Abstract
Exposure of the mature Arabidopsis (Arabidopsis thaliana) seed to water results in the rapid release of pectinaceous mucilage from the outer cells of the testa. Once released, mucilage completely envelops the seed in a gel-like capsule. The physical force required to rupture the outer cell wall of the testa comes from the swelling of the mucilage as it expands rapidly following hydration. In this study, we show that mutations in the transcriptional regulator LEUNIG_HOMOLOG (LUH) cause a mucilage extrusion defect due to altered mucilage swelling. Based on sugar linkage and immunomicroscopic analyses, we show that the structure of luh mucilage is altered, having both an increase in substituted rhamnogalacturonan I and in methyl-esterified homogalacturonan. Also correlated with the structural modification of luh mucilage is a significant decrease in MUCILAGE MODIFIED2 (MUM2; a β-galactosidase) expression in the luh seed coat, raising the possibility that reduced activity of this glycosidase is directly responsible for the luh mucilage defects. Consistent with this is the structural similarity between mum2 and luh mucilage as well as the observation that elevating MUM2 expression in luh mutants completely suppresses the mucilage extrusion defect. Suppression of the luh mutant phenotype was also observed when LEUNIG, a transcriptional corepressor closely related to LUH, was introduced in luh mutants under the control of the LUH promoter. Based on these data, we propose a new model for the regulation of pectin biosynthesis during plant growth and development.
Seed development in angiosperms is characterized by the formation of the embryo, endosperm, and seed coat. Unlike the embryo and endosperm, the seed coat is derived from the ovule integuments and therefore is of maternal origin. Seed coat differentiation is characterized by extensive modifications of integument cells that in many species involve the formation of thickened cell walls followed by cell death. These specialized cell layers protect the embryo from dehydration, physical damage, and pathogen attack as well as playing important roles in controlling dormancy, germination, and seed dispersal (Leon-Kloosterziel et al., 1994; Boesewinkel and Bouman, 1995).
In myxospermous species such as Arabidopsis (Arabidopsis thaliana), cells in the outer layer of the seed coat (testa) synthesize and secrete large quantities of mucilage into the apoplast between the radial and outer tangential cell walls (Beeckman et al., 2000; Western et al., 2000; Windsor et al., 2000). During the differentiation process, the internal structure of mucilage-secreting cells (MSC) also changes dramatically. Initially, vacuolar expansion drives the growth of MSC, but this is soon followed by a rapid reduction in vacuole volume as the cells begin to secrete mucilage into the apoplast. Accumulating mucilage eventually forces the cytoplasm into the center of the cell, where it forms a column. The last stages of MSC differentiation are characterized by the thickening of the radial cell walls and extensive deposition of cell wall material into the cytoplasmic column, resulting in its conversion into a volcano-shaped columella. Finally, as the seed becomes progressively desiccated, the MSC collapse, leaving a ring of dehydrated mucilage around the base of the columella. When the mature seed is next exposed to water following dispersal, mucilage swells rapidly, causing the rupture of the MSC. Released mucilage subsequently envelops the seed in a gelatinous gel (Western et al., 2000). While the function of seed mucilage is not well understood, suggested roles include aiding seed dispersal, protecting the germinating seed against dehydration, and, in some species, maintaining seed viability in harsh environments (Gutterman and Shemtov, 1996; Penfield et al., 2001).
Treating imbibed Arabidopsis seeds with ruthenium red, a dye that binds to carboxyl groups typical of acidic pectic polysaccharides, reveals two distinct layers of mucilage (Western et al., 2000, 2001). The outer layer is diffuse, stains poorly with ruthenium red, and is easily detached from the seed by agitation. Due to the ease of extraction, this layer is often referred to as the water-soluble layer. In contrast, the inner adherent layer directly adjacent to the testa stains more intensely with ruthenium red and cannot be easily detached from the seed. Structural analysis of the water-soluble mucilage layer has shown that it is primarily composed of unsubstituted rhamnogalacturonan I (RG-I), a pectin with an alternating α-1,4-linked GalUA (GalA) and α-1,2-linked Rha residue backbone (Western et al., 2000, 2004; Penfield et al., 2001; Usadel et al., 2004; Macquet et al., 2007a, 2007b). Use of more vigorous extraction methods also identified RG-I as the major pectin of the inner mucilage layer, although in this case, the presence of arabinans and (arabino)galactans suggests that this pectin is more highly substituted than its counterpart in the outer layer (Macquet et al., 2007a). Homogalacturonan (HG) represents only a small fraction of the pectin present in Arabidopsis mucilage and is mostly located within the inner adherent layer (Macquet et al., 2007a). The distribution of HG is not homogeneous, as highly methyl esterified HG is confined to the periphery of the inner layer while sparsely methyl esterified HG is found located in dense patches above the columella (Macquet et al., 2007a). Furthermore, the presence of cellulose in the inner domain of the inner adherent layer is thought to play a role in tethering mucilage to the seed coat (Macquet et al., 2007a).
Analysis of mutants has identified two groups of genes required for normal mucilage extrusion from the Arabidopsis seed coat. The first is a series of transcription factors, APETALA2, ENHANCER OF GLABRA3 (EGL3), GLABRA2 (GL2), MYB PROTEIN5 (MYB5), MYB61, TRANSPARENT TESTA8 (TT8), TRANSPARENT TESTA GLABRA1 (TTG1), and TTG2, that promote seed coat differentiation and hence mucilage biosynthesis as well as regulating a range of other developmental processes (Koornneef, 1981; Bowman et al., 1989; Nesi et al., 2000; Penfield et al., 2001; Western et al., 2001, 2004; Johnson et al., 2002; Zhang et al., 2003; Li et al., 2009). The second group of genes, called MUCILAGE MODIFIED (MUM), specifically affect the amount and/or structure of mucilage, having little or no effect on seed coat differentiation (Western et al., 2001). Of these, RHAMNOSE SYNTHASE2 (RHM2)/MUM4 encodes an enzyme involved in the synthesis of UDP-l-Rha, which presumably supplies most of the Rha required for RG-I synthesis in the mucilage-secreting cells of the testa (Usadel et al., 2004; Western et al., 2004; Oka et al., 2007). In addition to altering the quantity of mucilage, mum4 is the only mutant in this class to display overt columella defects, suggesting a link between pectin biosynthesis and cell wall thickening (Western et al., 2004). Although mum2 mutants fail to release mucilage when hydrated, chemically weakening the cell wall of the seed induces a small amount of mucilage extrusion (Western et al., 2004). However, mum2 mucilage expands poorly, indicating likely structural modifications that affect mucilage hydration. Consistent with a role in regulating mucilage structure, MUM2 encodes a β-galactosidase that removes galactosyl residues from the galactan side chains present on the RG-I backbone following secretion into the apoplast (Dean et al., 2007; Macquet et al., 2007b).
While not identified as a mum mutant, loss of bifunctional β-d-xylosidase/α-l-arabinofuranosidase (BXL1) activity also affects mucilage structure by increasing the proportion of α-1,5-l-arabinan (Ara) attached to the RG-I backbone (Arsovski et al., 2009). In this case, elevated RG-I substitution is associated with a slow and patchy release of mucilage from the seed following hydration (Arsovski et al., 2009). Another recent study has shown that mutations in GAUT11, a putative galacturonosyltransferase involved in RG-I biosynthesis, also affect the quantity and hydration properties of mucilage (Caffall et al., 2009). Interestingly, not all mucilage-deficient mutants have defects in RG-I structure. Mutations in the subtilisin-like Ser protease AtSBT1.7 are associated with a significant reduction in HG methyl esterification, which not only affects mucilage release from the seed but also changes the viscosity of the outer cell wall of the testa (Rautengarten et al., 2008).
Given the structural complexity of pectin, it is assumed that a large number of glycosyltransferases, sugar nucleotide-interconverting enzymes, methyltransferases, and acetyltransferases are involved in pectin biosynthesis. While recent progress has seen some of these enzymes identified (for review, see Mohnen, 2008), almost nothing is known about the molecular mechanisms regulating these biosynthetic pathways. Here, we present a detailed characterization of the mucilage extrusion defects associated with mutations in the transcriptional regulator LEUNIG_HOMOLOG (LUH). Using biochemical analysis, we show that the RG-I present in luh mucilage is more substituted than RG-I from wild-type mucilage and that this is associated with an increased proportion of terminal Gal residues attached to the RG-I backbone. We show that LUH is allelic to MUM1, and the β-galactosidase MUM2 is a likely target of LUH regulation. Finally, we present evidence that the transcriptional corepressor LEUNIG (LUG) is functionally interchangeable with LUH, raising the possibility that LUH functions as a repressor during seed coat maturation. We propose that LUH controls MUM2 activity indirectly via an as yet unidentified MUM2 negative regulator.
RESULTS
luh Mutants Display a Mucilage Extrusion Defect Associated with Altered Mucilage Hydration Properties
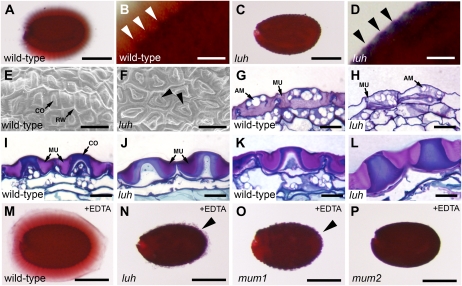
A characteristic feature of luh mutants is delayed germination when plated onto Murashige and Skoog medium (Sitaraman et al., 2008). As previous work has established a link between mucilage release (extrusion) and germination (Arsovski et al., 2009), we examined whether luh seeds release mucilage normally. When hydrated wild-type seeds are treated with the dye ruthenium red, released mucilage appears as a red halo around the seed (Fig. 1A) and the volcano-shaped columellae become more prominent due to rupture of the MSC (Fig. 1B). Absence of a ruthenium red halo surrounding imbibed luh seeds, together with a lack of distinguishable columellae (Fig. 1, C and D), indicate that the MSC fail to rupture when exposed to water.
Figure 1.
Structure and development of wild-type and luh mutant seed coats. A and B, Ruthenium red-stained mature wild-type seed (A) and close-up view of columellae (white arrowheads) protruding from the seed surface (B). C and D, Ruthenium red-stained luh-4 seed (C) and view of enclosed columellae (black arrowheads) within the MSC of the testa (D). E and F, Scanning electron micrographs showing the surface morphology of wild-type (E) and luh-1 (F) MSC. Arrowheads in F indicate abnormal doughnut-shaped columellae. G to L, Longitudinal sections through wild-type (G, I, and K) and luh-4 mutant (H, J, and L) seeds at 6 dpa (G and H), 9 dpa (I and J), and 12 dpa (K and L) stained with toluidine blue. M to P, Ruthenium red-stained seeds from wild-type (M), luh-4 (N), mum1-1 (O), and mum2-1 (P) plants following a 2-h treatment with 50 mm EDTA. Arrowheads indicate small amounts of mucilage release from luh and mum1 mutants. AM, Amyloplasts; CO, columella; MU, mucilage; RW, radial wall. Bars = 250 μm in A, C, and M to P and 50 μm in B, D, and E to L.
Possible reasons for this extrusion defect include disruptions to seed coat differentiation, reductions in mucilage biosynthesis, or alterations to mucilage fine structure (Western et al., 2001, 2004; Dean et al., 2007; Macquet et al., 2007b). Because the first two defects are associated with altered MSC morphology, we used scanning electron microscopy (SEM) to examine the surface of luh seeds. Like the wild type, luh MSC had a hexagonal appearance with thick radial cell walls and a central columella (Fig. 1, E and F). A depression in the center of the columella was frequently observed in the strong luh-1 mutant (Fig. 1F, arrowheads) but was not a consistent feature of other luh alleles (Supplemental Figure S1). Likewise, comparisons of histological sections through wild-type and luh MSC revealed few differences. At 6 d post anthesis (dpa), numerous amyloplasts were visible in the cytoplasm of both wild-type and luh cells, together with mucilage accumulation in the apoplastic space (Fig. 1, G and H). By 9 dpa, the cytoplasm of wild-type and luh cells was confined to a central column, in both cases exhibited cell wall thickening. This process was slightly more advanced in luh mutants, as cytoplasmic disintegration was apparent (Fig. 1, I and J). Formation of the columella was complete in 12-dpa luh seeds but was not as advanced in wild-type cells (Fig. 1, K and L). Despite the apparent acceleration of luh seed coat maturation, there was no obvious difference in the amount of pink-staining mucilage material seen in wild-type and luh MSC.
Absence of patterning or mucilage secretion defects in luh mutants led us to consider whether the hydration properties of luh mucilage were altered. To test this, we first weakened the outer seed coat cell wall by gently shaking seeds in a solution of weak alkali (1 m Na2CO3), a mild cation chelator (0.2% [w/v] ammonium oxalate), or a strong cation chelator (50 mm EDTA) for 2 h before staining with ruthenium red. All three treatments caused a small amount of mucilage release, with EDTA having the greatest effect (Fig. 1N; Supplemental Fig. S2). Interestingly, released luh mucilage did not expand properly and stained poorly with ruthenium red (Fig. 1, compare M and N). These observations indicate that the hydration properties of luh mucilage have been significantly altered. This phenotype is shared with previously described mum1 and mum2 mutants (Fig. 1, O and P; Western et al., 2001).
LUH and MUM1 Are Genetically Identical
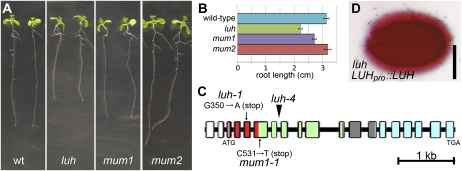
Given the similarity in mucilage extrusion defects, we next considered whether mum1 and mum2 mutants have other phenotypes in common with luh. As luh mutants have a notable reduction in root growth (Sitaraman et al., 2008), we compared the length of luh, mum1, and mum2 roots with the wild type after 10 d of growth on vertical plates (Fig. 2A). mum1 and luh roots were significantly shorter than wild-type roots (t test: luh, P < 0.001, n = 28; mum1, P < 0.004, n = 28; Fig. 2, A and B), whereas mum2 roots were similar in length to the wild type (t test: P > 0.7; Fig. 2, A and B).
Figure 2.
Phenotypic similarity between luh and mum mutants. A, Ten-day-old plants grown on vertical plates. B, Histogram showing root length after 6 d of growth on vertical plates. Error bars represent se. C, Structure of LUH, with boxes representing exons and lines between the boxes representing introns. Colors indicate the following features: 5′ untranslated region (white), LUFS domain (red), Gln-rich domain (green), variable region (gray), and WD40 domain (blue). The arrowhead indicates the T-DNA insertion allele used in this study, and arrows indicate ethyl methanesulfonate-induced mutations. For the ethyl methanesulfonate alleles, the positions of the altered nucleotides are given relative to the translation initiation site. D, Representative seed from a luh;LUHpro::LUH plant displaying a wild-type pattern of ruthenium red staining. Bar = 250 μm.
The striking similarity between the luh and mum1 phenotypes prompted us to consider whether MUM1 and LUH are genetically identical. To test for allelism, luh and mum1 mutants were crossed, and seeds produced by the F1 plants were examined for a mucilage extrusion defect. All 24 F1 plants tested produced seeds that failed to release mucilage, whereas plants derived from a cross between luh and mum2 produced seeds that extrude mucilage normally (data not shown). To confirm that the lesion in mum1 resides at the LUH locus, we amplified and sequenced approximately 4.6 kb of genomic DNA spanning the entire LUH coding region from mum1-1 mutants. This identified a C-to-T change at nucleotide 531 (as measured from the translational start) that is predicted to convert Glu-97 to a stop codon in the sixth exon (Fig. 2C). As this lesion is similar to the strong luh-1 allele (Fig. 2C; Sitaraman et al., 2008), it is likely that mum1-1 also conditions a strong loss of function.
To confirm that lesions at the LUH locus are responsible for the mucilage extrusion defects, we showed that expressing the LUH cDNA sequence from the previously characterized 2.6-kb LUH promoter (Stahle et al., 2009) was sufficient to restore mucilage extrusion in seeds derived from 38 of 41 primary luh transformants (Fig. 2D). The remainder showed partial or no complementation.
LUH Regulates MUM2 in the Developing Seed
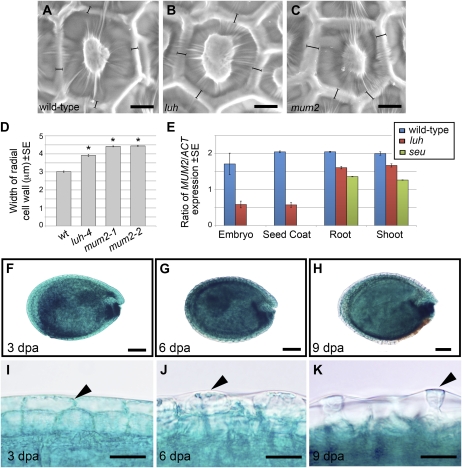
In addition to having similar mucilage extrusion defects, the MSC of luh and mum2 also have slightly thicker radial cell walls (Fig. 3, A–D). To test whether these phenotypic similarities are a consequence of LUH and MUM2 functioning in the same genetic pathway, we used quantitative reverse transcription (qRT)-PCR to examine the expression of MUM2 in developing wild-type and luh mutant seeds. To ensure that seed coat expression was assayed, we manually separated developing embryos from the seed coat and examined expression in near homogeneous pools of tissue. Detecting expression of the embryo-specific gene ASYMMETRIC LEAVES1 (Byrne et al., 2000) in two independent pools of embryonic tissue (E1/E2) but not seed coat tissue (SC1/SC2) confirmed the origin and purity of these samples (Supplemental Fig. S3).
Figure 3.
LUH regulates MUM2 in the developing seed. A to C, Scanning electron micrographs of wild-type (A), luh-4 (B), and mum2-1 (C) seeds showing the hexagonal MSC of the testa. Bracketed lines indicate measurements taken of radial wall width. D, Histogram showing the average width of wild-type (wt) and mutant radial cell walls (n > 50). Error bars represent se. Statistical differences between the wild type and mutants were calculated using Student’s t test, with P < 0.001 indicated by asterisks. E, qRT-PCR analysis of MUM2 expression in embryonic and seed coat tissue collected from 10-dpa wild-type and luh-4 siliques as well as shoot and root tissue of luh and seu mutants. F to H, Histochemical localization of LUHpro::GUS expression in seeds harvested from siliques at 3 dpa (F), 6 dpa (G), and 9 dpa (H). I to K, Higher magnification of seeds shown in F to H revealing GUS staining (arrowheads) in the epidermis of the testa. Bars = 10 μm in A to C, 100 μm in F to H, and 20 μm in I to K.
As well as the expected expression in embryos (Stahle et al., 2009), LUH expression was also detected in seed coat (testa) samples (Fig. 7D; Supplemental Fig. S3). Absence of PCR products in the luh samples indicates that the T-DNA insertion in luh-4 (Fig. 2C) causes a significant decrease in LUH transcript abundance and thus likely represents an RNA null allele (Fig. 7D; Supplemental Fig. S3). As reported previously, MUM2 expression was detected in both embryonic and testa tissue (Fig. 3E; Dean et al., 2007; Macquet et al., 2007b). Consistent with LUH regulating MUM2, there was an approximately 3-fold reduction of MUM2 expression in luh seed coat and embryonic samples (Fig. 3E). To determine whether MUM2 expression was affected in other tissues of the plant, we next assayed luh shoot and root tissue. While not as dramatic as the reduction seen in seeds, MUM2 expression was reduced by 16.5% in luh shoot tissue and by 21.5% in luh root tissue in comparison with the wild type (t test: shoot, P < 0.04; root, P < 0.007; Fig. 3E).
Figure 7.
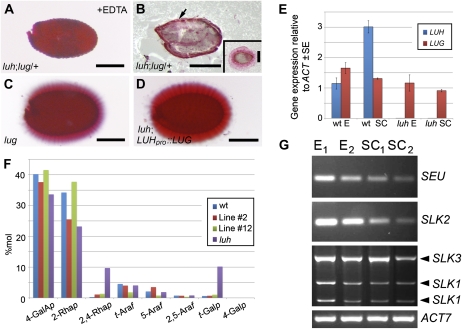
Redundancy between LUH and LUG. A, Ruthenium red-stained seed obtained from a luh;lug/+ plant following a 2-h EDTA treatment. B, Ruthenium red-stained section of a wax-embedded seed obtained from a luh;lug/+ plant. Mucilage staining is indicated with the arrow. The inset shows a wild-type seed section stained with ruthenium red. C and D, Ruthenium red-stained seeds from lug mutant (C) and luh;LUHpro::LUG transgenic (D) plants displaying wild-type levels of mucilage release. Bars = 250 μm. E, qRT-PCR analysis of LUH and LUG expression in embryo (E) and seed coat (SC) samples obtained from wild-type (wt) and luh 10-dpa siliques. F, Histogram showing mol % of sugar linkages associated with the RG-I backbone and side chains present in the alkali-soluble fraction of mucilage extracted from wild-type and transgenic luh;LUHpro::LUG lines. The complete data set is presented in Supplemental Table S1. G, RT-PCR analysis of SEU, SLK1-3, and ACT7 expression in embryo (E1 and E2) and seed coat (SC1 and SC2) samples obtained from wild-type 10-dpa siliques.
Using previously generated LUHpro::GUS plants, we examined LUH promoter activity in developing seeds. Consistent with published in situ data, LUHpro activity was detected at all stages of embryo development (data not shown; Stahle et al., 2009). In addition, GUS activity was also apparent throughout the maturing seed coat (Fig. 3, F–H). Higher magnification revealed blue GUS stain in the periphery of MSC at 3 dpa (Fig. 3I), whereas by 6 dpa, it had shifted centrally (Fig. 3J). At lower levels, stain was also apparent in the cytoplasmic column of MSC at 9 dpa (Fig. 3K).
Previous work has shown that LUH physically interacts with the coregulator SEUSS (SEU) in yeast (Sitaraman et al., 2008; Stahle et al., 2009), suggesting that such interactions may be important for LUH function. To determine whether SEU also functions upstream of MUM2, we used qRT-PCR to examine MUM2 expression in shoot and root tissue derived from seu mutants. This revealed a 36.8% reduction in shoot tissue and a 33.6% decrease in root tissue (t test: shoot, P < 0.005; root, P < 0.0005; Fig. 3E), which is consistent with SEU and LUH being part of the same regulatory complex.
In summary, our analysis shows that LUH/MUM1 is a major regulator of MUM2 expression in developing seeds and, to a lesser extent, in other tissues of the plant.
Altered HG Esterification Is Detected in luh and mum2 MSC
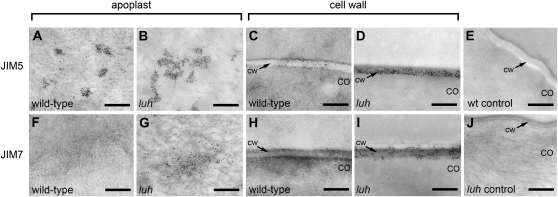
Previous characterization of mum1 and mum2 mucilage has identified several structural modifications that may influence the degree of pectin swelling following hydration (Western et al., 2001; Dean et al., 2007; Macquet et al., 2007b). The first is an apparent 6% to 8% increase in the level of pectin methyl esterification detected in ammonium oxalate-extracted mucilage from both mum mutant lines (Western et al., 2001). However, owing to the different extractability of mum and wild-type mucilage, direct comparisons between these samples is potentially misleading. Thus, to avoid issues associated with extraction, we used immunoelectron microscopy to examine the extent of methyl esterification present in wild-type and luh mutant MSC in seeds harvested from 12-dpa siliques. It is expected that, at this stage of development, mucilage modification has ceased, as the epidermal cells are fully differentiated and beginning to desiccate. The methyl esterification status of HG was selected for analysis because previous studies have found that the main component of mucilage, RG-I, is not substantially methyl esterified (Penfield et al., 2001; Macquet et al., 2007a). Monoclonal antibodies JIM5 and JIM7 were used for this analysis, as they recognize sparsely and heavily methyl esterified HG, respectively (Knox et al., 1990; Willats et al., 2000, 2001). A secondary antibody conjugated to gold (18 nm) was then used to visualize binding to HG epitopes.
JIM5 labeling of wild-type and luh mutant cells detected clumps of sparsely methyl esterified HG within the secreted mucilage present in the apoplastic space interior to the cell wall of seed coat epidermal cells (Fig. 4, A and B). This contrasts with a much more even distribution of epitopes within the primary cell wall (Fig. 4, C and D). Labeling with JIM7 revealed few heavily methyl esterified HG epitopes in either the apoplastic space or the primary cell wall of wild-type cells (Fig. 4, F and H). Interestingly, the frequency of JIM7 labeling was noticeably elevated in the luh mutant (Fig. 4, G and I), consistent with the increased methyl esterification previously reported for this line (Western et al., 2001).
Figure 4.
Distribution of methyl-esterified HG epitopes in epidermal cells of the testa. A to D and F to I, Transmission electron microscopy of sections through 12-dpa seeds labeled with JIM5 (A–D) and JIM7 (F–I) antibodies. HG epitopes bound by primary antibodies were visualized with a secondary antibody conjugated to gold (black dots). Micrographs show labeling of HG epitopes in the apoplasts of wild-type (A and F) and luh-4 (B and G) epidermal cells of the seed coat and labeling of HG epitopes in the primary cell walls (cw) of wild-type (C and H) and luh-4 (D and I) epidermal cells of the seed coat. CO, Columella. E and J, Micrographs showing background labeling with secondary antibody in the absence of primary antibody. Bars = 0.5 μm.
Increased RG-I Substitution Is Detected in luh Mucilage
In addition to altered pectin methyl esterification, increased RG-I substitution is also a feature of mum2 mucilage (Dean et al., 2007; Macquet et al., 2007b). Given that MUM2 expression is reduced in luh mutants, we used linkage (by methylation) analysis to characterize the sugar linkages present in mucilage extracted from luh seeds using hot acid and then strong alkali (see “Materials and Methods”). Based on ruthenium red staining of seeds following these treatments (Supplemental Fig. S4), the acid-soluble fraction contains loosely attached pectins from the outer water-soluble layer and possibly those from the inner layer. The remainder of the strongly associated pectins and cross-linking glycans (hemicellulose/cellulose) from the inner mucilage layer were largely solubilized following extended alkali treatment. These fractions were then subjected to carboxyl reduction and methylation before partially methylated alditol acetates were quantified by gas chromatography-mass spectrometry (GC-MS). Data are presented as mol % (Table I).
Table I. Sugar linkage composition of extracted mucilage from wild-type, luh, and mum2 seeds.
Soluble polysaccharides from intact seeds were extracted sequentially with acid (HCl soluble) and alkali (NaOH soluble). Samples were neutralized, and following methylation, the partially methylated alditol acetates were quantified by GC-MS. Results are given as mol % ± se calculated from two independent experiments (wild type and luh). ND, Not detected; tr, trace (less than 0.5 mol %).
| Sugar and Linkage | HCl Soluble | NaOH Soluble | ||||
| Wild Type | luh | mum2 | Wild Type | luh | mum2 | |
| Rha | ||||||
| t-Rhap | 0.9 ± 0.5 | tr | 0.5 | 1.0 ± 0.0 | 0.9 ± 0.1 | tr |
| 2-Rhap | 44.1 ± 4.6 | 28.3 ± 1.5 | 30.9 | 28.5 ± 3.9 | 22.8 ± 1.2 | 23.0 |
| 2,4-Rhap | 1.0 ± 0.5 | 7.0 ± 3.2 | 5.7 | 2.4 ± 0.2 | 9.2 ± 0.7 | 5.8 |
| Total | 46 | 35.3 | 37.1 | 31.9 | 32.9 | 28.8 |
| Ara | ||||||
| t-Araf | 2.4 ± 0.0 | 5.0 ± 2.0 | 3.0 | 5.9 ± 2.1 | 4.0 ± 1.9 | 2.0 |
| 2-Araf | 0.5 ± 0.2 | 0.8 ± 0.1 | 0.6 | 2.9 ± 2.5 | 2.2 ± 2.5 | 2.1 |
| 3-Araf | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.5 | 1.7 ± 0.8 | 1.0 ± 0.9 | 1.1 |
| 5-Araf | 0.8 ± 0.2 | 2.0 ± 1.8 | 1.4 | 2.0 ± 0.8 | 1.4 ± 1.4 | 1.0 |
| 2,5-Araf | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.8 | 1.1 ± 1.5 | 0.6 ± 0.9 | ND |
| 3,5-Araf | tr | tr | ND | tr | tr | ND |
| Total | 4.7 | 9 | 6.3 | 13.6 | 9.2 | 6.2 |
| Xyl | ||||||
| t-Xylp | tr | 0.7 ± 0.6 | 0.5 | 3.1 ± 1.4 | 2.5 ± 1.7 | 0.5 |
| 4-Xylp | tr | 1.6 ± 2.2 | 1.0 | 5.3 ± 4.2 | 49.2.9 | 5.4 |
| Total | 0 | 2.3 | 1.5 | 8.4 | 7.4 | 5.9 |
| Man | ||||||
| 4-Manp | 0.5 ± 0.7 | 1.7 ± 2.4 | 1.2 | 1.8 ± 2.5 | 1.5 ± 2.1 | 2.1 |
| Gal | ||||||
| t-Galp | 0.8 ± 0.3 | 8.1 ± 0.7 | 8.4 | 1.7 ± 0.3 | 8.9 ± 0.1 | 8.0 |
| 3-Galp | tr | 1.0 ± 0.7 | 0.8 | 1.3 ± 0.0 | 1.0 ± 0.2 | 4.0 |
| 4-Galp | 0.6 ± 0.3 | 0.9 ± 0.8 | tr | 1.1 ± 1.1 | 1.1 ± 1.1 | ND |
| 6-Galp | tr | tr | ND | 0.5 ± 0.8 | tr | ND |
| 3,6-Galp | tr | 0.6 ± 0.9 | ND | 0.5 ± 0.7 | 0.5 ± 0.7 | ND |
| Total | 1.4 | 10.6 | 9.2 | 5.1 | 11.5 | 12 |
| Glc | ||||||
| t-Glcp | tr | tr | tr | 1.6 ± 1.8 | 1.2 ± 1.1 | 2.1 |
| 2-Glcp | tr | tr | ND | tr | 1.2 ± 0.7 | 1.5 |
| 4-Glcp | 0.9 ± 0.3 | 1.9 ± 1.5 | 7.9 | 4.5 ± 1.4 | 2.5 ± 0.7 | 7.9 |
| 3,4-Glcp | tr | tr | ND | 0.7 ± 1.1 | 0.7 ± 1.0 | ND |
| Total | 0.9 | 1.9 | 7.9 | 6.8 | 5.6 | 11.5 |
| GalUA | ||||||
| t-GalAp | 1.0 ± 0.3 | 0.9 ± 0.7 | 1.5 | 1.0 ± 0.1 | 1.2 ± 0.4 | tr |
| 4-GalAp | 42.0 ± 1.3 | 35.1 ± 2.4 | 33.9 | 28.6 ± 0.7 | 30.1 ± 0.1 | 32.5 |
| Total | 43 | 36 | 35.4 | 29.6 | 31.3 | 32.5 |
| GlcA | ||||||
| t-GlcAp | tr | 0.7 ± 0.5 | 0.7 | 1.1 ± 1.6 | 0.5 ± 0.1 | tr |
As reported previously, the predominant linkages present in mucilage from wild-type seeds are 2-linked Rha (2-Rhap) and 4-linked GalA (4-GalAp) with a small quantity of 2,4-linked Rha (2,4-Rhap; Table I; Penfield et al., 2001; Western et al., 2004). Interestingly, the degree of RG-I substitution differed between fractions, with the substituted (2,4-Rha):unsubstituted (2-Rha) Rha ratio being approximately 1:42 in the acid-soluble fraction and approximately 1:14 in the alkali-soluble fraction (Table II). Modest increases in sugars associated with arabinan and (arabino)-3,6-galactan side chains were also observed in the alkali-soluble fraction, including terminal Ara (t-Araf), 5-Araf, 2,5-Araf, 3-Galp, 6-Galp, and 3,6-Galp residues. Similarly, increases in terminal Gal (t-Galp) and 4-Galp residues were also detected, suggesting that individual Gal residues and (arabino)-4-galactan side chains are attached to the RG-I backbone in alkali-extracted mucilage (Table I).
Table II. Calculated polysaccharide composition (mol %) of extracted mucilage from wild-type, luh, and mum2 seeds.
| Polysaccharide | HCl Soluble | NaOH Soluble | ||||
| Wild Type | luh | mum2 | Wild Type | luh | mum2 | |
| Arabinan | 2.7 | 4.6 | 4.0 | 8.8 | 5.9 | 4.3 |
| Type I arabinogalactan | 0.6 | 0.9 | 0.0 | 1.1 | 1.1 | 0.0 |
| Type II arabinogalactan | 2.3 | 2.8 | 4.0 | 2.0 | 0.8 | 0.0 |
| Arabinoxylan | 0 | 1.6 | 1.0 | 5.3 | 4.9 | 5.4 |
| Galacto(gluco)mannan | 0.5 | 1.7 | 1.2 | 1.8 | 1.5 | 2.1 |
| HG | 0 | 0 | 0 | 0 | 0.4 | 3.8 |
| RG-I (substituted:unsubstituted ratio) | 91.4 (1:42.3) | 77.6 (1:4.0) | 78.8 (1:5.5) | 63.1 (1:14.2) | 66.2 (1:3.3) | 63.2 (1:4.0) |
| Other | 2.5 | 10.8 | 11.0 | 18.0 | 19.3 | 21.2 |
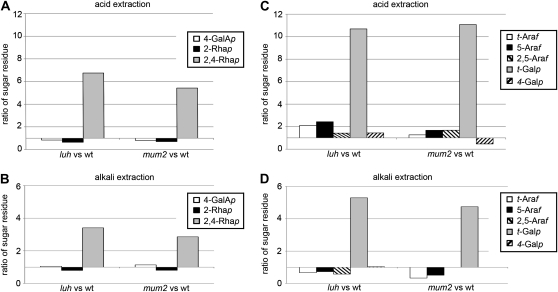
Analysis of mucilage extracted from luh seeds revealed near wild-type levels of 4-GalAp in the alkali-soluble fraction but reduced levels in the acid-soluble fraction (Table I). In comparison with wild-type mucilage, levels of the substituted 2,4-Rhap residues were significantly elevated and 2-Rhap residues were reduced in both the acid- and alkali-soluble mucilage fractions obtained from luh mutants (Table I; Fig. 5, A and B). As a result of these changes, the ratio of substituted (2,4-Rha) to unsubstituted (2-Rha) Rha in the acid- and alkali-soluble fractions of luh mutants was approximately 1:4 and approximately 1:3, respectively (Table II). Correlated with changes in luh RG-I structure was a substantial increase in t-Galp residues, with an approximately 10-fold increase observed in the acid-soluble fraction and an approximately 5-fold increase in the alkali-soluble fraction (Table I; Fig. 5, C and D). Moderate increases in t-Araf and 5-Araf residues were also detected in the acid-soluble fraction but not in the alkali-soluble fraction (Table I; Fig. 5, C and D). While changes to RG-I structure are the most obvious defect in luh mutant mucilage, small changes in the distribution of xylans, galacto(gluco)mannans, and residues belonging to arabino-3,6-galactan side chains of either RG-I or arabinogalactan proteins were also observed (Table II).
Figure 5.
Changes in the proportion of sugar linkages present in luh and mum2 mutant mucilage in comparison with the wild-type (wt). Histograms show the change in proportion of monosaccharide residues associated with the RG-I backbone (A and B) and arabinan/(arabino)galactan side chains (C and D) present in mucilage extracted with acid (A and C) and alkali (B and D) relative to the wild type as determined by GC-MS (the complete data set is shown in Table I). Inset legends indicate each monosaccharide and its linkage.
To confirm that the luh mucilage structure was similar to that of mum2 mutants, we determined the linkage composition of mum2 mucilage. The changes detected in RG-I structure closely paralleled those seen in luh mutants and were also similar to the published mum2 mucilage structure (Tables I and II; Fig. 5; Dean et al., 2007; Macquet et al., 2007b). Structural similarity between luh and mum2 mutant mucilage is consistent with a loss of MUM2 activity in luh mutant seeds.
Heterologous MUM2 Expression Restores Mucilage Release from luh Seeds
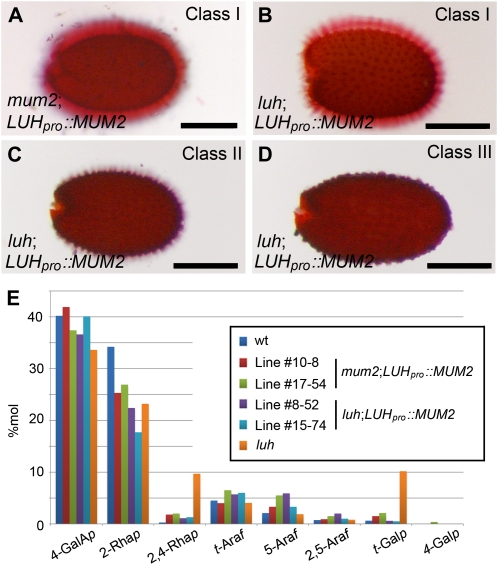
The striking similarity between luh and mum2 RG-I structure together with low-level MUM2 expression in luh mutant seeds strongly suggested that LUH regulates MUM2 activity in the developing seed coat. To further test this hypothesis, we used a transgenic approach to restore MUM2 expression in developing luh seeds. Because LUH and MUM2 have similar expression profiles (Fig. 3, I–K; Dean et al., 2007; Macquet et al., 2007b), we reasoned that the LUH promoter might be suitable to drive MUM2 expression in the developing seed coat. Therefore, we introduced a LUHpro::MUM2 construct into mum2 mutants and used ruthenium red staining to assess mucilage release from seeds produced by T1 transgenic plants. Based on the staining pattern, we distinguished four categories of transgenic plant. Class I plants produced seeds with wild-type levels of mucilage release that stained well with ruthenium red (n = 27; Fig. 6A). Class II plants produced seeds that released wild-type levels of mucilage but, unlike seeds from the first class, stained poorly with ruthenium red (n = 14). The next two classes of plants produced seeds that either released small quantities of mucilage (class III; n = 21) or completely failed to release mucilage (class IV; n = 28). Finding full mucilage release in seeds obtained from approximately one-third of mum2 transformants confirmed that the LUH promoter was sufficiently active to compensate for the loss of endogenous MUM2 activity in mum2 mutants. Having established the utility of the LUH promoter, we next introduced the LUHpro::MUM2 construct into luh mutants. In this case, approximately one-quarter of primary transformants displayed full complementation (class I; n = 33; Fig. 6B), while the remaining transformants produced seeds with a class II phenotype (n = 47; Fig. 6C), a class III phenotype (n = 31; Fig. 6D), or a class IV phenotype (n = 10; data not shown).
Figure 6.
Heterologous MUM2 expression in mutant lines. A, Seed obtained from a class I primary mum2;LUHpro::MUM2 transformant showing restored mucilage extrusion following ruthenium red staining. B to D, Seeds obtained from class I (B), class II (C), and class III (D) primary luh;LUHpro::MUM2 transformants. Bars = 250 μm. E, Histogram showing mol % of sugar linkages associated with the RG-I backbone and side chains present in the alkali-soluble fraction of mucilage extracted from wild-type (wt) and transgenic mutant lines. The complete data set is presented in Supplemental Table S1.
To confirm that heterologous MUM2 expression in developing mum2 and luh seeds produces a wild-type mucilage structure, methylation analysis was conducted on seed mucilage extracted from two independently derived mum2;LUHpro::MUM2 class I lines and two independently derived luh;LUHpro::MUM2 class I lines (Fig. 6E; Supplemental Table S1). Due to the large number of samples, we confined our analyses to alkali-soluble mucilage. Based on the relative abundance of residues associated with the RG-I backbone and its side chains, mucilage obtained from transgenic seeds had a structural profile similar to the wild type (Fig. 6E). For instance, the increased proportions of branched Rha residues (2,4-Rha) and t-Galp residues found in luh and mum2 mucilage (Fig. 5; Table I) were not observed in mucilage from transgenic lines (Fig. 6E; Supplemental Table S1). Subsequent qRT-PCR analysis confirmed that MUM2 expression was close to wild-type levels in 10-dpa seed tissue obtained from two independent class I luh;LUHpro::MUM2 lines (Table III). This confirms that elevating MUM2 expression in luh mutants restores RG-I substitution to wild-type levels and reestablishes mucilage extrusion.
Table III. qRT-PCR analysis of MUM2 expression in transgenic lines.
| Line | Ratio of MUM2 to ACT7 Expression | |
| Seed Coat | Embryo | |
| Wild type | 1.89 | 1.83 |
| luh;LUHpro::MUM2 #8-35 | 1.51 | 1.55 |
| luh;LUHpro::MUM2 #15-74 | 1.48 | 1.40 |
| luh;LUHpro::LUH #4-1 | 1.34 | 1.53 |
| luh;LUHpro::LUH #5-2 | 1.34 | 1.40 |
| luh;LUHpro::LUG #2 | 1.34 | 1.27 |
| luh;LUHpro::LUG #12 | 1.41 | 1.49 |
LUG Functions Redundantly with LUH to Promote Mucilage Extrusion from the Testa
Previous work has shown that LUG and LUH regulate overlapping processes in vegetative and floral development (Sitaraman et al., 2008; Stahle et al., 2009). To determine whether this is also the case in the seed coat epidermis, we characterized luh mutant seeds lacking LUG activity. Due to lug;luh double mutants being embryo lethal (Sitaraman et al., 2008), we focused on seeds derived from plants homozygous for luh and heterozygous for lug (luh;lug/+). Although lug segregation occurs embryonically, all seeds derived from this line have a luh;lug/+ seed coat genotype due to this tissue being maternal in origin.
In contrast to luh mutants, no mucilage was released from seeds arising from luh;lug/+ plants following EDTA treatment (compare Fig. 1N and Fig. 7A). The presence of mucilage in the luh;lug/+ seed coat was subsequently confirmed by sectioning wax-embedded seeds and staining with ruthenium red (Fig. 7B). As mucilage is released from lug mutant seeds following hydration (Fig. 7C), the role of LUG in the seed coat is only apparent when LUH activity is compromised. Consistent with LUG having a role in the developing seed, qRT-PCR assays detected LUG expression in both embryonic and seed coat tissue (Fig. 7D). However, when compared with LUH, LUG expression was substantially (approximately 3-fold) lower in the seed coat tissue.
Given that the enhanced extrusion defect of the luh;lug/+ seeds was not associated with an obvious reduction in mucilage accumulation within the MSC (Supplemental Fig. S5), we next addressed whether this phenotype was correlated with a further decrease in MUM2 expression. qRT-PCR analysis, however, failed to detected a significant expression difference between luh and luh;lug/+ seed coats (data not shown). As an alternative strategy to determine whether LUG has a role in seed coat development, we placed the LUG coding region under the control of the LUH promoter and introduced the construct into luh mutants. Of the 42 primary transformants, 39 plants produced seeds with a wild-type pattern of mucilage extrusion (Fig. 7E). To confirm that the luh;LUHpro::LUG transgenic plants produce mucilage with a wild-type structure, we performed linkage analysis on mucilage extracted from two independent luh;LUHpro::LUG transgenic lines (Supplemental Table S1). Consistent with LUG and LUH being functionally interchangeable, levels of sugar linkages normally associated with a wild-type RG-I backbone and side chains were present in the transgenic mucilage (Fig. 7F; Supplemental Table S1). Furthermore, qRT-PCR analysis detected similar levels of MUM2 expression in both luh;LUHpro::LUH and class I luh;LUHpro::LUG seed coat tissue (Table III). While these data confirm the functional equivalence of LUG and LUH, differences in expression level presumably account for their unequal roles within the seed coat.
Recruitment of the corepressor LUG, and presumably LUH, to regulatory sequences of target genes is dependent on physical interactions with the coregulator SEU. Therefore, we examined whether SEU and the closely related SEU-LIKE (SLK1–SLK3) genes (Stahle et al., 2009; Bao et al., 2010) are expressed in embryonic and seed coat tissue. Consistent with redundancy between these coregulators, RT-PCR analysis detected expression of all four genes in these tissues (Fig. 7G).
DISCUSSION
This study has established that mutations in LUH, a gene closely related to the transcriptional corepressor LUG (Liu and Karmarkar, 2008), affect mucilage release from the seed coat following contact with water. Mutants lacking mucilage extrusion can be grouped according to whether they either disrupt the differentiation of the seed coat or specifically interfere with mucilage biosynthesis and/or structure (for review, see Western, 2006). Based on our cytological analysis, luh mutants belong to this second group, as seed coat differentiation and mucilage secretion into the apoplast are largely unaltered.
Relationship between LUH and MUM Genes
Phenotypic similarity between luh and the well-characterized mucilage extrusion-defective mutants mum1 and mum2 (Western et al., 2001) suggested that these genes might function in the same pathway. Through a series of genetic crosses, we established that luh and mum1 mutations were allelic and subsequently identified a lesion at the LUH locus in mum1-1 mutants. Given that LUH encodes a transcriptional regulator (see below) and MUM2 encodes a β-galactosidase, we addressed whether LUH might regulate MUM2. Our analyses provide two lines of evidence in support of such regulatory arrangement. First, we found significant overlap in LUH promoter activity, as assessed by GUS assays, and MUM2 expression in both the developing seed coat and other tissues of the plant (Macquet et al., 2007b; Stahle et al., 2009; this study). Next, we showed by qRT-PCR that there is an approximately 3- to 4-fold reduction of MUM2 expression in both seed coat and embryo tissue of luh mutants as well as smaller changes in the shoots and roots of these mutants. Based on these observations, we propose that LUH is a global regulator of MUM2 and that loss of MUM2 activity from the luh seed coat causes the mucilage extrusion defect. Consistent with this hypothesis, restoring MUM2 expression in luh seeds results in normal mucilage release when exposed to water. Whether the root growth defect observed in luh mutants is also a consequence of reduced MUM2 activity remains to be seen, although lack of root growth defects in mum2 mutants makes this scenario seem unlikely.
Altered RG-I Structure in luh Mutants
Loss of MUM2 (β-galactosidase) activity in luh mutant seeds prompted us to examine the structure of luh mucilage. Analysis of mucilage extracted from luh mutants revealed significantly more side chain substitution of RG-I in both acid- and alkali-soluble fractions when compared with the wild type. The majority of side chain residues were terminal Gal residues, although residues associated with linear arabinan side chains were apparent in the acid-soluble fraction. Finding an almost identical RG-I substitution profile associated with mum2-extracted mucilage (Dean et al., 2007; Macquet et al., 2007b; this study) provides additional evidence for the structural changes in luh mucilage arising from a loss of MUM2 activity. This conclusion is further corroborated by a return to a wild-type ratio of substituted (2,4-Rha) to unsubstituted (2-Rha) Rha residues in mucilage of transgenic luh seeds in which MUM2 expression is restored.
Although weakening the cell wall of luh mutant seeds results in mucilage release, it does not swell to the same extent as wild-type mucilage. Finding similar defects in mum2 mucilage (Western et al., 2001; Dean et al., 2007; Macquet et al., 2007b) suggests that increased RG-I substitution affects the hydration properties of mucilage. Consistent with this observation, increased RG-I substitution with arabinan side chains is correlated with a slow and patchy release of mucilage from bxl1 mutant seeds (Arsovski et al., 2009). In contrast, removing galactan side chains from avocado (Persea americana) pectin using a β-d-galactosidase increased solubility by reducing the molecular size and aggregation potential of pectin molecules (De Veau et al., 1993). In this respect, it is interesting that the biophysical properties of the inner and outer mucilage layers of Arabidopsis seeds differ. Mucilage present in the outer layer has a smaller molecular mass and increased solubility compared with the inner layer, which forms an insoluble dense gel of high molecular mass (Macquet et al., 2007a). Given that the extent of RG-I substitution varies between the layers, it is likely that the biophysical properties of each layer are largely determined by their RG-I composition. Increased RG-I substitution observed in both luh and mum2 mucilage, therefore, is expected to increase the molecular mass and aggregation potential of pectin and substantially reduce mucilage solubility. As a consequence, mutant mucilage will not swell to the same extent as the wild type following hydration and, hence, will exert less physical force on the outer cell wall of the MSC, either severely restricting or preventing the rupturing of these cells.
Although little is known about how RG-I substitution influences the hydration properties of mucilage, it is clear that highly substituted RG-I has a larger molecular mass than unsubstituted RG-I (Macquet et al., 2007a). Based on the observation that the degree of RG-I substitution influences the activity of RG-I backbone-degrading enzymes such as RG hydrolase and RG lyase (Azadi et al., 1995; Mutter et al., 1998), we propose that RG-I backbone-degrading enzymes are also active in the apoplast of mucilage-secreting cells. Accordingly, substituted RG-I present in the inner layer will not be processed by the RG-I backbone-degrading enzymes to the same extent as unsubstitutedRG-I in the outer layer and thus will have a larger molecular mass. Although there are several classes of RG-I backbone-degrading enzymes, only RG lyases have been unambiguously identified in planta (Naran et al., 2007). Based on sequence alignments with bacterial and fungal lyase sequences, it is predicted that a small RG lyase family is present in the Arabidopsis genome (Coutinho and Henrissat, 1999). Thus, it is conceivable that these enzymes might be active in the developing seeds where they target RG-I for degradation. Greater substitution of the RG-I backbone in luh and mum2 mutant mucilage will likely block access of the RG-I backbone-degrading enzymes to the RG-I backbone, presumably as a result of steric hindrance. Failure to process the RG-I will result in the mutant mucilage having a larger molecular mass, which in turn is likely to alter its hydration properties. An important test of this model will be to determine whether the loss of RG-I backbone-degrading activity in the mucilage-secreting cells of the testa is associated with a mucilage extrusion defect. Presumably, this defect would arise from unsubstituted RG-I no longer being processed into smaller polymers.
Increased Methyl Esterification of HGs in luh Mucilage
In addition to alterations in RG-I substitution, changes in the pattern of HG methyl esterification were also detected within the apoplastic space and primary cell walls of luh seed coat epidermal cells using immunoelectron microscopy analysis. Assuming that the process of HG modification in the apoplast of mucilage-secreting cells is similar to that of cell walls (Schols and Voragen, 1996), it is likely that HG is secreted in a highly methyl esterified form and subsequently deesterified by a family of pectin methylesterases (PMEs) in the apoplast. The increased HG methyl esterification observed in luh mucilage, therefore, could reflect a role for LUH in promoting PME expression during seed coat maturation. However, as increased methyl esterification has been observed in extractable mum2 mucilage (Western et al., 2001), it is possible that PME activity is altered in response to RG-I modification. According to this possibility, LUH would not be a direct regulator of PME activity but would function indirectly via RG-I modification. Future work will need to distinguish between these possibilities.
On the basis of immunofluorescence studies, two distinct populations of HG are distinguishable in the inner mucilage layer. Heavily methyl esterified HG localizes in the periphery of the inner layer, whereas sparsely methyl esterified HG is enriched in a region directly adjacent to the epidermal cell wall. Thus, based on these observations, it is likely that the dense gel-like matrix formed by the inner mucilage layer is due in part to calcium-based cross-linking between sparsely esterified HG polymers (Willats et al., 2001; Macquet et al., 2007a). Consistent with reduced HG methyl esterification affecting the hydration properties of mucilage, increased PME activity in developing seeds of atsbt1.7 mutants results in a mucilage extrusion defect (Rautengarten et al., 2008). Conversely, increased methyl esterification of HG present in the inner layer of mum5 mutants (M. Facetter and C. Somerville, personal communication, cited in Western, 2006) did not adversely affect mucilage release from the testa but instead reduced the gelling properties of the inner layer. Based on these observations, it is unlikely that the increased HG methyl esterification observed in luh mucilage can explain the extrusion defects observed in this line.
Redundancy between LUH and LUG
Of the 13 Gro/Tup1 corepressors in Arabidopsis, LUG and LUH share the greatest similarity, with over 80% sequence identity in the N-terminal LUFS domain as well as extensive identity in the C-terminal WD repeats and adjacent sequences (Liu and Karmarkar, 2008). Thus, it is not surprising to find that these genes function redundantly in a number of processes, including early embryonic development and postembryonic leaf, shoot, and flower development (Sitaraman et al., 2008; Stahle et al., 2009). In postembryonic development, redundancy between LUG and LUH was inferred from the enhancement of lug phenotypes when luh/+ was present in the background, as lug;luh double mutants are embryo lethal. While the mucilage extrusion defects of seeds derived from lug mutants heterozygous for luh/+ could not be assessed due to infertility of lug;luh/+ flowers, seeds derived from luh mutants heterozygous for lug displayed an enhanced mucilage extrusion defect following treatment with EDTA. Furthermore, transgenic experiments clearly indicate that LUG and LUH are functionally equivalent, although mutations in these genes do not condition identical phenotypes. For instance, lug mutants release mucilage following contact with water, whereas luh mutants do not. A possible explanation for this difference emerged from our qRT-PCR analysis, which detected substantially higher levels of LUH expression in the developing seed coat, in comparison with LUG. This observation supports the view that the cis-regulatory elements of these genes have diverged so that LUG is no longer expressed at high levels within the seed coat. Analysis of the publicly available microarray data has also identified differences in the transcriptional responses of LUG and LUH to abiotic and biotic stress (Sitaraman et al., 2008), which also supports the view that the regulatory responses of these genes have diverged. While our data point to LUG and LUH being functionally interchangeable in the developing seed coat, the same is apparently not true in the developing flower, where constitutive expression of LUH fails to restore the floral patterning defects of lug mutants (Sitaraman et al., 2008). Why LUG and LUH should have identical functions in one tissue type but not another is unclear at present but could conceivably arise from a differing distribution of cofactors that are required for LUG and LUH function. Candidate cofactors are SEU and the SLK proteins, which display redundant functions in various plant tissues (Stahle et al., 2009; Bao et al., 2010). In this regard, it is interesting that while SEU clearly plays a role in MUM2 regulation within shoot and root tissue, seu mutants do not condition a mucilage extrusion defect. However, given that all three SLK genes are expressed in the seed coat (Fig. 7C), it is likely that there is extensive redundancy between these genes, as noted in previous studies (Stahle et al., 2009; Bao et al., 2010).
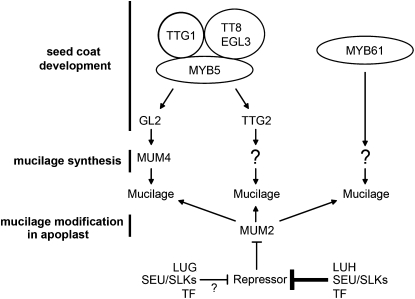
Model for LUH Function in the Seed Coat
On the basis of in vivo assays, LUG is found to act as a potent transcriptional repressor when bound to plant or yeast promoters (Sridhar et al., 2004). Given that the domains involved in repression are shared with LUH (Sridhar et al., 2004; Sitaraman et al., 2008), it is probable that LUH also functions as a negative regulator. This view is supported by genetic evidence showing that luh/+ enhances AGAMOUS misexpression in the outer whorl organs of lug mutants (M. Walker, M. Tehseen, M.S. Doblin, F.A. Pettolino, S.M. Wilson, A. Bacic, and J.F. Golz, unpublished data) and KNOX misexpression in lug mutant leaves (Stahle et al., 2009). However, in the absence of a direct biochemical test, attributing repressor activity to LUH remains speculative.
Nonetheless, finding that LUG restores the mucilage defects of luh mutants when expressed under the control of the LUH promoter raises the possibility that transcriptional repression is involved in MUM2 regulation. Taken together with the observed reduction of MUM2 expression in luh mutants, we propose that LUH regulates MUM2 indirectly. In this model, a LUH-containing complex, and to a lesser extent a LUG-containing complex, directly regulates a MUM2 repressor (Fig. 8). Loss of LUH activity, therefore, is expected to result in increased activity of the MUM2 repressor and reduced expression of MUM2. Testing this model will require the identification of the MUM2 repressor, which may be achieved by either defining the targets of the LUH regulatory complex within the developing testa or through the identification of the transcription factors bound by the LUH regulatory complex.
Figure 8.
A model for the role of LUH in regulating mucilage modification in seed coat epidermal cells. Epidermal seed coat differentiation is controlled by a multimeric complex that includes MYB5, bHLH proteins (TT8/EGL3), and a WD40 repeat protein, TTG1 (Li et al., 2009). This complex is thought to positively regulate GL2. GL2 in turn activates MUM4, an NDP-l-Rha synthase required for the synthesis of RG-I (Western et al., 2004). Although MYB61 also regulates seed coat differentiation and mucilage synthesis, it is thought to function via a separate pathway (Western et al., 2004). MUM2 encodes a secreted glycosidase that removes Gal residues from the RG-I side chains in the apoplast (Dean et al., 2007; Macquet et al., 2007b). We propose that LUH may promote MUM2 expression indirectly through the repression of a negative regulator (repressor). LUH is likely to perform this function by forming a regulatory complex with either SEU or SLK proteins and an as yet unidentified transcription factor (TF). According to this model, reduced LUH activity would lead to increased expression of the MUM2 repressor, and as a consequence, MUM2 expression would be reduced. While not unambiguously demonstrated, it is likely that LUG is also capable of regulating MUM2 activity.
In summary, this study, to our knowledge, is the first to identify a regulatory pathway involved in pectin modification. Although this work has focused on seed mucilage, it is possible given the broad expression pattern of LUH, LUG, SEU, and SLKs (Stahle et al., 2009) that this regulatory complex also controls cell wall pectin structure in other plant tissues. If the role of LUG and LUH as global regulators of pectin-modifying enzymes is confirmed, it will raise the intriguing possibility that some of the developmental defects associated with lug, luh, and seu mutants are caused by changes to pectin structure. This hypothesis is not without precedent, as previous work has shown that altering RG-I substitution in potato (Solanum tuberosum) plants causes a variety of developmental defects, including a reduction in shoot branching and a failure to form flowers, stolons, and tubers (Skjøt et al., 2002). As the effect of altering pectin structure is likely to be exacerbated during primary cell wall deposition, the LUG/LUH regulatory complex may also play a crucial role in elongating tissue.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type Arabidopsis (Arabidopsis thaliana) plants were either Columbia (Col) or Columbia erecta (Col er). luh-1 (seed stock no. CS91893), luh-3 (seed stock no. SALK_107245C), luh-4 (seed stock no. SALK_097509), mum1-1 (seed stock no. CS91893), mum2-1 (seed stock no. CS91893), and mum2-2 (seed stock no. CS91893) mutant lines were obtained from the Arabidopsis Biological Resource Center. With the exception of luh-1, which is in the Col er background, all mutant lines are in the Col background (Western et al., 2001; Sitaraman et al., 2008; Stahle et al., 2009).
Plants were either grown on soil or on one-half-strength Murashige and Skoog medium in a growth room at 18°C or in a growth cabinet kept at 21°C under lights for 16 h.
Staining of Seed Mucilage
Seeds were gently shaken in distilled, deionized water for 2 h and then stained with 0.01% (w/v) ruthenium red for 2 h. Following a brief wash in distilled, deionized water, seeds were then viewed under bright-field optics. To stain seed sections, seeds were first embedded in Paraplast before generating 20-μm sections and staining for 5 min. Sections were viewed under bright-field optics using a Nikon SMZ800 dissecting microscope, and images were captured with a Nikon digital DS-U1 camera. To soften the cell wall, seeds were treated with 1 m Na2CO3, 50 mm EDTA, or 0.2% (w/v) ammonium oxalate for 2 h, rinsed twice in distilled, deionized water, and then stained with ruthenium red as outlined above.
RT-PCR
Developing seeds from 10-dpa siliques were placed in 10% glycerol and pressure applied to force embryo release. Following the manual collection of naked embryos and embryoless seeds (designated seed coats), RNA was isolated using an RNeasy RNA purification kit (Qiagen). Contaminating DNA was removed using DNA-free DNase (Ambion), and first-strand cDNA was synthesized with SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. All cDNAs were amplified using standard PCR conditions with primers described in Supplemental Table S2, and products were separated by gel electrophoresis. ACTIN7 (ACT7; At5g09810) amplification involved 25 PCR cycles, whereas all other assays involved 30 PCR cycles. Due to near perfect sequence identity between SLK1 and SLK3, PCR products were distinguished on the basis of BglII restriction digest. For qRT-PCR analysis, RNA from shoot, root, and seed tissue was used for first-strand cDNA synthesis as described above. A Sensi-Mix dT kit (Quantace) was then used for real-time PCR analysis according to the manufacturer’s instructions. PCR was performed in the presence of SYBR-Green on a Rotor-Gene 3000 Real-Time Cycler (Corbett Research) with ACT7 as a housekeeping control.
Constructs
To generate LUH promoter constructs, 2.6 kb of genomic DNA upstream of the LUH coding sequence was amplified with high-fidelity Taq polymerase using oligonucleotides pLUH-F1/pLUH-R1, which incorporate PstI and KpnI restriction sites (Supplemental Table S2). The promoter fragment was then cloned into the PstI/KpnI sites of shuttle vector pMIGRO. LUHpro:LUG and LUHpro:LUH were made by placing the LUG and LUH coding sequences, which were generated by RT-PCR, downstream of the LUH promoter. Similarly, constructing LUHpro:MUM2 required amplification of the MUM2 coding sequence, which was achieved by RT-PCR using primer combination MUM2-FK/MUM2-RB (Supplemental Table S2). KpnI and BamHI sites present in the primers were subsequently used to insert the cDNA downstream of the LUH promoter in pMIGRO. NotI-containing promoter-cDNA cassettes were then cloned into the binary vector pMLBART.
Binary vectors were introduced into Agrobacterium tumefaciens (GV3101) by electroporation and then transferred into plants using Agrobacterium-mediated floral dip (Clough and Bent, 1998). Transformants were identified following BASTA treatment.
Histology and Microscopy
Siliques harvested at 6, 9, and 12 dpa were fixed in 2.5% glutaraldehyde overnight at 4°C and then treated with 1% osmium tetroxide. Tissue was passed through a graded ethanol series and subsequently embedded in LR-White resin. For histological analysis, 2-μm sections were stained with toluidine blue and examined under bright-field optics using a DM2500 Leica compound light microscope. For immunolabeling, 80-nm sections were processed according to established procedures (Burton et al., 2006). Grids were initially exposed to a 1:50 dilution of primary antibody (JIM5 and JIM7; Plant Probes), washed, and then treated with secondary antibody conjugated to 18-nm gold particles (Jackson ImmunoResearch). Samples were washed, treated with 2% aqueous uranyl acetate, and viewed by transmission electron microscopy as described previously (Burton et al., 2006).
SEM of whole seeds was performed with an FEI Quanta environmental SEM device at room temperature using an accelerating voltage of 12.5 kV.
Histochemical Analysis
The LUHpro::GUS construct has been described previously (Macquet et al., 2007b; Stahle et al., 2009). Siliques at 3, 6, and 9 dpa were sliced open, vacuum infiltrated for 1 h, and incubated overnight in 50 mm phosphate buffer containing 2 mm 5-bromo-4-chloro-3-indolyl-β-glucuronic acid and a mixture of 1 mm potassium ferricyanide and ferrocyanide at 37°C. Siliques were washed, fixed in formaldehyde-acetic acid, and passed through a graded ethanol series. Seeds were then placed in Hoyer’s solution before being viewed under differential interference contrast optics.
Linkage Analysis of Mucilage
Seed mucilage was extracted using an acid/alkali procedure (Macquet et al., 2007b) with minor modifications. Seeds were shaken vigorously (900 rpm) in 50 mm HCl at 85°C for 30 min, rinsed with distilled, deionized water, and then shaken again in 1 m NaOH containing 10 mg mL−1 NaBH4 at room temperature for 40 min. Seeds were then rinsed several times with distilled, deionized water. The acid and alkali fractions were neutralized and dialyzed extensively against deionized water for 24 h before being freeze dried.
Isolated mucilage polysaccharide was carboxyl reduced and subsequently methylated according to established methods (Kim and Carpita, 1992; Sims and Bacic, 1995). For GC-MS, samples were resuspended in dichloromethane and subsequently loaded onto a BPX70 column for analysis as described previously (Lau and Bacic, 1993).
Polysaccharide content was estimated from the methylation data essentially as described by Shea et al. (1989) and Zhu et al. (2005).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. SEM of wild-type and mutant seeds.
Supplemental Figure S2. Mucilage release from luh mutants following chemical treatment.
Supplemental Figure S3. RT-PCR analysis using wild-type and luh mutant seed tissue.
Supplemental Figure S4. Mucilage release from seed coats following acid/alkali treatment.
Supplemental Figure S5. Comparison of developing wild-type, luh, and luh;lug/+ mutant seed coats.
Supplemental Table S1. Linkage analysis of alkali-extracted mucilage from wild-type, mutant, and transgenic lines.
Supplemental Table S2. Primer sequences used for RT-PCR, real-time PCR, and cloning.
Acknowledgments
We thank Nicole Crequer for help with root growth assays, Roger Curtain for technical assistance with the environmental SEM, Cherie Walsh for technical assistance with the GC-MS, and members of the Golz and Plant Cell Biology Research Centre laboratories for critically reading the manuscript. We also thank George Haughn for pointing out the similarities between the mum1 and luh phenotypes.
References
- Arsovski AA, Popma TM, Haughn GW, Carpita NC, McCann MC, Western TL. (2009) AtBXL1 encodes a bifunctional β-d-xylosidase/α-l-arabinofuranosidase required for pectic arabinan modification in Arabidopsis mucilage secretory cells. Plant Physiol 150: 1219–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadi P, O’Neill MA, Bergmann C, Darvill AG, Albersheim P. (1995) The backbone of the pectic polysaccharide rhamnogalacturonan I is cleaved by an endohydrolase and an endolyase. Glycobiology 5: 783–789 [DOI] [PubMed] [Google Scholar]
- Bao F, Azhakanandam S, Franks RG. (2010) SEUSS and SEUSS-LIKE transcriptional adaptors regulate floral and embryonic development in Arabidopsis. Plant Physiol 152: 821–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckman T, De Rycke R, Viane R, Inze D. (2000) Histological study of seed coat development in Arabidopsis thaliana. J Plant Res 113: 139–148 [Google Scholar]
- Boesewinkel FD, Bouman F. (1995) The seed: structure and function. In J Kigel, G Galili, eds, Seed Development and Germination. Marcel Dekker, New York, pp 1–24 [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM. (1989) Genes directing flower development in Arabidopsis. Plant Cell 1: 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, Medhurst A, Stone BA, Newbigin EJ, Bacic A, Fincher GB. (2006) Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-β-D-glucans. Science 311: 1940–1942 [DOI] [PubMed] [Google Scholar]
- Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA. (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408: 967–971 [DOI] [PubMed] [Google Scholar]
- Caffall KH, Pattathil S, Phillips SE, Hahn MG, Mohnen D. (2009) Arabidopsis thaliana T-DNA mutants implicate GAUT genes in the biosynthesis of pectin and xylan in cell walls and seed testa. Mol Plant 2: 1000–1014 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Coutinho PM, Henrissat B. (1999) Carbohydrate-active enzymes: an integrated database approach. Gilbert HJ, Davies G, Henrissat B, Svensson B, , Recent Advances in Carbohydrate Bioengineering. Royal Society of Chemistry, Cambridge, UK, pp 3–12 [Google Scholar]
- Dean GH, Zheng H, Tewari J, Huang J, Young DS, Hwang YT, Western TL, Carpita NC, McCann MC, Mansfield SD, et al. (2007) The Arabidopsis MUM2 gene encodes a β-galactosidase required for the production of seed coat mucilage with correct hydration properties. Plant Cell 19: 4007–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veau EJI, Gross KC, Huber DJ, Watada AE. (1993) Degradation and solubilization of pectin by β-galactosidases purified from avocado mesocarp. Physiol Plant 87: 279–285 [Google Scholar]
- Gutterman Y, Shemtov S. (1996) Structure and function of the mucilaginous seed coats of Plantago coronopus inhabiting the Negev desert of Israel. Isr J Plant Sci 44: 125–133 [Google Scholar]
- Johnson CS, Kolevski B, Smyth DR. (2002) TRANSPARENT TESTA GLABRA2, a trichome and seed coat development gene of Arabidopsis, encodes a WRKY transcription factor. Plant Cell 14: 1359–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Carpita NC. (1992) Changes in esterification of the uronic acid groups of cell wall polysaccharides during elongation of maize coleoptiles. Plant Physiol 98: 646–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C, Roberts K. (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissue of root apices. Planta 181: 512–521 [DOI] [PubMed] [Google Scholar]
- Koornneef M. (1981) The complex syndrome of ttg mutants. Arabidopsis Inf Serv 18: 45–51 [Google Scholar]
- Lau E, Bacic A. (1993) Capillary gas chromatography of partially methylated alditol acetates on a high-polarity, cross-liked, fused-silica BPX70 column. J Chromatogr 637: 100–103 [Google Scholar]
- Leon-Kloosterziel KM, Keijzer CJ, Koornneef M. (1994) A seed shape mutant of Arabidopsis that is affected in integument development. Plant Cell 6: 385–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SF, Milliken ON, Pham H, Seyit R, Napoli R, Preston J, Koltunow AM, Parish RW. (2009) The Arabidopsis MYB5 transcription factor regulates mucilage synthesis, seed coat development, and trichome morphogenesis. Plant Cell 21: 72–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Karmarkar V. (2008) Groucho/Tup1 family co-repressors in plant development. Trends Plant Sci 13: 137–144 [DOI] [PubMed] [Google Scholar]
- Macquet A, Ralet MC, Kronenberger J, Marion-Poll A, North HM. (2007a) In situ, chemical and macromolecular study of the composition of Arabidopsis thaliana seed coat mucilage. Plant Cell Physiol 48: 984–999 [DOI] [PubMed] [Google Scholar]
- Macquet A, Ralet MC, Loudet O, Kronenberger J, Mouille G, Marion-Poll A, North HM. (2007b) A naturally occurring mutation in an Arabidopsis accession affects a β-d-galactosidase that increases the hydrophilic potential of rhamnogalacturonan I in seed mucilage. Plant Cell 19: 3990–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen D. (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11: 266–277 [DOI] [PubMed] [Google Scholar]
- Mutter M, Colquhoun IJ, Beldman G, Schols HA, Bakx EJ, Voragen AG. (1998) Characterization of recombinant rhamnogalacturonan α-l-rhamnopyranosyl-(1,4)-α-d-galactopyranosyluronide lyase from Aspergillus aculeatus: an enzyme that fragments rhamnogalacturonan I regions of pectin. Plant Physiol 117: 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naran R, Pierce ML, Mort AJ. (2007) Detection and identification of rhamnogalacturonan lyase activity in intercellular spaces of expanding cotton cotyledons. Plant J 50: 95–107 [DOI] [PubMed] [Google Scholar]
- Nesi N, Debeaujon I, Jond C, Pelletier G, Caboche M, Lepiniec L. (2000) The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell 12: 1863–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Nemoto T, Jigami Y. (2007) Functional analysis of Arabidopsis thaliana RHM2/MUM4, a multidomain protein involved in UDP-D-glucose to UDP-L-rhamnose conversion. J Biol Chem 282: 5389–5403 [DOI] [PubMed] [Google Scholar]
- Penfield S, Meissner RC, Shoue DA, Carpita NC, Bevan MW. (2001) MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell 13: 2777–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautengarten C, Usadel B, Neumetzler L, Hartmann J, Büssis D, Altmann T. (2008) A subtilisin-like serine protease essential for mucilage release from Arabidopsis seed coats. Plant J 54: 466–480 [DOI] [PubMed] [Google Scholar]
- Schols HA, Voragen AGJ. (1996) Complex pectins: structure elucidation using enzymes. Visser J, Voragen AGJ, , Pectins and Pectinases. Elsevier Science, Amsterdam, pp 3–19 [Google Scholar]
- Shea EM, Gibeaut DM, Carpita NC. (1989) Structural analysis of the cell walls regenerated by carrot protoplasts. Planta 179: 293–308 [DOI] [PubMed] [Google Scholar]
- Sims IM, Bacic A. (1995) Extracellular polysaccharides from suspension cultures of Nicotiana plumbaginifolia. Phytochemistry 38: 1397–1405 [Google Scholar]
- Sitaraman J, Bui M, Liu Z. (2008) LEUNIG_HOMOLOG and LEUNIG perform partially redundant functions during Arabidopsis embryo and floral development. Plant Physiol 147: 672–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjøt M, Pauly M, Bush MS, Borkhardt B, McCann MC, Ulvskov P. (2002) Direct interference with rhamnogalacturonan I biosynthesis in Golgi vesicles. Plant Physiol 129: 95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar VV, Surendrarao A, Gonzalez D, Conlan RS, Liu Z. (2004) Transcriptional repression of target genes by LEUNIG and SEUSS, two interacting regulatory proteins for Arabidopsis flower development. Proc Natl Acad Sci USA 101: 11494–11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahle MI, Kuehlich J, Staron L, von Arnim AG, Golz JF. (2009) YABBYs and the transcriptional corepressors LEUNIG and LEUNIG_HOMOLOG maintain leaf polarity and meristem activity in Arabidopsis. Plant Cell 21: 3105–3118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadel B, Kuschinsky AM, Rosso MG, Eckermann N, Pauly M. (2004) RHM2 is involved in mucilage pectin synthesis and is required for the development of the seed coat in Arabidopsis. Plant Physiol 134: 286–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western TL. (2006) Changing spaces: the Arabidopsis mucilage secretory cells as a novel system to dissect cell wall production in differentiating cells. Can J Bot 84: 622–630 [Google Scholar]
- Western TL, Burn J, Tan WL, Skinner DJ, Martin-McCaffrey L, Moffatt BA, Haughn GW. (2001) Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiol 127: 998–1011 [PMC free article] [PubMed] [Google Scholar]
- Western TL, Skinner DJ, Haughn GW. (2000) Differentiation of mucilage secretory cells of the Arabidopsis seed coat. Plant Physiol 122: 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western TL, Young DS, Dean GH, Tan WL, Samuels AL, Haughn GW. (2004) MUCILAGE-MODIFIED4 encodes a putative pectin biosynthetic enzyme developmentally regulated by APETALA2, TRANSPARENT TESTA GLABRA1, and GLABRA2 in the Arabidopsis seed coat. Plant Physiol 134: 296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WG, Limberg G, Buchholt HC, van Alebeek GJ, Benen J, Christensen TM, Visser J, Voragen A, Mikkelsen JD, Knox JP. (2000) Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res 327: 309–320 [DOI] [PubMed] [Google Scholar]
- Willats WG, McCartney L, Knox JP. (2001) In-situ analysis of pectic polysaccharides in seed mucilage and at the root surface of Arabidopsis thaliana. Planta 213: 37–44 [DOI] [PubMed] [Google Scholar]
- Windsor JB, Symonds VV, Mendenhall J, Lloyd AM. (2000) Arabidopsis seed coat development: morphological differentiation of the outer integument. Plant J 22: 483–493 [DOI] [PubMed] [Google Scholar]
- Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A. (2003) A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130: 4859–4869 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Pettolino F, Mau SL, Bacic A. (2005) Characterization of cell wall polysaccharides from the medicinal plant Panax notoginseng. Phytochemistry 66: 1067–1076 [DOI] [PubMed] [Google Scholar]