Abstract
Although radiation therapy (RT) is an integral component of treatment of patients with many types of cancer, inherent and/or acquired resistance to the cytotoxic effects of RT is increasingly recognized as a significant impediment to effective cancer treatment. Inherent resistance is mediated by constitutively activated oncogenic, proliferative and anti-apoptotic proteins/pathways whereas acquired resistance refers to transient induction of proteins/pathways following radiation exposure. To realize the full potential of RT, it is essential to understand the signaling pathways that mediate inducible radiation resistance, a poorly characterized phenomenon, and identify druggable targets for radiosensitization. Ionizing radiation induces a multi-layered signaling response in mammalian cells by activating many pro-survival pathways that converge to transiently activate a few important transcription factors (TFs), including nuclear factor kappa B (NF-κB) and signal transducers and activators of transcription (STATs), the central mediators of inflammatory and carcinogenic signaling. Together, these TFs activate a wide spectrum of pro-survival genes regulating inflammation, anti-apoptosis, invasion and angiogenesis pathways, which confer tumor cell radioresistance. Equally, radiation-induced activation of pro-inflammatory cytokine network (including interleukin (IL)-1β, IL-6 and tumor necrosis factor-α) has been shown to mediate symptom burden (pain, fatigue, local inflammation) in cancer patients. Thus, targeting radiation-induced inflammatory pathways may exert a dual effect of accentuating the tumor radioresponse and reducing normal tissue side-effects, thereby increasing the therapeutic window of cancer treatment. We review recent data demonstrating the pivotal role played by inflammatory pathways in cancer progression and modulation of radiation response.
Keywords: Radiation therapy, inflammation, NF-κB, STAT, radiosensitization
1. Introduction
The functional link between inflammation and cancer was postulated many years ago. In 1863, Virchow [1] noted the presence of leukocytes in the neoplastic tissue and hypothesized that cancers originated from the sites of chronic inflammation. In 1986, Dvorak illustrated that wound healing and tumor stroma formation share several salient features [2]. Since then, the notion that chronic inflammation provides a favorable environment for cancer formation and progression has been widely accepted. Whereas the critical correlation between tumor progression and inflammation is increasingly confirmed by evolving data, it is only recently that the potential significance of these associations has become apparent. The rapid expansion of our understanding of oncogenic molecular signaling pathways has revealed the intricate functional relationships that exist among the inflammatory pathways, tumor progression, invasion and metastasis and resistance of tumors to therapy [3]. These insights have led to the development of new anti-inflammatory therapeutic approaches for cancer treatment [4, 5].
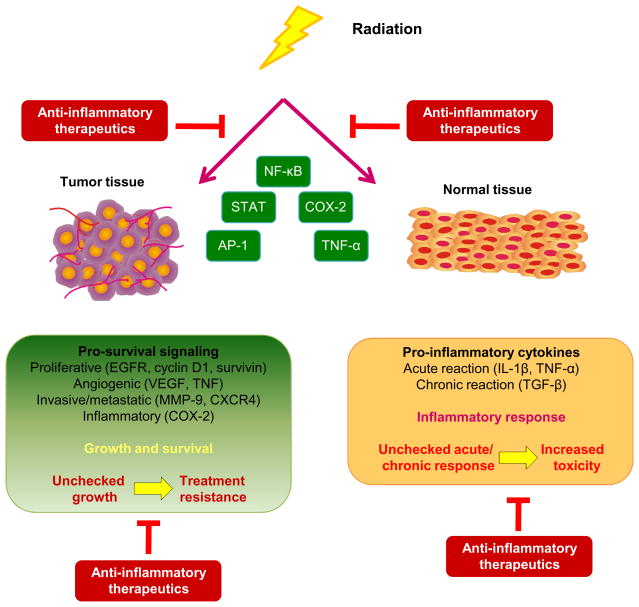
This review portrays the convergence of multiple lines of evidence that inflammatory signaling pathways modulate the response of tumors to radiation therapy (RT). The scope of this review is, however, not to detail the fundamental relationship between inflammatory pathways and tumor progression which has been thoroughly reviewed elsewhere [1, 3, 6, 7] or to elaborate on tumor immunology [8] and/or tumor immunotherapy [9, 10]. Instead, focusing on the intricate interplay between tumor responses to radiation and inflammation, this review offers insights into potential druggable targets to sensitize tumors to RT without significant collateral damage to normal tissue. It is now known that exposure to clinically relevant doses of ionizing radiation (IR) not only induces DNA damage in the nucleus, but also triggers a large network of intracellular signaling events. These include transient activation of prosurvival pathways [receptor tyrosine kinase (RTK) pathways such as the epidermal growth factor receptor (EGFR) pathway and the downstream Ras and phosphoinositide 3-kinase/atypical kinase (PI3K/Akt) signaling], activation of transcription factors [p53, nuclear factor kappa B (NF-κB), activator protein-1 (AP-1)], upregulation of the expression of chemokine receptors (CXC motif receptor 4; CXCR4) [11] and upregulation of the levels of a variety of cytokines [tumor necrosis factor alpha (TNF α), IL-6, IL-8, urokinase-type plasminogen activator (uPA), transforming growth factor (TGF)-β] [12–15], all of which are not only crucial mediators of inflammation but also control post-irradiation cell survival responses [16–20]. Whereas many of these inflammatory downstream responses to radiation are detrimental to normal tissue, they confer a survival advantage to tumor cells. Therefore, unlike many radiosensitization strategies that equally sensitize the tumor cells and the adjacent collaterally irradiated normal cells to RT [21–23], specific targeting of downstream components of this inducible inflammatory signaling response offer the possibility of seamlessly and simultaneously abrogating radioresistance within tumor cells and blocking deleterious inflammatory responses of normal tissues to RT (Fig. 1).
Fig. 1.
Conceptual framework of the topics discussed in this review. Radiation therapy induces pro-inflammatory responses in the tumor (which are largely mediated through activation of NF-κB). In turn, NF-κB can up-regulate the pro-survival, angiogenic, invasive and anti-apoptosis pathways which confer a radioresistant phenotype on tumor cells (beneficial to the tumor, detrimental to the patient) and/or it can trigger a pro-inflammatory network of cytokines which mediate radiation-induced early and late side effects in normal tissues (detrimental to the normal tissues and to the patient). By inhibiting these divergent pro-inflammatory responses in tumors and normal tissues, it may be possible to simultaneously achieve enhanced tumor kill and reduced toxicity.
2. Radiation therapy in current cancer management
RT remains an integral part of modern cancer management in both benign and malignant diseases. More than 50% of the newly diagnosed cancer patients worldwide receive RT (alone or in combination with chemotherapy or surgery) at some point in the course of their treatment [24]. Compared to both surgery and chemotherapy, RT is unique in that it is generally non-invasive and devoid of intense systemic toxicity, and therefore an integral component of organ preserving and adjuvant treatment strategies for primary tumors and palliative treatment strategies for advanced and metastatic tumors. In the past decade, there have been substantial improvements in radiation treatment outcomes attributable to advances in the clinical, physics, and biology realms [25]. In particular, the technological sophistication of imaging, planning and delivery of RT has enabled more cancers to be treated with higher and more tumoricidal doses of radiation with curative intent [26, 27]. Similarly, an improved (and to a certain degree, renewed) understanding of basic radiobiology has allowed investigation of the causes of tumor cell radioresistance (both inherent and acquired), which, in turn, leads to an increased likelihood of recurrence and treatment failure in many patients. A more nuanced understanding of the biological basis for the response (or lack thereof) of tumors to RT, placed within the larger context of an explosion of knowledge about the complex molecular mechanistic underpinnings of carcinogenesis, has paved the way for exploring novel and/or combinatorial therapeutic approaches for cancer treatment using IR [28, 29]. Whereas combination of IR with cytotoxic chemotherapeutic agents was the primary approach evaluated in the past [30, 31], more recently less toxic cytostatic biological therapies have been combined with RT. Most of these targeted therapies have largely resulted from the improved understanding of the molecular mechanisms underlying intrinsic tumor radioresistance and the systematic profiling of cellular radiation responses [23, 25]. It is increasingly recognized that IR not only damages DNA in the cell, but also affects several disparate cellular components and these collectively elicit a multilayered biological response in the irradiated tumor cell. Since some of these molecular signaling events are responsible for tumor radioresistance, numerous promising ‘druggable’ targets and new molecular drugs, are increasingly being tested in various clinical or preclinical settings for enhancing tumor radiosensitivity [28, 32].
3. Molecular Biology of Tumor Radioresistance-A Brief Overview
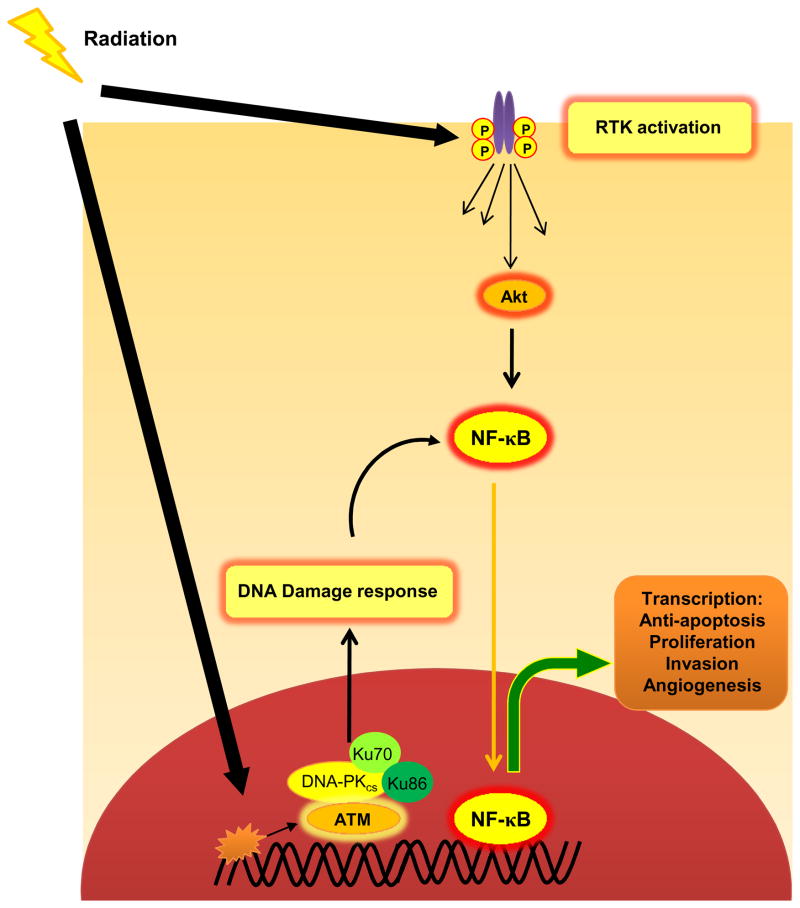
The concept that the intrinsic tumor radiosensitivity is governed by the balance between DNA damage and DNA repair following irradiation has dominated the conceptual framework of radiobiology for decades. Although there is an undisputable cause-function relationship between the extent of radiation-induced DNA damage and the cellular consequences, recent data indicate that this may not be the sole contributor to defining tumor radiosensitivity. Instead, the fate of damaged DNA as well as the cascade of radiation-induced cytoplasmic signaling events are both equally important determinants of tumor radiosensitivity [33]. The cellular signaling triggered by low doses of IR (1–5 Gy) occur at two distinct sites - a) Nuclear - Signaling events initiated by the damaged DNA, which halt cell cycle progression through G1, intra-S and G2/M and elicit a DNA damage response to potentially allow the repair of damaged DNA and b) Cytoplasmic -Signaling at the receptor level which is partly triggered by inactivation of phosphatases by reactive oxygen species (ROS) and consequently causes ligand-independent activation of RTKs (Fig. 2) [23, 33, 34]. In sublethally irradiated cells, both these events elicit pro-survival and anti-apoptotic responses (the inducible radioresistance paradigm) that can then become a potential target for radiosensitization.
Fig. 2.
Simplified depiction of cellular signaling events triggered by clinically relevant doses of radiation. Exposure to clinically relevant doses of ionizing radiation can elicit cellular signaling at two distinct sites - a) Nuclear - The damaged DNA activates the DNA repair machinery which includes activation of ATM, ATR, DNA-PKcs and other repair proteins which arrest the cell cycle b) Cytoplasmic - Radiation-induced disturbance of cellular redox homeostasis by generation of reactive oxygen species (ROS) can activate receptor tyrosine kinases (RTKs) [through inhibition of protein tyrosine phosphatases (PTPases)], which initiate downstream pro-survival signaling pathways mediated through multiple proteins such as Akt. These signaling cascades finally converge upon a small cadre of transcription factors such as NF-κB and STATs which regulate the expression of effector gene products.
3.1 Nuclear Events: Safeguarding the internal damage
The nuclear signaling governs sensing the DNA double strand breaks (DSBs), recruitment of the repair proteins at the sites of DNA DSBs (reviewed in [35, 36]), arresting the cell cycle to allow repair and triggering apoptosis if the repair of damaged DNA is not possible. Two kinases from the PI3K-related kinase (PIKK) family, ataxia-telangiectasia mutated (ATM) and ATR (ATM and RAD3-related), are at the heart of the cellular response to DSBs. ATM is activated when it is recruited to sites of DSB damage by the Mre11-Rad50-Nbs1 (MRN) complex [37–40]. When activated, ATM and ATR phosphorylate a multitude of proteins, which initiate a cascade inducing cell cycle arrest and facilitating DNA repair [41, 42]. Among others, substrates of activated ATM include p53 [43, 44], the kinases CHK1 and CHK2 [42, 45], the histone H2AX [46] and the MRN complex itself. Similarly, ATR phosphorylates CHK1 and DNA helicase BLM1. These proteins act as “transducers” that modulate apoptosis (p53 and CHK2), DNA repair (H2AX and MRN), and cell cycle arrest (p53, CHK1, and CHK2) [41]. Kinases CHK1 and CHK2 primarily cause cell cycle arrest at G2/M or S [47]. With cell cycle arrest, the core machinery of the DNA repair pathway is activated. Mammalian cells process DNA DSBs via two principal pathways - non-homologous end joining (NHEJ) [48, 49] or homologous recombination (HR) [50]. NHEJ is the major route for DSB repair throughout the cell cycle. In this process, the Ku70 and Ku80 proteins form a heterodimer that binds to the ends of a DSB and then recruit DNA-dependent protein kinase (DNA-PKcs), which, consecutively, recruits and activates protein Artemis that processes DNA ends before XRCC4 (X-ray-complementing Chinese hamster gene 4) and DNA ligase IV to facilitate the final ligation step [51, 52]. HR is generally restricted to late S and G2 phase and rejoins DSBs using a sister homologue as a template. An early step in HR involves the generation of a single-stranded region of DNA, followed by invasion of the template strand, which creates a Holliday junction. DNA synthesis using the sister strand as a template is followed by branch migration and subsequent resolution of the heteroduplex. Rad51, a central player in HR, is loaded onto ssDNA and promotes strand invasion, with BRCA2 having a role in delivering Rad51 to the DNA [53, 54]. The primary function of HR is to repair DSBs at the replication fork, whereas NHEJ chiefly repairs DSBs that have been generated elsewhere in the DNA (e.g. radiation-induced). The success of the repair process ultimately determines the fate of the cell (survival vs. apoptosis). The strategy of tumor radiosensitization via inhibition of DNA repair pathway(s) is addressed exhaustively in some recent reviews [22, 55–57] and will not be detailed here.
3.2 Cytoplasmic Events: Proliferative signaling downstream of plasma membrane receptors
In contrast to the nuclear signaling, radiation-induced cytoplasmic signaling events appear to be much more diverse and intricately hardwired together. The cytoplasmic signaling is primarily triggered by the redox imbalance generated by IR in the cytosol and amplified within the mitochondria to result in sufficient activation of ROS (reactive oxygen species) and RNS (reactive nitrogen species) to inhibit protein tyrosine phosphatases (PTPase) [58]. Additionally, larger doses of radiation also increase the production of ceramide by activating acidic sphingomyelinase. Inhibition of PTPases leads to activation of receptor and non-receptor tyrosine kinases and activation of downstream signaling pathways. Radiation-induced ceramide promotes membrane-associated receptor activation by facilitating the clustering of receptors within the lipid rafts [59]. The activation of RTKs (cell-surface receptors with intrinsic tyrosine kinase activity) [60, 61] initiates a cascade of proliferative signaling events activating a number of proteins. The best characterized among these is signaling through the members of the ErbB/epidermal growth factor receptor (EGFR) family [62, 63], which often determines the resistance of cells to chemotherapy or radiotherapy [63, 64]. Activated ErbBs stimulate many intracellular signaling pathways and different ErbBs preferentially modulate specific signaling pathways. Two of the main pathways activated by the receptors are the RAS-Raf-MAPK (mitogen-activated protein kinase) and the PI3K/Akt pathways [65, 66]. Other important ErbB signaling effectors are the signal transducer and activator of transcription proteins (STATs) [67]; SRC tyrosine kinase, the activity of which is increased in response to EGFR and ErbB2 signaling [68]; and mammalian target of rapamycin (mTOR), a serine/threonine kinase activated downstream of PI3K/Akt (reviewed in [69]). These downstream signaling networks play important roles in tumor radiosensitivity by eliciting pro-survival and pro-inflammatory responses.
3.3 Convergence of nuclear and cytoplasmic signaling: Activation of transcription factors
Signaling from the cell surface receptors and from the damaged DNA both lead to downstream pathways that ultimately result in activation of a variety of transcription factors (TFs) which are important in governing gene expression patterns [70]. Radiation-induced TFs chiefly include the dynamic NF-κB family of proteins [71–73], STAT3 [74], and components of the activator protein-1 (AP-1) complex [75–77], which govern various aspects of cellular proliferation, invasion, metastasis, chemo-resistance, radioresistance and inflammation. The first two are of prime importance as they both have been reported to be constitutively activated in many types of cancers [78–87] and linked to chemoresistance and radioresistance [88, 89].
3.4 Effector response: Altering the gene expression
Once activated, the TFs translocate to the nucleus, where they bind to specific DNA sites and trigger the expression of various genes, under various regulatory networks. NF-κB alone regulates the expression of genes involved in cell proliferation (Cyclin D1) [90], angiogenesis (vascular endothelial growth factor; VEGF) [91], invasion (matrix metalloproteinases; MMPs) [92] and a number of pro-inflammatory genes like TNF-α [93], IL-1 [94], IL-8 [95], and cyclooxygenase-2 (COX-2) [96], chemokine receptors (CXCR4) [89]. Activated STAT3 regulates the expression of a multitude of proteins such as cyclin D1 [97], Bcl-XL [98], VEGF [99] and IL-8 [89]. Production of these growth factors and angiogenic factors in response to radiotherapy by activated TFs is the principal mechanism of inducible radioresistance [100]. It is clear from above observations that radiation-induced multilayered signaling in tumor cell leads to proliferative and inflammatory responses, and provides clues for devising newer tumor radiosensitizing strategies.
4. Targeting pro-inflammatory signaling pathways for tumor radiosensitization
Among the plethora of proliferative signaling pathways triggered by sublethal doses of radiation within tumor cells (inducible signaling), inflammatory signaling cascades are unique in that they promote tumor cell survival but, when unchecked, tend to have detrimental effects in normal tissues. Therefore, not only is such an inflammatory signaling pathway inducible (as opposed to constitutive), but may also confer some degree of selectivity for the tumor cell. Consequently, targeting this inducible radioresistance pathway could radiosensitize tumor cells preferentially without sensitizing normal cells – a feature that would not be as readily apparent with targeting constitutive radioresistance pathways that are aberrantly hyper activated or deregulated in cancer cells but also vital for normal tissue homeostasis and proliferation. Therefore, proteins that are transiently activated by IR and play a central role in mediating pro-inflammatory signaling can serve as important targets for radiosensitizing strategies. As TFs activated by radiation are positioned at the convergence of outside-in signals from the cytoplasm and inside-out signals from the nucleus, and orchestrate the regulation of multiple pro-survival and pro-inflammatory signals, they and/or their critical upstream mediators could serve as promising targets for novel radiosensitization strategies.
4.1 NF-κB
4.1.1 Signaling through NF-κB
The transcription factor NF- κB is a family of closely related protein dimers that regulate inducible gene expression in various physiological settings [71, 101]. The mammalian NF-κB family consists of five related proteins: p65 (RelA), RelB, c-Rel, p50/p105 (NF-κB1) and p52 (NF-κB2), which all share an amino-terminal REL homology domain (RHD) [101]. Dimers of NF-κB proteins bind to common sequence motifs in DNA (the κB sites) in promoters or enhancers of target genes, the transcription of which is regulated through the recruitment of transcriptional co-activators and transcriptional co-repressors.
In unstimulated cells, the NF-κB dimers are sequestered in the cytoplasm by their association with inhibitors of κB (IκBs), which mask the nuclear localization sequence (NLS) of NF-κB and thereby prevent nuclear translocation of NF-κB dimers. These dimers can be activated by a plethora of factors that encompass free radicals, cytokines, γ-irradiation, ultraviolet light, growth factors, mitogenic stimuli and a variety of cellular stresses [102–104]. Activation signals for NF-κB can either come through cell surface receptors [104] or from the nucleus (through genotoxic stress) [105]. For example, in response to IR, DNA DSBs provoke a stress signal that is directed to the cytoplasm by NEMO (NF-κB essential modulator) and ATM. DNA DSBs promote SUMO modification of nuclear NEMO, which prevents its nuclear export. In parallel, the DSBs activate nuclear ATM kinase. SUMO-modified NEMO is phosphorylated by active ATM, and this leads to subsequent removal of SUMO and attachment of ubiquitin to NEMO. Modified NEMO that is associated with ATM exits the nucleus and then associates with, and activates, the IKK (IκB kinase) complex. Essentially, both the NF-κB activation pathways converge at activation of IKK [consisting of IKKα, IKKβ, and IKKγ/NEMO, with IKKβ as the catalytic subunit] [106, 107]. The primary step in this process is activation of IKK via phosphorylation of IKKβ. Activated IKK then phosphorylates IκB, triggering polyubiquitinylation of IκB and its rapid proteasomal degradation. This process exposes the NLS signal on p65 and p50, ensuring nuclear translocation of NF-κB to promote gene transcription. Activated NF-κB can induce expression of >200 genes that have been shown to suppress apoptosis, induce cellular transformation, proliferation, invasion, metastasis, chemo-resistance, radio-resistance and mediate inflammation in a wide variety of tumors [108].
4.1.2 Biological significance of NF-κB
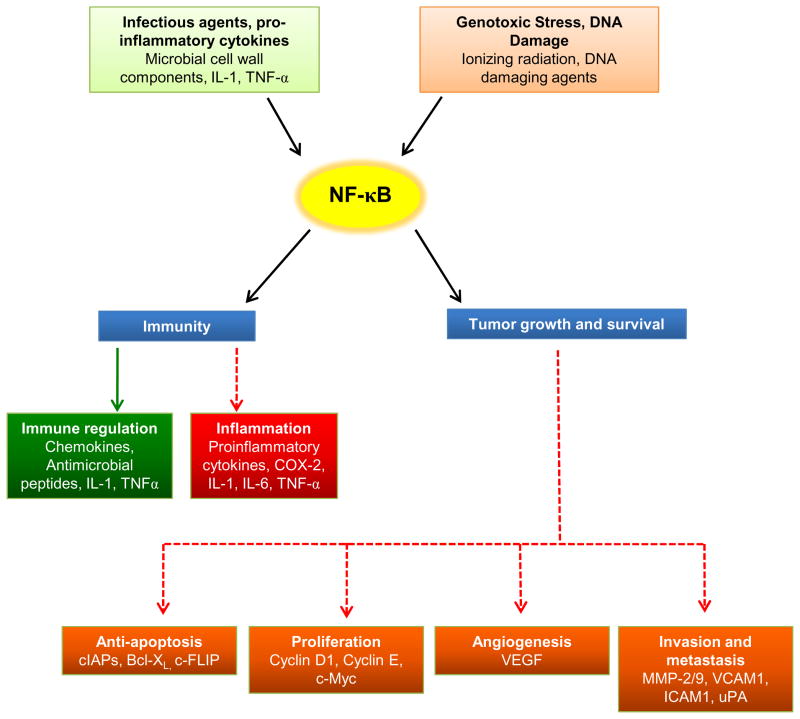
As activation of NF-κB can occur in response to infectious agents, inflammatory cytokines and proteins, DNA damaging agents, genotoxic stress and in turn regulate transcription of a myriad genes important in immunity, suppression of apoptosis, proliferation, invasion and angiogenesis, it is the key molecular linchpin that connects inflammation, carcinogenesis and tumor radioresistance [109, 110] (Fig. 3). In the proposed model for chronic inflammation and carcinogenesis [111, 112], persistent infections and chronic inflammation result in NF-κB activation and once induced, NF-κB target genes protect pre-neoplastic and fully malignant cells from apoptosis induced by surveillance mechanisms that are activated by DNA damage and chromosomal rearrangements or by genotoxic anti-cancer drugs and radiation. NF-κB is constitutively activated in diverse solid malignancies [78], but not their normal counterparts [82, 113]. Whereas, radiation-induced NF-κB in cancer cells is transient, this peaks within few minutes following exposure to IR and returns to baseline levels after few hours [114]. Nevertheless, this transiently activated NF-κB can provoke multiple radioresistance signals which attenuate the lethal effects of radiation [115]. Not surprisingly, inhibition of NF-κB is perceived to be an exciting strategy to enhance tumor radiosensitivity [110, 116–118].
Fig. 3.
NF-κB as the central mediator of inflammation, carcinogenesis and tumor radioresistance. NF-κB can be activated by microbial components or other pro-inflammatory cytokines which can positively modulate the host immune response by synthesizing chemokines and antimicrobial peptides or can lead to local inflammation. Similarly, NF-κB can be induced by anticancer therapeutics and genotoxic stress, and this activated NF-κB promotes cell survival, which forms the basis of carcinogenesis. However, the most peculiar feature of NF-κB is its ability to be transiently activated by anticancer therapeutics (such as ionizing radiation), which elicits a plethora of pro-survival signaling in the irradiated tumor cells. Thus, activated NF-κB could be beneficial to the host (indicated by solid green arrow) or be detrimental to the host (favoring tumor growth; pathways indicated by dotted red arrows).
4.1.3 Strategies targeting NF-κB for tumor radiosensitization
Several studies have investigated the impact of NF-κB inhibition on radiosensitivity in different models [119–122]. Using genetic approaches, it was shown that expression of the super-repressors of IκB enhanced radiation-induced apoptosis in cancer cells [123] and overexpression of wild type IκB sensitized human glioblastoma to radiation [124]. However, the more readily clinically translatable pharmacological approach of NF-κB inhibition for tumor radiosensitization is the use of a variety of synthetic drugs (highly targeted) and natural products (more broad spectrum in their activity). Since a number of different steps are involved in NF-κB activation, there are several potential methods to downregulate NF-κB in target tissues using a wide range of agents (corticosteroids, phytochemicals, proteasome inhibitors and synthetic peptides). These approaches can be roughly categorized as a) inhibition of the key steps of the NF-κB pathway, b) targeting the upstream components of the NF-κB pathway, or c) pharmacological inhibition of the key components of the effector response.
4.1.3.1 Broad-spectrum NF-κB inhibition
Popular for their broad spectrum anticancer and anti-inflammatory effects and low toxicity profile, plant phytochemicals have recently being exploited for tumor radiosensitization through NF-κB inhibition [125]. The rationale behind these studies was to render tumor cells more radiosensitive by concurrently suppressing radiation-induced pro-survival signaling at multiple levels (as opposed to targeting individual components) [125, 126]. Best studied amongst these is the plant polyphenol curcumin. We have shown that curcumin sensitizes colorectal cancer cells to gamma radiation in vitro [114] and in vivo [127] by suppressing radiation-induced NF-κB activation. Curcumin achieves this by inhibiting NF-κB activation directly (IKK activation, IκBα degradation), inhibition of upstream activators of this pathway (Akt) [114] and finally, suppressing the NF-κB regulated anti-apoptotic, proliferative, angiogenic, invasive and pro-inflammatory gene products [114, 127]. Curcumin also sensitizes cancer cells to radiation by suppressing radiation-induced TNF-α [12] or TNF superfamily genes [128]. Similarly, the radiosensitizing properties of the soy isoflavone genistein are mediated by suppression of radiation-induced NF-κB leading to altered expression of regulatory cell cycle proteins such as cyclin B and/or p21WAF1/Cip1, thus promoting G2/M arrest [129]. The sesquiterpene lactone parthenolide sensitizes human hybrid CGL1 cells to radiation by inhibiting NF-κB and enhancing apoptosis [130]. In prostate cancer cells, parthenolide mediates radiosensitization by inhibiting radiation-induced NF-κB activation and the expression of its downstream target sod2, the gene coding for an important antiapoptotic and antioxidant enzyme (manganese superoxide dismutase) [131]. Cepharanthin, the anti-inflammatory biscoclaurine alkaloid extracted from the roots of Stephania cepharantha hayata, enhances the radiosensitivity of oral squamous cell carcinoma cells by inhibiting the activation of radiation-induced NF-κB and the production of NF-κB-mediated downstream pro-inflammatory cytokines IL-6 and IL-8 [132]. Apart from plant phytochemicals, a few other compounds have been shown to exert their radiosensitizing effects through NF-κB suppression, like docosahexaenoic acid (DHA) [133] and pitavastatin [134], however, the precise molecular mechanism of NF-κB suppression has not been investigated in detail in either study. Although in all the above approaches the compounds used did not exhibit any specificity towards NF-κB pathway per se, they clearly demonstrated the rationale of targeting inflammatory pathways for tumor radiosensitization.
4.1.3.2 Proteasome inhibition
The ubiquitin-proteasome system (UPS) is the major protein degradation machinery for intracellular proteins which regulates protein turnover in a number of cell signaling pathways including cell proliferation, DNA repair, apoptosis and immune response [135]. Recent data have shown that pharmacological inhibition of the UPS system can be an attractive approach for cancer treatment [136–139]. As the UPS functioning is crucial in regulating the NF-κB pathway [140–142], this strategy has been exploited for radiosensitization via NF-κB inhibition. The proteasome inhibitor Velcade® (bortezomib or PS-341) has been shown to increase radiation-induced apoptosis and augment radiosensitivity in colorectal cancer cells in vitro and in vivo [117] through NF-κB inhibition. The variability in bortezomib-inducedradiosensitization of cervical cancer cell lines has been attributed to differential NF-κB signaling patterns among cell lines [143]. However, downstream biological effects of NF-κB suppression were not investigated in either study [117, 143].
4.1.3.3 Inhibition of upstream mediators PI3K/Akt/PKB
As discussed earlier, IKK activation acts as a converging point for NF-κB activation. However, NF-κB activation signals can be received from growth factor receptors and other pro-survival proteins, upstream of IKK. Although a number of factors can activate IKK, the PI3K/Akt/PKB pathway is probably the most relevant target with respect to tumor radiosensitization due to many reasons [144]. The PI3K/Akt pathway is constitutively activated in various cancers [145, 146], plays a critical role in promoting cell growth, and mediates TNFα-mediated NF-κB activation [144, 147]. Also, the PI3K/Akt pathway forms an important link in NF-κB mediated expression of COX-2 [148] and radiation-induced NF-κB-mediated expression of MMP-9 [149]. Additionally, inhibition of the PI3K/Akt pathway could attenuate NF-κB-regulated inflammatory gene expression [150]. Together, these reports suggest that the Akt/PKB plays a crucial role in NF-κB activation through various stimuli including radiation. Interestingly, although the importance of Akt/PKB in radioresistance is well known [151] and a number of reports have shown that specific inhibition of Akt/PKB can lead to potent radiosensitization of different types of tumors [152–155], these studies did not investigate the role of NF-κB in the observed radiosensitization. Nevertheless, in colorectal cancers, curcumin-mediated radiosensitization through NF-κB suppression was mediated via Akt inhibition [114].
4.2 STATs
The Jak–STAT signaling pathway is an evolutionarily conserved pathway essential for cytokine receptor signaling [156, 157] which plays an essential role in regulating the immune response. The STAT proteins are a family of latent cytoplasmic transcription factors involved in cytokine, hormone and growth factor signal transduction. The STAT proteins are activated by tyrosine phosphorylation and this modification serves as a molecular switch which alters their conformation to allow specific binding to DNA and alter gene expression. Tyrosine phosphorylation can be induced by a variety of factors like Jak (Janus kinase) tyrosine kinases or cytokine receptors [158], G-protein-coupled receptors or growth factor receptors (such as EGFR or platelet-derived growth factor receptor; PDGF) [159–163]. Biologic effects of STATs include promotion of cell survival through increased expression of anti-apoptotic proteins such as Bcl-2 and Bcl-XL. Persistent activation of STAT3 mediates tumor-promoting inflammation and promotes pro-oncogenic inflammatory pathways, including NF-κB and IL-6–GP130–Jak pathways [164]. Given its central role in inflammation and cancer, it is not surprising that STAT-mediated signaling (especially STAT3) exhibits multiple levels of cross-talk with the NF-κB pathway [164, 165]. Activated STAT3 can upregulate many pro-inflammatory genes such as COX-2 [166], IL-1β [167], IL-6 [168], and IL-8 [164, 169] and thus represents a promising therapeutic target for preventing inflammation-mediated cancers.
The role of STAT3 in radioresistance has been recently established. Stable transfection with shRNA against STAT3 results in enhanced radiosensitivity of human squamous cell carcinoma (A431) cells [170]. Studies investigating the altered proteomic profiles of radioresistant prostate cancer cells [171] confirm that the enhanced radioresistant phenotype of the tumor cells (enhanced cell survival, proliferation, invasion and motility) is accompanied by multiple mechanisms, with radiation-induced activation of the Jak-STAT pathway playing a significant role. In breast cancer cell lines, STAT3-mediated radiosensitization is mediated through downregulation of survivin [172]. However, other members of the STAT family also play a role in tumor radioresistance. For instance, downregulation of STAT1 sensitizes renal cell carcinoma to radiation [173]. Although pharmacological inhibition of STAT for radiosensitization is not as far along in development as that of NF-κB, inhibition of STATs could be one of the underlying mechanisms of radiosensitization by plant phytochemicals [125].
4.3 COX-2
Cyclooxygenase (COX) is the key enzyme required for the conversion of arachidonic acid to prostaglandins [174]. It exhibits two isoforms (COX-1 and COX-2), the latter being inducible (including by NF-κB [175]) and playing an important role in cancer-related inflammation [176, 177]. Overexpression of COX-2 has been shown in patients with various types of cancers [178]. Consequently, COX-2 has emerged as an important therapeutic target for anticancer and anti-inflammatory therapies. With the development of new COX-2 selective inhibitors (coxibs) it has been possible to target COX-2 for anticancer therapeutic approaches. Radiation is known to induce inflammation and NF-κB, which activates COX-2. Selective COX-2 inhibitors have been tested for their therapeutic efficacy when combined with radiation or chemotherapy [179–181]. Clinical translation of some of these investigations was thwarted by the identification of serious cardiac side effects of these drugs when used as chemopreventive agents. The COX-2 inhibitor SC-236 significantly enhances the radiosensitivity of sarcoma cells in vitro by arresting cells in the radiosensitive G2/M phase of the cell cycle [182]. Another COX-2 inhibitor, celecoxib, radiosensitizes the human head-and-neck cancer cell line HN5 by inhibition of DNA repair pathways [183]. Celecoxib downregulates the expression of Ku70 and inhibits the kinase activity of DNA-PK and interestingly, inhibits NF-κB activation [183]. Celecoxib also (independently of COX-2 inhibition) radiosensitizes cells via inhibition of nuclear translocation of EGFR which, in turn, results in inhibition of DNA repair [184]. Other COX-2 inhibitors also enhance radiosensitivity in various settings [185, 186], either through cell cycle arrest or through NF-κB suppression. In summary, COX-2 inhibition is a viable radiosensitization strategy that warrants continued investigation using safer therapeutic agents.
5. Targeting pro-inflammatory response to reduce the side-effects of radiation
5.1 Pro-inflammatory cytokines
Radiation is known to induce multiple biological responses at the cell and tissue level via the early activation of cytokine cascades [187]. Elevations in cancer-related and treatment-induced circulating inflammatory cytokines may be partially responsible for the development of clusters of symptoms (e.g., pain, fatigue, distress, and disturbed sleep; the symptom equivalent of tumor burden that is often referred to as ‘symptom burden’) during RT. For example, a positive correlation was found between IL-6 serum levels and severity of mucositis and dysphagia in head and neck cancer patients receiving combined chemoradiation therapy [13]. Also, pro-inflammatory cytokines (IL-6, -10 and TNFR1) are over-expressed in non-small cell lung cancer patients undergoing chemoradiation therapy [188].
In addition to mediating symptom burden acutely during a course of RT, many of the late sequelae of radiation are also attributable to deranged inflammatory cytokine signaling. Whereas the early local or loco-regional side effects of radiation occur within a few weeks (skin erythema, dry or moist desquamation of the skin, mucositis), late side effects occur after long latent periods of months or years (radiation-induced fibrosis, atrophy, vascular damage). Radiation-induced fibrosis, the most extensively studied amongst these, is characterized by reduced tissue flexibility, reduced compliance and/or strictures [189]. The early phases of fibrogenesis after irradiation can be seen as a wound-healing response characterized by an almost immediate upregulation of pro-inflammatory cytokines such as TNF-α, IL-1 and IL-6 and many growth factors in the irradiated tissue. Chemokines are released, which recruit inflammatory cells from the surrounding tissue into the irradiated area. In the entire process, TGF-β acts as a key fibrogenic cytokine.
The 25 kDa multifunctional cytokine TGF-β can be classified both as a cytokine and as a growth factor. The TGF-β family regulates a range of processes, including embryonic development, homeostasis, cell cycle control and wound healing [190] and its dysregulation has been associated with carcinogenesis. TGF-β is secreted in a latent form and is unable to bind to the receptor unless it is activated in the extracellular space by dissociation of the active mature TGF-β from the latency associated peptide (LAP) [191]. Following an appropriate trigger, a large pool of latent TGF-β can be rapidly mobilized. IR can induce TGF-β activation within minutes at doses as low as 0.1 Gy [192, 193]. Once activated, TGF-β binds to distinct receptor complexes to phosphorylate distinct intracellular effectors, R-Smads, which then form heteromeric complexes with Smad4 and these complexes translocate to the nucleus. Specific R-Smads recognize different DNA binding proteins, regulate distinct target genes, and thereby generate diverse biological responses [194]. Owing to its role in radiation-induced fibrosis, suppression of TGF-β can be used as a therapeutic strategy to protect normal tissues from radiation injury. For instance, a free-radical scavenging agent ambroxol ameliorates radiation-induced lung injury in patients by suppressing the radiation-induced TGF-β and TNF-α activation [15]. The plant-derived alkaloid, halofuginone, radiosensitizes tumor cells by inhibiting cell growth, arresting cell cycle progression, decreasing radiation-induced DNA damage repair, and decreasing TGF-β receptor II protein levels [195]. Although these strategies differ from the others discussed in this review, they represent a means to reduce the side effects of RT, thereby widening the therapeutic window. Newer agents capable of inhibiting the radiation-induced pro-inflammatory response in specific normal tissues are likely to further enhance our ability to deliver escalated doses of radiation to tumors while protecting surrounding normal tissues from harmful side effects, both acutely and chronically.
5.2 Chemokines
Small, pro-inflammatory peptides, the chemokines, are often expressed in response to the induction of NF-κB by cytokines or other stimuli [196], orchestrate immunologic and inflammatory processes such as leukocyte trafficking, adhesion, hematopoiesis, angiogenesis, and facilitate tumor growth and metastases. Many cancers have a complex chemokine network that influences immune-cell infiltration of tumor and tumor growth and survival. Although chemokine expression profiles on cancer cells vary across cancer types, the most commonly expressed receptor on most cancer cells is CXCR4 (CXC chemokine ligand receptor 4) [197, 198]. The ligand of CXCR4, CXCL12 (stromal cell-derived factor; SDF-1), is expressed at the sites of tumor metastases and is involved in homing of the tumors to different organs [199]. Up-regulation of CXCR4 has been associated with metastasis [200] and its suppression is associated with inhibition of CXCL12-induced invasion of tumor cells [201]. Interestingly, low doses of radiation increase the expression of chemokines (including CXCR4) in human endothelial cells [11]. Given that radiation can trigger angiogenic and/or metastatic cascades through up-regulation of NF-κB, which, in turn regulates the expression of chemokines, targeted disruption of this axis during RT may enhance the efficacy of RT.
6. Conclusions
Despite the technological advances in the field of radiation oncology, overcoming intrinsic and inducible tumor radioresistance still remains a major conceptual and therapeutic challenge. This is particularly true when the resistance mechanism is similar in tumor cells as well as in normal cells. Targeting inflammatory signaling pathways induced by radiation offers the promise of seamless integration of a means of enhancing the intrinsic sensitivity of tumors to radiation and a means of decreasing the normal tissue side-effects of radiation. Whereas uncontrolled radiation-induced inflammatory signaling in normal tissues characteristically produces the five cardinal signs of inflammation - redness, warmth, swelling, pain and loss of function (rubor, calor, tumor, dolor, and function laesa) - this can be detrimental in clinical RT. However, radiation-induced transient pro-inflammatory cascades within tumor cells may be beneficial to the cell for its survival from a sublethal dose of radiation. Therefore, blockade of this radiation-induced signaling pathway has the potential to kill two birds with one stone.
Among a diverse network of signaling cascades initiated by radiation and directed outside-in to the nucleus and inside-out from the nucleus, a common denominator are a relatively small number of transcription factors which act as linchpins that dynamically regulate the effector responses. Interestingly, NF-κB, a ‘master regulator’ of inflammatory signaling also serves as a modulator of the gene products that control tumor proliferation, invasion and angiogenesis. Thus, NF-κB suppression has the potential to enhance the effectiveness of RT by radiosensitizing tumor tissue while radioprotecting normal tissues. Apart from NF-κB, other mediators of pro-inflammatory responses such as STATs and COX-2 can also be targeted to achieve similar effects. A wide range of new drugs are emerging that are capable of inhibiting these inflammatory network and are increasingly being tested as radiosensitizing strategies in various clinical and pre-clinical settings.
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello CA. Anti-inflammatory Agents: Present and Future. Cell. 2010;140:935–50. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther. 2010;87:401–6. doi: 10.1038/clpt.2009.312. [DOI] [PubMed] [Google Scholar]
- 6.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 8.Mocellin S, Lise M, Nitti D. Tumor immunology. Adv Exp Med Biol. 2007;593:147–56. doi: 10.1007/978-0-387-39978-2_14. [DOI] [PubMed] [Google Scholar]
- 9.Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2:227–38. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 10.Begley J, Ribas A. Targeted therapies to improve tumor immunotherapy. Clin Cancer Res. 2008;14:4385–91. doi: 10.1158/1078-0432.CCR-07-4804. [DOI] [PubMed] [Google Scholar]
- 11.Chang CC, Lerman OZ, Thanik VD, Scharf CL, Greives MR, Schneider RJ, et al. Dose-dependent effect of radiation on angiogenic and angiostatic CXC chemokine expression in human endothelial cells. Cytokine. 2009;48:295–302. doi: 10.1016/j.cyto.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Chendil D, Ranga RS, Meigooni D, Sathishkumar S, Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene. 2004;23:1599–607. doi: 10.1038/sj.onc.1207284. [DOI] [PubMed] [Google Scholar]
- 13.Meirovitz A, Kuten M, Billan S, Abdah-Bortnyak R, Sharon A, Peretz T, et al. Cytokines levels, severity of acute mucositis and the need of PEG tube installation during chemo-radiation for head and neck cancer--a prospective pilot study. Radiat Oncol. 2010;5:16. doi: 10.1186/1748-717X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kargiotis O, Chetty C, Gogineni V, Gondi CS, Pulukuri SM, Kyritsis AP, et al. uPA/uPAR downregulation inhibits radiation-induced migration, invasion and angiogenesis in IOMM-Lee meningioma cells and decreases tumor growth in vivo. Int J Oncol. 2008;33:937–47. [PMC free article] [PubMed] [Google Scholar]
- 15.Xia DH, Xi L, Xv C, Mao WD, Shen WS, Shu ZQ, et al. The protective effects of ambroxol on radiation lung injury and influence on production of transforming growth factor β(1) and tumor necrosis factor α. Med Oncol. 2009:9271–73. doi: 10.1007/s12032-009-9271-3. [DOI] [PubMed] [Google Scholar]
- 16.Eichholtz-Wirth H, Sagan D. Altered signaling of TNFα-TNFR1 and SODD/BAG4 is responsible for radioresistance in human HT-R15 cells. Anticancer Res. 2002;22:235–40. [PubMed] [Google Scholar]
- 17.Legue F, Guitton N, Brouazin-Jousseaume V, Colleu-Durel S, Nourgalieva K, Chenal C. IL-6 a key cytokine in in vitro and in vivo response of Sertoli cells to external gamma irradiation. Cytokine. 2001;16:232–8. doi: 10.1006/cyto.2001.0970. [DOI] [PubMed] [Google Scholar]
- 18.Ma Z, Webb DJ, Jo M, Gonias SL. Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci. 2001;114:3387–96. doi: 10.1242/jcs.114.18.3387. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto Y, Hosotani R, Doi R, Wada M, Ida J, Tsuji S, et al. Interleukin-6 inhibits radiation induced apoptosis in pancreatic cancer cells. Anticancer Res. 2001;21:2449–56. [PubMed] [Google Scholar]
- 20.Zhou D, Yu T, Chen G, Brown SA, Yu Z, Mattson MP, et al. Effects of NF-kappaB1 (p50) targeted gene disruption on ionizing radiation-induced NF-κB activation and TNFα, IL-1α, IL-1β and IL-6 mRNA expression in vivo. Int J Radiat Biol. 2001;77:763–72. doi: 10.1080/09553000110050047. [DOI] [PubMed] [Google Scholar]
- 21.Chinnaiyan P, Allen GW, Harari PM. Radiation and new molecular agents, part II: targeting HDAC, HSP90, IGF-1R, PI3K, and Ras. Semin Radiat Oncol. 2006;16:59–64. doi: 10.1016/j.semradonc.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Choudhury A, Cuddihy A, Bristow RG. Radiation and new molecular agents part I: targeting ATM-ATR checkpoints, DNA repair, and the proteasome. Semin Radiat Oncol. 2006;16:51–8. doi: 10.1016/j.semradonc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, et al. Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther. 2007;6:789–801. doi: 10.1158/1535-7163.MCT-06-0596. [DOI] [PubMed] [Google Scholar]
- 24.Ringborg U, Bergqvist D, Brorsson B, Cavallin-Stahl E, Ceberg J, Einhorn N, et al. The Swedish Council on Technology Assessment in Health Care (SBU) systematic overview of radiotherapy for cancer including a prospective survey of radiotherapy practice in Sweden 2001-summary and conclusions. Acta Oncol. 2003;42:357–65. doi: 10.1080/02841860310010826. [DOI] [PubMed] [Google Scholar]
- 25.Elshaikh M, Ljungman M, Ten Haken R, Lichter AS. Advances in radiation oncology. Annu Rev Med. 2006;57:19–31. doi: 10.1146/annurev.med.57.121304.131431. [DOI] [PubMed] [Google Scholar]
- 26.James D, Cox KKA. Technique Results. Philadelphia, PA: Mosby Elsevier; 2010. Radiation Oncology: Rationale. [Google Scholar]
- 27.Søren M, Bentzen PMH, Tomé Wolfgang, Mehta Minesh P, editors. Radiation Oncology Advances. Chicago, IL: Springer; 2008. [Google Scholar]
- 28.Oehler C, Dickinson DJ, Broggini-Tenzer A, Hofstetter B, Hollenstein A, Riesterer O, et al. Current concepts for the combined treatment modality of ionizing radiation with anticancer agents. Curr Pharm Des. 2007;13:519–35. doi: 10.2174/138161207780162935. [DOI] [PubMed] [Google Scholar]
- 29.Bentzen SM, Harari PM, Bernier J. Exploitable mechanisms for combining drugs with radiation: concepts, achievements and future directions. Nat Clin Pract Oncol. 2007;4:172–80. doi: 10.1038/ncponc0744. [DOI] [PubMed] [Google Scholar]
- 30.Jaffe N, Paed D, Traggis D, Salian S, Cassady JR. Improved outlook for Ewing’s sarcoma with combination chemotherapy (vincristine, actinomycin D and cyclophosphamide) and radiation therapy. Cancer. 1976;38:1925–30. doi: 10.1002/1097-0142(197611)38:5<1925::aid-cncr2820380510>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 31.Bernier J. Alteration of radiotherapy fractionation and concurrent chemotherapy: a new frontier in head and neck oncology? Nat Clin Pract Oncol. 2005;2:305–14. doi: 10.1038/ncponc0201. [DOI] [PubMed] [Google Scholar]
- 32.Ma BB, Bristow RG, Kim J, Siu LL. Combined-modality treatment of solid tumors using radiotherapy and molecular targeted agents. J Clin Oncol. 2003;21:2760–76. doi: 10.1200/JCO.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 33.Szumiel I. Intrinsic radiation sensitivity: cellular signaling is the key. Radiat Res. 2008;169:249–58. doi: 10.1667/RR1239.1. [DOI] [PubMed] [Google Scholar]
- 34.Goldkorn T, Balaban N, Shannon M, Matsukuma K. EGF receptor phosphorylation is affected by ionizing radiation. Biochim Biophys Acta. 1997;1358:289–99. doi: 10.1016/s0167-4889(97)00063-3. [DOI] [PubMed] [Google Scholar]
- 35.Su TT. Cellular responses to DNA damage: one signal, multiple choices. Annu Rev Genet. 2006;40:187–208. doi: 10.1146/annurev.genet.40.110405.090428. [DOI] [PubMed] [Google Scholar]
- 36.Lord CJ, Garrett MD, Ashworth A. Targeting the double-strand DNA break repair pathway as a therapeutic strategy. Clin Cancer Res. 2006;12:4463–8. doi: 10.1158/1078-0432.CCR-06-1269. [DOI] [PubMed] [Google Scholar]
- 37.Bakkenist CJ, Kastan MB. Phosphatases join kinases in DNA-damage response pathways. Trends Cell Biol. 2004;14:339–41. doi: 10.1016/j.tcb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–11. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 39.Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, et al. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–4. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 41.Andreassen PR, Ho GP, D’Andrea AD. DNA damage responses and their many interactions with the replication fork. Carcinogenesis. 2006;27:883–92. doi: 10.1093/carcin/bgi319. [DOI] [PubMed] [Google Scholar]
- 42.Gatei M, Sloper K, Sorensen C, Syljuasen R, Falck J, Hobson K, et al. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J Biol Chem. 2003;278:14806–11. doi: 10.1074/jbc.M210862200. [DOI] [PubMed] [Google Scholar]
- 43.Khanna KK, Lavin MF. Ionizing radiation and UV induction of p53 protein by different pathways in ataxia-telangiectasia cells. Oncogene. 1993;8:3307–12. [PubMed] [Google Scholar]
- 44.Canman CE, Lim DS, Cimprich KA, Taya Y, Tamai K, Sakaguchi K, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science. 1998;281:1677–9. doi: 10.1126/science.281.5383.1677. [DOI] [PubMed] [Google Scholar]
- 45.Zhou BB, Chaturvedi P, Spring K, Scott SP, Johanson RA, Mishra R, et al. Caffeine abolishes the mammalian G(2)/M DNA damage checkpoint by inhibiting ataxia-telangiectasia-mutated kinase activity. J Biol Chem. 2000;275:10342–8. doi: 10.1074/jbc.275.14.10342. [DOI] [PubMed] [Google Scholar]
- 46.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–7. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 47.Zhou BB, Bartek J. Targeting the checkpoint kinases: chemosensitization versus chemoprotection. Nat Rev Cancer. 2004;4:216–25. doi: 10.1038/nrc1296. [DOI] [PubMed] [Google Scholar]
- 48.van Gent DC, van der Burg M. Non-homologous end-joining, a sticky affair. Oncogene. 2007;26:7731–40. doi: 10.1038/sj.onc.1210871. [DOI] [PubMed] [Google Scholar]
- 49.Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417:639–50. doi: 10.1042/BJ20080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Heyer WD. Homologous recombination in DNA repair and DNA damage tolerance. Cell Res. 2008;18:99–113. doi: 10.1038/cr.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burma S, Chen BP, Chen DJ. Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair (Amst) 2006;5:1042–8. doi: 10.1016/j.dnarep.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 52.Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol. 2003;4:712–20. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 53.O’Driscoll M, Jeggo PA. The role of double-strand break repair - insights from human genetics. Nat Rev Genet. 2006;7:45–54. doi: 10.1038/nrg1746. [DOI] [PubMed] [Google Scholar]
- 54.Pellegrini L, Yu DS, Lo T, Anand S, Lee M, Blundell TL, et al. Insights into DNA recombination from the structure of a RAD51-BRCA2 complex. Nature. 2002;420:287–93. doi: 10.1038/nature01230. [DOI] [PubMed] [Google Scholar]
- 55.Schaue D, McBride WH. Counteracting tumor radioresistance by targeting DNA repair. Mol Cancer Ther. 2005;4:1548–50. doi: 10.1158/1535-7163.MCT-05-CO1. [DOI] [PubMed] [Google Scholar]
- 56.Sakata K, Someya M, Matsumoto Y, Hareyama M. Ability to repair DNA double-strand breaks related to cancer susceptibility and radiosensitivity. Radiat Med. 2007;25:433–8. doi: 10.1007/s11604-007-0161-3. [DOI] [PubMed] [Google Scholar]
- 57.Tofilon PJ, Camphausen K. Molecular targets for tumor radiosensitization. Chem Rev. 2009;109:2974–88. doi: 10.1021/cr800504x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiarugi P, Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28:509–14. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 59.Galabova-Kovacs G, Kolbus A, Matzen D, Meissl K, Piazzolla D, Rubiolo C, et al. ERK and beyond: insights from B-Raf and Raf-1 conditional knockouts. Cell Cycle. 2006;5:1514–8. doi: 10.4161/cc.5.14.2981. [DOI] [PubMed] [Google Scholar]
- 60.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 61.Schlessinger J, Lemmon MA. Nuclear signaling by receptor tyrosine kinases: the first robin of spring. Cell. 2006;127:45–8. doi: 10.1016/j.cell.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 62.Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, et al. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003;159:283–300. doi: 10.1667/0033-7587(2003)159[0283:sariao]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 63.Nyati MK, Morgan MA, Feng FY, Lawrence TS. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006;6:876–85. doi: 10.1038/nrc1953. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, et al. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–7. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 65.Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–7. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- 66.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 67.Yu H, Jove R. The STATs of cancer-new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 68.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–14. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 70.Criswell T, Leskov K, Miyamoto S, Luo G, Boothman DA. Transcription factors activated in mammalian cells after clinically relevant doses of ionizing radiation. Oncogene. 2003;22:5813–27. doi: 10.1038/sj.onc.1206680. [DOI] [PubMed] [Google Scholar]
- 71.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–8. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Brach MA, Gruss HJ, Kaisho T, Asano Y, Hirano T, Herrmann F. Ionizing radiation induces expression of interleukin 6 by human fibroblasts involving activation of nuclear factor-κB. J Biol Chem. 1993;268:8466–72. [PubMed] [Google Scholar]
- 73.Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991;88:691–5. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amorino GP, Hamilton VM, Valerie K, Dent P, Lammering G, Schmidt-Ullrich RK. Epidermal growth factor receptor dependence of radiation-induced transcription factor activation in human breast carcinoma cells. Mol Biol Cell. 2002;13:2233–44. doi: 10.1091/mbc.01-12-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hallahan DE, Virudachalam S, Beckett M, Sherman ML, Kufe D, Weichselbaum RR. Mechanisms of X-ray-mediated protooncogene c-jun expression in radiation-induced human sarcoma cell lines. Int J Radiat Oncol Biol Phys. 1991;21:1677–81. doi: 10.1016/0360-3016(91)90352-5. [DOI] [PubMed] [Google Scholar]
- 76.Sherman ML, Datta R, Hallahan DE, Weichselbaum RR, Kufe DW. Ionizing radiation regulates expression of the c-jun protooncogene. Proc Natl Acad Sci U S A. 1990;87:5663–6. doi: 10.1073/pnas.87.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woloschak GE, Chang-Liu CM. Differential modulation of specific gene expression following high- and low-LET radiations. Radiat Res. 1990;124:183–7. [PubMed] [Google Scholar]
- 78.Pacifico F, Leonardi A. NF-κB in solid tumors. Biochem Pharmacol. 2006;72:1142–52. doi: 10.1016/j.bcp.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 79.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, et al. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–69. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 80.Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952–60. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-κB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–27. [PubMed] [Google Scholar]
- 82.Kojima M, Morisaki T, Sasaki N, Nakano K, Mibu R, Tanaka M, et al. Increased nuclear factor-κB activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res. 2004;24:675–81. [PubMed] [Google Scholar]
- 83.Sartor CI, Dziubinski ML, Yu CL, Jove R, Ethier SP. Role of epidermal growth factor receptor and STAT-3 activation in autonomous proliferation of SUM-102PT human breast cancer cells. Cancer Res. 1997;57:978–87. [PubMed] [Google Scholar]
- 84.Lin Q, Lai R, Chirieac LR, Li C, Thomazy VA, Grammatikakis I, et al. Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines: inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am J Pathol. 2005;167:969–80. doi: 10.1016/S0002-9440(10)61187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.To KF, Chan MW, Leung WK, Ng EK, Yu J, Bai AH, et al. Constitutional activation of IL-6-mediated JAK/STAT pathway through hypermethylation of SOCS-1 in human gastric cancer cell line. Br J Cancer. 2004;91:1335–41. doi: 10.1038/sj.bjc.6602133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang M, Page C, Reynolds RK, Lin J. Constitutive activation of stat 3 oncogene product in human ovarian carcinoma cells. Gynecol Oncol. 2000;79:67–73. doi: 10.1006/gyno.2000.5931. [DOI] [PubMed] [Google Scholar]
- 87.Greten FR, Weber CK, Greten TF, Schneider G, Wagner M, Adler G, et al. Stat3 and NF-κB activation prevents apoptosis in pancreatic carcinogenesis. Gastroenterology. 2002;123:2052–63. doi: 10.1053/gast.2002.37075. [DOI] [PubMed] [Google Scholar]
- 88.Ahn KS, Sethi G, Aggarwal BB. Nuclear factor-κB: from clone to clinic. Curr Mol Med. 2007;7:619–37. doi: 10.2174/156652407782564363. [DOI] [PubMed] [Google Scholar]
- 89.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–30. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 90.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–8. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang S, Robinson JB, Deguzman A, Bucana CD, Fidler IJ. Blockade of nuclear factor-κB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 2000;60:5334–9. [PubMed] [Google Scholar]
- 92.Yokoo T, Kitamura M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-κB and AP-1. Am J Physiol. 1996;270:F123–30. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- 93.Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor α transcription in macrophages: involvement of four κB-like motifs and of constitutive and inducible forms of NF-κB. Mol Cell Biol. 1990;10:1498–506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Betts JC, Cheshire JK, Akira S, Kishimoto T, Woo P. The role of NF-kappa B and NF-IL6 transactivating factors in the synergistic activation of human serum amyloid A gene expression by interleukin-1 and interleukin-6. J Biol Chem. 1993;268:25624–31. [PubMed] [Google Scholar]
- 95.Harant H, de Martin R, Andrew PJ, Foglar E, Dittrich C, Lindley IJ. Synergistic activation of interleukin-8 gene transcription by all-trans-retinoic acid and tumor necrosis factor-α involves the transcription factor NF-κB. J Biol Chem. 1996;271:26954–61. doi: 10.1074/jbc.271.43.26954. [DOI] [PubMed] [Google Scholar]
- 96.Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601–8. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 97.Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–5. [PubMed] [Google Scholar]
- 98.Zushi S, Shinomura Y, Kiyohara T, Miyazaki Y, Kondo S, Sugimachi M, et al. STAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cells. Int J Cancer. 1998;78:326–30. doi: 10.1002/(SICI)1097-0215(19981029)78:3<326::AID-IJC12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 99.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–8. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 100.Tamatani T, Azuma M, Ashida Y, Motegi K, Takashima R, Harada K, et al. Enhanced radiosensitization and chemosensitization in NF-κB-suppressed human oral cancer cells via the inhibition of gamma-irradiation- and 5-FU-induced production of IL-6 and IL-8. Int J Cancer. 2004;108:912–21. doi: 10.1002/ijc.11640. [DOI] [PubMed] [Google Scholar]
- 101.Ghosh S, Hayden MS. New regulators of NF-κB in inflammation. Nat Rev Immunol. 2008;8:837–48. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 102.Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 103.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109 (Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 104.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 105.Wu ZH, Miyamoto S. Many faces of NF-κB signaling induced by genotoxic stress. J Mol Med. 2007;85:1187–202. doi: 10.1007/s00109-007-0227-9. [DOI] [PubMed] [Google Scholar]
- 106.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–54. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 107.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, et al. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–6. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 108.Lin Y, Bai L, Chen W, Xu S. The NF-κB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14:45–55. doi: 10.1517/14728220903431069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karin M, Cao Y, Greten FR, Li ZW. NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–10. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 110.Magne N, Toillon RA, Bottero V, Didelot C, Houtte PV, Gerard JP, et al. NF-κB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett. 2006;231:158–68. doi: 10.1016/j.canlet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 111.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 112.Karin M. The IκB kinase - a bridge between inflammation and cancer. Cell Res. 2008;18:334–42. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 113.Yu LL, Yu HG, Yu JP, Luo HS, Xu XM, Li JH. Nuclear factor-κB p65 (RelA) transcription factor is constitutively activated in human colorectal carcinoma tissue. World J Gastroenterol. 2004;10:3255–60. doi: 10.3748/wjg.v10.i22.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sandur SK, Deorukhkar A, Pandey MK, Pabon AM, Shentu S, Guha S, et al. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-κB activity. Int J Radiat Oncol Biol Phys. 2009;75:534–42. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–7. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 116.Voboril R, Weberova-Voborilova J. Constitutive NF-κB activity in colorectal cancer cells: impact on radiation-induced NF-κB activity, radiosensitivity, and apoptosis. Neoplasma. 2006;53:518–23. [PubMed] [Google Scholar]
- 117.Russo SM, Tepper JE, Baldwin AS, Jr, Liu R, Adams J, Elliott P, et al. Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-κB. Int J Radiat Oncol Biol Phys. 2001;50:183–93. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 118.Ding GR, Honda N, Nakahara T, Tian F, Yoshida M, Hirose H, et al. Radiosensitization by inhibition of IκBα phosphorylation in human glioma cells. Radiat Res. 2003;160:232–7. doi: 10.1667/rr3018. [DOI] [PubMed] [Google Scholar]
- 119.Pajonk F, Pajonk K, McBride WH. Apoptosis and radiosensitization of hodgkin cells by proteasome inhibition. Int J Radiat Oncol Biol Phys. 2000;47:1025–32. doi: 10.1016/s0360-3016(00)00516-2. [DOI] [PubMed] [Google Scholar]
- 120.Raju U, Gumin GJ, Tofilon PJ. NF kappa B activity and target gene expression in the rat brain after one and two exposures to ionizing radiation. Radiat Oncol Investig. 1999;7:145–52. doi: 10.1002/(SICI)1520-6823(1999)7:3<145::AID-ROI2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 121.Raju U, Lu R, Noel F, Gumin GJ, Tofilon PJ. Failure of a second X-ray dose to activate nuclear factor κB in normal rat astrocytes. J Biol Chem. 1997;272:24624–30. doi: 10.1074/jbc.272.39.24624. [DOI] [PubMed] [Google Scholar]
- 122.Yang CR, Wilson-Van Patten C, Planchon SM, Wuerzberger-Davis SM, Davis TW, Cuthill S, et al. Coordinate modulation of Sp1, NF-κB, and p53 in confluent human malignant melanoma cells after ionizing radiation. FASEB J. 2000;14:379–90. doi: 10.1096/fasebj.14.2.379. [DOI] [PubMed] [Google Scholar]
- 123.Didelot C, Barberi-Heyob M, Bianchi A, Becuwe P, Mirjolet JF, Dauca M, et al. Constitutive NF-κB activity influences basal apoptosis and radiosensitivity of head-and-neck carcinoma cell lines. Int J Radiat Oncol Biol Phys. 2001;51:1354–60. doi: 10.1016/s0360-3016(01)02608-6. [DOI] [PubMed] [Google Scholar]
- 124.Yamagishi N, Miyakoshi J, Takebe H. Enhanced radiosensitivity by inhibition of nuclear factor κB activation in human malignant glioma cells. Int J Radiat Biol. 1997;72:157–62. doi: 10.1080/095530097143374. [DOI] [PubMed] [Google Scholar]
- 125.Deorukhkar A, Krishnan S, Sethi G, Aggarwal BB. Back to basics: how natural products can provide the basis for new therapeutics. Expert Opin Investig Drugs. 2007;16:1753–73. doi: 10.1517/13543784.16.11.1753. [DOI] [PubMed] [Google Scholar]
- 126.Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–47. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 127.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to γ-radiation by targeting nuclear factor-κB-regulated gene products. Clin Cancer Res. 2008;14:2128–36. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 128.Aravindan N, Madhusoodhanan R, Ahmad S, Johnson D, Herman TS. Curcumin inhibits NFκB mediated radioprotection and modulate apoptosis related genes in human neuroblastoma cells. Cancer Biol Ther. 2008;7:569–76. doi: 10.4161/cbt.7.4.5534. [DOI] [PubMed] [Google Scholar]
- 129.Raffoul JJ, Wang Y, Kucuk O, Forman JD, Sarkar FH, Hillman GG. Genistein inhibits radiation-induced activation of NF-κB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer. 2006;6:107. doi: 10.1186/1471-2407-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mendonca MS, Chin-Sinex H, Gomez-Millan J, Datzman N, Hardacre M, Comerford K, et al. Parthenolide sensitizes cells to X-ray-induced cell killing through inhibition of NF-κB and split-dose repair. Radiat Res. 2007;168:689–97. doi: 10.1667/RR1128.1. [DOI] [PubMed] [Google Scholar]
- 131.Sun Y, St Clair DK, Fang F, Warren GW, Rangnekar VM, Crooks PA, et al. The radiosensitization effect of parthenolide in prostate cancer cells is mediated by nuclear factor-κB inhibition and enhanced by the presence of PTEN. Mol Cancer Ther. 2007;6:2477–86. doi: 10.1158/1535-7163.MCT-07-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tamatani T, Azuma M, Motegi K, Takamaru N, Kawashima Y, Bando T. Cepharanthin-enhanced radiosensitivity through the inhibition of radiation-induced nuclear factor-κB activity in human oral squamous cell carcinoma cells. Int J Oncol. 2007;31:761–8. [PubMed] [Google Scholar]
- 133.Zand H, Rahimipour A, Salimi S, Shafiee SM. Docosahexaenoic acid sensitizes Ramos cells to Gamma-irradiation-induced apoptosis through involvement of PPAR-γ activation and NF-κB suppression. Mol Cell Biochem. 2008;317:113–20. doi: 10.1007/s11010-008-9838-x. [DOI] [PubMed] [Google Scholar]
- 134.Tsuboi Y, Kurimoto M, Nagai S, Hayakawa Y, Kamiyama H, Hayashi N, et al. Induction of autophagic cell death and radiosensitization by the pharmacological inhibition of nuclear factor-κ B activation in human glioma cell lines. J Neurosurg. 2009;110:594–604. doi: 10.3171/2008.8.JNS17648. [DOI] [PubMed] [Google Scholar]
- 135.Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov. 2006;5:596–613. doi: 10.1038/nrd2056. [DOI] [PubMed] [Google Scholar]
- 136.Eldridge AG, O’Brien T. Therapeutic strategies within the ubiquitin proteasome system. Cell Death Differ. 2010;17:4–13. doi: 10.1038/cdd.2009.82. [DOI] [PubMed] [Google Scholar]
- 137.Cusack JC, Jr, Liu R, Houston M, Abendroth K, Elliott PJ, Adams J, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res. 2001;61:3535–40. [PubMed] [Google Scholar]
- 138.Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J Surg Res. 2001;100:11–7. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- 139.Shah SA, Potter MW, McDade TP, Ricciardi R, Perugini RA, Elliott PJ, et al. 26S proteasome inhibition induces apoptosis and limits growth of human pancreatic cancer. J Cell Biochem. 2001;82:110–22. doi: 10.1002/jcb.1150. [DOI] [PubMed] [Google Scholar]
- 140.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, et al. Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–97. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 141.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–62. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 142.Hayashi T, Faustman D. Essential role of human leukocyte antigen-encoded proteasome subunits in NF-κB activation and prevention of tumor necrosis factor-α-induced apoptosis. J Biol Chem. 2000;275:5238–47. doi: 10.1074/jbc.275.7.5238. [DOI] [PubMed] [Google Scholar]
- 143.Kamer S, Ren Q, Dicker AP. Differential radiation sensitization of human cervical cancer cell lines by the proteasome inhibitor velcade (bortezomib, PS-341) Arch Gynecol Obstet. 2009;279:41–6. doi: 10.1007/s00404-008-0667-7. [DOI] [PubMed] [Google Scholar]
- 144.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 145.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 146.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 147.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 148.Yang CM, Lee IT, Lin CC, Yang YL, Luo SF, Kou YR, et al. Cigarette smoke extract induces COX-2 expression via a PKCalpha/c-Src/EGFR, PDGFR/PI3K/Akt/NF-κB pathway and p300 in tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L892–902. doi: 10.1152/ajplung.00151.2009. [DOI] [PubMed] [Google Scholar]
- 149.Cheng JC, Chou CH, Kuo ML, Hsieh CY. Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-κB signal transduction pathway. Oncogene. 2006;25:7009–18. doi: 10.1038/sj.onc.1209706. [DOI] [PubMed] [Google Scholar]
- 150.Kim JH, Lee G, Cho YL, Kim CK, Han S, Lee H, et al. Desmethylanhydroicaritin inhibits NF-κB-regulated inflammatory gene expression by modulating the redox-sensitive PI3K/PTEN/Akt pathway. Eur J Pharmacol. 2009;602:422–31. doi: 10.1016/j.ejphar.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 151.Bussink J, van der Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9:288–96. doi: 10.1016/S1470-2045(08)70073-1. [DOI] [PubMed] [Google Scholar]
- 152.Nakamura JL, Karlsson A, Arvold ND, Gottschalk AR, Pieper RO, Stokoe D, et al. PKB/Akt mediates radiosensitization by the signaling inhibitor LY294002 in human malignant gliomas. J Neurooncol. 2005;71:215–22. doi: 10.1007/s11060-004-1718-y. [DOI] [PubMed] [Google Scholar]
- 153.Lee CM, Fuhrman CB, Planelles V, Peltier MR, Gaffney DK, Soisson AP, et al. Phosphatidylinositol 3-kinase inhibition by LY294002 radiosensitizes human cervical cancer cell lines. Clin Cancer Res. 2006;12:250–6. doi: 10.1158/1078-0432.CCR-05-1084. [DOI] [PubMed] [Google Scholar]
- 154.Albert JM, Kim KW, Cao C, Lu B. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol Cancer Ther. 2006;5:1183–9. doi: 10.1158/1535-7163.MCT-05-0400. [DOI] [PubMed] [Google Scholar]
- 155.Kao GD, Jiang Z, Fernandes AM, Gupta AK, Maity A. Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. J Biol Chem. 2007;282:21206–12. doi: 10.1074/jbc.M703042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reich NC, Liu L. Tracking STAT nuclear traffic. Nat Rev Immunol. 2006;6:602–12. doi: 10.1038/nri1885. [DOI] [PubMed] [Google Scholar]
- 157.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–9. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 158.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334 ( Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem. 2007;102:311–9. doi: 10.1002/jcb.21475. [DOI] [PubMed] [Google Scholar]
- 160.David M, Wong L, Flavell R, Thompson SA, Wells A, Larner AC, et al. STAT activation by epidermal growth factor (EGF) and amphiregulin. Requirement for the EGF receptor kinase but not for tyrosine phosphorylation sites or JAK1. J Biol Chem. 1996;271:9185–8. doi: 10.1074/jbc.271.16.9185. [DOI] [PubMed] [Google Scholar]
- 161.Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE. ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J Biol Chem. 1999;274:17209–18. doi: 10.1074/jbc.274.24.17209. [DOI] [PubMed] [Google Scholar]
- 162.Park OK, Schaefer TS, Nathans D. In vitro activation of Stat3 by epidermal growth factor receptor kinase. Proc Natl Acad Sci U S A. 1996;93:13704–8. doi: 10.1073/pnas.93.24.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vignais ML, Sadowski HB, Watling D, Rogers NC, Gilman M. Platelet-derived growth factor induces phosphorylation of multiple JAK family kinases and STAT proteins. Mol Cell Biol. 1996;16:1759–69. doi: 10.1128/mcb.16.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yang J, Liao X, Agarwal MK, Barnes L, Auron PE, Stark GR. Unphosphorylated STAT3 accumulates in response to IL-6 and activates transcription by binding to NFkappaB. Genes Dev. 2007;21:1396–408. doi: 10.1101/gad.1553707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lo HW, Cao X, Zhu H, Ali-Osman F. Cyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Mol Cancer Res. 2010;8:232–45. doi: 10.1158/1541-7786.MCR-09-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 168.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 169.Trevino JG, Gray MJ, Nawrocki ST, Summy JM, Lesslie DP, Evans DB, et al. Src activation of Stat3 is an independent requirement from NF-κB activation for constitutive IL-8 expression in human pancreatic adenocarcinoma cells. Angiogenesis. 2006;9:101–10. doi: 10.1007/s10456-006-9038-9. [DOI] [PubMed] [Google Scholar]
- 170.Bonner JA, Trummell HQ, Willey CD, Plants BA, Raisch KP. Inhibition of STAT-3 results in radiosensitization of human squamous cell carcinoma. Radiother Oncol. 2009;92:339–44. doi: 10.1016/j.radonc.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Skvortsova I, Skvortsov S, Stasyk T, Raju U, Popper BA, Schiestl B, et al. Intracellular signaling pathways regulating radioresistance of human prostate carcinoma cells. Proteomics. 2008;8:4521–33. doi: 10.1002/pmic.200800113. [DOI] [PubMed] [Google Scholar]
- 172.Kim KW, Mutter RW, Cao C, Albert JM, Shinohara ET, Sekhar KR, et al. Inhibition of signal transducer and activator of transcription 3 activity results in down-regulation of Survivin following irradiation. Mol Cancer Ther. 2006;5:2659–65. doi: 10.1158/1535-7163.MCT-06-0261. [DOI] [PubMed] [Google Scholar]
- 173.Hui Z, Tretiakova M, Zhang Z, Li Y, Wang X, Zhu JX, et al. Radiosensitization by inhibiting STAT1 in renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:288–95. doi: 10.1016/j.ijrobp.2008.08.043. [DOI] [PubMed] [Google Scholar]
- 174.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 175.Ahn KS, Aggarwal BB. Transcription factor NF-κB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–33. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- 176.FitzGerald GA. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov. 2003;2:879–90. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- 177.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–16. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 178.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–69. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Koki AT, Leahy KM, Masferrer JL. Potential utility of COX-2 inhibitors in chemoprevention and chemotherapy. Expert Opin Investig Drugs. 1999;8:1623–38. doi: 10.1517/13543784.8.10.1623. [DOI] [PubMed] [Google Scholar]
- 180.Milas L, Kishi K, Hunter N, Mason K, Masferrer JL, Tofilon PJ. Enhancement of tumor response to gamma-radiation by an inhibitor of cyclooxygenase-2 enzyme. J Natl Cancer Inst. 1999;91:1501–4. doi: 10.1093/jnci/91.17.1501. [DOI] [PubMed] [Google Scholar]
- 181.Davis TW, Hunter N, Trifan OC, Milas L, Masferrer JL. COX-2 inhibitors as radiosensitizing agents for cancer therapy. Am J Clin Oncol. 2003;26:S58–61. doi: 10.1097/01.COC.0000074158.59269.9F. [DOI] [PubMed] [Google Scholar]
- 182.Raju U, Nakata E, Yang P, Newman RA, Ang KK, Milas L. In vitro enhancement of tumor cell radiosensitivity by a selective inhibitor of cyclooxygenase-2 enzyme: mechanistic considerations. Int J Radiat Oncol Biol Phys. 2002;54:886–94. doi: 10.1016/s0360-3016(02)03023-7. [DOI] [PubMed] [Google Scholar]
- 183.Raju U, Ariga H, Dittmann K, Nakata E, Ang KK, Milas L. Inhibition of DNA repair as a mechanism of enhanced radioresponse of head and neck carcinoma cells by a selective cyclooxygenase-2 inhibitor, celecoxib. Int J Radiat Oncol Biol Phys. 2005;63:520–8. doi: 10.1016/j.ijrobp.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 184.Dittmann KH, Mayer C, Ohneseit PA, Raju U, Andratschke NH, Milas L, et al. Celecoxib induced tumor cell radiosensitization by inhibiting radiation induced nuclear EGFR transport and DNA-repair: a COX-2 independent mechanism. Int J Radiat Oncol Biol Phys. 2008;70:203–12. doi: 10.1016/j.ijrobp.2007.08.065. [DOI] [PubMed] [Google Scholar]
- 185.Bijnsdorp IV, van den Berg J, Kuipers GK, Wedekind LE, Slotman BJ, van Rijn J, et al. Radiosensitizing potential of the selective cyclooygenase-2 (COX-2) inhibitor meloxicam on human glioma cells. J Neurooncol. 2007;85:25–31. doi: 10.1007/s11060-007-9385-4. [DOI] [PubMed] [Google Scholar]
- 186.Grimes KR, Warren GW, Fang F, Xu Y, St Clair WH. Cyclooxygenase-2 inhibitor, nimesulide, improves radiation treatment against non-small cell lung cancer both in vitro and in vivo. Oncol Rep. 2006;16:771–6. [PubMed] [Google Scholar]
- 187.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–13. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 188.Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.03.009. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bentzen SM, Overgaard M, Thames HD. Fractionation sensitivity of a functional endpoint: impaired shoulder movement after post-mastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 1989;17:531–7. doi: 10.1016/0360-3016(89)90103-x. [DOI] [PubMed] [Google Scholar]
- 190.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 191.Lawrence DA. Latent-TGF-β: an overview. Mol Cell Biochem. 2001;219:163–70. doi: 10.1023/a:1010819716023. [DOI] [PubMed] [Google Scholar]
- 192.Ewan KB, Henshall-Powell RL, Ravani SA, Pajares MJ, Arteaga C, Warters R, et al. Transforming growth factor-β1 mediates cellular response to DNA damage in situ. Cancer Res. 2002;62:5627–31. [PubMed] [Google Scholar]
- 193.Ehrhart EJ, Segarini P, Tsang ML, Carroll AG, Barcellos-Hoff MH. Latent transforming growth factor β1 activation in situ: quantitative and functional evidence after low-dose gamma-irradiation. FASEB J. 1997;11:991–1002. doi: 10.1096/fasebj.11.12.9337152. [DOI] [PubMed] [Google Scholar]
- 194.Attisano L, Wrana JL. Signal transduction by the TGF-β superfamily. Science. 2002;296:1646–7. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 195.Cook JA, Choudhuri R, Degraff W, Gamson J, Mitchell JB. Halofuginone enhances the radiation sensitivity of human tumor cell lines. Cancer Lett. 2010;289:119–26. doi: 10.1016/j.canlet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664–74. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–9. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 198.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 199.Epstein RJ. The CXCL12-CXCR4 chemotactic pathway as a target of adjuvant breast cancer therapies. Nat Rev Cancer. 2004;4:901–9. doi: 10.1038/nrc1473. [DOI] [PubMed] [Google Scholar]
- 200.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–69. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 201.Sung B, Jhurani S, Ahn KS, Mastuo Y, Yi T, Guha S, et al. Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008;68:8938–44. doi: 10.1158/0008-5472.CAN-08-2155. [DOI] [PubMed] [Google Scholar]