Summary
The process of gastrulation is highly conserved across vertebrates on both the genetic and morphological levels, despite great variety in embryonic shape and speed of development. This mechanism spatially separates the germ layers and establishes the organizational foundation for future development. Mesodermal identity is specified in a superficial layer of cells, the epiblast, where cells maintain an epithelioid morphology. These cells involute to join the deeper hypoblast layer where they adopt a migratory, mesenchymal morphology. Expression of a cascade of related transcription factors orchestrates the parallel genetic transition from primitive to mature mesoderm. Although the early and late stages of this process are increasingly well understood, the transition between them has remained largely mysterious. We present here the first high resolution in vivo observations of the blebby transitional morphology of involuting mesodermal cells in a vertebrate embryo. We further demonstrate that the zebrafish spadetail mutation creates a reversible block in the maturation program, stalling cells in the transition state. This mutation creates an ideal system for dissecting the specific properties of cells undergoing the morphological transition of maturing mesoderm, as we demonstrate with a direct measurement of cell-cell adhesion.
Keywords: Mesoderm, Gastrulation, Involution, Adhesion, Bleb, spadetail, Cadherin, Myosin
Introduction
Gastrulation lays down the organizational foundation of the embryo by separating the endodermal, mesodermal and ectodermal germ layers (Keller, 2002; Shook and Keller, 2008). Cells in each germ layer undergo a characteristic series of molecular and morphological changes following their initial specification. In the case of zebrafish mesoderm, for example, cells first migrate collectively as an epithelioid sheet towards a site of involution, where they migrate underneath the outer cell layer (the epiblast) to populate a deeper layer (the hypoblast). Cells in the hypoblast exhibit a mesenchymal morphology, extending actin-based protrusions (lamellipodia and filopodia) as well as occasional blebs, which form when pressure in the cell inflates a blister of membrane at sites where the membrane and cortical actin are uncoupled (Reviewed in Charras and Paluch, 2008; Fackler and Grosse, 2008). Our work, and that of others, has demonstrated that regulated blebbing is a crucial aspect of embryonic cell migration, most notably in the case of germ cells, which migrate exclusively by blebbing (Blaser et al., 2006; Kardash et al., 2010; Weiser et al., 2007; Weiser et al., 2009). These various protrusive activities act coordinately to allow zebrafish mesendodermal cells to converge toward the dorsal side of the embryo during gastrulation.
An extensive body of literature describes the pathways that initially specify mesodermal identity in the epiblast (reviewed in Kimelman, 2006; Schier and Talbot, 2005), and the factors that regulate cell behavior in the hypoblast are increasingly well understood (Arboleda-Estudillo et al., 2010; Kai et al., 2008; Keller, 2005; Myers et al., 2002; Ulrich et al., 2005; von der Hardt et al., 2007). Elegant studies have demonstrated the means by which the directed migration of many mesodermal cells shapes the gastrulating embryo (Carmany-Rampey and Schier, 2001; Concha and Adams, 1998; Warga and Kimmel, 1990; Yin et al., 2009). Such studies have focused on the movement of cells within the hypoblast or epiblast. However, the transition between epiblast and hypoblast has remained mysterious, both on the molecular level and in understanding the process of involution. Because this transition represents such a dramatic and crucial change in cell morphology we wanted to dissect the regulatory mechanisms at work. Two barriers to dissecting the transition state are the brief time each cell spends moving between the epiblast and hypoblast, and the difficulty of making in vivo observations. Zebrafish embryos have several advantages for studying this dynamic process including optical clarity, rapid and external development, and thinness of the tissue. Using time-lapse DIC microscopy we made high resolution observations of cells transitioning from epiblast to hypoblast and observed a dramatic change in behavior in which cells undergoing involution pass through a transition state in which they bleb extensively.
We reasoned that the transition state between epiblast and hypoblast would be more tractable for study if we could find a way to hold cells in this state. The spadetail (spt) mutation was a good candidate to introduce a temporary blockade in the morphogenetic transition from epiblast to hypoblast (Kimmel et al., 1989). Mesodermal cells that contribute to the somites express specific T-box transcription factors in series as they progress from specification through their differentiation program (Amacher et al., 2002; Goering et al., 2003; Griffin and Kimelman, 2002). These mesodermal progenitors express the brachyury factor no tail (ntl) from the onset of their fate specification, then subsequently activate spt and tbx6 as they begin the differentiation process, entering a region that we have called the maturation zone (Griffin and Kimelman, 2002). As cells leave the maturation zone and enter the presomitic mesoderm, they turn off ntl, and activate tbx24. We previously showed by examining gene expression that spt mutant cells enter the maturation zone state but remain trapped there, retaining expression of progenitor genes but failing to activate downstream genes such as tbx24. These results, however, did not explain why spt mutant cells fail to migrate properly, although defective cell adhesion has been a commonly held view (Warga and Nusslein-volhard, 1998; Yamamoto et al., 1998). Here we show that mesodermal cells in spt mutant embryos enter the blebby transition state as normal but are unable to complete the morphological transition of normal cells in the hypoblast. Whereas normal cells reduce blebbing as they leave the transition state and migrate away, spt cells continue the rapid blebbing and fail to move away from the transition zone. Crucially we show that this phenotype represents a temporary, reversible interruption in the maturation program rather than a permanent change in cell fate. Thus, mesodermal cells pass through a morphological as well as genetic transition stage between epiblast and hypoblast, with Spadetail required to complete the transition.
We utilized mesodermal cells lacking Spadetail to probe aspects of the epiblast-to-hypoblast transition state. Using a single-cell adhesion assay we demonstrate that non-axial mesoderm lacking Spadetail is significantly more adhesive than wild-type mesoderm, ruling out the possibility that cells fails to leave the maturation zone because of an inability to adhere to their neighbors. In support of this, we show that surface levels of the classical cadherins, the major adhesion factors in the early embryo, are not affected by a loss of Spadetail. Interestingly, we also observed identical levels of phosphorylated (activated) myosin in cells with and without Spadetail. This result is surprising since previous work showed that zebrafish mesodermal cells adopt a highly blebby state in response to increases in myosin phosphorylation (Weiser et al., 2009). We conclude that wild-type cells activate the highly blebby state as they enter the maturation zone, and that Spadetail inhibits this activity in a myosin-independent manner. Our results demonstrate that spt mutant embryos are a valuable system for probing the dynamics of the epiblast to hypoblast transition since they reversibly hold cells in the transition state.
Materials and Methods
Zebrafish lines, Heat Shocks and morpholinos
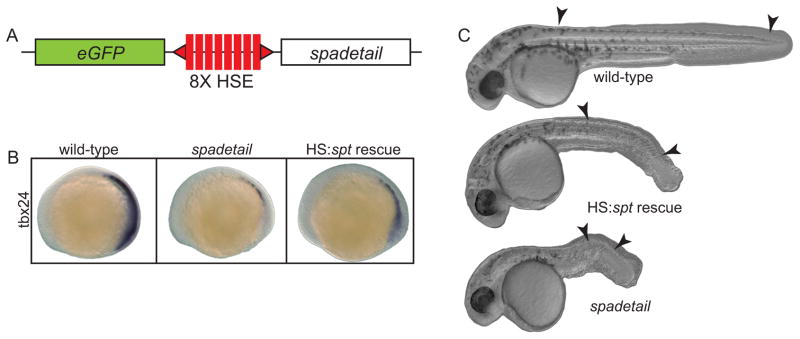
The Tg(HS:spt, eGFP) line was created by placing the coding sequence of zebrafish spt (Spadetail-myc fusion, a kind gift from David Grunwald) on one side of a multimerized heat shock promoter (Bajoghli et al., 2004) with a Green Fluorescent Protein (eGFP) gene on the opposite side (Fig. 4A). This was flanked by two Tol2 elements and used to generate stable transgenics in the WIK/AB background according to Kawakami (2004). Heat shocks were at 40.5°C for thirty minutes, in pre-warmed embryo rearing media (EM). spt morpholinos were the same as in Lewis and Eisen (2004). A mixture of 1.5 ng of MO1 and 0.75 ng MO2 was injected into each embryo.
Fig. 4. Restoring Spadetail significantly rescues the spt phenotypes.
(A) A genetic construct was incorporated into the zebrafish genome by Tol2-mediated transgenesis. The spt and eGFP genes were placed downstream of a bi-directional heat shock-sensitive promoter. (B) Expressing spt from this construct partially restored expression of tbx24 in spt morphant embryos. (C) Significant phenotypic rescue was also observed by inducing transgene expression early in gastrulation. Arrowheads mark the position of the first and last formed somite in each embryo.
Induced ventral/lateral mesoderm
To induce ventral and lateral mesoderm, embryos were injected with 5 pg synthetic cyc mRNA at the 1-cell stage. At dome stage, embryos were treated with the GSK3 inhibitor BIO (CalBiochem) at 3 μM.
Surface biotinylation, immunoprecipitation and Western blotting
Surface-exposed proteins were biotinylated immediately after the onset of gastrulation in induced ventral/lateral mesoderm embryos. Dechorionated embryos were gently de-yolked by pipetting, leaving the blastoderms intact. They were incubated in 1 mg/mL EZ-Link Sulfo-NHS-Biotin (Pierce) in 0.1X MMR without EDTA (0.1M NaCl, 2mM KCl, 1mM MgSO4, 2mM CaCl2, 5mM HEPES, pH 7.8). Biotinylation was carried out at 4°C for 10 minutes, then quenched with two washes of 5mM glycine in embryo rearing media. Cells were lysed in buffer containing 1% Triton X-100 and HALT protease/phosphatase inhibitor cocktail (Pierce; 1.5 μL/embryo lysed). This method was adapted from Chen et. al. (1997).
Total embryo lysate was separated by polyacrylamide gel electrophoresis, blotted onto nitrocellulose, and probed with anti-actin (MAB1501R, Chemicon) or anti-pMLC2 (Cell Signaling, 3671) antibodies. Biotinylated proteins were immunoprecipitated with E-cadherin or N-cadherin antibodies (raised against synthetic peptides: DKDLPPFAGPFKVEPQGDTSKN for Cdh1 (as described in Babb and Marrs, 2004), and CNAGPYAFELPNRPSDIRRNWTL for Cdh2), and detected on Western blots with an anti-biotin antibody (Thermo Pierce, MA1-37172).
In Situ Hybridization and Immunohistochemistry
Single probe whole-mount in situ hybridization was performed as described in Griffin, et al. (1995).
Cell Transplantation
Donor embryos obtained from an outcross of Tg(HS:spt, eGFP) hemizygotes to WIK/AB wild-types were injected with spt morpholinos and 1% rhodamine dextran at the 1-cell stage. Transplants were from donors at sphere stage into hosts at shield stage, targeted to the ventral mesoderm. Hosts were heat shocked at the 1-somite or 8-somite stage and photographed at 36 hpf.
Analysis of cell movement
An Axiovert 200M microscope (Zeiss) and 40X objective with DIC optics were used to make in vivo observations of lateral mesodermal cells (mesoderm 90° from the shield) at 55–60% epiboly. Observations were made of at least four separate embryos for all conditions. Images were acquired every three seconds to observe cell protrusions, or every 15 seconds for cell movement tracking. Tracking was performed using ImageJ (Rasband, 1997–2009).
Dual Micropipette Aspiration Assay
The Dual Micropipette Aspiration Assay was performed as previously described (Daoudi et al., 2004). Cells were prepared as by Krieg et al. (2008) from embryos induced to ventral/lateral mesoderm, with and without prior injection of spt MOs. The cells were manipulated at 25°C with two micropipettes, each one held by a micromanipulator connected to a microfluidic pump (Fluigent). Micropipettes with an internal diameter of 6–7μm were pulled (model P-97; Sutter Instrument), cut, and bent with a homemade microforge. Cells were imaged using an inverted epifluorescence microscope (Zeiss) equipped with a 40X objective (Zeiss LD PlanNeoFluar 0.6 numerical aperture Ph2 Korr) and a cooled charged-coupled device CoolSnap HQ (Photometrics). Images were acquired using Metamorph software.
Results
Mesodermal cells pass through a blebby intermediate state between epiblast and hypoblast
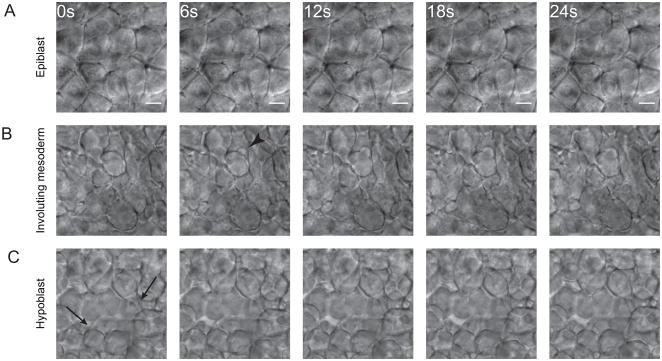
In order to observe changes in cell behavior during the epiblast to hypoblast transition, we examined the morphology of zebrafish mesodermal cells during gastrulation with high resolution time-lapse movies made with a computer-controlled Differential Interference Contrast (DIC) microscope. DIC optics allow observation of both superficial and deeper cells in living zebrafish embryos. Cells in the epiblast displayed an epithelioid morphology (Fig. 1A, Supplemental Movie 1) and migrated as a sheet towards the margin, the site of involution. They maintained long, straight and stable contacts with neighbors, except during mitoses. In the hypoblast we observed a typical mesenchymal morphology in cells that had completed involution and migrated away from the margin (Fig. 1C, Supplemental Movie 1). In contrast with the epiblast, these cells extended many protrusions and made only transient adhesions at discrete points of membrane contact. Actin-based protrusions like lamellipodia and filopodia were observed as well as membrane blebs. At the stage we studied (55–60% epiboly), cells had not yet begun rapidly migrating towards the dorsal pole so only migration away from the margin was observed.
Fig. 1. Cell morphology changes during mesoderm maturation.
Cell morphology was observed using time-lapse DIC microscopy of the lateral margin during early gastrulation. (A) Cells in the outer (epiblast) layer did not extend protrusions and maintained long, stable, edge-to-edge contacts with their neighbors. (B) As cells underwent involution at the margin they remained tightly packed but extended many protrusions, primarily blebs (arrowheads). (C) Cells in the hypoblast displayed a mesenchymal morphology. They extended actin-based protrusions (arrows) as well as blebs, did not pack tightly, and formed only transient adhesions at discrete points of contact. All images are at the same magnification, scale bar is 10 μM.
Cells in the intermediate zone between epiblast and hypoblast, which we refer to as the transition zone, were observed to have a surprising morphology. Throughout involution these cells remained densely packed and blebbed extensively (Fig. 1B, Supplemental Movie 1). We quantified membrane protrusions in involuting mesodermal cells and cells already in the hypoblast, as shown in Figure 2B. Involuting cells blebbed significantly more than deeper cells, which mostly extended actin-based protrusions. These movies provide the first high resolution observations of cells undergoing the transition from epiblast to hypoblast in living vertebrate embryos, and demonstrate that the transition state morphology is different from the epiblast and hypoblast morphologies.
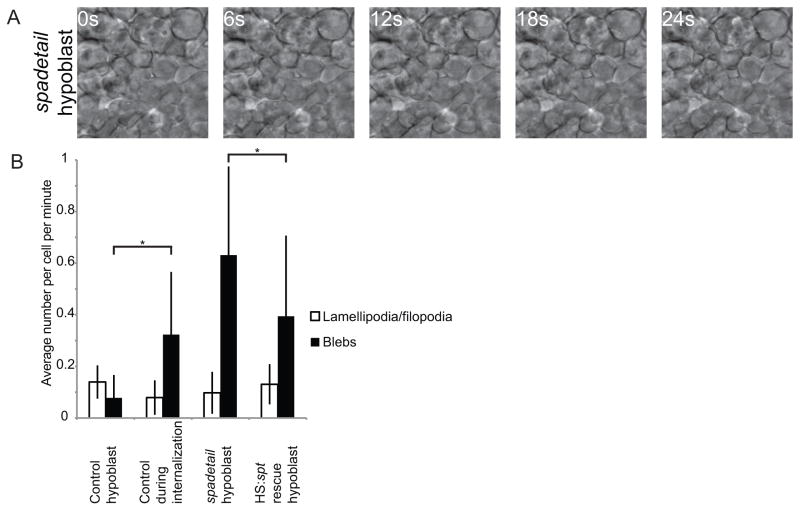
Fig. 2. Mesoderm in spt morphants displays abnormal morphology.
(A) Cells in the hypoblast of spt morphant embryos blebbed extensively and failed to adopt the proper mesenchymal morphology. (B) Cell protrusions were quantified in wild-type embryos during and after involution, in the hypoblast of spt morphants and in spt morphants expressing spt under the control of a heat shock-sensitive promoter. Mesodermal cells in spt morphants blebbed far more than in wild-type hypoblast, whereas restoring Spadetail significantly reduced this blebbing. Error bars indicate one standard deviation. At least 22 cells were quantified per condition. p<0.05 for data marked with an asterisk.
The spt mutation traps cells in the blebby intermediate state and disrupts normal migration
Because spt mutant cells are trapped in an early state in the differentiation process as shown by gene expression (Griffin and Kimelman, 2002), we wondered if they might also be morphologically stalled in the transition between epiblast and hypoblast. Cells in spt morphant embryos were observed by DIC time-lapse microscopy as for wild-type cells. Cells in the epiblast of these embryos had wild-type morphology, and no change from wild-type was observed in cells undergoing internalization. However, cells in the hypoblast of spt morphant embryos were observed to bleb to a much greater extent than those in the hypoblast of wild-type embryos, whereas the number of lamellipodia and filopodia was unchanged (Fig. 2A and B, Supplemental Movie 2). The extensive blebbing observed in these cells resembles the behavior of wild-type cells undergoing internalization, although cells in the hypoblast of spt morphant cells formed even more blebs than those cells. Since cells begin to express spt just prior to undergoing the epiblast to hypoblast transition (Amacher et al., 2002; Griffin et al., 1998), Spadetail may dampen down blebbing even in the transition state, as well as in the hypoblast where spt is strongly expressed. Importantly, identical cell morphological changes were observed in spt mutant embryos as in morphants (data not shown). For convenience, we have used morphants in our subsequent analyses since the morphants completely recapitulate the mutant phenotype with every embryo showing the mutant phenotype (as opposed to 25% when crossing heterozygous adults). In summary, our results show that spt morphant cells enter the epiblast to hypoblast transition normally, but fail to turn down the hyper-blebbing phenotype as they move into the hypoblast layer. As we demonstrate below, restoring expression of spadetail significantly rescues hypoblast cells from the hyper-blebbing phenotype.
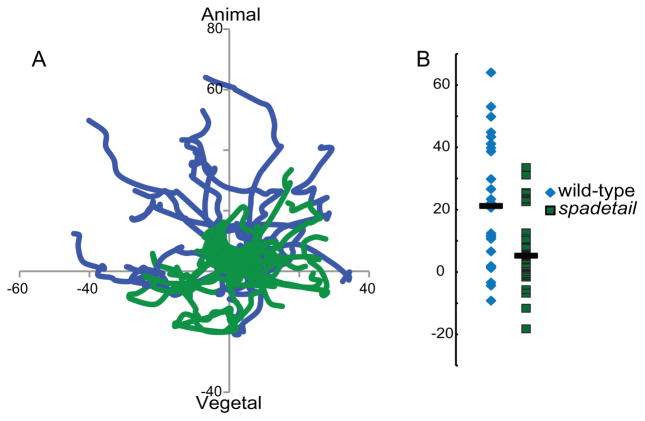
The failure of spt mutant mesoderm to undergo convergence movements towards the dorsal pole during gastrulation has been well documented (Ho and Kane, 1990). We observed that cells in the hypoblast of early gastrula stage spt morphants appeared to be packed more densely than in wild-type embryos. This suggested an additional defect in the animal-ward migration of hypoblast cells away from the transition zone immediately after involution. To test this hypothesis we tracked cell migration in hypoblast cells that had recently completed internalization. Cell movements were tracked for 30 minutes, producing tracks such as those shown in Figure 3A, with the margin parallel to the x-axis and all starting points set to (0,0). Wild-type cells (blue traces) mostly migrated towards the animal pole (Fig. 3B), without a dorsal or ventral bias at this stage. In contrast, spt morphant mesodermal cells migrated randomly, resulting in only a slight median net displacement towards the animal pole (Fig. 3A, B). Interestingly, the median velocity of both wild-type and spt morphant cells was identical during the observed periods at 0.03μm/sec (standard deviation of +/− 0.024 for both conditions). Therefore a loss of directionality of movement is the cause of the spt defect, not an inability to migrate.
Fig. 3. spt morphant mesoderm migrates abnormally.
(A) Recently involuted cells were tracked for 30 minutes in wild-type (blue traces) and spt morphant (green traces) embryos at 55–60% epiboly, lateral to the shield. The x-axis is aligned with the margin of the embryo. spt morphant cells failed to exhibit the animal-ward bias of migration observed in wild-type mesoderm. A representative set of tracks from one wild-type and one spt morphant is shown. (B) The final positions of all tracked cells on the y-axis are marked, with bars indicating median positions. Wild-type cells had a significant migration bias towards the animal pole, much greater than was observed in spt morphant cells. All axes are labeled with μm of displacement from the starting position. These results are taken from tracking 26 cells from 3 embryos, per condition.
spt defects can be rescued by restoring Spadetail
The absence of an essential transcription factor can cause differentiating cells to adopt alternate genetic programs, or to remain stalled in an intermediate state, waiting for the correct stimulus to proceed. To test these possibilities we generated a transgenic zebrafish line containing the coding region of the spt gene downstream of a multimerized heat shock-sensitive promoter (Bajoghli et al., 2004). As shown in Figure 4A, this bidirectional promoter also expresses eGFP upon induction allowing embryos carrying the transgene to be identified after heat shock. We found that defects caused by knocking down Spadetail levels by morpholino injection could be significantly rescued by restoring Spadetail with the transgenic line after heat shock induction at shield stage. Although spt expression from the transgene was not directly measured, our previous experiments using this heat shock construct (Row and Kimelman, 2009) demonstrated significant expression within one hour of the end of the heat shock. Genes such as tbx24 (Fig. 4B) or paraxial protocadherin (not shown) that are dependent on Spadetail function for their expression (Griffin and Kimelman, 2002 and data not shown) could be partially restored by inducing spt expression with the transgene. Partial rescue of the spt phenotype was also observed with somites forming in the posterior trunk, which is not observed in spt mutants or morphants (Fig. 4C). Heat shock induction at bud stage resulted in a much less significant phenotypic rescue (not shown). Finally, we also found that restoring Spadetail by inducing transgene expression in spt morphant embryos significantly reduced blebbing in hypoblast cells (Fig. 5A and Fig. 2B, Supplemental Movie 2). The partial rescue effects we observed are likely due to low expression levels of the transgene. Compared to other transgenic lines we have produced, the level of GFP fluorescence after heat shock in our heat shock transgenic line is considerably weaker, and we were not able to obtain a line with a strong heat shock response. Nonetheless, the rescue of gene expression and phenotype demonstrates that this line is useful for analysis of Spadetail function, especially since injection of mRNA encoding Spadetail causes aberrations in gastrulation that preclude many experiments (our unpublished results), whereas early gastrula or later activation of Spadetail by heat shock does not cause these defects.
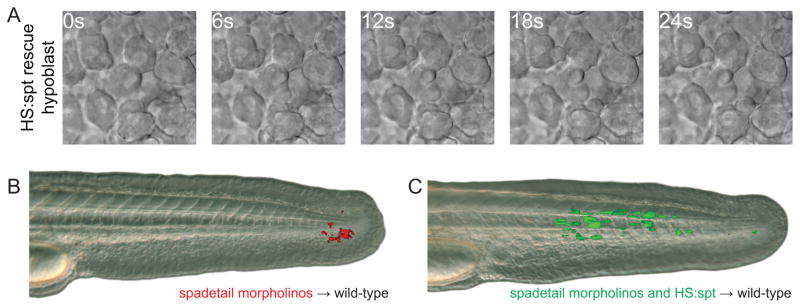
Fig. 5. Cell morphology and cell migration are rescued by restoring Spadetail.
(A) Cells in the hypoblast of spt morphant cells expressing spt after transgene induction blebbed less frequently than with morpholino alone (see Fig. 2B for quantification). (B) When spt morphant cells were transplanted into the ventral mesoderm of a wild-type embryo at shield stage they were unable to contribute to somites. (C) Restoring Spadetail by inducing transgene expression allowed cells to migrate out of the tailbud and contribute to developing somites. Each panel is representative of at least 16 successful transplants.
As shown in the elegant experiments of Ho and Kane (1990), spt mesoderm suffers a cell-autonomous migration defect that is clearly evident when transplanted into the mesoderm of a wild-type host. Wild-type mesoderm progenitors in the tailbud continuously contribute to pre-somitic mesoderm and subsequently join newly-forming somites (Cambray and Wilson, 2002; Cambray and Wilson, 2007; Davis and Kirschner, 2000; Martin and Kimelman, 2008). Transplanted spt cells populate a specific niche in the tailbud from the end of gastrulation to the completion of somitogenesis (Figure 5B), when they begin contributing to fin mesenchyme. Maturing mesodermal cells normally transit rapidly through this region, an area between the posterior progenitor zone and the pre-somitic mesoderm. When spt expression is restored to transplanted spt morphant cells by heat shock activation of a transgene at the 1-somite (Fig. 5C) or 8-somite (data not shown) stages, the ability of these cells to complete the maturation program is restored. The cells leave the ventral posterior niche, join the somites and differentiate into elongated muscle cells. We noticed that cells do not immediately leave the niche after heat shock, presumably because they require time for sufficient Spadetail protein to accumulate, similar to what was observed in the phenotype rescue experiments described above. In summary, these results demonstrate that mesodermal cells remain in a semi-mature transition state for an extended time if they lack Spadetail, and can be rescued when new Spadetail protein is supplied.
spt mutant mesoderm fails to downregulate cell-cell adhesion
One of the most striking characteristics of the spt mutant phenotype is the failure of cells to leave the posterior end of the embryo, resulting in the formation of a spade-like structure at the posterior end. A common hypothesis for this effect is that spt cells have a cell adhesion defect, preventing them from adhering to the presomitic mesoderm. An alternate possibility is a failure to receive or interpret a necessary guidance cue for directed migration. We decided to test the hypothesis that mesodermal cells lacking Spadetail have defective adhesion. For previous studies of adhesion of axial cells, zebrafish embryos were injected with the RNA encoding the Nodal factor Cyclops (Cyc, also called Ndr2), which primarily converts all cells to a head mesoderm fate, and then adhesion in dissociated cells was measured (Krieg et al., 2008). In order to study adhesion of the ventral and lateral mesoderm, we developed a new method for converting embryonic cells to this fate. RNA encoding Cyc was injected into 1-cell embryos at a lower dose than used in the previous studies, and then the GSK3 inhibitor BIO (Meijer et al., 2003) was added after the mid-blastula transition (MBT). Inhibition of GSK3 elevates canonical Wnt signaling (Yost et al., 1996), which after the MBT acts to promote ventral/lateral mesoderm and to repress axial fates (Glinka et al., 1998; Hashimoto et al., 2000; Leyns et al., 1997; Ramel and Lekven, 2004; Salic et al., 1997; Wang et al., 1997). In situ hybridization confirmed that most of the embryonic cells express the ventral/lateral mesodermal markers ntl (ntla), spt, papc and tbx24, whereas expression of the axial mesodermal markers goosecoid and floating head was eliminated (Supplemental Figure 1 and data not shown). We call these cells induced ventral/lateral mesoderm (IVLM).
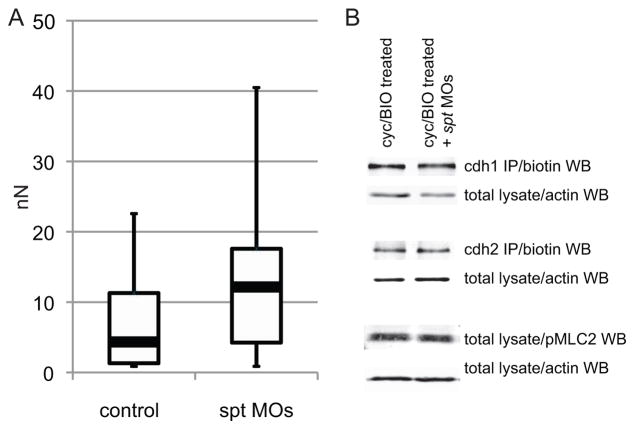
In order to quantitatively measure cell adhesion we utilized a dual micropipette assay, modified from the method of Daoudi et al. (2004). In this approach, embryos were treated as above and the cells were dissociated at late blastula stages. Two cells were randomly chosen and each was attached to a micropipette with gentle suction. The two cells were then placed in contact for 60 seconds, and then gently pulled apart. Varying the degree of suction on one of the pipettes allowed quantification of the force necessary to pull the two cells apart. As shown in Figure 6A the adhesion between induced ventral/lateral mesodermal cells lacking Spadetail is significantly higher than in wild-type IVLM cells. Thus, a lack of Spadetail does not result in reduced cell adhesion, and instead suggests that one role of Spadetail is to reduce cell adhesion as cells leave the transition state.
Fig. 6. Lateral mesodermal cells lacking Spadetail are more adhesive than wild-type cells.
(A) Cell-cell adhesion was measured in control and spt morpholino-injected cells induced to a ventral/lateral mesodermal identity. Cells lacking Spadetail were significantly more adhesive than wild-type controls. Black bars indicate mean values, white boxes label the 2nd and 3rd quartiles, and black lines indicate the range of observed values. 37 or 38 cell pairs were measured for IVLM and IVLM + spt MOs, respectively. (B) Major mediators of cell-cell adhesion and blebbing were not changed by the absence of Spadetail. Levels of surface-exposed E-cadherin and N-cadherin, and total phosphorylated MLC2 were measured by western blot.
We utilized the IVLM system to probe several molecules known to be crucial regulators of adhesion and blebbing. The major regulators of cell-cell adhesion in the early embryo are the classical cadherins, E-cadherin (Cdh1) and N-cadherin (Cdh2) [(Kane et al., 2005; Shimizu et al., 2005; Warga and Kane, 2007); reviewed in (Hammerschmidt and Wedlich, 2008; Solnica-Krezel, 2006)]. Total levels of Cdh1 and Cdh2 were unchanged with and without Spadetail (data not shown). Since Cadherins function at the cell surface, we induced ventral/lateral mesoderm and then biotinylated cell surface proteins using established protocols. The biotinylated protein was purified from total lysate by immunoprecipitation and subjected to western blots. Surface levels of both cadherins were not changed in embryos lacking Spadetail (Fig. 6B), suggesting a change in the downstream regulation of adhesion.
Phosphorylation of Myosin Regulatory Light Chain 2 (MLC2) is a key regulatory step in the cellular control of blebbing (Mills et al., 1998). This phosphorylation event increases hydrostatic pressure within the cell by increasing acto-myosin contractility, leading to enhanced tension on the cortical actin (Tinevez et al., 2009). In zebrafish, we previously found that inhibition of the myosin light chain phosphatase (Mypt1) results in acquisition of a highly blebby state within the hypoblast, demonstrating the essential role of MLC phosphorylation in regulating blebbing within the mesoderm (Weiser et al., 2009). Surprisingly, despite the fact that mesodermal cells lacking Spadetail are in a highly blebby state, we observed no change in the levels of activated MLC2 in the absence of Spadetail (Fig. 6B). These results reveal that wild-type cells have sufficient phosphorylated MLC2 in the hypoblast to bleb extensively, and support the model that Spadetail functions through an MLC-independent pathway to dampen blebbing in cells as they leave the transition state and enter the hypoblast.
Discussion
We present here the first high resolution in vivo observations of a highly blebby transition state in mesodermal cells undergoing involution during zebrafish gastrulation. This dramatic morphology is in stark contrast to the behavior of cells at earlier and later stages of the maturation program. Mesodermal cells in the epiblast are epithelioid with stable cell-cell contacts whereas they adopt a mesenchymal morphology after joining the hypoblast, producing lamellipodia, filopodia and some blebs. In the intermediate state cells stay tightly packed but extend many blebs during the brief time they are undergoing involution. This change in cell morphology is the first visible marker of cells that have committed to the differentiation program and is likely a consequence of cells remodeling their adhesive and migratory machinery prior to initiating the directed cytoskeletal behavior of the deep cells.
Dissecting the molecular players in the mesoderm maturation program has been performed in multiple model organisms, but correlating morphological and genetic changes is difficult using fixed tissues (Hashimoto and Nakatsuji, 1989; Vakaet, 1984). Zebrafish embryos are an ideal system to study this process in vivo. Microscopic observations of living embryos are straightforward and a wide array of genetic and molecular tools are available. It is crucial to make observations at high resolution in terms of both time and image quality, both of which requirements are addressed with modern computer-controlled microscopes and digital cameras. As we demonstrate here, the spt mutation further enhances the utility of zebrafish for dissecting the mesodermal maturation pathway by stalling cells part-way through the process.
It is worth noting two differences in this process between zebrafish embryos and embryos of amniotes such as chicken or mouse (Kane and Warga, 2004; Stern, 2004; Tam and Gad, 2004). First, the cells in the zebrafish epiblast are not a true polarized epithelium, in contrast to what is found in amniotes. In chicken embryos, epiblast cells need to dissociate basal contacts while preserving apical contacts because the epithelium must maintain integrity to prevent the contents of lower layers from leaking out. Zebrafish embryos are surrounded by an enveloping layer of tightly joined cells that maintains the inside/outside boundary, so the cells of the epiblast need not adhere so rigidly. Second, zebrafish gastrulae lack a basement membrane separating epiblast and hypoblast. Involuting cells in the zebrafish gastrula are able to move away from the epiblast without the directional cue of a basement membrane, and maintain a separation between the epiblast and hypoblast without a physical barrier. Even with these differences, homologous transcription factors are expressed in the same patterns across taxa. Based on the high conservation of known genetic players in this pathway such as the spt orthologs vegT/tbx6 in other vertebrates (Chapman et al., 1996; Horb and Thomsen, 1997; Knezevic et al., 1997; Lustig et al., 1996; Stennard et al., 1996; Zhang and King, 1996), and similar cellular movements (Solnica-Krezel, 2005), we anticipate that aspects of the newly-observed transition state will also be present in other vertebrate species, and therefore these findings will be broadly applicable.
The spt mutation stalls the maturation program
Even with improved visualization techniques, it is still difficult to study the epiblast to hypoblast transition because it is short-lived and involves only a small number of cells at any given time. We found that spt cells enter the highly blebby transition state normally, but fail to shut down this process and enter the hypoblast. This result parallels our earlier studies on gene expression in spt mutants in which we showed that spt mutant cells initiate the mesodermal differentiation program but fail to complete it, and thus remain stalled in the genetic program of the maturation zone, a region where cells first express early markers of differentiation (Griffin and Kimelman, 2002)(Fig. 7). Thus, we suggest that the cause of the migration defect in spt mesodermal cells is that they initiate the morphogenetic property of the epiblast to hypoblast transition, but lack the genetic instructions to leave the morphological transition state. In other words, spt cells do not migrate abnormally because they have acquired an aberrant property (as, for example, if they lost a key adhesion factor), but instead they remain stuck at the margin during gastrulation and in the posterior end of the embryo during somitogenesis because they persist in a behavior that they should only perform transiently. Importantly, we find that cells that lack Spadetail are able to complete the differentiation and morphogenesis program when Spadetail is introduced from a heat shock construct, demonstrating that cells do not immediately embark on an alternate differentiation and morphogenetic course when they lack Spadetail, and instead remain stalled in the differentiation and morphogenesis programs.
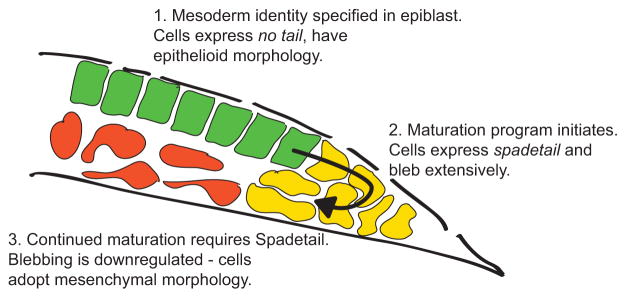
Fig. 7. Model for mesoderm maturation and the role of Spadetail.
Schematic illustration of lateral mesoderm during gastrulation, with cell shapes exaggerated for clarity. (1) Mesodermal progenitors are located in the epiblast layer and marked by expression of ntl. (2) As cells approach the margin the maturation program is initiated, which includes transcription of spt. As cells involute to join the deeper cell layers they enter the transition state and bleb extensively. (3) Cells require Spadetail to reduce blebbing and leave the transition state. Cells down-regulate ntl and other progenitor genes, and activate the expression of Spadetail targets such as tbx24 and papc.
Since the spt cells are stalled in the transition state this mutant will be very valuable for understanding the mesodermal maturation program. We took advantage of this to probe the adhesive properties of maturing mesodermal cells. Previously it was assumed that cells lacking Spadetail migrated abnormally because their cell-cell adhesion was reduced. Using a quantitative assay to measure adhesion directly we demonstrate that the opposite is true, with mesodermal cells lacking Spadetail being more adhesive than wild-type cells. Interestingly, E-cadherin and N-cadherin levels are unchanged at the cell surface with and without Spadetail, suggesting that Spadetail might regulate adhesion through intracellular events. We also observed that spt morphant mesodermal cells migrate at the same speed as wild-type cells during early gastrulation, but fail to move away from the transition zone in gastrula-stage embryos, and remain close to the margin. This raises the possibility that a directional guidance cue is not being properly interpreted or acted upon by cells lacking Spadetail. Thus, our results suggest that targets of Spadetail dampen adhesion in the cells leaving the transition state, and detect or act upon guidance cues necessary for directed migration. We do not believe, however, that increased adhesion alone is sufficient to explain the spt phenotype since experiments reducing adhesion using E-cadherin MOs failed to rescue the spt phenotype (unpublished results).
Regulation of blebbing
Although most studies on cell movements have focused on lamellipodia and filopodia, many new studies have pointed to the importance of blebbing both for the movement of embryonic cells and for the motility of metastatic cancer cells. Blebs extend from the membrane by an entirely different mechanism from the actin polymerization-driven protrusions, yet many of the same factors are used in bleb-based movements as in movements involving lamellipodia and filopodia, including actin, myosin and RhoA. While zebrafish germ cells migrate entirely using bleb-based movements (Blaser et al., 2006), paraxial mesodermal cells use all forms of movement with a single cell frequently alternating between blebbing, and producing lamellipodia and filopodia (Concha and Adams, 1998; Sepich and Solnica-Krezel, 2005). Yet, the protrusive activity must be carefully regulated since mesodermal cells that bleb too extensively can not undergo normal convergence-extension movements (Weiser et al., 2007). How mesodermal cells regulate the types of protrusive activity they use is largely unknown.
Since wild-type hypoblast cells have reduced blebbing whereas spt cells continue to bleb, an attractive hypothesis was that Spadetail would act to reduce myosin phosphorylation by acting on any of the upstream regulators of this process such as Rho or Rho-associated kinase [(Amano et al., 1996; Kimura et al., 1996); reviewed in (Fackler and Grosse, 2008)]. However, we found that spt cells have identical levels of myosin phosphorylation as wild-type cells, indicating that Spadetail must suppress blebbing through an alternative mechanism. Studies in cultured zebrafish cells have revealed that blebbing can be induced by changes in actin nucleation and actin cortex-membrane interactions (Diz-Muñoz et al., 2010). For example, positive and negative regulation of the formin family of actin nucleators can induce blebbing (Eisenmann et al., 2007). Since cell adhesion factors such as the cadherins are connected to the actin cytoskeleton, changes in cortical actin could also underlie the changes in adhesion when Spadetail is not present in the mesodermal cells, as well as being the reason that spadetail mutant cells to act upon guidance cues. With the induced ventral/lateral mesoderm system described here, it will be tractable to investigate these possibilities in embryonic cells as has been done in cultured cells.
Conclusions
Our studies demonstrate that zebrafish mesodermal cells enter a highly blebby state as the first morphological indication that they have begun the differentiation process. This observation suggests a new level of cell-autonomous regulation of morphology, to allow a rapid change in cell behavior by quickly tuning the activities of major adhesion and cytoskeletal regulators. Initiation of this process is independent of Spadetail, and is presumably connected to the decision by cells to leave the progenitor state, although it is not yet clear how cells know when to commence maturation. Spadetail is necessary to repress the highly blebby state and allow cells to leave the transition zone, as well as to initiate the transcriptional program of the PSM (Fig. 7). While many of the transcriptional targets of Spadetail involved in the differentiation program are known, a downstream factor involved in completing the morphological transition is at this point not clear, and is a subject of ongoing studies. Since regulated blebbing is now widely understood to be a common mechanism for cell migration in three-dimensional environments, spt mutants will shed new light on the transcriptional control of this crucial behavior in embryonic development.
Supplementary Material
Mesodermal cell behavior was observed in the epiblast, involuting mesoderm or hypoblast of gastrulating embryos. A computer-controlled microscope equipped with DIC optics acquired an image every 3 seconds for 15 minutes.
Lateral mesoderm movements in wild-type (the same movie as in Supplemental Movie 1 is shown for comparison), spt morphant and HS:spt rescue of spt morphant embryos. Mesodermal cell behavior was observed in the hypoblast as in Supplemental Movie 1.
The utility of the induced ventral/lateral mesoderm system was demonstrated by analyzing marker gene expression in treated embryos. Mesodermal identity was assayed by in situ hybridization for transcripts typical of ventral/lateral mesoderm fate (no tail (ntl), spadetail (spt) and paraxial protocadherin (papc)) or axial mesoderm fate (floating head (flh)). Treated embryos greatly expand their expression of ventral/lateral marker genes while axial fates are repressed. Blocking expression of spt with morpholino injection expands the domain of ntl expression and partially blocks papc expression, similar to what is observed in the mesoderm of spt embryos. The view is lateral with the dorsal pole to the right when it could be identified.
Acknowledgments
We thank David Grunwald for providing the spadetail-myc fusion construct. This work was supported by an NIH grant (GM079203) to D.K. and grants from the Austrian Academy of Sciences to P.S., and from the DFG, MPG and IST Austria to C.-P.H. R.R. was supported by a Developmental Biology Predoctoral Training Grant, T32HD007183, from the National Institute of Child Health and Human Development. B.L.M. was supported by an American Cancer Society fellowship (PF-07-048-01-DDC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amacher SL, Draper BW, Summers BR, Kimmel CB. The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development. 2002;129:3311–23. doi: 10.1242/dev.129.14.3311. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–9. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Arboleda-Estudillo Y, Krieg M, Stuhmer J, Licata NA, Muller DJ, Heisenberg CP. Movement directionality in collective migration of germ layer progenitors. Curr Biol. 2010;20:161–9. doi: 10.1016/j.cub.2009.11.036. [DOI] [PubMed] [Google Scholar]
- Babb SG, Marrs JA. E-cadherin regulates cell movements and tissue formation in early zebrafish embryos. Dev Dyn. 2004;230:263–77. doi: 10.1002/dvdy.20057. [DOI] [PubMed] [Google Scholar]
- Bajoghli B, Aghaallaei N, Heimbucher T, Czerny T. An artificial promoter construct for heat-inducible misexpression during fish embryogenesis. Dev Biol. 2004;271:416–30. doi: 10.1016/j.ydbio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Blaser H, Reichman-Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, Solnica-Krezel L, Heisenberg CP, Raz E. Migration of zebrafish primordial germ cells: a role for myosin contraction and cytoplasmic flow. Dev Cell. 2006;11:613–27. doi: 10.1016/j.devcel.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Cambray N, Wilson V. Axial progenitors with extensive potency are localised to the mouse chordoneural hinge. Development. 2002;129:4855–66. doi: 10.1242/dev.129.20.4855. [DOI] [PubMed] [Google Scholar]
- Cambray N, Wilson V. Two distinct sources for a population of maturing axial progenitors. Development. 2007;134:2829–40. doi: 10.1242/dev.02877. [DOI] [PubMed] [Google Scholar]
- Carmany-Rampey A, Schier AF. Single-cell internalization during zebrafish gastrulation. Curr Biol. 2001;11:1261–5. doi: 10.1016/s0960-9822(01)00353-0. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Agulnik I, Hancock S, Silver LM, Papaioannou VE. Tbx6, a mouse T-Box gene implicated in paraxial mesoderm formation at gastrulation. Dev Biol. 1996;180:534–42. doi: 10.1006/dbio.1996.0326. [DOI] [PubMed] [Google Scholar]
- Charras G, Paluch E. Blebs lead the way: how to migrate without lamellipodia. Nat Rev Mol Cell Biol. 2008;9:730–6. doi: 10.1038/nrm2453. [DOI] [PubMed] [Google Scholar]
- Chen Y, Merzdorf C, Paul DL, Goodenough DA. COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J Cell Biol. 1997;138:891–9. doi: 10.1083/jcb.138.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha ML, Adams RJ. Oriented cell divisions and cellular morphogenesis in the zebrafish gastrula and neurula: a time-lapse analysis. Development. 1998;125:983–94. doi: 10.1242/dev.125.6.983. [DOI] [PubMed] [Google Scholar]
- Daoudi M, Lavergne E, Garin A, Tarantino N, Debre P, Pincet F, Combadiere C, Deterre P. Enhanced adhesive capacities of the naturally occurring Ile249-Met280 variant of the chemokine receptor CX3CR1. J Biol Chem. 2004;279:19649–57. doi: 10.1074/jbc.M313457200. [DOI] [PubMed] [Google Scholar]
- Davis RL, Kirschner MW. The fate of cells in the tailbud of Xenopus laevis. Development. 2000;127:255–67. doi: 10.1242/dev.127.2.255. [DOI] [PubMed] [Google Scholar]
- Diz-Muñoz A, Krieg M, Bergert M, Ibarlucea-Benitez I, Muller DJ, Paluch E, Heisenberg CP. Control of Directed Cell Migration In Vivo by Membrane-to-Cortex Attachment. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann KM, Harris ES, Kitchen SM, Holman HA, Higgs HN, Alberts AS. Dia-interacting protein modulates formin-mediated actin assembly at the cell cortex. Curr Biol. 2007;17:579–91. doi: 10.1016/j.cub.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–84. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–62. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Goering LM, Hoshijima K, Hug B, Bisgrove B, Kispert A, Grunwald DJ. An interacting network of T-box genes directs gene expression and fate in the zebrafish mesoderm. Proc Natl Acad Sci U S A. 2003;100:9410–5. doi: 10.1073/pnas.1633548100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin K, Patient R, Holder N. Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development. 1995;121:2983–94. doi: 10.1242/dev.121.9.2983. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Amacher SL, Kimmel CB, Kimelman D. Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development. 1998;125:3379–88. doi: 10.1242/dev.125.17.3379. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Kimelman D. One-Eyed Pinhead and Spadetail are essential for heart and somite formation. Nat Cell Biol. 2002;4:821–5. doi: 10.1038/ncb862. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Wedlich D. Regulated adhesion as a driving force of gastrulation movements. Development. 2008;135:3625–41. doi: 10.1242/dev.015701. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Itoh M, Yamanaka Y, Yamashita S, Shimizu T, Solnica-Krezel L, Hibi M, Hirano T. Zebrafish Dkk1 functions in forebrain specification and axial mesendoderm formation. Dev Biol. 2000;217:138–52. doi: 10.1006/dbio.1999.9537. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Nakatsuji N. Formation of the Primitive Streak and Mesoderm Cells in Mouse Embryos - Detailed Scanning Electron Microscopical Study. Develop Growth & Differ. 1989;31 doi: 10.1111/j.1440-169X.1989.00209.x. [DOI] [PubMed] [Google Scholar]
- Ho RK, Kane DA. Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature. 1990;348:728–30. doi: 10.1038/348728a0. [DOI] [PubMed] [Google Scholar]
- Horb ME, Thomsen GH. A vegetally localized T-box transcription factor in Xenopus eggs specifies mesoderm and endoderm and is essential for embryonic mesoderm formation. Development. 1997;124:1689–98. doi: 10.1242/dev.124.9.1689. [DOI] [PubMed] [Google Scholar]
- Kai M, Heisenberg CP, Tada M. Sphingosine-1-phosphate receptors regulate individual cell behaviours underlying the directed migration of prechordal plate progenitor cells during zebrafish gastrulation. Development. 2008;135:3043–51. doi: 10.1242/dev.020396. [DOI] [PubMed] [Google Scholar]
- Kane DA, McFarland KN, Warga RM. Mutations in half baked/E-cadherin block cell behaviors that are necessary for teleost epiboly. Development. 2005;132:1105–16. doi: 10.1242/dev.01668. [DOI] [PubMed] [Google Scholar]
- Kane DA, Warga RM. Teleost Gastrulation. In: Stern CD, editor. Gastrulation. Cold Spring Harbor Laboratory Press; New York: 2004. pp. 157–170. [Google Scholar]
- Kardash E, Reichman-Fried M, Maître JL, Boldajipour B, Papusheva E, Messerschmidt EM, Heisenberg CP, Raz E. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat Cell Biol. 2010;12:47–53. doi: 10.1038/ncb2003. [DOI] [PubMed] [Google Scholar]
- Kawakami K. Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol. 2004;77:201–22. doi: 10.1016/s0091-679x(04)77011-9. [DOI] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Keller R. Cell migration during gastrulation. Curr Opin Cell Biol. 2005;17:533–41. doi: 10.1016/j.ceb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Kimelman D. Mesoderm induction: from caps to chips. Nat Rev Genet. 2006;7:360–72. doi: 10.1038/nrg1837. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Kane DA, Walker C, Warga RM, Rothman MB. A mutation that changes cell movement and cell fate in the zebrafish embryo. Nature. 1989;337:358–362. doi: 10.1038/337358a0. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–8. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Knezevic V, De Santo R, Mackem S. Two novel chick T-box genes related to mouse Brachyury are expressed in different, non-overlapping mesodermal domains during gastrulation. Development. 1997;124:411–9. doi: 10.1242/dev.124.2.411. [DOI] [PubMed] [Google Scholar]
- Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–36. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- Lewis KE, Eisen JS. Paraxial mesoderm specifies zebrafish primary motoneuron subtype identity. Development. 2004;131:891–902. doi: 10.1242/dev.00981. [DOI] [PubMed] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–56. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig KD, Kroll KL, Sun EE, Kirschner MW. Expression cloning of a Xenopus T-related gene (Xombi) involved in mesodermal patterning and blastopore lip formation. Development. 1996;122:4001–12. doi: 10.1242/dev.122.12.4001. [DOI] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Regulation of canonical Wnt signaling by Brachyury is essential for posterior mesoderm formation. Dev Cell. 2008;15:121–33. doi: 10.1016/j.devcel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–66. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol. 1998;140:627–36. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. Bmp activity gradient regulates convergent extension during zebrafish gastrulation. Dev Biol. 2002;243:81–98. doi: 10.1006/dbio.2001.0523. [DOI] [PubMed] [Google Scholar]
- Ramel MC, Lekven AC. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development. 2004;131:3991–4000. doi: 10.1242/dev.01277. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, MD, USA: 1997–2009. http://rsb.info.nih.gov/ij. [Google Scholar]
- Row RH, Kimelman D. Bmp inhibition is necessary for post-gastrulation patterning and morphogenesis of the zebrafish tailbud. Dev Biol. 2009;329:55–63. doi: 10.1016/j.ydbio.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic AN, Kroll KL, Evans LM, Kirschner MW. Sizzled: a secreted Xwnt8 antagonist expressed in the ventral marginal zone of Xenopus embryos. Development. 1997;124:4739–48. doi: 10.1242/dev.124.23.4739. [DOI] [PubMed] [Google Scholar]
- Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu Rev Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- Sepich DS, Solnica-Krezel L. Analysis of cell movements in zebrafish embryos: morphometrics and measuring movement of labeled cell populations in vivo. Methods Mol Biol. 2005;294:211–33. doi: 10.1385/1-59259-860-9:211. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yabe T, Muraoka O, Yonemura S, Aramaki S, Hatta K, Bae YK, Nojima H, Hibi M. E-cadherin is required for gastrulation cell movements in zebrafish. Mech Dev. 2005;122:747–63. doi: 10.1016/j.mod.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Shook DR, Keller R. Epithelial type, ingression, blastopore architecture and the evolution of chordate mesoderm morphogenesis. J Exp Zool B Mol Dev Evol. 2008;310:85–110. doi: 10.1002/jez.b.21198. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–28. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L. Gastrulation in zebrafish -- all just about adhesion? Curr Opin Genet Dev. 2006;16:433–41. doi: 10.1016/j.gde.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Stennard F, Carnac G, Gurdon JB. The Xenopus T-box gene, Antipodean, encodes a vegetally localised maternal mRNA and can trigger mesoderm formation. Development. 1996;122:4179–88. doi: 10.1242/dev.122.12.4179. [DOI] [PubMed] [Google Scholar]
- Stern CD. Gastrulation in the Chick. In: Stern CD, editor. Gastrulation. Cold Spring Harbor Laboratory Press; New York: 2004. pp. 219–232. [Google Scholar]
- Tam PPL, Gad JM. Gastrulation in the Mouse Embryo. In: Stern CD, editor. Gastrulation. Cold Spring Harbor Laboratory Press; New York: 2004. pp. 233–262. [Google Scholar]
- Tinevez JY, Schulze U, Salbreux G, Roensch J, Joanny JF, Paluch E. Role of cortical tension in bleb growth. Proc Natl Acad Sci U S A. 2009;106:18581–6. doi: 10.1073/pnas.0903353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–64. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Vakaet L. The initiation of gastrular ingression in the chick blastoderm. Am Zool. 1984;24 [Google Scholar]
- von der Hardt S, Bakkers J, Inbal A, Carvalho L, Solnica-Krezel L, Heisenberg CP, Hammerschmidt M. The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Curr Biol. 2007;17:475–87. doi: 10.1016/j.cub.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M., Jr Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell. 1997;88:757–66. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- Warga RM, Kane DA. A role for N-cadherin in mesodermal morphogenesis during gastrulation. Dev Biol. 2007;310:211–25. doi: 10.1016/j.ydbio.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Warga RM, Kimmel CB. Cell movements during epiboly and gastrulation in zebrafish. Development. 1990;108:569–80. doi: 10.1242/dev.108.4.569. [DOI] [PubMed] [Google Scholar]
- Warga RM, Nusslein-volhard C. spadetail-dependent cell compaction of the dorsal zebrafish blastula. Dev Biol. 1998;203:116–21. doi: 10.1006/dbio.1998.9022. [DOI] [PubMed] [Google Scholar]
- Weiser DC, Pyati UJ, Kimelman D. Gravin regulates mesodermal cell behavior changes required for axis elongation during zebrafish gastrulation. Genes Dev. 2007;21:1559–71. doi: 10.1101/gad.1535007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser DC, Row RH, Kimelman D. Rho-regulated myosin phosphatase establishes the level of protrusive activity required for cell movements during zebrafish gastrulation. Development. 2009;136:2375–84. doi: 10.1242/dev.034892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Amacher SL, Kim SH, Geissert D, Kimmel CB, De Robertis EM. Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm. Development. 1998;125:3389–97. doi: 10.1242/dev.125.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Ciruna B, Solnica-Krezel L. Convergence and extension movements during vertebrate gastrulation. Curr Top Dev Biol. 2009;89:163–92. doi: 10.1016/S0070-2153(09)89007-8. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–54. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zhang J, King ML. Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning. Development. 1996;122:4119–29. doi: 10.1242/dev.122.12.4119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mesodermal cell behavior was observed in the epiblast, involuting mesoderm or hypoblast of gastrulating embryos. A computer-controlled microscope equipped with DIC optics acquired an image every 3 seconds for 15 minutes.
Lateral mesoderm movements in wild-type (the same movie as in Supplemental Movie 1 is shown for comparison), spt morphant and HS:spt rescue of spt morphant embryos. Mesodermal cell behavior was observed in the hypoblast as in Supplemental Movie 1.
The utility of the induced ventral/lateral mesoderm system was demonstrated by analyzing marker gene expression in treated embryos. Mesodermal identity was assayed by in situ hybridization for transcripts typical of ventral/lateral mesoderm fate (no tail (ntl), spadetail (spt) and paraxial protocadherin (papc)) or axial mesoderm fate (floating head (flh)). Treated embryos greatly expand their expression of ventral/lateral marker genes while axial fates are repressed. Blocking expression of spt with morpholino injection expands the domain of ntl expression and partially blocks papc expression, similar to what is observed in the mesoderm of spt embryos. The view is lateral with the dorsal pole to the right when it could be identified.