosarum bv. viciae (for a review, see reference 183). Several quorum-sensing systems (rai, rhi, cin, and tra) have been identified and are intertwined in a complex regulatory network (Fig. 3) (29, 95, 139, 180, 184). Early work focused on the rhi system, composed of rhiR (a luxR homolog), rhiI (a luxI homolog), and the rhiABC operon, all of which are located on the symbiotic plasmid pRL1JI (29, 139). It was demonstrated that rhiABC was controlled by RhiR and that flavonoids repressed the expression of both rhiR and rhiABC (29). Although the function of rhiABC is unknown, rhiA was shown to be highly expressed in the rhizosphere but not in bacteroids (36). Moreover, mutations in either rhiA or rhiR led to a decrease in the number of nodules, but only in combination with a nodFE mutant, leading to the hypothesis that the rhi operon may play a role in the early stages of the symbiotic process, as do the nod genes (29). Further investigation identified rhiI (139) and showed that it was responsible for the synthesis of several short-chain AHLs, including C6-HSL, C8-HSL, and another compound comigrating with C7-HSL (139) (Table 2).
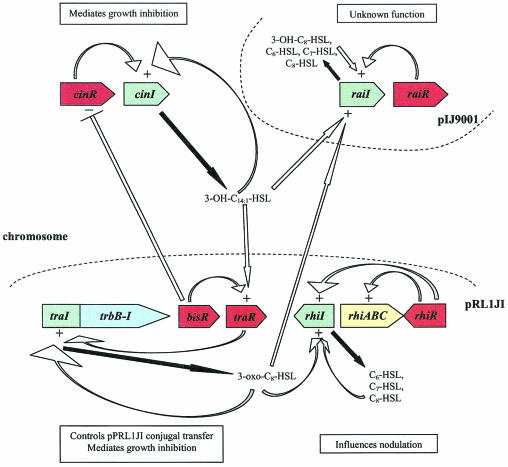
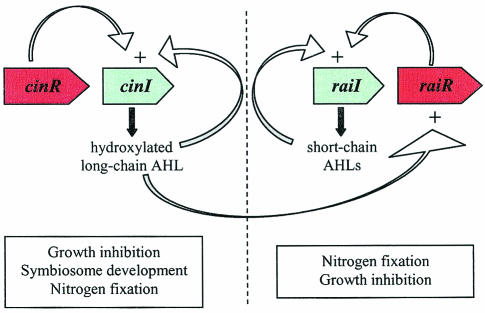
FIG. 3.
R. leguminosarum bv. viciae quorum-sensing network. R. leguminosarum harbors four known quorum-sensing systems. The cinRI system resides on the chromosome and produces 3-OH-C14:1-HSL, which positively influences the tra and rai systems. BisR plays a dual role in activating traR and repressing cinR in response to 3-OH-C14:1-HSL, thereby linking the cin and tra systems. pRL1JI harbors both the tra and rhi systems, as well as the genes that confer growth sensitivity in response to 3-OH-C14:1-HSL. The tra system is responsible for the production of 3-oxo-C8-HSL and controls conjugal plasmid transfer, while the rhi system produces several short-chain AHLs and influences nodulation efficiency by an unknown mechanism. The raiRI locus resides on pIJ9001 and also produces several short-chain AHLs; however, little is known about the role of this quorum-sensing system.
TABLE 2.
AHL production in rhizobia
| Organism | AHL(s) present | System responsible for AHL production | Phenomenon regulated by quorum sensing | Reference(s) |
|---|---|---|---|---|
| A. tumefaciens | 3-Oxo-C8-HSL | traR/traI | Plasmid transfer | 62, 84, 130 |
| R. leguminosarum bv. viciae | C6-HSL | rhiR/rhiI | Nodulation efficiency | 29, 139 |
| C7-HSL | ||||
| C8-HSL | ||||
| 3-OH-C8-HSL | raiR/raiI | Unknown | 184 | |
| C6-HSL | ||||
| C7-HSL | ||||
| C8-HSL | ||||
| 3-Oxo-C8-HSLa | traR/traI | Plasmid transfer | 180 | |
| C8-HSLa | ||||
| 3-OH-C14:1-HSL | cinR/cinI | Growth inhibition | 95 | |
| R. etli CNPAF512 | Hydroxylated long chain AHL | cinR/cinI | Nitrogen fixation, symbiosome development, growth inhibition | 141 |
| Short-chain AHLs | raiR/raiI | Nitrogen fixation, growth inhibition | 30, 141 | |
| R. etli CFN42 | 3-Oxo-C8-HSLa | traR/traI | Plasmid transfer | 170 |
| 3-OH-C8-HSL | Unknown | Unknown | 170 | |
| Rhizobium sp. strain NGR234 | 3-Oxo-C8-HSLa | traR/traI | Plasmid transfer, growth inhibition | 78 |
| Other short-chain and long-chain AHLs | Unknown | Unknown | 78 | |
| S. meliloti Rm1021 | C12-HSL | sinR/sinI | Exopolysaccharide EPS II production | 107, 108; Marketon et al., submitted |
| 3-Oxo-C14-HSL | ||||
| C16:1-HSL | ||||
| 3-Oxo-C16:1-HSL | ||||
| C18-HSL | ||||
| C8-HSL | mel (putative) | Unknown | 108 | |
| Other short-chain AHLs | ||||
| S. meliloti Rm41 | 3-Oxo-C14-HSL | sinR/sinI | Exopolysaccharide EPS II production | 107; Marketon et al., submitted |
| C16-HSL | ||||
| C16:1-HSL | ||||
| 3-Oxo-C16:1-HSL | ||||
| 3-Oxo-C8-HSL | traR/traI | Plasmid transfer | 107; Marketon et al., submitted | |
| 3-OH-C8-HSL | ||||
| C8-HSL | ||||
| Other short-chain AHLs | mel (putative) | Unknown | Marketon et al., submitted | |
| Bradyrhizobium sp. | Bradyoxetinb | Unknown | Activation of nod genes | 96, 98, 99 |
AHL designation is based on thin-layer chromatography characteristics but has not been confirmed by mass spectrometry or nuclear magnetic resonance spectroscopy analysis.
Not a AHL.
In addition to short-chain AHLs, a long-chain AHL, originally termed small for its bacteriocin-like activity (81, 179), was identified as 3-OH-C14:1-HSL (71, 150) (Table 2). Initial experiments showed 3-OH-C14:1-HSL to be an inducer of the rhi genes (71), but later data showing that rhiI and rhiA could be induced by short-chain AHLs (C6-HSL, C8-HSL, and 3-oxo-C8-HSL) but not 3-OH-C14:1-HSL suggested that the induction was indirect (139). The production of 3-OH-C14:1-HSL is a result of the cinRI locus, located on the chromosome (95). cinI, the AHL synthase gene, is positively autoregulated by CinR and 3-OH-C14:1-HSL. The mechanism of the regulation of cinR is unclear, since mutations in cinR or cinI or even the lack of the rhi and tra systems do not seem to affect cinR expression (95). However, cinR expression is cell density dependent (95). More important, though, is the observation that mutations in cinR and cinI led to decreased levels of all of the short-chain AHLs, suggesting that the cin system is situated at the top of the quorum-sensing network (95).
An additional plasmid (pIJ9001)-located locus, raiRI, has recently been identified (184); this gene synthesizes 3-OH-C8-HSL as its major product, with C6-HSL, C7-HSL, and C8-HSL as minor products (Table 2) (95). R. leguminosarum RaiI and RaiR have 93 and 88% identity to R. etli CNPAF512 RaiI and RaiR, respectively (184). As expected, raiI is positively autoregulated by RaiR and 3-OH-C8-HSL but is also induced to a lesser extent by 3-OH-C14:1-HSL and 3-oxo-C8-HSL (184). These observations help explain the effect of cinRI mutations on the production of some of the short-chain AHLs and also suggest that an additional 3-oxo-C8-HSL-producing locus may contribute to regulation of raiRI (184).
Recent work has also identified a cluster of genes on pRL1JI with homology to the trb operon of A. tumefaciens (180). Wilkinson et al. identified traI (an AHL synthase), followed by the trb genes (which function in mating pore formation), as in A. tumefaciens and Rhizobium NGR234 (see below) (51, 57, 62, 130, 180). Interestingly, in addition to a traR regulator, R. leguminosarum carries a second regulatory gene, bisR, downstream of the trb operon. BisR has 59% identity to CinR, while TraR has 64% identity to NGR234 TraR. Furthermore, traR expression seems to be controlled by cinRI through the BisR regulator, presumably through the binding of BisR to 3-OH-C14:1-HSL. TraR then goes on to control the conjugal transfer of pRL1JI by inducing the trb operon. Unpublished data from the laboratory of Alan Downie also suggests that traI is responsible for the synthesis of 3-oxo-C8-HSL, as in A. tumefaciens and NGR234, which could link activation of the tra system to a small induction of the rai system. Another link in the regulatory network is the observation that BisR can repress cinI, suggesting a negative-feedback loop between the cin and tra systems.
Although much work has gone into characterizing the quorum-sensing network of R. leguminosarum, little is known about the role of these systems in the life cycle of the organism. Mutations in the rai, cin, and tra systems do not have any apparent defects in nodulation (95, 180, 184). The rhi system seems to play a role in nodulation efficiency, but no dramatic defect has been observed for rhi mutants that might suggest a possible mechanism (29, 139). The only system with a defined role is the tra system, since it was clearly shown to regulate the conjugal transfer of pRL1JI, a symbiotic plasmid (180). However, the advantage of having plasmid transfer under the control of the cin system is not apparent. Lastly, the growth-inhibitory role of OH-C14:1-HSL and cinRI is still a mystery. This AHL-mediated growth inhibition was shown by Gray et al. (71) to result from an early induction of the stationary phase, but only strains carrying pRL1JI are sensitive to the growth inhibition. Furthermore, addition of OH-C14:1-HSL has been shown to promote starvation survival of R. leguminosarum cultures that enter stationary phase at low cell density (168). Wilkinson et al. later showed that BisR, TraR, and the tra AHLs conferred sensitivity to OH-C14:1-HSL, thus further complicating the reasoning for having the cin and tra systems intertwined (180).
R. etli CNPAF512
In contrast to R. leguminosarum, quorum sensing in R. etli is less well characterized but also seems less complex (Fig. 4). Although R. etli makes up to seven AHLs, only two quorum-sensing systems have been identified: raiRI and cinRI (30, 141). These two systems are responsible for the synthesis of all of the AHLs, since a raiI cinI double mutant produced no detectable AHLs (30). Rosemeyer et al. first identified raiI (a luxI homolog) and raiR (a luxR homolog) and demonstrated the role of raiI in the synthesis of multiple, unidentified short-chain AHLs (141). In addition, this study provided some of the first evidence linking quorum sensing to symbiosis by showing that raiI mutants actually had a slight increase in the number of nodules per plant but that no other defects were apparent. Recent unpublished work suggests a more general role for the rai quorum-sensing system in nitrogen fixation (R. Daniels, personal communication).
FIG. 4.
Interaction of the cinRI and raiRI quorum-sensing systems in R. etli CNPAF512. R. etli CNPAF512 carries two quorum-sensing systems, cinRI and raiRI, both of which are located on the chromosome. The cinRI locus encodes a long-chain AHL and is involved in growth inhibition, as in R. leguminosarum. This locus is also required for efficient nitrogen fixation and proper symbiosome development. The raiRI system is positively regulated by cinI-produced AHL and is, in turn, responsible for the production of several short-chain AHLs. The raiRI locus also controls nitrogen fixation and mediates growth inhibition.
In addition to the smaller AHLs, Rosemeyer et al. provided evidence that R. etli produced a long-chain AHL, related to the R. leguminosarum small (141). Although the structure of the R. etli AHL is still unknown, later work characterized it as a hydroxylated long-chain AHL, lacking the double bond seen in the 3-OH-C14:1-HSL of R. leguminosarum (30) (Table 2). This long-chain AHL is synthesized by the cinRI locus, which has 96 and 95% identity to the R. leguminosarum CinR and CinI proteins, respectively (30). This 3-hydroxylated long-chain AHL has a growth-inhibitory effect on R. leguminosarum bv. viciae 248 (30).
Although the rai and cin systems affect the growth of R. etli, the most exciting data on these two systems came from in planta studies (30). Daniels et al. reported the first analysis of AHL production by bacteroids (30). These authors demonstrated that compounds comigrating with the cinI AHL and some of the raiI AHLs could be extracted from R. etli bacteroids. Moreover, mutations in cinI and cinR, while causing no readily observable defect in nodulation, resulted in decreased nitrogen fixation as well as abnormal symbiosome development. Additionally, a cinI raiI double mutant had an exacarbation of the nitrogen-fixing defect. These observations provide some of the most compelling evidence supporting the idea that quorum sensing is involved in symbiosis, even though the mechanisms through which quorum sensing acts is still a mystery.
R. etli CFN42
R. etli strain CFN42 contains one chromosome and six plasmids (p42a to p42f). Plasmid p42a is self-transmissible at a high frequency (10−2) and is required for mobilization of the symbiotic plasmid, p42d (19). Recently, a traI-trb operon, with high similarity to the transfer genes of pTi and pNGR234a, has been localized on p42a (170). Four regulatory genes, traI, traR, cinR, and traM, have also been found on this plasmid (Fig. 5). Only two AHLs have been described in R. etli CFN42, 3-oxo-C8-HSL, synthesized by TraI, and a putative 3-OH-C8-HSL, whose function and synthase remain to be identified (Table 2).
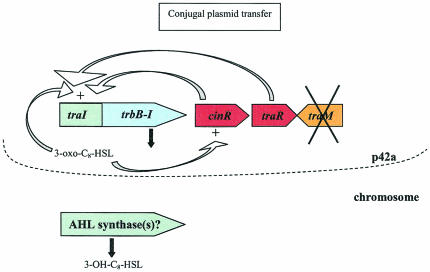
FIG. 5.
Quorum sensing in R. etli CFN42. Quorum sensing in R. etli CFN42 controls conjugal plasmid transfer through the action of the tra genes, which are located on p42a. In this system, traI produces 3-oxo-C8-HSL and is regulated by both CinR and TraR. The traM gene is present but does not seem to be expressed and therefore does not play a regulatory role in strain CFN42. A second AHL has also been described, but the locus responsible for its production has not been identified.
Transfer of p42a is regulated by the products of traI, traR, and cinR. TraI expression is dependent on itself and on the presence of TraR and CinR. The traR gene seems to be expressed constitutively, but expression of cinR requires an active TraI. The p42a plasmid also encodes a putative TraM-like antiactivator, but no expression of this gene was detected under the experimental conditions tested. Therefore, it seems that conjugal transfer of p42a is derepressed, which could account for its high transfer frequency (170). The R. etli CFN42 quorum-sensing system does not seem to be directly involved in the symbiotic process (derivatives of CFN42 lacking p42a are able to effectively nodulate bean plants) (18), but it probably plays an indirect role by regulating the conjugative transfer of the p42d symbiotic plasmid.
Rhizobium sp. Strain NGR234
Rhizobium sp. strain NGR234 is unique among the rhizobia for its unusual ability to nodulate over 100 different legume species and at least one nonlegume (133). Sequencing of the symbiotic plasmid pNGR234a (57) showed a cluster of genes (traI-trb operon) with significant homology to the conjugal plasmid transfer (tra) system of A. tumefaciens (1, 52, 57, 93). In addition to these genes, which are required for the mating pore and DNA transfer, there were the quorum-sensing regulators, traI, traR, and traM, all of which are orthologues of the A. tumefaciens quorum-sensing regulators discussed above. Although there were no previous reports on quorum sensing in NGR234, the identification of these genes suggested that a quorum-sensing system might exist to regulate the transfer of pNGR234a, similar to the regulation of Ti plasmid transfer in A. tumefaciens (Fig. 6).
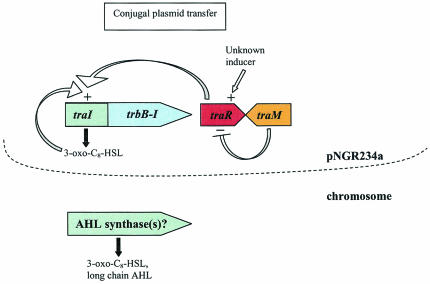
FIG. 6.
Quorum sensing in Rhizobium sp. strain NGR234. NGR234 carries a large symbiotic plasmid, pNGR234a, which harbors the tra quorum-sensing system. The tra genes control the production of 3-oxo-C8-HSL as well as the conjugal transfer of pNGR234a. At least one other quorum-sensing system may be present elsewhere in the genome and controls the production of 3-oxo-C8-HSL and another long-chain AHL.
Recent work by He et al. showed that indeed the NGR234 tra system has striking similarities to that of A. tumefaciens (78). These authors showed that traI is responsible for the synthesis of an AHL that is likely to be 3-oxo-C8-HSL. However, a traI mutant still produces a compound comigrating with 3-oxo-C8-HSL and another that seems to be a long-chain AHL, suggesting that one or more additional AHL synthases may be present (Table 2). Since these remaining putative AHLs are also seen in a strain lacking pNGR234a, the synthase gene(s) must reside elsewhere in the genome. As expected, traI seems to be autoregulated by TraR and 3-oxo-C8-HSL and TraR activity is inhibited by TraM, which corresponds to properties of the A. tumefaciens tra system. However, there are some interesting differences between the two systems. Whereas transfer of the Ti plasmid normally occurs at a frequency of 10−2, transfer of pNGR234a occurs at a frequency of 10−9 (78). While the cause of this extremely low transfer frequency is unknown, the authors speculated that an environmental signal, perhaps analogous to A. tumefaciens opines, may be required to induce higher levels of plasmid transfer. This finding is also in contrast to the transfer frequency (10−2) seen for R. leguminosarum and R. etli, which apparently does not require an extra signal to induce plasmid transfer. Therefore, although these three organisms carry a conserved cluster of genes involved in plasmid transfer, distinct forms of regulation have been imposed on the quorum-sensing regulators through evolution in order to customize the systems to the organisms' particular niches.
In addition to an extremely low plasmid transfer frequency, other interesting differences between the A. tumefaciens and NGR234 tra systems were observed (78). Through expression analysis of the expected TraR targets (traI-trb), He et al. found that the traAFB operon was not significantly expressed. This observation thus provides a possible explanation for the low transfer frequency of pNGR234a (78). Another possible explanation lies in the fact that the trbE coding sequence on pNGR234 is split into two separate reading frames, trbE1 and trbE2. Although each has a reasonable start codon and Shine-Dalgarno sequence, it is possible that these do not function in conjugation, leading to the overall conjugal deficiency (C. Fuqua, personal communication). Furthermore, it appears that there may be differences in the role of TraR. Expression of TraR increased the production of the other AHLs produced by NGR234 and, in the presence of 3-oxo-C8-HSL, resulted in growth inhibition, an observation reminiscent of the bacteriocin-like activity in R. leguminosarum bv. viciae (71, 180). Although the mechanism of the growth inhibition is unknown, data indicated that it requires one or more genes, which are not located on pNGR234a, in addition to traI, traR, and traM (78).
S. meliloti
The well-characterized S. meliloti strain Rm1021 harbors at least two quorum-sensing systems (Fig. 7) (107, 108). The sinR/sinI locus is responsible for the production of several novel AHLs, ranging in size from C12-HSL to C18-HSL (108) (Table 2). Some of these are the longest AHLs identified so far. Disruption of the sin system correlates with a delay in the appearance of nitrogen-fixing nodules, as well as with an overall decrease in the number of pink nodules, suggesting a role for quorum sensing in establishing a successful symbiosis with Medicago sativa (108). More recently, it was shown that the sinR and sinI genes were required for synthesis of EPS II by a strain proficient in the production of this exopolysaccharide (106). The Rm1021 strain, which normally does not produce EPS II, has an insertion sequence that results in the disruption of expR (a luxR homologue) (125). Strains proficient in EPS II production, such as Rm41 and Rm8530 (Rm1021 expR+), possess an intact expR gene (106, 125). ExpR is a positive regulator of the exp genes, which are responsible for EPS II biosynthesis; however, it is unclear whether this regulatory effect is direct or indirect (66). In a sinI mutant, expression of several of the exp genes is abolished, and this deficiency can be fully complemented by the addition of either crude AHL extracts from wild-type Rm1021 or synthetic C16:1-HSL (106). Therefore, it seems that the sinRI locus controls EPS II production via ExpR. Regulation of EPS II production by sinRI was shown to be important for nodule invasion, since a strain that produces exclusively EPS II, combined with a sinI mutation, is no longer capable of forming nitrogen-fixing nodules. These results provide the first details of a mechanism for quorum sensing control in the development of symbiosis (106).
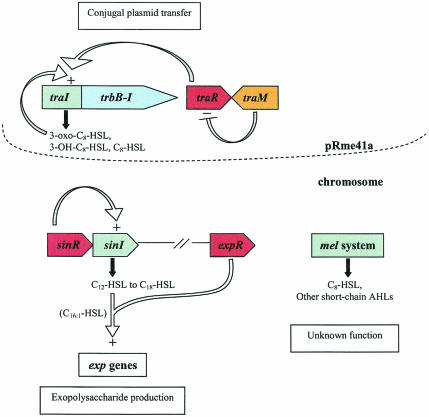
FIG. 7.
S. meliloti quorum-sensing systems. S. meliloti strain Rm41 harbors three quorum-sensing systems, while strain Rm1021 carries only two systems. The tra system, which is carried on pRme41a and is present only in strain Rm41, produces several short-chain AHLs, including 3-oxo-C8-HSL, and controls the conjugal transfer of pRme41a. The sinRI system resides on the chromosome and is present in both strains. SinI produces several long-chain AHLs, and at least one of them (C16:1-HSL), along with ExpR, is required for the production of a symbiotically important exopolysaccharide, EPS II. The mel system, also present in both strains, produces several short-chain AHLs, but the corresponding genes remain to be identified.
Disruption of the sinI gene abolishes the production of only the long-chain AHLs, while synthesis of short-chain AHLs, one of which was identified as C8-HSL, remains unaffected (Table 2). It was proposed that these short-chain AHLs are part of a second quorum-sensing system in Rm1021, termed the mel system (108). While the components of the mel system have not yet been identified, a search of the Rm1021 genome database did not reveal any additional LuxI homologues. However, Rm1021 does carry a gene homologous to the P. fluorescens HdtS AHL synthase. Whether this HdtS homolog synthesizes AHLs in S. meliloti remains to be determined.
In addition to the sin and mel systems, a third quorum-sensing system has been identified in another commonly used S. meliloti strain, Rm41 (107). The tra system, named for its homology to the tra systems in A. tumefaciens and Rhizobium strain NGR234, resides on a plasmid called pRme41a. This plasmid is unique to Rm41 and therefore represents a quorum-sensing system that is present in Rm41 but not Rm1021. At least three regulatory genes (traR, traI, and traM), in addition to genes with homology to the trb operon, have been identified in pRme41a (M. M. Marketon, M. R. Gronquist, A. Eberhard, and J. E. González, submitted). TraI is an AHL synthase responsible for the production of at least three different AHLs, 3-oxo-C8-HSL, 3-OH-C8-HSL, and C8-HSL (Marketon et al., submitted) (Table 2). This activity is regulated through the transcriptional activator TraR. TraM was shown to negatively regulate TraR activity in a manner analogous to the tra system in A. tumefaciens, to ensure that the tra system is active only at high cell densities. The S. meliloti Rm41 tra system also controls conjugal plasmid transfer, as in other organisms, by mediating the transfer of pRme41a (Marketon et al., submitted). Disruption of the traR, traI, or the trb genes abolishes plasmid transfer. Interestingly, transfer frequency, as in NGR234, also occurs at a fairly low frequency (about 10−7) (Marketon et al., submitted).
B. japonicum
While all the other nitrogen-fixing rhizobia characterized thus far utilize AHLs to mediate a quorum-sensing response, Bradyrhizobium japonicum appears to be unique (Fig. 8). Early work showed that nod genes seemed to be repressed at high cell densities, suggesting a quorum-sensing phenomenon, but to date no evidence of AHL production has been found (97, 99, 187). This population density-dependent control appeared to be mediated by an extracellular signal molecule termed CDF, for “cell density factor” (97, 98). The chemical structure of CDF was recently elucidated and shown to be 2-{4-[[4-(3-aminooxetan-2-yl)phenyl](imino)methyl]phenyl}oxetan-3-ylamine, also designated bradyoxetin (96-98) (Table 2). Bradyoxetin has structural similarity to certain antibiotics and to siderophores. Synthesis of bradyoxetin was found to be iron regulated, with maximal production under iron-depleted conditions (96, 98). Loh et al. identified a potential response regulator, nwsB, which is part of a two-component system and is required for detection of CDF (97). NwsB responds to a rise in cell density and autoinducer levels by inducing nolA (97), which then activates the nodD2 regulator (99). NodD2 is required for repression of the nod genes at high cell densities in the presence of flavonoids (97, 99). In planta studies showed that nolA and nwsB were required for the repression of nod genes in plants (97), suggesting that quorum sensing in B. japonicum could mediate signaling events in the early to intermediate stages of the symbiotic process. Interestingly, bradyoxetin activity has been found in extracts of all the α-proteobacteria tested (96, 98). This suggests that compounds similar to bradyoxetin may play an important role not only in rhizobial symbiosis but also in other plant- and animal-bacterium interactions.
FIG. 8.
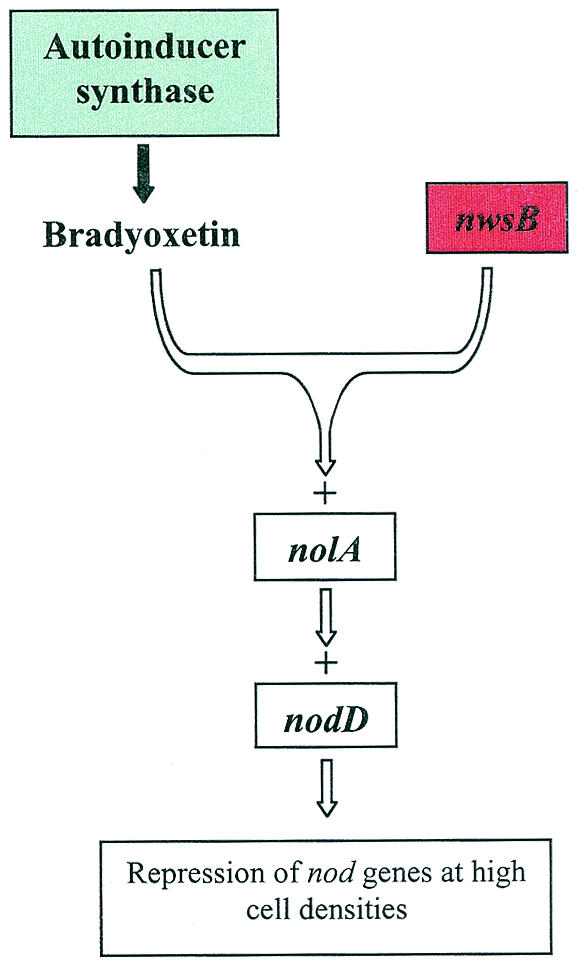
Quorum sensing in B. japonicum. Quorum sensing in B. japonicum is involved in repressing the nodulation genes at high cell densities. The system, unlike other rhizobial quorum-sensing systems, is not controlled by AHLs. Instead, the autoinducer is a low-molecular-weight compound termed bradyoxetin. NwsB is part of a two-component system and is required for the detection of bradyoxetin. NwsB then induces nolA, which in turn induces nodD2, leading to repression of the nod genes.
CONCLUSIONS AND PERSPECTIVES
Establishment of symbiosis between rhizobia and their legume hosts is a poorly understood and complex process. For example, bacterial exopolysaccharides are required for successful nodule invasion but their exact function is still unknown. Another area of uncertainty is the regulation of bacterial differentiation and host-bacterium signal exchange once the bacteria are inside the nodule. It is known, however, that the cell density of Rhizobium species must reach a threshold level around the plant roots before nodulation can occur (21). Therefore, it seemed likely that quorum sensing plays a role in regulating one or more stages of symbiosis. Nodule invasion requires the clustering of bacteria around the root hairs; it is therefore possible that the rise in cell density around the root hairs alters the expression of some genes. In addition, selected rhizobial strains are able to synthesize rhizopines, opine-like compounds reminiscent of those produced by Agrobacterium species. These compounds are synthesized by the bacteroids within the plant nodule and can be catabolized as a nutrient source by the free-living cognate rhizobia (113). These rhizopine-producing strains could potentially affect the dynamics of rhizobial soil populations.
In R. leguminosarum and R. etli, quorum sensing seems to be involved in restricting the number of nodules and in symbiosome development, although very little is known about the mechanism. In S. meliloti, quorum sensing controls the production of EPS II, an exopolysaccharide shown to be involved in the nodule invasion process. Most of the nitrogen-fixing rhizobia also seem to regulate conjugal plasmid transfer via quorum-sensing systems, as in A. tumefaciens. While these tra systems do not seem to be essential for nodulation, they may play a role in rhizosphere survival.
Recently, AHL-dependent gene regulation has received increasing recognition as an important form of cell-cell communication in gram-negative bacteria. Often, the genes controlled by LuxR-LuxI-type quorum sensors are involved in important microbial processes, including microbe-host interactions such as pathogenesis and symbiosis. Quorum sensing also seems to regulate virulence in many human and plant pathogens. Presumably, in an attempt to avoid alerting the host immune system to their presence, quorum-sensing bacteria delay virulence factor production until their cell number is large enough that secretion of virulence factors will result in a productive infection. As our understanding of the biochemical mechanism by which bacteria synthesize and respond to AHLs increases, human intervention designed to manipulate this regulation and to harness or mollify quorum-dependent gene products will become possible. Furthermore, the observation that AHL-mediated regulation can act in a global fashion suggests that a wide range of functions may be targeted with a single strategy. It is therefore important to develop rigorous analyses of how bacteria communicate within and between species and how eukaryotic hosts talk back. The rhizobia serve as an excellent quorum-sensing model system for such studies. It seems that the seminal work of understanding the signal molecules and the regulatory systems involved in the production of those signals is now in place. The next few years should provide a wealth of information on how these quorum-sensing systems participate in the nodulation process. This information will help us elucidate bacterial cell-cell signaling in addition to the insufficiently understood prokaryotic-eukaryotic cell-signaling systems.
Acknowledgments
We thank Clay Fuqua, Ruth Daniels, Cristina Tun-Garrido, and Steve Winans for sharing unpublished data and for helpful comments. We also thank the members of this laboratory for their helpful discussions on and insights into this the work in this review.
The work in our laboratory was supported by the National Science Foundation grant MCB-9733532 and by the Texas Advanced Research Program under grant 009741-0022-2001 to J.E.G.
REFERENCES
- 1.Alt-Morbe, J., J. L. Stryker, C. Fuqua, P. L. Li, S. K. Farrand, and S. C. Winans. 1996. The conjugal transfer system of Agrobacterium tumefaciens octopine-type Ti plasmids is closely related to the transfer system of an IncP plasmid and distantly related to Ti plasmid vir genes. J. Bacteriol. 178:4248-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, R. A., A. R. Eriksson, R. Heikinheimo, A. Mae, M. Pirhonen, V. Koiv, H. Hyytiainen, A. Tuikkala, and E. T. Palva. 2000. Quorum sensing in the plant pathogen Erwinia carotovora subsp. carotovora: the role of expR(Ecc). Mol. Plant-Microbe Interact. 13:384-393. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson, E. M., M. M. Palcic, O. Hindsgaul, and S. R. Long. 1994. Biosynthesis of Rhizobium meliloti lipooligosaccharide Nod factors: NodA is required for an N-acyltransferase activity. Proc. Natl. Acad. Sci. USA 91:8418-8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banfalvi, Z., E. Kondorosi, and A. Kondorosi. 1985. Rhizobium meliloti carries two megaplasmids. Plasmid 13:129-138. [DOI] [PubMed] [Google Scholar]
- 5.Banfalvi, Z., V. Sakanyan, C. Koncz, A. Kiss, I. Dusha, and A. Kondorosi. 1981. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of Rhizobium meliloti. Mol. Gen. Genet. 184:318-325. [DOI] [PubMed] [Google Scholar]
- 6.Barloy-Hubler, F., D. Capela, J. Batut, and F. Galibert. 2000. High-resolution physical map of the pSymb megaplasmid and comparison of the three replicons of Sinorhizobium meliloti strain 1021. Curr. Microbiol. 41:109-113. [DOI] [PubMed] [Google Scholar]
- 7.Bassler, B. L. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582-587. [DOI] [PubMed] [Google Scholar]
- 8.Bassler, B. L. 2002. Small talk. Cell-to-cell communication in bacteria. Cell 109:421-424. [DOI] [PubMed] [Google Scholar]
- 9.Bassler, B. L., E. P. Greenberg, and A. M. Stevens. 1997. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 179:4043-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassler, B. L., M. Wright, R. E. Showalter, and M. R. Silverman. 1993. Intercellular signaling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9:773-786. [DOI] [PubMed] [Google Scholar]
- 11.Bassler, B. L., M. Wright, and M. R. Silverman. 1994. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 13:273-286. [DOI] [PubMed] [Google Scholar]
- 12.Bauer, W. D. 1981. Infection of legumes by rhizobia. Annu. Rev. Plant Physiol. 32:407-449. [Google Scholar]
- 13.Beck von Bodman, S., and S. K. Farrand. 1995. Capsular polysaccharide biosynthesis and pathogenicity in Erwinia stewartii require induction by an N-acyl homoserine lactone autoinducer. J. Bacteriol. 177:5000-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck von Bodman, S., G. T. Hayman, and S. K. Farrand. 1992. Opine catabolism and conjugal transfer of the nopaline Ti plasmid pTiC58 are coordinately regulated by a single repressor. Proc. Natl. Acad. Sci. USA 89:643-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck von Bodman, S., D. R. Majerczak, and D. L. Coplin. 1998. A negative regulator mediates quorum-sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc. Natl. Acad. Sci. USA 95:7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beynon, J. L., J. E. Beringer, and A. W. B. Johnston. 1980. Plasmids and host range in Rhizobium leguminosarum and Rhizobium phaseoli. J. Gen. Microbiol. 120:421-429. [Google Scholar]
- 17.Bono, J. J., J. Riond, K. C. Nicolaou, N. J. Bockovich, V. A. Estevez, J. V. Cullimore, and R. Ranjeva. 1995. Characterization of a binding site for chemically synthesized lipo-oligosaccharidic NodRm factors in particulate fractions prepared from roots. Plant J. 7:253-260. [DOI] [PubMed] [Google Scholar]
- 18.Brewin, N. J. 1991. Development of the legume root nodule. Annu. Rev. Cell Biol. 7:191-226. [DOI] [PubMed] [Google Scholar]
- 19.Brom, S., A. Garcia de los Santos, T. Stepkowsky, M. Flores, G. Davila, D. Romero, and R. Palacios. 1992. Different plasmids of Rhizobium leguminosarum bv. phaseoli are required for optimal symbiotic performance. J. Bacteriol. 174:5183-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brom, S., A. Garcia-de los Santos, L. Cervantes, R. Palacios, and D. Romero. 2000. In Rhizobium etli symbiotic plasmid transfer, nodulation competitivity and cellular growth require interaction among different replicons. Plasmid 44:34-43. [DOI] [PubMed] [Google Scholar]
- 21.Caetano-Anolles, G., and P. M. Gresshoff. 1991. Plant genetic control of nodulation. Annu. Rev. Microbiol. 45:345-382. [DOI] [PubMed] [Google Scholar]
- 22.Carlson, R. W., and B. S. Krishnaiah. 1992. Structures of the oligosaccharides obtained from the core regions of the lipopolysaccharides of Bradyrhizobium japonicum 61A101c and its symbiotically defective lipopolysaccharide mutant, JS314. Carbohydr. Res. 231:205-219. [DOI] [PubMed] [Google Scholar]
- 23.Carlson, R. W., N. P. Price, and G. Stacey. 1994. The biosynthesis of rhizobial lipo-oligosaccharide nodulation signal molecules. Mol. Plant-Microbe Interact. 7:684-695. [DOI] [PubMed] [Google Scholar]
- 24.Cha, C., P. Gao, Y. C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 25.Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler, and F. M. Hughson. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545-549. [DOI] [PubMed] [Google Scholar]
- 26.Cheng, H. P., and G. C. Walker. 1998. Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J. Bacteriol. 180:5183-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi, S. H., and E. P. Greenberg. 1991. The C-terminal region of the Vibrio fischeri LuxR protein contains an inducer-independent lux gene activating domain. Proc. Natl. Acad. Sci. USA 88:11115-11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi, S. H., and E. P. Greenberg. 1992. Genetic dissection of DNA binding and luminescence gene activation by the Vibrio fischeri LuxR protein. J. Bacteriol. 174:4064-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cubo, M. T., A. Economou, G. Murphy, A. W. Johnston, and J. A. Downie. 1992. Molecular characterization and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum biovar viciae. J. Bacteriol. 174:4026-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniels, R., D. E. De Vos, J. Desair, G. Raedschelders, E. Luyten, V. Rosemeyer, C. Verreth, E. Schoeters, J. Vanderleyden, and J. Michiels. 2002. The cin quorum sensing locus of Rhizobium etli CNPAF512 affects growth and symbiotic nitrogen fixation. J. Biol. Chem. 277:462-468. [DOI] [PubMed] [Google Scholar]
- 31.Demont-Caulet, N., F. Maillet, D. Tailler, J. C. Jacquinet, J. C. Prome, K. C. Nicolaou, G. Truchet, J. M. Beau, and J. Denarie. 1999. Nodule-inducing activity of synthetic Sinorhizobium meliloti nodulation factors and related lipo-chitooligosaccharides on alfalfa. Importance of the acyl chain structure. Plant Physiol. 120:83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denarie, J., and J. Cullimore. 1993. Lipo-oligosaccharide nodulation factors: a new class of signaling molecules mediating recognition and morphogenesis. Cell 74:951-954. [DOI] [PubMed] [Google Scholar]
- 33.Denarie, J., F. Debelle, and C. Rosenberg. 1992. Signaling and host range variation in nodulation. Annu. Rev. Microbiol. 46:497-531. [DOI] [PubMed] [Google Scholar]
- 34.Devine, J. H., G. S. Shadel, and T. O. Baldwin. 1989. Identification of the operator of the lux regulon from the Vibrio fischeri strain ATCC7744. Proc. Natl. Acad. Sci. USA 86:5688-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Haeze, W., and M. Holsters. 2002. Nod factor structures, responses, and perception during initiation of nodule development. Glycobiology 12:79R-105R. [DOI] [PubMed] [Google Scholar]
- 36.Dibb, N. J., J. A. Downie, and N. J. Brewin. 1984. Identification of a rhizosphere protein encoded by the symbiotic plasmid of Rhizobium leguminosarum. J. Bacteriol. 158:621-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolan, K. M., and E. P. Greenberg. 1992. Evidence that GroEL, not sigma 32, is involved in transcriptional regulation of the Vibrio fischeri luminescence genes in Escherichia coli. J. Bacteriol. 174:5132-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong, Y. H., L. H. Wang, J. L. Xu, H. B. Zhang, X. F. Zhang, and L. H. Zhang. 2001. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411:813-817. [DOI] [PubMed] [Google Scholar]
- 39.Dong, Y. H., J. L. Xu, X. Z. Li, and L. H. Zhang. 2000. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 97:3526-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Downie, J. A., and S. A. Walker. 1999. Plant responses to nodulation factors. Curr. Opin. Plant Biol. 2:483-489. [DOI] [PubMed] [Google Scholar]
- 41.Dunlap, P. V., and E. P. Greenberg. 1985. Control of Vibrio fischeri luminescence gene expression in Escherichia coli by cyclic AMP and cyclic AMP receptor protein. J. Bacteriol. 164:45-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eberhard, A., T. Longin, C. A. Widrig, and S. J. Stranick. 1991. Synthesis of the lux gene autoinducer in Vibrio fischeri is positively autoregulated. Arch. Microbiol. 155:294-297. [Google Scholar]
- 43.Eberl, L., M. K. Winson, C. Sternberg, G. S. Stewart, G. Christiansen, S. R. Chhabra, B. Bycroft, P. Williams, S. Molin, and M. Givskov. 1996. Involvement of N-acyl-l-hormoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol. Microbiol. 20:127-136. [DOI] [PubMed] [Google Scholar]
- 44.Egland, K. A., and E. P. Greenberg. 1999. Quorum sensing in Vibrio fischeri: elements of the luxl promoter. Mol. Microbiol. 31:1197-1204. [DOI] [PubMed] [Google Scholar]
- 45.Ehrhardt, D. W., E. M. Atkinson, K. F. Faull, D. I. Freedberg, D. P. Sutherlin, R. Armstrong, and S. R. Long. 1995. In vitro sulfotransferase activity of NodH, a nodulation protein of Rhizobium meliloti required for host-specific nodulation. J. Bacteriol. 177:6237-6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elasri, M., S. Delorme, P. Lemanceau, G. Stewart, B. Laue, E. Glickmann, P. M. Oger, and Y. Dessaux. 2001. Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soilborne Pseudomonas spp. Appl. Environ. Microbiol. 67:1198-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Endre, G., A. Kereszt, Z. Kevei, S. Mihacea, P. Kalo, and G. B. Kiss. 2002. A receptor kinase gene regulating symbiotic nodule development. Nature 417:962-966. [DOI] [PubMed] [Google Scholar]
- 48.Engebrecht, J., K. Nealson, and M. Silverman. 1983. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32:773-781. [DOI] [PubMed] [Google Scholar]
- 49.Engebrecht, J., and M. Silverman. 1984. Identification of genes and gene products necessary for bacterial bioluminescence. Proc. Natl. Acad. Sci. USA 81:4154-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Etzler, M. E., G. Kalsi, N. N. Ewing, N. J. Roberts, R. B. Day, and J. B. Murphy. 1999. A nod factor binding lectin with apyrase activity from legume roots. Proc. Natl. Acad. Sci. USA 96:5856-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farrand, S. K. 1998. Conjugation in Rhizobiaceae, p. 199-233. In H. P. Spaink, A. Kondorosi, and P. J. J. Hooykaas (ed.), The Rhizobiaceae: molecular biology of model plant-associated bacteria. Kluwer Academic Publishing, Dordrecht, The Netherlands.
- 52.Farrand, S. K., I. Hwang, and D. M. Cook. 1996. The tra region of the nopaline-type Ti plasmid is a chimera with elements related to the transfer systems of RSF1010, RP4, and F. J. Bacteriol. 178:4233-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finan, T. M., A. M. Hirsch, J. A. Leigh, E. Johansen, G. A. Kuldau, S. Deegan, G. C. Walker, and E. R. Signer. 1985. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell 40:869-877. [DOI] [PubMed] [Google Scholar]
- 54.Fisher, R. F., H. L. Brierley, J. T. Mulligan, and S. R. Long. 1987. Transcription of Rhizobium meliloti nodulation genes. Identification of a nodD transcription initiation site in vitro and in vivo. J. Biol. Chem. 262:6849-6855. [PubMed] [Google Scholar]
- 55.Fisher, R. F., and S. R. Long. 1992. Rhizobium-plant signal exchange. Nature 357:655-660. [DOI] [PubMed] [Google Scholar]
- 56.Flores, M., P. Mavingul, L. Girard, X. Perret, W. J. Broughton, E. Martinez-Romero, G. Davila, and R. Palacios. 1998. Three replicons of Rhizobium sp. strain NGR234 harbor symbiotic gene sequences. J. Bacteriol. 180:6052-6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freiberg, C., R. Fellay, A. Bairoch, W. J. Broughton, A. Rosenthal, and X. Perret. 1997. Molecular basis of symbiosis between Rhizobium and legumes. Nature 387:394-401. [DOI] [PubMed] [Google Scholar]
- 58.Fuqua, C., M. Burbea, and S. C. Winans. 1995. Activity of the Agrobacterium Ti plasmid conjugal transfer regulator TraR is inhibited by the product of the traM gene. J. Bacteriol. 177:1367-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fuqua, C., and E. P. Greenberg. 1998. Self perception in bacteria: quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1:183-189. [DOI] [PubMed] [Google Scholar]
- 60.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 61.Fuqua, C., and S. C. Winans. 1996. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J. Bacteriol. 178:435-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuqua, W. C., and S. C. Winans. 1994. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J. Bacteriol. 176:2796-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuqua, W. C., S. C. Winans, and E. P. Greenberg. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilson, L., A. Kuo, and P. V. Dunlap. 1995. AinS and a new family of autoinducer synthesis proteins. J. Bacteriol. 177:6946-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Givskov, M., R. de Nys, M. Manefield, L. Gram, R. Maximilien, L. Eberl, S. Molin, P. D. Steinberg, and S. Kjelleberg. 1996. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J. Bacteriol. 178:6618-6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glazebrook, J., and G. C. Walker. 1989. A novel exopolysaccharide can function in place of the Calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell 56:661-672. [DOI] [PubMed] [Google Scholar]
- 67.González, J. E., B. L. Reuhs, and G. C. Walker. 1996. Low molecular weight EPS II of Rhizobium meliloti allows nodule invasion in Medicago sativa. Proc. Natl. Acad. Sci. USA 93:8636-8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.González, J. E., G. M. York, and G. C. Walker. 1996. Rhizobium meliloti exopolysaccharides: synthesis and symbiotic function. Gene 179:141-146. [DOI] [PubMed] [Google Scholar]
- 69.Gray, K. M. 1997. Intercellular communication and group behavior in bacteria. Trends Microbiol. 5:184-188. [DOI] [PubMed] [Google Scholar]
- 70.Gray, K. M., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1994. Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. J. Bacteriol. 176:3076-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gray, K. M., J. P. Pearson, J. A. Downie, B. E. Boboye, and E. P. Greenberg. 1996. Cell-to-cell signaling in the symbiotic nitrogen-fixing bacterium Rhizobium leguminosarum: autoinduction of a stationary phase and rhizosphere-expressed genes. J. Bacteriol. 178:372-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Habeeb, L. F., L. Wang, and S. C. Winans. 1991. Transcription of the octopine catabolism operon of the Agrobacterium tumor-inducing plasmid pTiA6 is activated by a LysR-type regulatory protein. Mol. Plant-Microbe Interact. 4:379-385. [DOI] [PubMed] [Google Scholar]
- 73.Hanzelka, B. L., and E. P. Greenberg. 1995. Evidence that the N-terminal region of the Vibrio fischeri LuxR protein constitutes an autoinducer-binding domain. J. Bacteriol. 177:815-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanzelka, B. L., and E. P. Greenberg. 1996. Quorum sensing in Vibrio fischeri: evidence that S-adenosylmethionine is the amino acid substrate for autoinducer synthesis. J. Bacteriol. 178:5291-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hanzelka, B. L., M. R. Parsek, D. L. Val, P. V. Dunlap, J. E. Cronan, Jr., and E. P. Greenberg. 1999. Acylhomoserine lactone synthase activity of the Vibrio fischeri AinS protein. J. Bacteriol. 181:5766-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hanzelka, B. L., A. M. Stevens, M. R. Parsek, T. J. Crone, and E. P. Greenberg. 1997. Mutational analysis of the Vibrio fischeri LuxI polypeptide: critical regions of an autoinducer synthase. J. Bacteriol. 179:4882-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hardman, A. M., G. S. Stewart, and P. Williams. 1998. Quorum sensing and the cell-cell communication dependent regulation of gene expression in pathogenic and non-pathogenic bacteria. Antonie Leeuwenhoek 74:199-210. [DOI] [PubMed] [Google Scholar]
- 78.He, X., W. Chang, D. L. Pierce, L. O. Seib, J. Wagner, and C. Fuqua. 2003. Quorum sensing in Rhizobium sp. strain NGR234 regulates conjugal transfer (tra) gene expression and influences growth rate. J. Bacteriol. 185:809-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirsch, A. M., S. R. Long, M. Bang, N. Haskins, and F. M. Ausubel. 1982. Structural studies of alfalfa roots infected with nodulation mutants of Rhizobium meliloti. J. Bacteriol. 151:411-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirsch, A. M., M. R. Lum, and J. A. Downie. 2001. What makes the rhizobia-legume symbiosis so special? Plant Physiol. 127:1484-1492. [PMC free article] [PubMed] [Google Scholar]
- 81.Hirsch, P. R. 1979. Plasmid-determined bacteriocin production by Rhizobium leguminosarum. J. Gen. Microbiol. 113:219-228. [Google Scholar]
- 82.Huguet, T., C. Rosenberg, F. Casse-Delbart, P. De Lajudie, L. Jouanin, J. Batut, P. Boistard, J.-S. Julliot, and J. Denarie. 1983. Studies on Rhizobium meliloti plasmids and on their role in the control of nodule formation and nitrogen fixation: the pSym megaplasmids and the other large plasmids, p. 35-45. In A. Pühler (ed.), Molecular genetics of the bacteria-plant interaction. Springer-Verlag, Berlin, Germany.
- 83.Hwang, I., D. M. Cook, and S. K. Farrand. 1995. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J. Bacteriol. 177:449-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hwang, I., P. L. Li, L. Zhang, K. R. Piper, D. M. Cook, M. E. Tate, and S. K. Farrand. 1994. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc. Natl. Acad. Sci. USA 91:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hwang, I., A. J. Smyth, Z. Q. Luo, and S. K. Farrand. 1999. Modulating quorum sensing by antiactivation: TraM interacts with TraR to inhibit activation of Ti plasmid conjugal transfer genes. Mol. Microbiol. 34:282-294. [DOI] [PubMed] [Google Scholar]
- 86.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163:1210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laue, B. E., Y. Jiang, S. R. Chhabra, S. Jacob, G. S. Stewart, A. Hardman, J. A. Downie, F. O'Gara, and P. Williams. 2000. The biocontrol strain Pseudomonas fluorescens F113 produces the Rhizobium small bacteriocin, N-(3-hydroxy-7-cis-tetradecenoyl)homoserine lactone, via HdtS, a putative novel N-acylhomoserine lactone synthase. Microbiology 146:2469-2480. [DOI] [PubMed] [Google Scholar]
- 88.Leadbetter, J. R., and E. P. Greenberg. 2000. Metabolism of acyl-homoserine lactone quorum-sensing signals by Variovorax paradoxus. J. Bacteriol. 182:6921-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leigh, J. A., and D. L. Coplin. 1992. Exopolysaccharides in plant-bacterial interactions. Annu. Rev. Microbiol. 46:307-346. [DOI] [PubMed] [Google Scholar]
- 90.Leigh, J. A., J. W. Reed, J. F. Hanks, A. M. Hirsch, and G. C. Walker. 1987. Rhizobium meliloti mutants that fail to succinylate their calcofluor-binding exopolysaccharide are defective in nodule invasion. Cell 51:579-587. [DOI] [PubMed] [Google Scholar]
- 91.Leigh, J. A., E. R. Signer, and G. C. Walker. 1985. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. USA 82:6231-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lerouge, P., P. Roche, C. Faucher, F. Maillet, G. Truchet, J. C. Promé, and J. Dénarié. 1990. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature 344:781-784. [DOI] [PubMed] [Google Scholar]
- 93.Li, P. L., D. M. Everhart, and S. K. Farrand. 1998. Genetic and sequence analysis of the pTiC58 trb locus, encoding a mating-pair formation system related to members of the type IV secretion family. J. Bacteriol. 180:6164-6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li, P. L., and S. K. Farrand. 2000. The replicator of the nopaline-type Ti plasmid pTiC58 is a member of the repABC family and is influenced by the TraR-dependent quorum-sensing regulatory system. J. Bacteriol. 182:179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lithgow, J. K., A. Wilkinson, A. Hardman, B. Rodelas, F. Wisniewski-Dye, P. Williams, and J. A. Downie. 2000. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol. Microbiol. 37:81-97. [DOI] [PubMed] [Google Scholar]
- 96.Loh, J., R. W. Carlson, W. S. York, and G. Stacey. 2002. Bradyoxetin, a unique chemical signal involved in symbiotic gene regulation. Proc. Natl. Acad. Sci. USA 99:14446-14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loh, J., D. P. Lohar, B. Andersen, and G. Stacey. 2002. A two-component regulator mediates population-density-dependent expression of the Bradyrhizobium japonicum nodulation genes. J. Bacteriol. 184:1759-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Loh, J., and G. Stacey. 2003. Nodulation Gene Regulation in Bradyrhizobium japonicum: a unique integration of global regulatory circuits. Appl. Environ. Microbiol. 69:10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loh, J. T., J. P. Yuen-Tsai, M. G. Stacey, D. Lohar, A. Welborn, and G. Stacey. 2001. Population density-dependent regulation of the Bradyrhizobium japonicum nodulation genes. Mol. Microbiol. 42:37-46. [DOI] [PubMed] [Google Scholar]
- 100.Long, S. R. 1996. Rhizobium symbiosis: nod factors in perspective. Plant Cell 8:1885-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Long, S. R. 1989. Rhizobium-legume nodulation: life together in the underground. Cell 56:203-214. [DOI] [PubMed] [Google Scholar]
- 102.Long, S. R., and E. M. Atkinson. 1990. Rhizobium sweet-talking. Nature 344:712-713. [Google Scholar]
- 103.Long, S. R., and B. J. Staskawicz. 1993. Prokaryotic plant parasites. Cell 73:921-935. [DOI] [PubMed] [Google Scholar]
- 104.Luo, Z. Q., Y. Qin, and S. K. Farrand. 2000. The antiactivator TraM interferes with the autoinducer-dependent binding of TraR to DNA by interacting with the C-terminal region of the quorum-sensing activator. J. Biol. Chem. 275:7713-7722. [DOI] [PubMed] [Google Scholar]
- 105.Manefield, M., T. B. Rasmussen, M. Henzter, J. B. Andersen, P. Steinberg, S. Kjelleberg, and M. Givskov. 2002. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148:1119-1127. [DOI] [PubMed] [Google Scholar]
- 106.Marketon, M. M., S. A. Glenn, A. Eberhard, and J. E. González. 2003. Quorum sensing controls exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 185:325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marketon, M. M., and J. E. González. 2002. Identification of two quorum-sensing systems in Sinorhizobium meliloti. J. Bacteriol. 184:3466-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marketon, M. M., M. R. Gronquist, A. Eberhard, and J. E. González. 2002. Characterization of the Sinorhizobium meliloti sinR/sinI locus and the production of novel N-acyl homoserine lactones. J. Bacteriol. 184:5686-5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reference deleted.
- 110.McKenney, D., K. E. Brown, and D. G. Allison. 1995. Influence of Pseudomonas aeruginosa exoproducts on virulence factor production in Burkholderia cepacia: evidence of interspecies communication. J. Bacteriol. 177:6989-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.More, M. I., L. D. Finger, J. L. Stryker, A. C. Fuqua, Eberhard, and S. C. Winans. 1996. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 272:1655-1658. [DOI] [PubMed] [Google Scholar]
- 112.Mulligan, J. T., and S. R. Long. 1985. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc. Natl. Acad. Sci. USA 82:6609-6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Murphy, P. J., N. Heycke, Z. Banfalvi, M. E. Tate, F. de Bruijn, A. Kondorosi, J. Tempe, and J. Schell. 1987. Genes for the catabolism and synthesis of an opine-like compound in Rhizobium meliloti are closely linked and on the Sym plasmid. Proc. Natl. Acad. Sci. USA 84:493-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nealson, K. H., T. Platt, and J. W. Hastings. 1970. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 104:313-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Niebel, A., J.-J. Bono, R. Ranjeva, and J. V. Cillimore. 1997. Identification of a high affinity binding site for lipooligosaccharidic NodRm factors in the microsomal fraction of Medicago cell suspension cultures. Mol. Plant-Microb Interact. 10:132-134. [Google Scholar]
- 116.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 178:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oresnik, I. J., S. L. Liu, C. K. Yost, and M. F. Hynes. 2000. Megaplasmid pRme2011a of Sinorhizobium meliloti is not required for viability. J. Bacteriol. 182:3582-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pappas, K. M., and S. C. Winans. 2003. A LuxR-type regulator from Agrobacterium tumefaciens elevates Ti plasmid copy number by activating transcription of plasmid replication genes. Mol. Microbiol. 48:1059-1073. [DOI] [PubMed] [Google Scholar]
- 119.Pappas, K. M., and S. C. Winans. 2003. The RepA and RepB autorepressors and TraR play opposing roles in the regulation of a Ti plasmid repABC operon. Mol. Microbiol. 49:1365-2958. [DOI] [PubMed] [Google Scholar]
- 120.Parsek, M. R., and E. P. Greenberg. 2000. Acyl-homoserine lactone quorum sensing in Gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc. Natl. Acad. Sci. USA 97:8789-8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Parsek, M. R., A. L. Schaefer, and E. P. Greenberg. 1997. Analysis of random and site-directed mutations in rhII, a Pseudomonas aeruginosa gene encoding an acylhomoserine lactone synthase. Mol. Microbiol. 26:301-310. [DOI] [PubMed] [Google Scholar]
- 122.Parsek, M. R., D. L. Val, B. L. Hanzelka, J. E. Cronan, Jr., and E. P. Greenberg. 1999. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA 96:4360-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paulsen, I., M. Brown, and R. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pearson, J. P., C. Van Delden, and B. H. Iglewski. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pellock, B. J., M. Teplitski, R. P. Boinay, W. D. Bauer, and G. C. Walker. 2002. A LuxR homolog controls production of symbiotically active extracellular polysaccharide II by Sinorhizobium meliloti. J. Bacteriol. 184:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Perret, X., C. Staehelin, and W. J. Broughton. 2000. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 64:180-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peters, N. K., J. W. Frost, and S. R. Long. 1986. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233:977-980. [DOI] [PubMed] [Google Scholar]
- 128.Petit, A., A. Kerr, M. Holsters, M. van Montagu, and J. Schell. 1978. Substrate induction of conjugative activity of Agrobacterium tumefaciens Ti plasmids. Nature 271:570-572. [Google Scholar]
- 129.Petrovics, G., P. Putnoky, B. Reuhs, J. Kim, T. A. Thorp, K. D. Noel, R. W. Carlson, and A. Kondorosi. 1993. The presence of a novel type of surface polysaccharide in Rhizobium meliloti requires a new fatty acid synthase-like gene cluster involved in symbiotic nodule development. Mol. Microbiol. 8:1083-1094. [DOI] [PubMed] [Google Scholar]
- 130.Piper, K. R., S. Beck von Bodman, and S. K. Farrand. 1993. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature 362:448-450. [DOI] [PubMed] [Google Scholar]
- 131.Piper, K. R., S. Beck Von Bodman, I. Hwang, and S. K. Farrand. 1999. Hierarchical gene regulatory systems arising from fortuitous gene associations: controlling quorum sensing by the opine regulon in Agrobacterium. Mol. Microbiol. 32:1077-1089. [DOI] [PubMed] [Google Scholar]
- 132.Piper, K. R., and S. K. Farrand. 2000. Quorum sensing but not autoinduction of Ti plasmid conjugal transfer requires control by the opine regulon and the antiactivator TraM. J. Bacteriol. 182:1080-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pueppke, S. G., and W. J. Broughton. 1999. Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol. Plant-Microbe Interact. 12:293-318. [DOI] [PubMed] [Google Scholar]
- 134.Puskas, A., E. P. Greenberg, S. Kaplan, and A. L. Schaefer. 1997. A quorum-sensing system in the free-living photosynthetic bacterium Rhodobacter sphaeroides. J. Bacteriol. 179:7530-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Qin, Y., Z. Q. Luo, A. J. Smyth, P. Gao, S. Beck von Bodman, and S. K. Farrand. 2000. Quorum-sensing signal binding results in dimerization of TraR and its release from membranes into the cytoplasm. EMBO J. 19:5212-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ramirez-Romero, M. A., N. Soberon, A. Perez-Oseguera, J. Tellez-Sosa, and M. A. Cevallos. 2000. Structural elements required for replication and incompatibility of the Rhizobium etli symbiotic plasmid. J. Bacteriol. 182:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Redfield, R. J. 2002. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 10:365-370. [DOI] [PubMed] [Google Scholar]
- 138.Roche, P., F. Debellé, F. Maillet, P. Lerouge, C. Faucher, G. Truchet, and J. Dénarié. 1991. Molecular basis of symbiotic host specificity in Rhizobium meliloti: nodH and nodPQ genes encode the sulfation of lipo-oligosaccharide signals. Cell 67:1131-1143. [DOI] [PubMed] [Google Scholar]
- 139.Rodelas, B., J. K. Lithgow, F. Wisniewski-Dye, A. Hardman, A. Wilkinson, A. Economou, P. Williams, and J. A. Downie. 1999. Analysis of quorum-sensing-dependent control of rhizosphere-expressed (rhi) genes in Rhizobium leguminosarum bv.viciae. J. Bacteriol. 181:3816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rolfe, B. G., and P. M. Gresshoff. 1988. Genetic analysis of legume nodule initiation. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 39:297-319. [Google Scholar]
- 141.Rosemeyer, V., J. Michiels, C. Verreth, and J. Vanderleyden. 1998. luxI- and luxR-homologous genes of Rhizobium etli CNPAF512 contribute to synthesis of autoinducer molecules and nodulation of Phaseolus vulgaris. J. Bacteriol. 180:815-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ruby, E. G. 1999. The Euprymna scolopes-Vibrio fischeri symbiosis: a biomedical model for the study of bacterial colonization of animal tissue. J. Mol. Microbiol. Biotechnol. 1:13-21. [PubMed] [Google Scholar]
- 143.Ruby, E. G., and L. M. Asato. 1993. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch. Microbiol. 159:160-167. [DOI] [PubMed] [Google Scholar]
- 144.Saier, M. H., Jr., R. Tam, A. Reizer, and J. Reizer. 1994. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol. Microbiol. 11:841-847. [DOI] [PubMed] [Google Scholar]
- 145.Salmond, G. P., B. W. Bycroft, G. S. Stewart, and P. Williams. 1995. The bacterial “enigma”: cracking the code of cell-cell communication. Mol. Microbiol. 16:615-624. [DOI] [PubMed] [Google Scholar]
- 146.Schaefer, A. L., T. A. Taylor, J. T. Beatty, and E. P. Greenberg. 2002. Long-chain acyl-homoserine lactone quorum-sensing regulation of Rhodobacter capsulatus gene transfer agent production. J. Bacteriol. 184:6515-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schaefer, A. L., D. L. Val, B. L. Hanzelka, J. E. Cronan, Jr., and E. P. Greenberg. 1996. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc. Natl. Acad. Sci. USA 93:9505-9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schauder, S., K. Shokat, M. G. Surette, and B. L. Bassler. 2001. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 41:463-476. [DOI] [PubMed] [Google Scholar]
- 149.Schneider, A., S. A. Walker, S. Poyser, M. Sagan, T. H. Ellis, and J. A. Downie. 1999. Genetic mapping and functional analysis of a nodulation-defective mutant (sym19) of pea (Pisum sativum L.). Mol. Gen. Genet. 262:1-11. [DOI] [PubMed] [Google Scholar]
- 150.Schripsema, J., K. E. de Rudder, T. B. van Vliet, P. P. Lankhorst, E. de Vroom, J. W. Kijne, and A. A. van Brussel. 1996. Bacteriocin small of Rhizobium leguminosarum belongs to the class of N-acyl-l-homoserine lactone molecules, known as autoinducers and as quorum-sensing cotranscription factors. J. Bacteriol. 178:366-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schwedock, J. S., C. Liu, T. S. Leyh, and S. R. Long. 1994. Rhizobium meliloti NodP and NodQ form a multifunctional sulfate-activating complex requiring GTP for activity. J. Bacteriol. 176:7055-7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shadel, G. S., and T. O. Baldwin. 1992. Positive autoregulation of the Vibrio fischeri luxR gene. LuxR and autoinducer activate cAMP-catabolite gene activator protein complex-independent and -dependent luxR transcription. J. Biol. Chem. 267:7696-7702. [PubMed] [Google Scholar]
- 153.Shadel, G. S., and T. O. Baldwin. 1991. The Vibrio fischeri LuxR protein is capable of bidirectional stimulation of transcription and both positive and negative regulation of the luxR gene. J. Bacteriol. 173:568-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shadel, G. S., R. Young, and T. O. Baldwin. 1990. Use of regulated cell lysis in a lethal genetic selection in Escherichia coli: identification of the autoinducer-binding region of the LuxR protein from Vibrio fischeri ATCC 7744. J. Bacteriol. 172:3980-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Slock, J., D. VanRiet, D. Kolibachuk, and E. P. Greenberg. 1990. Critical regions of the Vibrio fischeri LuxR protein defined by mutational analysis. J. Bacteriol. 172:3974-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Steidle, A., K. Sigl, R. Schuhegger, A. Ihring, M. Schmid, S. Gantner, M. Stoffels, K. Riedel, M. Givskov, A. Hartmann, C. Langebartels, and L. Eberl. 2001. Visualization of N-acylhomoserine lactone-mediated cell-cell communication between bacteria colonizing the tomato rhizosphere. Appl. Environ. Microbiol. 67:5761-5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Stevens, A. M., K. M. Dolan, and E. P. Greenberg. 1994. Synergistic binding of the Vibrio fischeri LuxR transcriptional activator domain and RNA polymerase to the lux promoter region. Proc. Natl. Acad. Sci. USA 91:12619-12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stevens, A. M., N. Fujita, A. Ishihama, and E. P. Greenberg. 1999. Involvement of the RNA polymerase alpha-subunit C-terminal domain in LuxR-dependent activation of the Vibrio fischeri luminescence genes. J. Bacteriol. 181:4704-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stevens, A. M., and E. P. Greenberg. 1997. Quorum sensing in Vibrio fischeri: essential elements for activation of the luminescence genes. J. Bacteriol. 179:557-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Stougaard, J. 2001. Genetics and genomics of root symbiosis. Curr. Opin. Plant Biol. 4:328-335. [DOI] [PubMed] [Google Scholar]
- 161.Sullivan, J. T., and C. W. Ronson. 1998. Evolution of rhizobia by acquisition of a 500-kb symbiosis island that integrates into a phe-tRNA gene. Proc. Natl. Acad. Sci. USA 95:5145-5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Surette, M. G., and B. L. Bassler. 1999. Regulation of autoinducer production in Salmonella typhimurium. Mol. Microbiol. 31:585-595. [DOI] [PubMed] [Google Scholar]
- 164.Swartzman, A., S. Kapoor, A. F. Graham, and E. A. Meighen. 1990. A new Vibrio fischeri lux gene precedes a bidirectional termination site for the lux operon. J. Bacteriol. 172:6797-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Swiderska, A., A. K. Berndtson, M. R. Cha, L. Li, G. M. Beaudoin III, J. Zhu, and C. Fuqua. 2001. Inhibition of the Agrobacterium tumefaciens TraR quorum-sensing regulator. Interactions with the TraM anti-activator. J. Biol. Chem. 276:49449-49458. [DOI] [PubMed] [Google Scholar]
- 166.Swift, S., J. P. Throup, P. Williams, G. P. Salmond, and G. S. Stewart. 1996. Quorum sensing: a population-density component in the determination of bacterial phenotype. Trends Biochem. Sci. 21:214-219. [PubMed] [Google Scholar]
- 167.Teplitski, M., J. B. Robinson, and W. D. Bauer. 2000. Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol. Plant-Microbe Interact. 13:637-648. [DOI] [PubMed] [Google Scholar]
- 168.Thorne, S. H., and H. D. Williams. 1999. Cell density-dependent starvation survival of Rhizobium leguminosarum bv. phaseoli: identification of the role of an N-acyl homoserine lactone in adaptation to stationary-phase survival. J. Bacteriol. 181:981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Truchet, G., P. Roche, P. Lerouge, J. Vasse, S. Camut, F. de Billy, J.-C. Promé, and J. Dénarié. 1991. Sulphated lipo-oligosaccharide signals of Rhizobium meliloti elicit root nodule organogenesis in alfalfa. Nature 351:670-673. [Google Scholar]
- 170.Tun-Garrido, C., P. Bustos, V. Gonzalez, and S. Brom. 2003. Conjugative transfer of p42a from Rhizobium etli CFN42, which is required for mobilization of the symbiotic plasmid, is regulated by quorum sensing. J. Bacteriol. 185:1681-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vannini, A., C. Volpari, C. Gargioli, E. Muraglia, R. Cortese, R. De Francesco, P. Neddermann, and S. D. Marco. 2002. The crystal structure of the quorum sensing protein TraR bound to its autoinducer and target DNA. EMBO J. 21:4393-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Verma, D. P. S., and S. Long. 1983. The molecular biology of Rhizobium-legume symbiosis. Int. Rev. Cytol. 14:211-245. [Google Scholar]
- 173.Visick, K. L., and M. J. McFall-Ngai. 2000. An exclusive contract: specificity in the Vibrio fischeri-Euprymna scolopes partnership. J. Bacteriol. 182:1779-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Walker, S. A., V. Viprey, and J. A. Downie. 2000. Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by nod factors and chitin oligomers. Proc. Natl. Acad. Sci. USA 97:13413-13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Watson, W. T., T. D. Minogue, D. L. Val, S. B. von Bodman, and M. E. Churchill. 2002. Structural basis and specificity of acyl-homoserine lactone signal production in bacterial quorum sensing. Mol. Cell 9:685-694. [DOI] [PubMed] [Google Scholar]
- 176.Watson, W. T., F. V. Murphy IV, T. A. Gould, P. Jambeck, D. L. Val, J. E. Cronan, Jr., S. Beck von Bodman, and M. E. Churchill. 2001. Crystallization and rhenium MAD phasing of the acyl-homoserinelactone synthase EsaI. Acta Crystallogr. Ser. D 57:1945-1949. [DOI] [PubMed] [Google Scholar]
- 177.Welch, M., D. E. Todd, N. A. Whitehead, S. J. McGowan, B. W. Bycroft, and G. P. Salmond. 2000. N-Acyl homoserine lactone binding to the CarR receptor determines quorum-sensing specificity in Erwinia. EMBO J. 19:631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Whitehead, N. A., A. M. Barnard, H. Slater, N. J. Simpson, and G. P. Salmond. 2001. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 25:365-404. [DOI] [PubMed] [Google Scholar]
- 179.Wijffelman, C. A. P., E. Brussel, and A. A. N. van Hooykaas. 1983. Repression of small bacteriocin excretion in Rhizobium leguminosarum and Rhizobium trifolii by transmissible plasmids. Mol. Gen. Genet. 192:171-176. [Google Scholar]
- 180.Wilkinson, A., V. Danino, F. Wisniewski-Dye, J. K. Lithgow, and J. A. Downie. 2002. N-Acyl-homoserine lactone inhibition of rhizobial growth is mediated by two quorum-sensing genes that regulate plasmid transfer. J. Bacteriol. 184:4510-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Winans, S. C. 1992. Two-way chemical signaling in Agrobacterium-plant interactions. Microbiol. Rev. 56:12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Winzer, K., K. R. Hardie, and P. Williams. 2002. Bacterial cell-to-cell communication: sorry, can't talk now—gone to lunch! Curr. Opin. Microbiol. 5:216-222. [DOI] [PubMed] [Google Scholar]
- 183.Wisniewski-Dye, F., and J. A. Downie. 2002. Quorum-sensing in Rhizobium. Antonie Leeuwenhoek 81:397-407. [DOI] [PubMed] [Google Scholar]
- 184.Wisniewski-Dye, F., J. Jones, S. R. Chhabra, and J. A. Downie. 2002. raiIR genes are part of a quorum-sensing network controlled by cinI and cinR in Rhizobium leguminosarum. J. Bacteriol. 184:1597-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Withers, H., S. Swift, and P. Williams. 2001. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr. Opin. Microbiol. 4:186-193. [DOI] [PubMed] [Google Scholar]
- 186.Yang, C., E. R. Signer, and A. M. Hirsch. 1992. Nodules initiated by Rhizobium meliloti exopolysaccharide mutants lack a discrete, persistent nodule meristem. Plant Physiol. 98:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yuen, J. P.-Y., and G. Stacey. 1996. Inhibition of nod gene expression in Bradyrhizobium japonicum by organic acids. Mol. Plant-Microb Interact. 9:424-428. [Google Scholar]
- 188.Zhang, L., P. J. Murphy, A. Kerr, and M. E. Tate. 1993. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature 362:446-448. [DOI] [PubMed] [Google Scholar]
- 189.Zhang, R. G., T. Pappas, J. L. Brace, P. C. Miller, T. Oulmassov, J. M. Molyneaux, J. C. Anderson, J. K. Bashkin, S. C. Winans, and A. Joachimiak. 2002. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature 417:971-974. [DOI] [PubMed] [Google Scholar]
- 190.Zhu, J., P. M. Oger, B. Schrammeijer, P. J. Hooykaas, S. K. Farrand, and S. C. Winans. 2000. The bases of crown gall tumorigenesis. J. Bacteriol. 182:3885-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhu, J., and S. C. Winans. 1999. Autoinducer binding by the quorum-sensing regulator TraR increases affinity for target promoters in vitro and decreases TraR turnover rates in whole cells. Proc. Natl. Acad. Sci. USA 96:4832-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhu, J., and S. C. Winans. 2001. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc. Natl. Acad. Sci. USA 98:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]