Abstract
Gene-targeted deletion of the predominant Shaker potassium channel, Kv1.3, in the mitral cells of the olfactory bulb, decreases the number of presynaptic, odorant receptor (OR)-identified olfactory sensory neurons (OSNs) in the main olfactory epithelium (MOE) and alters the nature of their postsynaptic connections to mitral cell targets. The current study examined whether OSN density was state-dependent by examining the impact of 1) odor enrichment, 2) sensory deprivation, and 3) aging upon the number of P2- or M72-expressing neurons. Histological approaches were used to quantify the number of OSNs across entire epithelia for wildtype (WT) vs. Kv1.3-null (KO) mice bred onto an ORtauLacZ reporter background. Following either odor-enrichment or early unilateral naris-occlusion, the number of M72-expressing OSNs was significantly decreased in WT mice, but was unchanged in KO animals. Following naris-occlusion, the number of P2-expressing OSNs was decreased regardless of genotype. Animals that were reared to 2 years of age demonstrated loss of both P2- and M72-expressing OSNs in WT mice and a concomitant loss of only M72-expressing neurons in KO mice. These findings suggest that voltage-gated activity of the mitral cells is important for OSN plasticity, and can prevent neuronal loss via sensory- and OR-dependent mechanisms.
Keywords: potassium channel, odor enrichment, naris-occlusion, aging, odorant receptor
Olfactory sensory neurons (OSNs) are the cells in the main olfactory epithelium (MOE) that express odorant receptors (ORs), which bind to various chemical features of volatile molecules. Changing the number or the morphology of OSNs can alter the sensory input to the olfactory bulb (OB) thereby modifying odor perception via changing the signal-to-noise ratio [17]. Changing mitral cell activity, by gene-targeted deletion of the voltage-gated potassium channel, Kv1.3, has been found to alter the number and synaptic convergence of OSNs to OR-identified glomeruli in the OB [1;9]. Kv1.3-null mice have smaller, heterogeneous, and more numerous glomeruli, while the abundance of OSNs expressing either P2 or M72 ORs is significantly reduced [1;9]. Since Kv1.3-null animals also have a “super-smeller” behavioral phenotype [9] and enhanced longevity [26], we sought to understand how modified post-synaptic activity could impact the olfactory system via anatomical changes at the periphery. Our strategy was to utilize P2-IRES-tau-LacZ and M72-IRES-tau-LacZ mice [1;22] in order to visualize changes in OR expression in response to different environmental treatments or physiological states (odor enrichment, odor deprivation, and age), while altering the basal activity level of the mitral cells in the OB via gene-targeted deletion of the Kv1.3 ion channel [9].
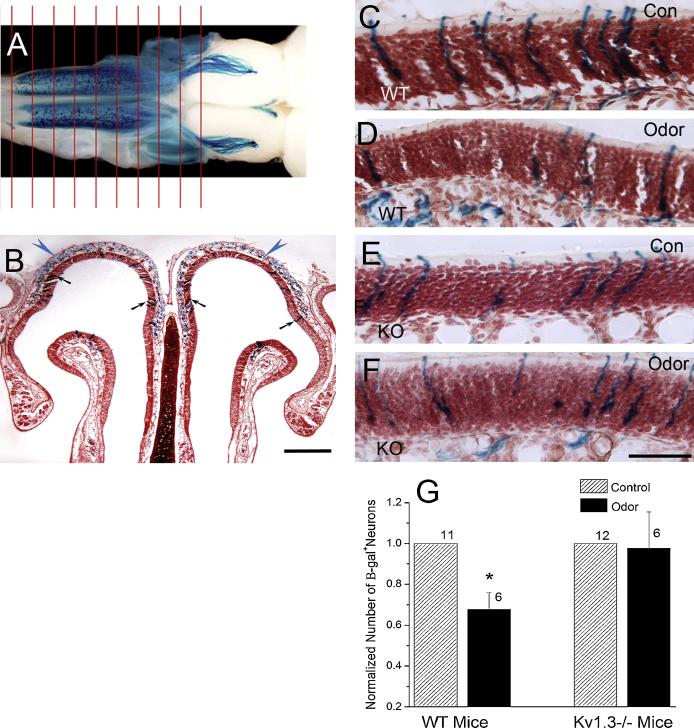
Studies on the effects of odor enrichment on the olfactory system have centered upon assessing changes in olfactory discrimination [20;21;24], anatomical and functional changes in the OB [4;14;19;25], and changes in cell survival and turnover in the MOE and OB [24;27;28]. Generally, there is dramatic improvement in olfactory discrimination ability following odor enrichment [20;21], while increasing both number and activity of interneurons in the OB [19;25], resulting in attenuation of mitral cell responses to odors [4;19;25]. There is little known about how odor enrichment alters OSNs, but there is evidence that enrichment accelerates the refinement of the glomerular map [14], targeting of OSN projections [6], and survival of OSNs [27]. We hypothesized that odor enrichment may not affect the already sensitized olfactory system of the Kv1.3−/− mice. In our study, P2-IRES-tau-LacZ (Olfr17) and M72-IRES-tau-LacZ (Olfr160) mice were generated and provided by Dr. Peter Mombaerts (Rockefeller University, New York, NY) [22]. All mice were of similar background strain (C57BL/6) of both sexes and were housed at the Florida State University vivarium in accordance with guidelines set by the National Institutes of Health. Following weaning, all mice were housed individually in conventional rodent cages with food and water provided ad libitum. We generated odorant receptor-tagged mice in a Kv1.3−/− background [15] by breeding homozygous P2- or M72-IRES-tau-LacZ mice with homozygous Kv1.3−/− mice as described previously [1]. Olfactory sensory neurons (OSNs) expressing the modified P2 or M72 odorant receptor in the Kv1.3-null background were visualized by histological staining for β-galactosidase activity [22]. For simplicity throughout the manuscript, odorant receptor-tagged, IRES-tau-LacZ mice will be identified as expressing Kv1.3 (wildtype; WT) or as Kv1.3−/− (knock-out; KO) preceding the OR type (i.e. WT M72 vs. KO M72). One month and 4-6 month old WT and KO M72 mice were exposed to cotton swabs soaked with 100 μl acetophenone (1 μM; Fluka Chemicals catalog # 42163, Sigma Aldrich); the ligand for the M72 odorant receptor [11], five sessions per day for 30 days. Water-soaked cotton swabs served as the control treatment. Since the ligand for the P2 OR is not known, P2-IRES-tau-LacZ mice were not tested for odor enrichment. An odor enrichment session was created by introducing cotton swabs to the testing cage for five, 10-minute trials separated by a 10-minute recovery interval [27]. Prior to the first session and following the fifth session, the litter in each cage was replaced with 60 g of unsoiled litter and a controlled 20 g of food so that there was an equivalent odor space for each animal. The numbers of positively identified OSNs in the control and odor-stimulated groups were quantified for each animal by manually counting β-galactosidase-positive neurons across serial sections of entire MOE (~500 sections/mouse; Fig. 1A). An example of a single MOE tissue section is shown in Fig. 1B. While experimental condition varied across Figures 1-3, histological preparation and method of analysis were the same. Mice were euthanized with an overdose of sodium pentobarbital and perfused with PBS followed by 4% paraformaldehyde. The heads were decalcified in 0.3M EDTA for 72–95 hours and cryoprotected in 10% then 30% sucrose PBS at 4°C. Serial coronal sections (16 μm; Leica CM1850, Wetzlar, Germany) were transferred to 1% gelatin-coated slides and stored at −20°C until use. The β-galactosidase reaction and neutral red counterstaining procedure was similar to that described previously [1;22]. Digital images were acquired using a Zeiss Axiocam with AxioVision software on a Axiovert S100 upright microscope (Carl Zeiss, Oberkochen, Germany) and then images were processed with Adobe Photoshop CS (San Jose, CA). Since the neuronal shape and nucleus position within the neural epithelium has previously been shown not to vary across the WT and KO mice [1], we elected to focus our study on neuronal abundance. Our criterion for identifying a β-galactosidase+ neuron was to only include OSNs with a defined nucleus, so as to prevent overestimate across adjacent sections. There was no significant difference in the magnitude of M72 OSN loss with respect to time of odor enrichment (1 month or 6 month), therefore, analysis only compared the effects of genotype. Repeated odor stimulation decreased the number of M72 OSNs in WT (p ≤ 0.05; Fig. 1C, D, G) but not KO mice (Fig. 1E-G), suggesting that the loss of Kv1.3 in mitral cells is correlated to OSN survival.
Figure 1.
Absence of the Kv1.3 ion channel prevents the loss of M72-expressing olfactory sensory neurons (OSNs) caused by odor enrichment. A: Whole-mount of a WT mouse main olfactory epithelium (MOE) and olfactory bulb (OB) processed to visualize the M72-expressing OSNs across the epithelium. Whole epithelia were sectioned coronally (red lines) at 16 μm thickness to produce ~500 sections that were then manually scored for positive β-galactosidase –identified OR expression. B: Representative photomicrograph of a section of the MOE processed to visualize M72-expressing OSNs (scale bar = 500 μm). C-F: Representative photomicrographs of the OSN layer of the MOE from WT (wildtype; C, D) and KO ( Kv1.3−/−; E, F) mice under control (con; C, E) and odor enriched (odor; D, F) conditions (scale bar = 50 μm). G: Histogram of the mean (+/− standard error of the mean; s.e.m.) number of tau β-galactosidase+ OSNs across the entire epithelium normalized to the control condition (hatched bar). Black bar = odor enriched condition. * = Significantly-different by Student’s t-test, p ≤ 0.05. Sample size (number of mice): Control WT (11), Odor WT (6), Control KO (12), Odor KO (6). Arrows: M72 OSNs (black) and axons (blue).
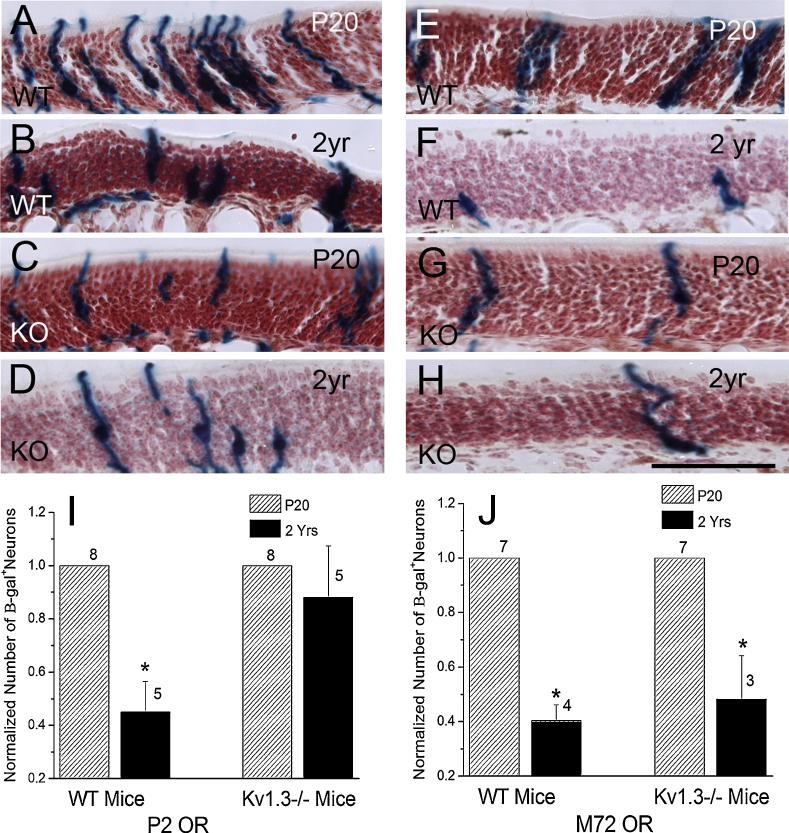
Figure 3.
Aging decreases the number of M72-expressing OSNs regardless of genotype, while P2-expressing OSNs are only decreased in WT mice. A-H: Representative photomicrographs of the OSN layer of the MOE from P2- (A-D) and M72-tagged (E-H) WT (wildtype; A, B, E, F) and KO (Kv1.3−/−; C, D, G, H) mice aged 20 days (A, C, E, G) and 2 years (B, D, F, H; scale bar = 50 μm). Fewer P2-expressing OSNs are observed in 2 year old vs. 20 day old mice for both genotypes (WT: A vs. B; KO: C vs. D). There is only an age-dependent loss of M72-expressing OSNs for WT mice (E vs. F), while the number of OSNs is unchanged in the KO mice (G vs. H). I, J: Histograms representing the mean (+/− s.e.m.) number of tau β-galactosidase+ OSNs as prepared in figure 1 and normalized to the P20 mice. Hatched bar = 20 days old (P20). Black bar = 2 years old (2yr). * = Significantly different by Student’s t-test, p ≤ 0.05. Sample size for the P2 mice: P20 WT (8), 2yr WT (5), P20 KO (8), 2yr KO (5). Sample size for the M72 mice: P20 WT (7), 2yr WT (4), P20 KO (7), 2yr KO (3).
Several laboratories have explored the effects of odor deprivation, by unilateral naris-occlusion, at the level of the OB [7] as well as in the MOE [7;10]. Odor deprivation decreases the volume of the OB [7] and the thickness of the neuron layer of the MOE [7;10] ipsilateral to the occlusion. Farbman and colleagues [10] also observed a decrease in the number of neurons on the occluded side, while the supporting cells remained unchanged. We hypothesized that sensory deprivation may have more impact on mice with a sensitized olfactory system (Kv1.3−/−). Our study examined the affects of naris occlusion on two specific subpopulations of genetically-identified OSNs. Permanent, unilateral naris-occlusion was performed on postnatal day 1, as described previously [2]. At postnatal day 20, the number of X-Gal positive OSNs across the epithelium on the occluded-naris half (closed) was compared to that on the non-occluded half (open) of the same animal using a paired t-test (α ≤ 0.05). Odor deprivation decreased the number of P2 OSNs on the closed-naris side vs. the open-naris side for both WT (p ≤ 0.05; Fig. 2A, B, I) and KO (p ≤ 0.05; Fig. 2C, D, I) mice. In contrast, the number of M72 OSNs was significantly decreased on the occluded side for WT mice (p ≤ 0.05; Fig. 2E, F, J), while the KO mice (Fig. 2G, H, J) showed no change in the number of M72 OSNs following odor deprivation. These results again suggest altering mitral cell activity (Kv1.3 −/−) may be promoting OSN survival. However, this increased survival is not universal for all OSN subpopulations because P2-expressing OSNs are lost in KO animals, albeit to a lesser extent than that in WT animals. Therefore, OSN survival in response to environmental stimuli appears to be OR dependent.
Figure 2.
Kv1.3-null mice are resistant to odor deprivation-induced loss of M72-expressing OSNs, but not loss of P2-expressing OSNs. A-H: Representative photomicrographs of the OSN layer of the MOE from P2- (A-D) and M72-tagged (E-H) WT (wildtype; A, B, E, F) and KO (Kv1.3−/−; C, D, G, H) mice 20 days following unilateral naris-occlusion (scale bar = 50 μm). The number of P2 OSNs is decreased on the naris-occluded half for both WT (B) and KO (D) mice (closed) when compared to the non-occluded half (A and C respectively; open). There is only a decrease in the number of M72 OSNs on the occluded-naris side of WT mice (E = open; F = closed), while the number of M72 OSNs is similar on both sides for KO mice (G = open; H = closed). I, J: Histograms representing the mean (+/− s.e.m.) number of tau β-galactosidase+ OSNs after odor deprivation as prepared in figure 1 and normalized to the open side of the MOE. Hatched bar = open naris. Black bar = closed naris. * = Significantly-different by paired t-test, p ≤ 0.05. Sample size for the number of P2 mice: WT (3), KO (4) and M72 mice: WT (5), KO (5).
Aging is known to be correlated with deficits in the sense of smell in humans, but the mechanisms underlying the effect are not known [3]. The loss of olfactory function has been mirrored in studies with rodents [8], where there is a decrease in the size of the mitral cell somas [13], a decrease in the number of ORs and OSNs [13], and an increase in the number of synapses per OSN [13]. Given that Kv1.3-null animals have an increased life span of up to 950 days compared with wild-type animals (~750 days) [26], we hypothesized that their increased longevity might abrogate anatomical signs of aging on these neuronal populations. In comparing 20 day old WT vs. KO mice, we replicated our previous findings [1] that KO mice have fewer P2 and M72 OSNs than do WT mice (P2: 5996 +/− 1156 vs. 11333 +/− 588 neurons; M72: 3490 +/− 216 vs. 6358 +/− 193 neurons). We observed that the number of P2 OSNs was decreased in 2 year old vs. 20 day old WT mice (p ≤ 0.05; Fig. 3A, B, I), while KO mice appeared to be resistant to this age-induced neuron loss (Fig. 3C, D, I). There was interestingly a large amount of inter-animal variability in terms of P2 expression in the older mice regardless of genotype (Fig. 3I). On the other hand, both aged (2 yr) WT (Fig. 3 E, F, J) and KO (Fig. 3G, H, J) mice had significantly fewer M72 OSNs than 20 day old mice (p ≤ 0.05), providing further evidence that OSN survival is dependent on the OR being expressed by the neuron in addition to the physiological state of the animal.
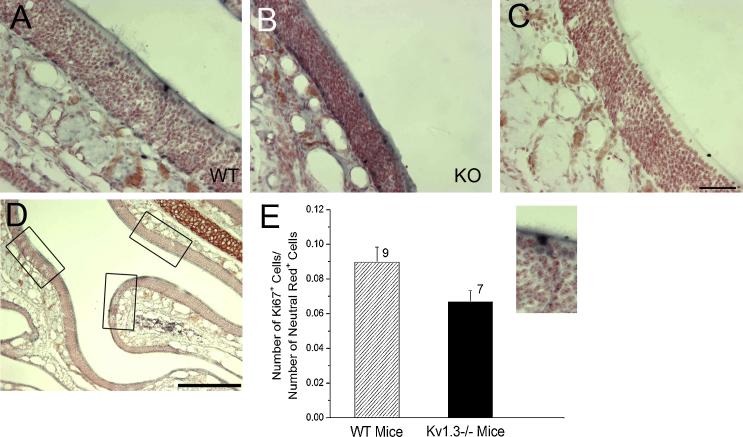
To determine if the resistance to loss of OSNs observed in the KO mice was due to changes in the proliferation of basal cells (OSN precursors) in the MOE, we next used separate groups of one month old WT and KO mice, without the odorant receptor tag, to quantify the number of Ki67+ OSNs (αKi67; 1:5000 dilution) [12;23] via avidin-biotin-peroxidase methods [2]. Ki67 is a marker for cell proliferation [12]; Dr. Stanley Watson (University of Michigan, Ann Arbor, MI) generously provided the Ki67 antiserum [23]. Three regions within a coronal section in a similar zone of the MOE were used for counts (Fig. 4D) for both WT (Fig. 4A, E) and KO (Fig. 4B, E) mice [5]. The data were plotted as a ratio of Ki67+: neutral red+ cells for WT vs. KO mice and statistically compared with a Student’s t-test with an arc-sine transformation (α ≤ 0.05). There were similar levels of Ki67 immunoreactivity (bluish black staining) observed in the basal cell layer just above the lamina propria of both WT (Fig. 4A) and KO (Fig. 4B) mice, while no immunoreactivity was observed without primary antiserum (Fig. 4C). There was no significant difference in basal cell proliferation for WT and KO mice (Fig. 4E).
Figure 4.
The number of proliferating basal cells in the MOE is not different in WT and KO mice. A-C: Representative photomicrographs of the MOE processed to visualize Ki67+ proliferating cells in one month old WT (A) vs. KO (B) mice. Note there is no immunoreactivity (lack of bluish black staining) when the primary antiserum is omitted (C; scale bar = 50 μm). D: Representative micrograph of the MOE, highlighting the three 200 μm regions (boxes) analyzed for number of Ki67+ cells across 3 different locations throughout the MOE (scale bar = 200 μm). E: Bar graph of the number of Ki67+ cells normalized to the total number of neutral red stained OSNs for WT (wildtype; black bar; n=9) and KO (Kv1.3−/−; open bar; n=6) mice. Not significantly-different by Student’s t-test with arc-sine transformation.
Our current results suggest a novel regulation of OSN abundance in response to differing environmental or physiological states that is influenced by the activity level of the mitral cells in the OB. Odor enrichment, odor deprivation, and aging all decrease the number of OSNs in WT mice. Increasing mitral cell activity, however, by gene-targeted deletion of Kv1.3, affects OSN abundance in a combined OR- and state-dependent manner. P2-expressing OSNs are lost following unilateral naris-occlusion in KO mice but are resistant to age-induced loss. While M72-expressing OSNs in KO mice are subject to age-induced loss, they are resistant to the effects of both odor enrichment and odor deprivation. Thus, it appears that increased mitral cell activity can increase OSN survival. However, this survival cannot be explained by changes in the proliferation of MOE basal cells.
Little is known regarding how odor enrichment regulates OSNs [14;27]. Since Kv1.3-null mice have increased basal mitral cell activity, this may compensate for the effects of odor enrichment on the mitral cells and subsequently protect the OSNs from pruning due to a decrease in the activity of the postsynaptic target cell. While we observed a decrease in the number of M72-expressing OSNs following repeated exposure to acetophenone in WT mice, Kerr and Belluscio [14] did not find significant differences in rI7/M71-expressing OSN abundance following octanal exposure. This result supports our conclusion that OSN abundance is regulated not only by environmental state but OR type as well.
Odor deprivation caused by unilateral naris-occlusion decreases the thickness of the OSN layer [7;10], which is likely due to decreased proliferation and increased apoptosis in the OSNs [10]. The fact that we observe a reduction in M72-expressing OSNs with both naris-occlusion and also following odor enrichment with acetophenone must indicate that the cellular processes controlling neuronal abundance through enrichment and deprivation are not simply inverse pathways.
The mechanism responsible for the loss of olfactory sensation in aging humans is not known. Olfactory bulb and epithelium changes include smaller mitral cell somas, decreased neurogenesis, and attenuated electro-olfactogram responses to odorants [8;13]. While we observe an age-induced loss of P2-expressing OSNs in 2 year old WT mice, Lee and colleagues [16] report no change in P2 mRNA over development. Therefore, OR protein expression may not necessarily be predicted by mRNA expression. There is also evidence for differential effects of age on OSN populations based on their location in the MOE. The posterior MOE shows less signs of damage with aging and the OSNs are mature and more similar to that found in young counterparts [18]. In contrast, OSNs in the anterior MOE are in an immature form [18]. This may be of significance because we typically observe M72-expressing OSNs on more anterior sections (zone 1) than that of P2-expressing OSNs (data not shown). Therefore, the age-induced decrease in the number of M72-expressing OSNs and the age-resistant P2-expressing OSNs may be due to position in the epithelium. Interestingly, we have previously quantified that both P2- and M72-axonal projections remain supernumerary across development in the KO animals and that the number of axonal projections from P2-expressing OSNs increases with aging but that from M72-expressing OSNs remains stable, regardless of the Kv channel genotype [1]. Thus in the WT animals our current data demonstrate that there is OSN loss at the periphery regardless of OR type, but centrally, M72 projections are stable while that of P2 are increasing their glomerular targets. The resistance to OSN loss observed in response to environmental treatments in the KO mice is not due to a difference in the proliferation of the basal cells in the MOE when compared to WT mice and there may be changes in the rate of apoptosis that may account for the survival of OSNs observed in KO mice. Future studies will focus on analyzing the numbers of apoptotic cells under different environmental or physiological states.
Acknowledgements
We would like to thank our FSU undergraduate scholars for their assistance with changing of the mice cages, odor stimulation, neuron counting, or routine technical assistance. We also thank Drs. Peter Mombaerts and Leonard Kazcmarek for the donation of the odor receptor-tagged and Kv1.3−/− mice, respectively, and Dr. Stanley Watson for his generous gift of the Ki67 antiserum. This work was supported by grants DC03387 and DC00044 from the National Institute of Deafness and Communication Disorders at the National Institutes of Health.
References
- [1].Biju KC, Marks DR, Mast TG, Fadool DA. Deletion of voltage-gated channel affects glomerular refinement and odorant receptor expression in the mouse olfactory system. J. Comp Neurol. 2008;506:161–179. doi: 10.1002/cne.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Biju KC, Mast TG, Fadool DA. Olfactory sensory deprivation increases the number of proBDNF-immunoreactive mitral cells in the olfactory bulb of mice. Neurosci. Lett. 2008;447:42–47. doi: 10.1016/j.neulet.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boyce JM, Shone GR. Effects of ageing on smell and taste. Postgrad. Med. J. 2006;82:239–241. doi: 10.1136/pgmj.2005.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Buonviso N, Gervais R, Chalansonnet M, Chaput M. Short-lasting exposure to one odour decreases general reactivity in the olfactory bulb of adult rats. Eur. J. Neurosci. 1998;10:2472–2475. doi: 10.1046/j.1460-9568.1998.00266.x. [DOI] [PubMed] [Google Scholar]
- [5].Carson C, Saleh M, Fung FW, Nicholson DW, Roskams AJ. Axonal dynactin p150Glued transports caspase-8 to drive retrograde olfactory receptor neuron apoptosis. J. Neurosci. 2005;25:6092–6104. doi: 10.1523/JNEUROSCI.0707-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Col JA, Matsuo T, Storm DR, Rodriguez I. Adenylyl cyclase-dependent axonal targeting in the olfactory system. Development. 2007;134:2481–2489. doi: 10.1242/dev.006346. [DOI] [PubMed] [Google Scholar]
- [7].Cummings DM, Henning HE, Brunjes PC. Olfactory bulb recovery after early sensory deprivation. J. Neurosci. 1997;17:7433–7440. doi: 10.1523/JNEUROSCI.17-19-07433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, Parsons AD, Overton JM, Koni PA, Flavell RA, Kaczmarek LK. Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron. 2004;41:389–404. doi: 10.1016/s0896-6273(03)00844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Farbman AI, Brunjes PC, Rentfro L, Michas J, Ritz S. The effect of unilateral naris occlusion on cell dynamics in the developing rat olfactory epithelium. J. Neurosci. 1988;8:3290–3295. doi: 10.1523/JNEUROSCI.08-09-03290.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feinstein P, Bozza T, Rodriguez I, Vassalli A, Mombaerts P. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell. 2004;117:833–846. doi: 10.1016/j.cell.2004.05.013. [DOI] [PubMed] [Google Scholar]
- [12].Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- [13].Hinds JW, McNelly NA. Aging in the rat olfactory system: correlation of changes in the olfactory epithelium and olfactory bulb. J. Comp Neurol. 1981;203:441–453. doi: 10.1002/cne.902030308. [DOI] [PubMed] [Google Scholar]
- [14].Kerr MA, Belluscio L. Olfactory experience accelerates glomerular refinement in the mammalian olfactory bulb. Nat. Neurosci. 2006;9:484–486. doi: 10.1038/nn1673. [DOI] [PubMed] [Google Scholar]
- [15].Koni PA, Khanna R, Chang MC, Tang MD, Kaczmarek LK, Schlichter LC, Flavella RA. Compensatory anion currents in Kv1.3 channel-deficient thymocytes. J. Biol. Chem. 2003;278:39443–39451. doi: 10.1074/jbc.M304879200. [DOI] [PubMed] [Google Scholar]
- [16].Lee AC, Tian H, Grosmaitre X, Ma M. Expression patterns of odorant receptors and response properties of olfactory sensory neurons in aged mice. Chem. Senses. 2009;34:695–703. doi: 10.1093/chemse/bjp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lledo PM, Gheusi G, Vincent JD. Information processing in the mammalian olfactory system. Physiol Rev. 2005;85:281–317. doi: 10.1152/physrev.00008.2004. [DOI] [PubMed] [Google Scholar]
- [18].Loo AT, Youngentob SL, Kent PF, Schwob JE. The aging olfactory epithelium: neurogenesis, response to damage, and odorant-induced activity. Int. J. Dev. Neurosci. 1996;14:881–900. doi: 10.1016/s0736-5748(96)00046-9. [DOI] [PubMed] [Google Scholar]
- [19].Mandairon N, Didier A, Linster C. Odor enrichment increases interneurons responsiveness in spatially defined regions of the olfactory bulb correlated with perception. Neurobiol. Learn. Mem. 2008;90:178–184. doi: 10.1016/j.nlm.2008.02.008. [DOI] [PubMed] [Google Scholar]
- [20].Mandairon N, Stack C, Kiselycznyk C, Linster C. Enrichment to odors improves olfactory discrimination in adult rats. Behav. Neurosci. 2006;120:173–179. doi: 10.1037/0735-7044.120.1.173. [DOI] [PubMed] [Google Scholar]
- [21].Mandairon N, Stack C, Linster C. Olfactory enrichment improves the recognition of individual components in mixtures. Physiol Behav. 2006;89:379–384. doi: 10.1016/j.physbeh.2006.07.013. [DOI] [PubMed] [Google Scholar]
- [22].Mombaerts P. Targeting olfaction. Curr. Opin. Neurobiol. 1996;6:481–486. doi: 10.1016/s0959-4388(96)80053-5. [DOI] [PubMed] [Google Scholar]
- [23].Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H. A new role for FGF2 as an endogenous inhibitor of anxiety. J. Neurosci. 2009;29:6379–6387. doi: 10.1523/JNEUROSCI.4829-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J. Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sachse S, Rueckert E, Keller A, Okada R, Tanaka NK, Ito K, Vosshall LB. Activity-dependent plasticity in an olfactory circuit. Neuron. 2007;56:838–850. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- [26].Tucker K, Overton JM, Fadool DA. Kv1.3 gene-targeted deletion alters longevity and reduces adiposity by increasing locomotion and metabolism in melanocortin-4 receptor-null mice. Int. J. Obes. (Lond) 2008;32:1222–1232. doi: 10.1038/ijo.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Watt WC, Sakano H, Lee ZY, Reusch JE, Trinh K, Storm DR. Odorant stimulation enhances survival of olfactory sensory neurons via MAPK and CREB. Neuron. 2004;41:955–967. doi: 10.1016/s0896-6273(04)00075-3. [DOI] [PubMed] [Google Scholar]
- [28].Woo CC, Hingco EE, Taylor GE, Leon M. Exposure to a broad range of odorants decreases cell mortality in the olfactory bulb. Neuroreport. 2006;17:817–821. doi: 10.1097/01.wnr.0000215780.84226.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]