Abstract
The neuroadaptation theory of addiction suggests that, similar to the development of most memories, exposure to drugs of abuse induces adaptive molecular and cellular changes in the brain which likely mediate addiction-related memories or the addictive state. Compared to other types of memories, addiction-related memories develop fast and last extremely long, suggesting that the cellular and molecular processes that mediate addiction-related memories are exceptionally adept and efficient. We recently demonstrated that repeated exposure to cocaine generated a large portion of “silent” glutamatergic synapses within the nucleus accumbens (NAc). Silent glutamatergic synapses are synaptic connections in which only N-methyl-D-aspartic acid receptor (NMDAR)-mediated responses are readily detected whereas alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) are absent or highly labile. Extensive experimental evidence suggests that silent synapses are conspicuously efficient plasticity sites at which long-lasting plastic changes can be more easily induced and maintained. Thus, generation of silent synapses can be regarded as a process of metaplasticity, which primes the NAc for subsequent durable and robust plasticity for addiction-related memories. Focusing on silent synapse-based metaplasticity, this review discusses how key brain regions, such as the NAc, utilize the metaplasticity mechanism to optimize the plasticity machineries to achieve fast and durable plastic changes following exposure to cocaine. A summary of recent related results suggests that upon cocaine exposure, newly generated silent synapses may prime excitatory synapses within the NAc for long-term potentiation (LTP), thus setting the direction of future plasticity. Furthermore, because cocaine-generated silent synapses are enriched in NMDARs containing the NR2B subunit, the enhanced NR2B-signaling may set up a selective recruitment of certain types of AMPARs. Thus, silent synapse-based metaplasticity may lead to not only quantitative but also qualitative alterations in excitatory synapses within the NAc. This review is one of the first systematic analyses regarding the hypothesis that drugs of abuse induce metaplasticity, which regulates the susceptibility, the direction, and the molecular details of subsequent plastic changes. Taken together, metaplasticity ultimately serves as a key step in mediating cascades of addiction-related plastic alterations.
Keywords: metaplasticity, silent synapses, cocaine, NMDA, AMPA, membrane excitability
1. Introduction
One of the leading hypotheses guiding current molecular and cellular researches of drug addiction is the neuroadaptation theory (Hyman, 1996; Berke and Hyman, 2000; Hyman and Malenka, 2001; Hyman et al., 2006). This theory suggests that the brain utilizes similar molecular and cellular mechanisms to form addiction-related memories as it does to form normal memories (Hyman and Malenka, 2001; Hyman et al., 2006). Thus, drug addiction is considered a form of memory--an extreme one that develops faster and lasts longer than most other types of memories. These exceptionally efficient properties of the addiction memory suggest that the development and maintenance of addiction-related neural plasticity involve highly adept cellular and molecular processes.
Metaplasticity, the plasticity of synaptic plasticity, is an exceptionally effective and efficient cellular mechanism that primes synapses for subsequent long-lasting plastic changes, such as long-term potentiation (LTP) or long-term depression (LTD) (Abraham and Bear, 1996; Bear, 1996; Malenka and Bear, 2004). Metaplasticity does not necessarily affect the efficacy of synaptic transmission per se. Rather, it can significantly improve the susceptibility of synapses for the subsequent induction of more permanent plasticity and pre-set the direction of the future plasticity at these synapses (i.e., LTP vs. LTD). As such, metaplasticity can act as a powerful prelude that primes synapses to be more effective and efficient for the induction and maintenance of subsequent plasticity.
Our recent work demonstrates that during exposure to cocaine, a large portion of silent glutamatergic synapses are generated in the nucleus accumbens (NAc) (Huang et al., 2009). Generation of silent synapses may not affect the strength of excitatory synaptic transmission to the NAc, but it does provide a large number of plasticity substrates that are highly susceptible to the induction of LTP (Kerchner and Nicoll, 2008). As such, cocaine-induced generation of silent synapses can be regarded as a form of metaplasticity, priming excitatory synapses within the NAc for LTP or LTP-like postsynaptic strengthening. Focusing on silent synapse-based metaplasticity, this review will discuss i) potential molecular mechanisms which mediate cocaine-induced generation of silent synapses in the NAc; ii) subsequent synaptic consequences dictated by cocaine-generated silent synapses and how they affect excitatory synaptic transmission in the NAc; iii) impacts of cocaine-generated silent synapses on other cellular targets within the NAc; and iv) roles for other forms of cocaine-induced metaplasticity. These analyses are among the first discussions that provide a metaplasticity point of view to understanding the extremely durable molecular and cellular alterations induced by cocaine and other drugs of abuse.
Experimental results discussed in this manuscript are mainly from studies using non-contingent drug procedures (e.g., intraperitoneal injection by experimenter) in the NAc shell (simply referred to as "NAc" throughout). A wide variety of results are seen in studies using other drug procedures or regions in the brain, such as the NAc core, but it is difficult to reach a coherent conclusion in many of these cases, and thus they are not extensively discussed.
2. Silent synapses as metaplasticity substrates in the NAc
2.1 Metaplasticity at excitatory synapses
The term "metaplasticity" was coined by Abraham and Bear to describe the plastic change in the ability of synapses to undergo future plasticity (Abraham and Bear, 1996). It has long been known that experience-dependent plasticity can be difficult to experimentally induce and short-lived when induced at naïve synapses (synapses that have not undergone a prior priming process) when the induction protocol is not sufficiently strong (Malenka, 1991; Volianskis and Jensen, 2003). However, if these synapses are previously primed by neuromodulators (Schimanski et al., 2007; Sun et al., 2008), in vitro-activities (Huang et al., 1992), or in vivo experience (Philpot et al., 2003), the same relatively "weak" induction protocol can trigger more robust and long-lasting plastic changes. These observations suggest that in addition to directly regulating synaptic transmission, experience can also affect the ability of synapses to undergo plastic changes. With metaplasticity, synapses can be primed such that certain types of plasticity (e.g., LTP or LTD) can be preferentially induced. Therefore, inductions of plasticity can be more effective, and acquired plasticity can be more durable. It is thus conceivable that experience-dependent synaptic plasticity, if preceded by metaplasticity, results in more robust and durable memories.
Several forms of metaplasticity have been documented at different types of synapses in several brain regions including the hippocampus (Chevaleyre and Castillo, 2004), visual cortex (Philpot et al., 2003), cerebellum (Schweighofer and Arbib, 1998) and hypothalamus (Kuzmiski et al., 2009). Among these, NMDAR-based metaplasticity, which can be either inhibitory or potentiating, at excitatory synapses has been most systemically examined. The inhibitory role of NMDAR-based metaplasticity was first demonstrated in the hippocampus, where it has been shown that low-level activity of NMDARs increases the induction threshold of subsequent LTP (Izumi et al., 1992; Larkman et al., 1992; Christie et al., 1995; Moody et al., 1999). This form of inhibitory metaplasticity seems to be mediated either by activation of the high affinity Ca2+-signaling, upregulation of the converging inhibitory synaptic input, or desensitization of LTP-associated molecular machineries (Davies et al., 1991; Komatsu, 1994; Chard et al., 1995; Malenka and Nicoll, 1999). On the other hand, the facilitative role of NMDAR-based metaplasticity has been systematically illustrated in the visual cortex, in which the induction threshold of LTP at excitatory synapses is substantially decreased in animals with little visual stimulation (animals reared in dark) (Kirkwood et al., 1996; Philpot et al., 2003). This facilitative form of metaplasticity appears to be mediated by alterations in the relative weights of NR2A-and NR2B-containing NMDARs because the NR2A/NR2B ratio at visual cortical excitatory synapses is decreased in dark-reared animals. Additionally, preventing changes in the NR2A/NR2B ratio by knocking out NR2A subunits erases the shift of the LTP threshold in dark-reared animals (Quinlan et al., 1999a; Quinlan et al., 1999b; Philpot et al., 2003; Philpot et al., 2007). Thus, a potential extrapolation of these results is that an increase in the relative weight of NR2B subunits in synaptic NMDARs facilitates the induction of LTP at excitatory synapses. This notion has, however, been highly debatable in the current synaptic plasticity field.
2.2. Silent synapses
Typical glutamatergic synapses contain two types of ionotropic glutamate receptors, AMPARs and NMDARs. The number and/or function of AMPARs is often regarded as being indicative of the strength of excitatory synapses because AMPAR-mediated currents contribute most of the fast excitatory postsynaptic currents (EPSCs). NMDARs also contribute to EPSCs, but the contribution is relatively limited; the more physiologically-significant role of NMDARs is to sense the activation state of excitatory synapses and, in turn, to regulate the function of AMPARs or other cellular targets via NMDAR-coupled signaling. A well known example demonstrating the differential roles of AMPARs and NMDARs is LTP at excitatory synapses. At near-resting membrane potentials, EPSCs from a set of synapses are primarily mediated by AMPARs, whereas NMDARs are largely inactive due to the voltage-dependent Mg2+ block (Mayer et al., 1984). But, if NMDARs are strongly activated, typically by strong activation of AMPARs from high frequency stimulation, NMDAR-coupled signaling positively regulates synaptic AMPARs, resulting in long-lasting potentiation of AMPAR-mediated EPSCs, or LTP (Malenka and Nicoll, 1999). NMDAR-induced upregulation of AMPAR function can be achieved through several different molecular processes, among which postsynaptic recruitment of new AMPARs, thus increasing in the overall number of functional AMPARs, has been proven to be one of the major means (Malinow et al., 2000).
Silent synapses are a unique type of glutamatergic synapses in that they only express NMDAR-mediated responses whereas AMPAR-mediated responses are either absent or highly labile (Liao et al., 1995; Isaac et al., 1997). When measured electrophysiologically, only NMDAR-mediated currents can be reliably detected in silent synapses. Because of the voltage-dependent Mg2+ block of NMDARs at hyperpolarized voltages, these AMPAR-deficient silent synapses conduct little current at near-resting membrane potentials. Thus, they are termed “silent synapses” (Merrill and Wall, 1972). In theory, the AMPAR-silent nature of silent synapses can reside in both pre- and postsynaptic loci. At the presynaptic site, because AMPARs have lower affinity to glutamate than NMDARs (Dingledine et al., 1999), a potential low release probability of glutamate may contribute to the insufficient activation of postsynaptic AMPARs (Kullmann et al., 1996; Gasparini et al., 2000). However, presynaptic involvement can be at least partially excluded by focally applying uncaged glutamate directly to the dendrites and showing there is still a large number of dendritic spines exhibiting only NMDAR-signaling (Busetto et al., 2008). Thus, the silent nature of silent synapses is at least partially mediated by postsynaptic mechanisms. At the postsynaptic site, a debate remains whether silent synapses only express NMDARs. Whereas the NMDAR-only synapses are observed in some morphological, biochemical, and electrophysiological studies (Constantine-Paton and Cline, 1998; Liao et al., 1999; Petralia et al., 1999; Washbourne et al., 2002), other biochemical and molecular results support that both AMPARs and NMDARs are approximately simultaneously synthesized, translocated, clustered, and equipped at newly formed synapses (Groc et al., 2002; Hanse et al., 2009). Despite this controversy, it is in agreement that postsynaptic AMPARs, if any, are highly unstable at silent synapses and tend to be functionally silent when measured by evoked EPSCs (Groc et al., 2006; Kerchner and Nicoll, 2008). Given that an important mechanism for LTP induction is postsynaptic recruitment of new AMPARs (Shi et al., 1999; Malinow et al., 2000; Shi et al., 2001), silent synapses with their unique structural properties may have great potential for housing incoming AMPARs or stabilizing labile AMPARs upon the induction of LTP. Indeed, the un-silencing of silent synapses by recruitment/stabilization of AMPARs has been shown to be a highly effective and efficient mechanism for the expression of LTP (Isaac et al., 1995; Liao et al., 1995; Isaac et al., 1997; Kerchner and Nicoll, 2008).
Because silent synapses themselves add little to the excitatory synaptic strength but are highly susceptible to the induction of LTP, generation of silent synapses can be a powerful form of metaplasticity to strengthen selective sets of excitatory synaptic connections quickly and durably. However, exploration of experience-induced generation of silent synapses has been slow, partially due to a popular notion that silent synapses are merely an early developmental phenomenon. Silent synapses have been readily detected throughout the central nervous system in the developing brain (Kerchner and Nicoll, 2008), and as the brain matures, the number of silent synapses drops significantly (Durand et al., 1996; Isaac et al., 1997). Thus, it has been a general belief that silent synapses are developmental endowments that simply contribute to the enhanced learning ability in juvenile organisms.
Recently, we demonstrated that a highly salient experience such as in vivo cocaine exposure generates a large portion of silent synapses within the NAc (Huang et al., 2009), a forebrain region essential for developing addiction-related memories (Wolf, 2010). This finding demonstrates that silent synapses are not exclusively present during development, and shows they can be generated de novo in the developed brain by experience. As such, experience-dependent generation of silent synapses can be an important form of metaplasticity that the brain uses to establish certain forms of subsequent plasticity more quickly and more durably. Below we will discuss this form of metaplasticity in more detail and speculate that the subsequent plasticity stemmed from cocaine-generated silent synapses.
2.3. Cocaine-induced generation of silent synapses in the NAc
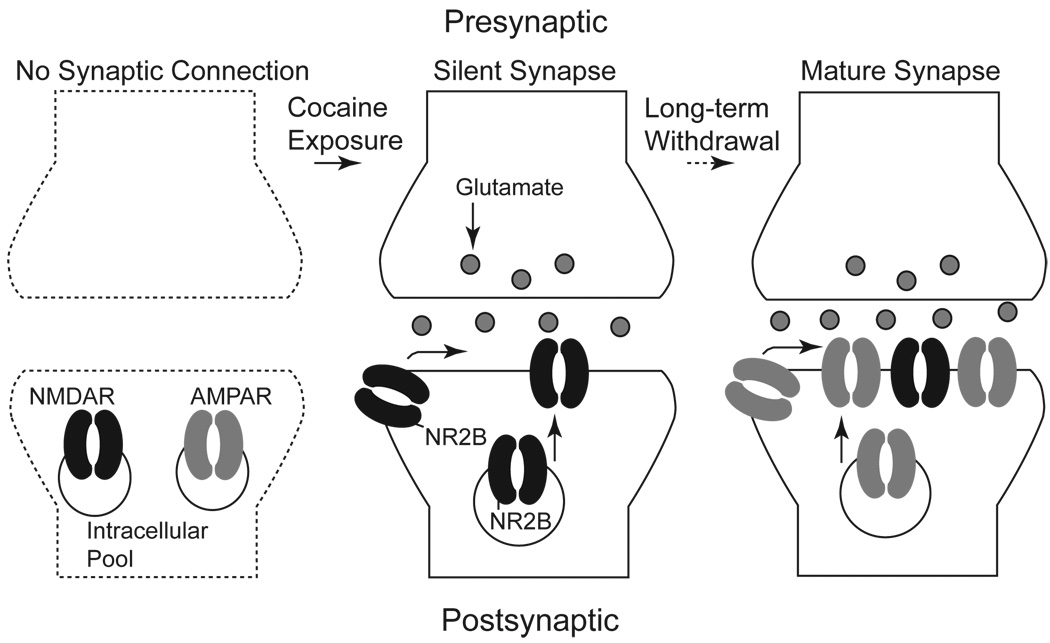
It has long been understood that addiction-associated memories are developed in such a way that they are much more durable than most other memories acquired during adulthood. To explore the unique mechanisms underlying the development of addiction-associated memories, we have been focusing on the NAc, an essential brain site for the development of drug addiction. Our recent results suggest that silent synapses can be produced by in vivo cocaine experience. Specifically, using the minimal stimulation assay (Isaac et al., 1995; Liao et al., 1995) combined with the analysis of coefficient of variation of amplitudes of AMPAR- and NMDAR-mediated EPSCs (Kullmann et al., 1996; Marie et al., 2005), we observed that during or shortly after repeated exposure to cocaine (i.p. injection, 15 mg/kg/day, 5 days, with 1–2 days of withdrawal), a large portion of silent synapses was generated in NAc medium spiny neurons (MSNs) (Huang et al., 2009). Additional molecular manipulations demonstrate that, accompanying the generation of silent synapses, NR2B-containing NMDARs are selectively inserted into synaptic locations within NAc MSNs. Furthermore, in the presence of selective antagonists of NR2B-containing NMDARs, the increased portion of silent synapses in cocaine-treated animals is no longer detected (Huang et al., 2009). These results taken together suggest that exposure to cocaine generates silent synapses by loading NR2B-containing NMDARs to potentially new synaptic sites in NAc MSNs (Fig 1).
Figure 1.
A hypothesized diagram demonstrating the cellular processes of the development and maturation of cocaine-generated silent synapses. (Left) The proposed state before the generation of a silent synapse. The synaptic connection is absent, demonstrated by the dotted lines. (Middle) After repeated exposure to cocaine, silent synapses are generated by the insertion of new NMDARs enriched in the NR2B subunit via lateral diffusion or exocytosis. (Right) Long-term withdrawal is a potential trigger of synaptic insertion of AMPARs thus maturation of silent synapses.
The finding that cocaine induces the generation of silent synapses may provide a new angle for understanding the identified cocaine-induced synaptic alterations. For example, an electrophysiological study shows that during short-term (1 d) withdrawal from repeated exposure to cocaine (a similar time point at which cocaine-generated silent synapses are detected), the AMPAR/NMDAR ratio at excitatory synapse of NAc MSNs is decreased (Kourrich et al., 2007). This result was previously interpreted as indicating a decrease in AMPARs. Conversely, a biochemical study demonstrates that at a similar withdrawal time point, the surface level of AMPAR subunits remains largely unchanged (Boudreau and Wolf, 2005). If surface AMPARs are predominantly synaptic, this biochemical result would suggest no change in synaptic AMPARs. These seemingly contradicting electrophysiological and biochemical results are now well reconciled as cocaine-induced generation of silent synapses does not necessarily change AMPARs but does increase the number of functional NMDARs (Huang et al., 2009), contributing to the observed decrease in the AMPAR/NMDAR ratio.
At the conceptual level, demonstration of cocaine-induced generation of silent synapses provides at least three lines of insights for the synaptic plasticity field. First, it has been highly debated whether the ‘silent’ nature of silent synapses originates pre or postsynaptically (Kerchner and Nicoll, 2008), and whether or not AMPARs are present in silent synapses (Groc et al., 2006). Detailed characterizations of NMDARs upon cocaine exposure (Huang et al., 2009) suggests that postsynaptic NMDARs play a central role, at least in cocaine-generated silent synapses. Second, although un-silencing of silent synapses serves as a prominent model for LTP of excitatory synaptic transmission (Isaac et al., 1995; Liao et al., 1995; Kerchner and Nicoll, 2008), silent synapses are not normally abundant in the developed brain (Groc et al., 2006; Kerchner and Nicoll, 2008). Demonstration of experience-dependent generation of silent synapses in the developed brain provides a conceptual basis for silent synapse-based metaplasticity as a prelude for the adult brain to undergo specific types of synaptic plasticity. Third, silent synapses are the characteristic structures in the developing brain, and may, arguably, be created through synaptogenesis (Kerchner and Nicoll, 2008; Zito et al., 2009). Thus, a broader view would be that some strong in vivo experiences may selectively ‘rejuvenate’ the related neural circuits/synapses through silent synapse-based metaplasticity for more robust synaptic plasticity upon subsequent experience.
2.4 Synaptic consequences of cocaine-induced silent synapse-based metaplasticity
A clear prediction for cocaine-induced generation of silent synapses in NAc MSNs would be that these neurons have an increased ability to undergo LTP during the short-term withdrawal period. Such enhanced LTP was indeed observed in NAc MSNs from cocaine-pre-exposed animals, although the data were interpreted differently at that time (Yao et al., 2004). Our laboratory, however, has not been able to reliably induce LTP in NAc MSNs from naïve, saline-or cocaine-treated animals with the ex vivo preparations, despite rigorous attempts with many types of induction protocols. We thus have not been able to directly confirm the above prediction ourselves. Regardless of our “unlucky” attempts, LTP in NAc MSNs has been successfully induced by several other laboratories using ex vivo conditions (Kombian and Malenka, 1994; Li and Kauer, 2004; Yao et al., 2004; Schotanus and Chergui, 2008). Thus, it is very likely that LTP is inducible in NAc MSNs, and a failure of induction may reflect the importance of the microenvironment surrounding the affected synapses, which can differ in each experimental condition. For example, NAc MSNs sit in a rich modulatory environment constituted by a large number of neuromodulators including dopamine, adenosine, and endocannabinoids. These neuromodulator systems can be essential for LTP induction (Centonze et al., 2001) and are better preserved under some experimental conditions but not others. Consistent with this notion, LTP in NAc MSNs appears to be more reliably induced under in vivo conditions (Goto and Grace, 2005; Moussawi et al., 2009).
Nonetheless, the facilitative effect of silent synapse-based metaplasticity, if any, should only occur during or shortly after short-term withdrawal (e.g., 1–2 days) from repeated exposure to cocaine because after this time window, the number of silent synapses gradually returns to baseline levels (Huang et al., 2009). If cocaine-induced generation of silent synapses is indeed a form of metaplasticity that primes NAc MSNs for subsequent LTP induction, what would be the in vivo induction stimulation/trigger? An apparent candidate is the withdrawal experience. After repeated exposure to drugs of abuse, withdrawal, a termination of access to drug exposure, is no longer a passive process. Withdrawal is accompanied by a comprehensive set of stimulations involving many types of symptoms, both physiologically (e.g., abnormal blood pressures) and psychologically (e.g., emotional state of craving). Thus, withdrawal can be regarded as the “trigger” procedure for subsequent plasticity.
During long-term withdrawal, both the total (Churchill et al., 1999) and surface (Boudreau and Wolf, 2005; Boudreau et al., 2007) levels of AMPARs are substantially increased in animals that previously exhibit cocaine-induced locomotor sensitization. If it is assumed that surface AMPARs are mostly synaptic, these results would suggest that the overall excitatory synaptic strength is potentiated during long-term withdrawal (Fig 1). The detailed induction mechanism of this potentiation is not clear (Wolf and Ferrario, 2010). One possibility, as mentioned above, is that withdrawal or withdrawal-associated stimulations serve as an in vivo “induction protocol” that triggers an AMPAR trafficking-based LTP-like process, resulting in more synaptic AMPARs in NAc MSNs. This potential process is somewhat similar to Hebbian plasticity. And if this is the case, previously generated silent synapses could greatly facilitate synaptic insertion of AMPARs. Evidence supporting this scenario comes from two in vivo studies (Goto and Grace, 2005; Moussawi et al., 2009), which show that during long-term withdrawal from repeated cocaine exposure, LTP at excitatory synapses could no longer be induced in the NAc. As with most regulatory processes, the excitatory synaptic strength is regulated within a range. If a set of synapses previously undergoes a strong LTP-like process and their synaptic strength has been maximized, further attempts at induction could no longer cause additional up-regulation. Thus, one explanation of the lack of NAc LTP during withdrawal is that the excitatory synaptic strength has already been saturated.
Another possibility is that the increased number of synaptic AMPARs is a result of homeostatic plasticity (thus, non-Hebbian plasticity), namely synaptic scaling (Boudreau and Wolf, 2005; Boudreau et al., 2007). The scenario is that following repeated exposure to cocaine, presynaptic glutamatergic terminals may become less active due to the cocaine-induced hypoactivity of the medial prefrontal cortex or other glutamatergic projection areas (Goldstein and Volkow, 2002; Porrino et al., 2002; Porrino et al., 2007). The reduced presynaptic activity may homeostatically scale up postsynaptic responsiveness (Boudreau and Wolf, 2005; Boudreau et al., 2007). In this case, silent synapses can also be facilitative; they provide open “slots” for the upcoming AMPARs. There are other possibilities related to withdrawal-induced up-regulation of AMPARs which will be explored in the next section (2.5). Nonetheless, un-silencing of silent synapses can be one of the key mechanisms by which excitatory synapses within the NAc are strengthened during long-term withdrawal.
It is important to note that the strengthening of excitatory synapses in the NAc is not likely to be exclusively mediated by the un-silencing of silent synapses. Thomas and colleagues demonstrated that the AMPAR/NMDAR ratio at excitatory synapses of NAc MSNs is substantially increased during long-term withdrawal (Kourrich et al., 2007), suggesting that the number and/or function of AMPARs is also up-regulated at non-silent synapses. Indeed, extensive mechanistic studies in the hippocampus demonstrate that the expression of LTP can be mediated by modification of pre-existing synaptic AMPARs, insertion of new AMPARs to pre-existing synapses, un-silencing silent synapses, or enhancement of presynaptic release of glutamate. And, it is not uncommon that some or all of these mechanisms occur simultaneously during the expression of LTP (Malenka and Bear, 2004; Shepherd and Huganir, 2007; Kessels and Malinow, 2009). As such, withdrawal from repeated cocaine administration may strengthen excitatory synaptic transmission in the NAc by employing several cellular and/or molecular mechanisms in parallel, including un-silencing silent synapses.
Then, why does exposure to cocaine utilize more than one means to increase the excitatory synaptic strength in NAc MSNs? The NAc receives extensive glutamatergic synaptic inputs from a variety of brain regions including the limbic components of the prefrontal cortex, the basolateral amygdala, and the hippocampus (Shepard, 2004). It is possible that these different LTP expression mechanisms are differentially involved in different glutamatergic afferents to the NAc upon withdrawal from cocaine administration. Furthermore, via different LTP expression mechanisms, different afferents may recruit different subtypes of AMPARs. Cocaine-generated silent synapses are distinctly different from other synapses in that they not only have greater potential for recruiting AMPARs, but also are enriched in NR2B-containing NMDARs, by which specific plasticity properties (e.g., recruiting a specific type of AMPAR) may be bestowed upon these synapses. One candidate for such specific AMPAR recruitment is GluR2-lacking receptors, which exhibit several unique properties: permeability to Ca2+, relatively higher single channel conductance, and inward rectification at positive potentials (Cull-Candy et al., 2006; Isaac et al., 2007; Liu and Zukin, 2007; Thiagarajan et al., 2007). A potential recruitment of GluR2-lacking AMPARs not only quantitatively, but also qualitatively alters the synaptic properties. During long-term withdrawal from a passive cocaine procedure, the appearance of GluR2-lacking AMPARs has indeed been detected in one study (Mameli et al., 2009), whereas it has not been detected in another (Kourrich et al., 2007). Besides various technical reasons, a possible explanation for these inconsistent results is that GluR2-lacking AMPARs are selectively inserted into certain afferents, and synaptic transmission from these selected afferents is preferentially sampled in some studies but not others. On the other hand, GluR2-lacking AMPARs are expressed in the NAc during long-term withdrawal from prolonged cocaine self-administration, and selectively inhibiting GluR2-lacking AMPARs within the NAc inhibits cocaine-craving in animal models (Conrad et al., 2008). Two open questions are whether silent synapses are selectively generated within certain afferents and whether GluR2-lacking AMPARs are selectively inserted to silent synapses during cocaine withdrawal.
Additionally, the seemingly de novo nature of cocaine-generated silent synapses (Huang et al., 2009) presents the more speculative possibility that these newly-created silent synapses are new, premature synaptic connections that are in the process of forming entirely novel brain circuitry. The presynaptic terminals of cocaine-induced silent synapses may project from one of the well known afferents to the NAc, or alternatively, project from a brain region that does not normally have a connection with the NAc. Un-silencing these silent synapses would consolidate this new circuitry, re-organize the neural network, and reshape the pattern of information processing within the NAc, resulting in fundamental alterations of NAc-based behaviors.
It is also worth mentioning that re-exposure (challenge) to cocaine after long-term withdrawal appears to restore the excitatory synaptic strength of NAc MSNs back down to normal levels. Upon a single exposure to cocaine following a long-term withdrawal, withdrawal-induced up-regulation of AMPARs (Boudreau and Wolf, 2005) is no longer observed; it falls even below control levels (Boudreau et al., 2007) in previously cocaine-sensitized animals. Furthermore, the AMPAR/NMDAR ratio at excitatory synapses of NAc MSNs, which is increased during long-term withdrawal (Kourrich et al., 2007), returns to (Kourrich et al., 2007) or becomes lower (Thomas et al., 2001) than the baseline level (i.e., the ratio from naïve or saline-treated animals). This re-exposure-induced effect cannot be explained by the acute effect of cocaine as a single cocaine injection to naïve animals does not affect the AMPAR/NMDAR ratio (Kourrich et al., 2007). More likely, re-exposure acts as a de-potentiation procedure that induces a de-potentiation of LTP previously induced during the withdrawal period. It is also important to note that the surface levels of AMPARs become higher again following additional 6–7 days after the challenge injection of cocaine (Bachtell and Self, 2008; Ferrario et al., 2010). These results suggest that excitatory synaptic transmission to NAc neurons undergoes dynamic potentiation, depression/de-potentiation, re-potentiation throughout the exposure, withdrawal, and re-exposure period.
2.5 Other cellular consequences of cocaine-induced silent synapses
Although termed “silent” synapses, silent synapses are not truly silent in vivo, especially in NAc MSNs. The membrane potential of in vivo NAc MSNs (measured from cell bodies) frequently climbs to, if not always dwelling at, relatively depolarized voltage plateaus (i.e., ~−55 mV) (Wilson and Groves, 1981; Mahon et al., 2006), at which Mg2+-mediated blockade of NMDARs is incomplete (Jahr and Stevens, 1990). These depolarization plateaus likely originate from dendrites, where the temporal synchronization of excitatory synaptic inputs occurs (Wilson and Groves, 1981; O'Donnell and Grace, 1995). When also factoring in the spatial effect, the depolarization should be more significant in dendrites where synaptic NMDARs reside. Thus, synaptic NMDARs, including those in cocaine-generated silent synapses, can be activated in in vivo NAc MSNs; they may contribute to transmission and mediate NMDAR-signaling, which together influence the NAc-based cellular and behavioral outputs. One such broad cellular impact of cocaine-generated silent synapses is the selective enhancement of NMDAR NR2B-coupled signaling. NMDARs in the adult forebrain are thought to exist as tetramers primarily consisting of NR1 and NR2 (mostly 2A and 2B) subunits (Monyer et al., 1994; Standaert et al., 1994; Carroll and Zukin, 2002). Compared to NR2A-containing NMDARs, NR2B-containing NMDARs exhibit a slower time course of inactivation and a higher binding affinity to calcium/calmodulin kinase II (CaMKII). Thus upon activation, they may result in greater and longer stimulation of the NMDAR/CaMKII-signaling cascade (Strack and Colbran, 1998; Leonard et al., 1999; Strack et al., 2000; Mayadevi et al., 2002). Below we discuss two cellular/behavioral consequences that may result from the up-regulation of NR2B/CaMKII-signaling.
The first of these potential cellular consequences may occur at the signaling level. It has been shown that CaMKII-signaling is upregulated within the NAc upon repeated exposure to cocaine (McClung and Nestler, 2003), and activation of CaMKII-signaling may critically contribute to the strengthening of excitatory synaptic transmission to the NAc during cocaine withdrawal (Anderson et al., 2008; Sun et al., 2008). At the behavioral level, activation of NAc CaMKII-signaling is required for the expression of cocaine-induced locomotor sensitization (Pierce et al., 1998) and reinstatement of cocaine following withdrawal from cocaine self-administration (Anderson et al., 2008). Thus, the newly inserted NR2B-containing NMDARs from cocaine-generated silent synapses may act with other molecular processes to initiate and intensify these CaMKII-mediated cellular and behavioral alterations in cocaine-treated animals.
A second potential cellular consequence involves homeostatic regulation and dysregulation between excitatory synaptic input and membrane excitability of NAc MSNs. Excitatory synaptic input and membrane excitability are the two key physiological parameters that determine the functional output of NAc MSNs. Recently, our laboratory demonstrated a form of homeostatic crosstalk in NAc MSNs between excitatory synapses and membrane excitability, so-called homeostatic synapse-driven membrane plasticity (hSMP) (Ishikawa et al., 2009; Huang et al., 2010). hSMP is usually triggered by a chronic increase or decrease in the strength of excitatory synaptic transmission, and it is expressed as a decrease or increase in membrane excitability, respectively. As such, hSMP may homeostatically maintain the overall functional output of the NAc. Further studies demonstrate that hSMP is mediated by synaptic NMDARs. The activity level of excitatory synapses can be detected and translated by synaptic NMDARs into different degrees of activation, and NMDAR-coupled signaling (e.g., CaMKII-signaling), in turn, regulates ion channels (e.g., SK channels) to change the membrane excitability (Ishikawa et al., 2009). Our subsequent preliminary studies show that persistent activation of NR2B-containing, but not NR2A-containing, NMDARs induces a homeostatic decrease in membrane excitability of NAc MSNs (unpublished data). As discussed above (section 2.3), it is likely that the excitatory synaptic strength of NAc MSNs remains unchanged or is slightly decreased (Boudreau and Wolf, 2005; Boudreau et al., 2007; Kourrich et al., 2007) during late stage of repeated exposure to cocaine or short-term withdrawal from the exposure. However, during these periods, generation of silent synapses substantially up-regulates NMDAR NR2B-signaling. Thus, even though the actual incoming excitatory synaptic strength is not altered, the increased activity of NR2B-containing NMDARs may provide a false homeostatic signal, misleading hSMP to decrease the membrane excitability of NAc MSNs. Indeed, the membrane excitability of NAc MSNs is decreased during short- and long-term withdrawal from repeated exposure to cocaine (Dong et al., 2006; Ishikawa et al., 2009; Mu et al., 2010).
Two lines of evidence from our previous and ongoing studies support the idea that the decreased membrane excitability of NAc MSNs during cocaine withdrawal is mediated, at least in part, by silent synapse-associated hSMP. First, the hSMP-mediated decrease in membrane excitability of NAc MSNs is expressed by up-regulation of SK type calcium-activated potassium channels, and inhibiting SK channels partially reverse this membrane effect of cocaine (Ishikawa et al., 2009). Second, in transgenic mice in which NR2B-CaMKII-signaling is disabled, repeated exposure to cocaine no longer induces a decrease in membrane excitability of NAc MSNs (unpublished data). Thus, in addition to the tremendous synaptic impact, cocaine-generated silent synapses may also impinge upon other important cellular substrates within the NAc MSNs.
Moreover, studies in different neuronal types show that there is also a reverse form of hSMP, a homeostatic membrane-to-synapse regulation (Turrigiano and Nelson, 2004; Frank et al., 2006; Ibata et al., 2008). Specifically, a manipulation that decreases the membrane excitability of a neuron causes a homeostatic increase in postsynaptic AMPARs (Ibata et al., 2008). In NAc MSNs, the decreased membrane excitability lasts throughout the long-term withdrawal from repeated administration of cocaine (Mu et al., 2010). If the membrane-to-synapse homeostatic regulation also exists in NAc MSNs, it is possible that the observed up-regulation of postsynaptic AMPARs during long-term withdrawal (Boudreau and Wolf, 2005; Boudreau et al., 2007; Kourrich et al., 2007) is partially achieved by homeostatic up-scaling of AMPARs as a result of prolonged decrease in membrane excitability (Fig 2) (Wolf, 2010).
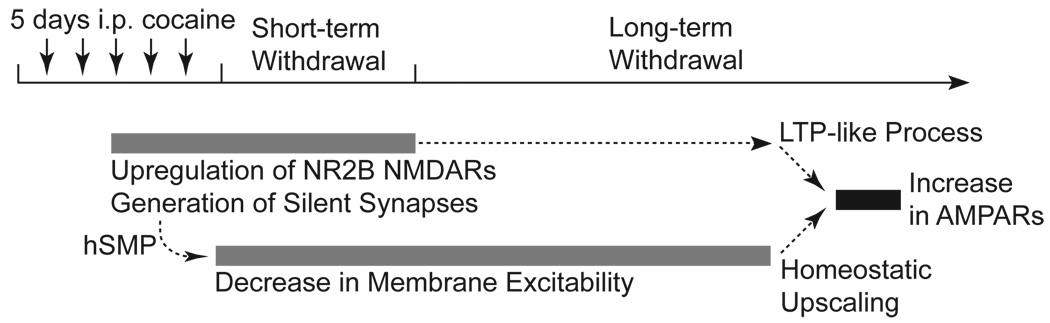
Figure 2.
Summary of synaptic and membrane alterations in NAc neurons during withdrawal from 5 day non-contingent exposure to cocaine. An important synaptic alteration in NAc neurons during the late phase of cocaine exposure and short-term withdrawal is the appearance of silent synapses enriched in NR2B-containing NMDARs. Immediately after repeated cocaine exposure the intrinsic membrane excitability of NAc neurons is decreased and persists throughout short-term and long-term withdrawal. Based on the timing of the cocaine-induced synaptic and membrane alterations, we hypothesize that these cellular alterations are linked. The increased NMDAR-mediated activity may create a false signal of increased synaptic strength to trigger hSMP, resulting in the observed decrease in membrane excitability. Newly generated silent synapses provide extra synaptic slots that may facilitate the recruitment of new AMPARs in a LTP-like process during long-term withdrawal. Simultaneously, this observed upregulation of AMPARs during long-term withdrawal may also be mediated by homeostatic up-scaling of AMPARs as a result of the prolonged decrease in membrane excitability.
Thus, in addition to LTP-like processes as discussed in section 2.4, the non-Hebbian homeostatic plasticity may also critically contribute to the strengthened excitatory synaptic transmission in the NAc during long-term withdrawal. Indeed, this potential homeostatic mechanism may fit better with the robust and “across-the-board” effect of cocaine. Hebbian LTP or LTP-like processes are typically highly pathway-specific (Malenka and Nicoll, 1999), suggesting that a small set of afferents and thus a small portion of synapses are affected by Hebbian LTP. As such, when sampled at the tissue level, these Hebbian LTP-associated changes may not always be detected. Despite the experimental and theoretical demonstration that a large portion of NAc MSNs do not respond to cocaine-related stimulations (Pennartz et al., 1994; Carelli, 2002), a robust potentiation at excitatory synapses is detected during long-term withdrawal in NAc tissue (Churchill et al., 1999; Boudreau and Wolf, 2005; Boudreau et al., 2007; Conrad et al., 2008) as well as across NAc MSNs (Kourrich et al., 2007; Mameli et al., 2009). Unlike Hebbian plasticity, homeostatic synaptic plasticity tends to be a more global effect (Turrigiano and Nelson, 2004), by which most synapses are affected in a sweeping fashion. Thus, for this specific aspect, the homeostatic mechanism seems to be favored. Nonetheless, because synaptic insertion of AMPARs serves as a key expression means for both homeostatic and Hebbian strengthening of excitatory synapses (Nelson and Turrigiano, 2008; Kessels and Malinow, 2009), previously generated silent synapses can facilitate either of these processes by providing open insertion “slots.”
3. Other cocaine-associated metaplasticity
3.1 NMDAR-based metaplasticity
As one of the key metaplasticity substrates, NMDARs are dynamically regulated in the NAc. Specifically for NR2B subunits, depending on the specific cocaine procedure or withdrawal time, a repertoire of seemingly highly inconsistent results have been reported. Some results show an increase in the number of these subunits, while others show a decrease or no change (Fitzgerald et al., 1996; Churchill et al., 1999; Loftis and Janowsky, 2000; Crespo et al., 2002; Scheggi et al., 2002; Yamaguchi et al., 2002; Tang et al., 2004; Hemby et al., 2005a; Hemby et al., 2005b; Lu et al., 2005; Ary and Szumlinski, 2007; Zhang et al., 2007; Conrad et al., 2008; Ben-Shahar et al., 2009; Ghasemzadeh et al., 2009; Schumann and Yaka, 2009; Ferrario et al., 2010). It is important to note that some of these studies measure the total number, rather than surface number of NMDAR subunits. Nonetheless, these results taken together may reflect that NR2B subunits within the NAc are differentially regulated in various subcellular compartments by either different patterns of cocaine administration, different exposure durations, or different withdrawal periods following cocaine administration. Each particular condition may set up a different direction and intensity of NMDAR-based metaplasticity in the NAc, leading to different cellular consequences.
In addition to the NAc, exposure to cocaine also alters NMDARs in other regions within or associated with the mesolimbic dopamine system. In the prefrontal cortex, withdrawal from cocaine self-administration substantially increases the total levels of both NR2A and NR2B subunits (Ben-Shahar et al., 2009). In the ventral tegmental area (VTA), repeated non-contingent exposure to cocaine not only increases the synaptic level of NR2B-containing NMDARs (Schilstrom et al., 2006), but also up-regulate the function of NR2A-containing NMDARs via Src-mediated phosphorylation (Schumann et al., 2009). The up-regulation of NMDARs has been proposed as a mechanism (Schilstrom et al., 2006) mediating cocaine-induced LTP-like processes in VTA dopamine neurons (Ungless et al., 2001; Saal et al., 2003; Borgland et al., 2004), an important form of cocaine-induced synaptic plasticity that has been implicated in addiction-related behaviors (Borgland et al., 2004; Dong et al., 2004). In the amygdala, the total levels of NR1 and NR2B NMDAR subunits are increased at different withdrawal time points following cocaine self-administration (Lu et al., 2005).
In addition to cocaine, other drugs of abuse also dynamically regulate NMDARs within the NAc. Upon repeated exposure to amphetamine, a decrease in NMDARs, particularly NR2B-contianing NMDARs, is observed in some (Mao et al., 2009) but not other studies (Nelson et al., 2009). Following alcohol self-administration, the total levels of NR2A and NR2B subunits of NMDARs in the NAc are increased and decreased, respectively (Obara et al., 2009). Conversely, NR2B subunits, at least at the functional level, appear to increase in the dorsal striatum following alcohol self-administration (Wang et al., 2007). Following exposure to morphine, although NR2B subunits are essential for the reinstatement of morphine administration (Ma et al., 2007), the total levels of NR1 and NR2A, but not NR2B, subunits are increased in the NAc (Murray et al., 2007).
Taken together, these drug-induced alterations in NMDARs may differentially set up metaplasticity processes, facilitating the formation of more persistent cellular changes in specific directions in the NAc and other key brain sites following exposure to a variety of drugs of abuse.
3.2 Other forms of cocaine-associated metaplasticity
GABAergic synaptic transmission can also be affected by drugs of abuse, influencing the susceptibility of excitatory synapses to subsequent plastic changes. Typically, an LTP-like process at excitatory synapses is triggered by co-activation of pre and postsynaptic terminals, and the induction efficacy of LTP is sensitive to the magnitude of this co-activation. As such, a decrease in the GABAergic tone, which disinhibits the excitatory synaptic activation, may increase the likelihood of LTP induction. In VTA neurons, the spike timing LTP at excitatory synapses, a type of plasticity that highly depends on the temporal contingency of pre and postsynaptic activation, is enhanced following repeated exposure to cocaine as a result of cocaine-induced inhibition of GABAergic synaptic transmission (Liu et al., 2005). In the NAc, GABA-mediated metaplasticity has not been examined but is likely to also exist. Specifically, it has been demonstrated in the hippocampus that activation of cannabinoid 1 receptors (CB1Rs) can be a highly localized event selective to GABAergic presynaptic terminals. Activation of these peri-GABAergic synaptic CB1Rs facilitates the induction of LTP at excitatory synapses (Freund and Hajos, 2003; Chevaleyre and Castillo, 2004). If this effect of CB1Rs is compromised, as has been suggested in a study showing that CB1R-mediated synaptic regulation is abolished by a single exposure to cocaine (Fourgeaud et al., 2004), CB1R/GABA-mediated metaplasticity might be disrupted by exposure to cocaine in NAc neurons. Therefore, exposure to cocaine may induce or enhance some forms of metaplasticity while simultaneously compromising others. And by doing this, certain subcellular substrates or certain plastic changes are favored over others, leading to more polarized changes in the affected brain regions following exposure to drugs of abuse.
Another potentially important metaplasticity factor stems from the activity of glial cells. In addition to supportive functions, glial cells, especially astrocytes, also dynamically regulate neural activity by releasing a variety of chemicals (Haydon et al., 2009). Among these glia-released molecules, D-serine is indeed the endogenous agonist for the glycine-binding site of NMDARs (Wolosker et al., 1999; Mothet et al., 2000). D-serine has been demonstrated at the extracellular level to act as a metaplasticity factor gating the induction of LTP and LTD at excitatory synapses (Panatier et al., 2006). Several lines of evidence suggest that exposure to cocaine modulates astrocytes regulatory processes (Bowers and Kalivas, 2003; Narita et al., 2006). However, it remains to be determined whether the astrocyte-mediated release of D-serine or other key factors (e.g., ATP and glutamate) are altered in the NAc accordingly.
4. Concluding remarks
Mainly focusing on NMDAR-dependent metaplasticity, we have discussed the potential mechanisms through which cocaine-associated memories are effectively and efficiently formed. These discussions emphasize that drug-induced metaplasticity acts as an important priming process that may facilitate a specific set of plasticity while it inhibits others. As such, the cellular history encoded in metaplasticity during or shortly after exposure to drugs of abuse may functionally pre-define the nature of subsequent more durable plastic changes (even before they actually occur). Thus, the initial transiently occurring cellular changes following drug exposure may serve as an important metaplasticity process that begins a long-lasting impact on the overall development of drug addiction.
Acknowledgements
We thank Peter Neumann for suggestions on the manuscript. The related work of these authors was supported by the Hope Foundation for Depression Research (YD), NIH DA023206 (YD), NIH DA028020 (BRL), and the Humboldt Foundation (YD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: a two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Self DW. Renewed cocaine exposure produces transient alterations in nucleus accumbens AMPA receptor-mediated behavior. J Neurosci. 2008;28:12808–12814. doi: 10.1523/JNEUROSCI.2060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF. A synaptic basis for memory storage in the cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:13453–13459. doi: 10.1073/pnas.93.24.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Kalivas PW. Forebrain astroglial plasticity is induced following withdrawal from repeated cocaine administration. Eur J Neurosci. 2003;17:1273–1278. doi: 10.1046/j.1460-9568.2003.02537.x. [DOI] [PubMed] [Google Scholar]
- Busetto G, Higley MJ, Sabatini BL. Developmental presence and disappearance of postsynaptically silent synapses on dendritic spines of rat layer 2/3 pyramidal neurons. J Physiol. 2008;586:1519–1527. doi: 10.1113/jphysiol.2007.149336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev. 2002;1:281–296. doi: 10.1177/1534582302238338. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13:1071–1077. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- Chard PS, Jordan J, Marcuccilli CJ, Miller RJ, Leiden JM, Roos RP, Ghadge GD. Regulation of excitatory transmission at hippocampal synapses by calbindin D28k. Proc Natl Acad Sci U S A. 1995;92:5144–5148. doi: 10.1073/pnas.92.11.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Christie BR, Stellwagen D, Abraham WC. Reduction of the threshold for long-term potentiation by prior theta-frequency synaptic activity. Hippocampus. 1995;5:52–59. doi: 10.1002/hipo.450050107. [DOI] [PubMed] [Google Scholar]
- Churchill L, Swanson CJ, Urbina M, Kalivas PW. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem. 1999;72:2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Curr Opin Neurobiol. 1998;8:139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- Crespo JA, Oliva JM, Ghasemzadeh MB, Kalivas PW, Ambrosio E. Neuroadaptive changes in NMDAR1 gene expression after extinction of cocaine self-administration. Ann N Y Acad Sci. 2002;965:78–91. doi: 10.1111/j.1749-6632.2002.tb04153.x. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC. Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(−/−) mice. Proc Natl Acad Sci U S A. 2004;101:14282–14287. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, Malenka RC. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourgeaud L, Mato S, Bouchet D, Hemar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–6945. doi: 10.1523/JNEUROSCI.0671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Hajos N. Excitement reduces inhibition via endocannabinoids. Neuron. 2003;38:362–365. doi: 10.1016/s0896-6273(03)00262-9. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Saviane C, Voronin LL, Cherubini E. Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release? Proc Natl Acad Sci U S A. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2009;159:414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Groc L, Gustafsson B, Hanse E. Spontaneous unitary synaptic activity in CA1 pyramidal neurons during early postnatal development: constant contribution of AMPA and NMDA receptors. J Neurosci. 2002;22:5552–5562. doi: 10.1523/JNEUROSCI.22-13-05552.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Gustafsson B, Hanse E. AMPA signalling in nascent glutamatergic synapses: there and not there! Trends Neurosci. 2006;29:132–139. doi: 10.1016/j.tins.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Hanse E, Taira T, Lauri S, Groc L. Glutamate synapse in developing brain: an integrative perspective beyond the silent state. Trends Neurosci. 2009;32:532–537. doi: 10.1016/j.tins.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Blendy J, Moss SJ, Rob Jackson F. Astrocytic control of synaptic transmission and plasticity: a target for drugs of abuse? Neuropharmacology. 2009;56 Suppl 1:83–90. doi: 10.1016/j.neuropharm.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain Res. 2005a;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Tang W, Muly EC, Kuhar MJ, Howell L, Mash DC. Cocaine-induced alterations in nucleus accumbens ionotropic glutamate receptor subunits in human and non-human primates. J Neurochem. 2005b;95:1785–1793. doi: 10.1111/j.1471-4159.2005.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Schluter OM, Dong Y. Cocaine-induced homeostatic regulation and dysregulation of nucleus accumbens neurons. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, Schluter OM, Zukin RS, Dong Y. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Colino A, Selig DK, Malenka RC. The influence of prior synaptic activity on the induction of long-term potentiation. Science. 1992;255:730–733. doi: 10.1126/science.1346729. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction to cocaine and amphetamine. Neuron. 1996;16:901–904. doi: 10.1016/s0896-6273(00)80111-7. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, Hu XT, Schluter OM, Dong Y. Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J Neurosci. 2009;29:5820–5831. doi: 10.1523/JNEUROSCI.5703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Clifford DB, Zorumski CF. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science. 1992;257:1273–1276. doi: 10.1126/science.1519065. [DOI] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J Neurosci. 1990;10:3178–3182. doi: 10.1523/JNEUROSCI.10-09-03178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- Komatsu Y. Age-dependent long-term potentiation of inhibitory synaptic transmission in rat visual cortex. J Neurosci. 1994;14:6488–6499. doi: 10.1523/JNEUROSCI.14-11-06488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombian SB, Malenka RC. Simultaneous LTP of non-NMDA-and LTD of NMDA-receptor-mediated responses in the nucleus accumbens. Nature. 1994;368:242–246. doi: 10.1038/368242a0. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Erdemli G, Asztely F. LTP of AMPA and NMDA receptor-mediated signals: evidence for presynaptic expression and extrasynaptic glutamate spill-over. Neuron. 1996;17:461–474. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- Kuzmiski JB, Pittman QJ, Bains JS. Metaplasticity of hypothalamic synapses following in vivo challenge. Neuron. 2009;62:839–849. doi: 10.1016/j.neuron.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman A, Hannay T, Stratford K, Jack J. Presynaptic release probability influences the locus of long-term potentiation. Nature. 1992;360:70–73. doi: 10.1038/360070a0. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1999;96:3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kauer JA. Repeated exposure to amphetamine disrupts dopaminergic modulation of excitatory synaptic plasticity and neurotransmission in nucleus accumbens. Synapse. 2004;51:1–10. doi: 10.1002/syn.10270. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. Regulation of NMDA receptor subunits and nitric oxide synthase expression during cocaine withdrawal. J Neurochem. 2000;75:2040–2050. doi: 10.1046/j.1471-4159.2000.0752040.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Shaham Y, Hope BT. Differential long-term neuroadaptations of glutamate receptors in the basolateral and central amygdala after withdrawal from cocaine self-administration in rats. J Neurochem. 2005;94:161–168. doi: 10.1111/j.1471-4159.2005.03178.x. [DOI] [PubMed] [Google Scholar]
- Ma YY, Chu NN, Guo CY, Han JS, Cui CL. NR2B-containing NMDA receptor is required for morphine-but not stress-induced reinstatement. Exp Neurol. 2007;203:309–319. doi: 10.1016/j.expneurol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau JM, Chouvet G, Charpier S. Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. J Neurosci. 2006;26:12587–12595. doi: 10.1523/JNEUROSCI.3987-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. Postsynaptic factors control the duration of synaptic enhancement in area CA1 of the hippocampus. Neuron. 1991;6:53–60. doi: 10.1016/0896-6273(91)90121-f. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Curr Opin Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie H, Morishita W, Yu X, Calakos N, Malenka RC. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron. 2005;45:741–752. doi: 10.1016/j.neuron.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Mayadevi M, Praseeda M, Kumar KS, Omkumar RV. Sequence determinants on the NR2A and NR2B subunits of NMDA receptor responsible for specificity of phosphorylation by CaMKII. Biochim Biophys Acta. 2002;1598:40–45. doi: 10.1016/s0167-4838(02)00315-1. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- Merrill EG, Wall PD. Factors forming the edge of a receptive field: the presence of relatively ineffective afferent terminals. J Physiol. 1972;226:825–846. doi: 10.1113/jphysiol.1972.sp010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Moody TD, Carlisle HJ, O'Dell TJ. A nitric oxide-independent and beta-adrenergic receptor-sensitive form of metaplasticity limits theta-frequency stimulation-induced LTP in the hippocampal CA1 region. Learn Mem. 1999;6:619–633. doi: 10.1101/lm.6.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA, Schluter OM, Dong Y. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci. 2010;30:3689–3699. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray F, Harrison NJ, Grimwood S, Bristow LJ, Hutson PH. Nucleus accumbens NMDA receptor subunit expression and function is enhanced in morphine-dependent rats. Eur J Pharmacol. 2007;562:191–197. doi: 10.1016/j.ejphar.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Milovanovic M, Wetter JB, Ford KA, Wolf ME. Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J Neurochem. 2009;109:35–51. doi: 10.1111/j.1471-4159.2009.05911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33:1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Espinosa JS, Bear MF. Evidence for altered NMDA receptor function as a basis for metaplasticity in visual cortex. J Neurosci. 2003;23:5583–5588. doi: 10.1523/JNEUROSCI.23-13-05583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot BD, Cho KK, Bear MF. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron. 2007;53:495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002;22:7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci U S A. 1999a;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999b;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Scheggi S, Mangiavacchi S, Masi F, Gambarana C, Tagliamonte A, De Montis MG. Dizocilpine infusion has a different effect in the development of morphine and cocaine sensitization: behavioral and neurochemical aspects. Neuroscience. 2002;109:267–274. doi: 10.1016/s0306-4522(01)00483-3. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, Bonci A. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci. 2006;26:8549–8558. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski LA, Ali DW, Baker GB, Nguyen PV. Impaired hippocampal LTP in inbred mouse strains can be rescued by beta-adrenergic receptor activation. Eur J Neurosci. 2007;25:1589–1598. doi: 10.1111/j.1460-9568.2007.05376.x. [DOI] [PubMed] [Google Scholar]
- Schotanus SM, Chergui K. Long-term potentiation in the nucleus accumbens requires both NR2A- and NR2B-containing N-methyl-D-aspartate receptors. Eur J Neurosci. 2008;27:1957–1964. doi: 10.1111/j.1460-9568.2008.06173.x. [DOI] [PubMed] [Google Scholar]
- Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Michaeli A, Yaka R. Src-protein tyrosine kinases are required for cocaine-induced increase in the expression and function of the NMDA receptor in the ventral tegmental area. J Neurochem. 2009;108:697–706. doi: 10.1111/j.1471-4159.2008.05794.x. [DOI] [PubMed] [Google Scholar]
- Schweighofer N, Arbib MA. A model of cerebellar metaplasticity. Learn Mem. 1998;4:421–428. doi: 10.1101/lm.4.5.421. [DOI] [PubMed] [Google Scholar]
- Shepard GM. The synaptic organization of the brain. 5th Edition. Oxford University Press; 2004. [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Standaert DG, Testa CM, Young AB, Penney JB., Jr Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J Comp Neurol. 1994;343:1–16. doi: 10.1002/cne.903430102. [DOI] [PubMed] [Google Scholar]
- Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- Strack S, McNeill RB, Colbran RJ. Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2000;275:23798–23806. doi: 10.1074/jbc.M001471200. [DOI] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem. 2004;89:1021–1033. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: overlapping mechanisms and implications for metaplasticity. Neuropharmacology. 2007;52:156–175. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Volianskis A, Jensen MS. Transient and sustained types of long-term potentiation in the CA1 area of the rat hippocampus. J Physiol. 2003;550:459–492. doi: 10.1113/jphysiol.2003.044214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Bennett JE, McAllister AK. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5:751–759. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Spontaneous firing patterns of identified spiny neurons in the rat neostriatum. Brain Res. 1981;220:67–80. doi: 10.1016/0006-8993(81)90211-0. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010 doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci U S A. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Suzuki T, Abe S, Hori T, Kurita H, Asada T, Okado N, Arai H. Repeated cocaine administration differentially affects NMDA receptor subunit (NR1, NR2A-C) mRNAs in rat brain. Synapse. 2002;46:157–169. doi: 10.1002/syn.10132. [DOI] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lee TH, Davidson C, Lazarus C, Wetsel WC, Ellinwood EH. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacology. 2007;32:377–387. doi: 10.1038/sj.npp.1301101. [DOI] [PubMed] [Google Scholar]
- Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61:247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]