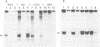Abstract
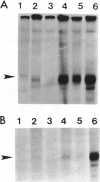
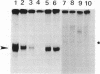
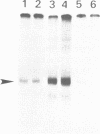
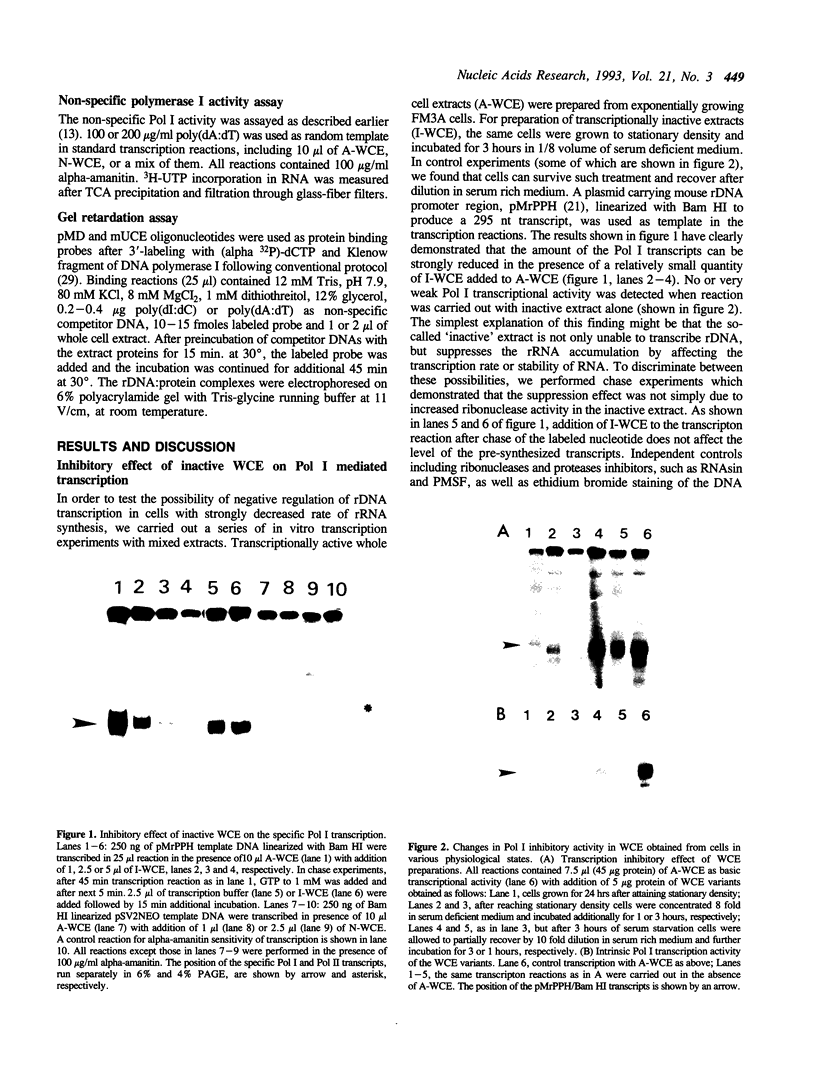
Extracts obtained from mouse cells growth arrested at stationary phase or under serum starvation exhibit no specific rDNA transcription activity. Experiments with mixed transcriptionally active and inactive whole cell extracts (WCE) obtained from rapidly dividing or growth arrested cells, respectively, demonstrate that rRNA synthesis in vitro can be suppressed by a polymerase I transcription inhibitory activity (PIN), present in inactive extracts. This inhibition effect is not related to increased nuclease activity and affects neither the non-specific Pol I transcription, nor a polymerase II promoter. A comparison of WCE isolated under different growth conditions indicates that PIN changes according to the physiological state of the cell. It reaches a maximal level soon after serum depletion and disappears rapidly when cells are allowed to recover in serum-rich medium. PIN can be clearly demonstrated in WCE but not in nuclear or cytoplasmic extracts and can be also obtained by an additional high salt extraction of nuclei. Furthermore, gel retardation and transcription-in-pellet assays demonstrate that rDNA promoter binding and preinitiation complex stability are similar in active and inactive WCE. This indicates that some later stage(s) of rDNA transcription, rather than the preinitiation complex formation, are attenuated by inactive extracts. Analysis of partially fractionated extracts suggests that PIN is not associated with but can be separated from polymerase I.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman E., Paule M. R. Regulation of eukaryotic ribosomal RNA transcription by RNA polymerase modification. Cell. 1986 Nov 7;47(3):445–450. doi: 10.1016/0092-8674(86)90601-x. [DOI] [PubMed] [Google Scholar]
- Bell S. P., Jantzen H. M., Tjian R. Assembly of alternative multiprotein complexes directs rRNA promoter selectivity. Genes Dev. 1990 Jun;4(6):943–954. doi: 10.1101/gad.4.6.943. [DOI] [PubMed] [Google Scholar]
- Bouche G., Caizergues-Ferrer M., Bugler B., Amalric F. Interrelations between the maturation of a 100 kDa nucleolar protein and pre rRNA synthesis in CHO cells. Nucleic Acids Res. 1984 Apr 11;12(7):3025–3035. doi: 10.1093/nar/12.7.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman L. H. rDNA transcription and pre-rRNA processing during the differentiation of a mouse myoblast cell line. Dev Biol. 1987 Jan;119(1):152–163. doi: 10.1016/0012-1606(87)90217-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Culotta V. C., Wides R. J., Sollner-Webb B. Eucaryotic transcription complexes are specifically associated in large sedimentable structures: rapid isolation of polymerase I, II, and III transcription factors. Mol Cell Biol. 1985 Jul;5(7):1582–1590. doi: 10.1128/mcb.5.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabeva M. D., Ikonomova R. N. Acceleration of ribosome formation in rat liver in response to hydrocortisone. Mol Cell Endocrinol. 1982 Nov-Dec;28(3):263–273. doi: 10.1016/0303-7207(82)90125-3. [DOI] [PubMed] [Google Scholar]
- Dauphinais C. The control of ribosomal RNA transcription in lymphocytes. Evidence that the rate of chain elongation is the limiting factor. Eur J Biochem. 1981 Mar;114(3):487–492. doi: 10.1111/j.1432-1033.1981.tb05171.x. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyhazi E., Pigon A., Chang J. H., Ghaffari S. H., Dreesen T. D., Wellman S. E., Case S. T., Olson M. O. Effects of anti-C23 (nucleolin) antibody on transcription of ribosomal DNA in Chironomus salivary gland cells. Exp Cell Res. 1988 Oct;178(2):264–272. doi: 10.1016/0014-4827(88)90397-7. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Sassone-Corsi P. More is better: activators and repressors from the same gene. Cell. 1992 Feb 7;68(3):411–414. doi: 10.1016/0092-8674(92)90178-f. [DOI] [PubMed] [Google Scholar]
- Gokal P. K., Mahajan P. B., Thompson E. A. Hormonal regulation of transcription of rDNA. Formation of initiated complexes by RNA polymerase I in vitro. J Biol Chem. 1990 Sep 25;265(27):16234–16243. [PubMed] [Google Scholar]
- Hammond M. L., Bowman L. H. Insulin stimulates the translation of ribosomal proteins and the transcription of rDNA in mouse myoblasts. J Biol Chem. 1988 Nov 25;263(33):17785–17791. [PubMed] [Google Scholar]
- Kato H., Nagamine M., Kominami R., Muramatsu M. Formation of the transcription initiation complex on mammalian rDNA. Mol Cell Biol. 1986 Oct;6(10):3418–3427. doi: 10.1128/mcb.6.10.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A., Grummt I. Dual role of the nucleolar transcription factor UBF: trans-activator and antirepressor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7340–7344. doi: 10.1073/pnas.89.16.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D. E., Zahradka P., Sells B. H. Control points in eucaryotic ribosome biogenesis. Biochem Cell Biol. 1991 Jan;69(1):5–22. doi: 10.1139/o91-002. [DOI] [PubMed] [Google Scholar]
- Mahajan P. B., Gokal P. K., Thompson E. A. Hormonal regulation of transcription of rDNA. The role of TFIC in formation of initiation complexes. J Biol Chem. 1990 Sep 25;265(27):16244–16247. [PubMed] [Google Scholar]
- Mahajan P. B., Thompson E. A. Hormonal regulation of transcription of rDNA. Purification and characterization of the hormone-regulated transcription factor IC. J Biol Chem. 1990 Sep 25;265(27):16225–16233. [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y., Financsek I., Kominami R., Muramatsu M. Fractionation and reconstitution of factors required for accurate transcription of mammalian ribosomal RNA genes: identification of a species-dependent initiation factor. Nucleic Acids Res. 1982 Nov 11;10(21):6659–6670. doi: 10.1093/nar/10.21.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine M., Kishimoto T., Aono J., Kato H., Kominami R., Muramatsu M. Sequestration analysis for RNA polymerase I transcription factors with various deletion and point mutations reveals different functional regions of the mouse rRNA gene promoter. Mol Cell Biol. 1987 Apr;7(4):1486–1495. doi: 10.1128/mcb.7.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. A., Bond U. Analysis of pre-rRNAs in heat-shocked HeLa cells allows identification of the upstream termination site of human polymerase I transcription. Mol Cell Biol. 1989 Jun;9(6):2500–2512. doi: 10.1128/mcb.9.6.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikaard C. S., Pape L. K., Henderson S. L., Ryan K., Paalman M. H., Lopata M. A., Reeder R. H., Sollner-Webb B. Enhancers for RNA polymerase I in mouse ribosomal DNA. Mol Cell Biol. 1990 Sep;10(9):4816–4825. doi: 10.1128/mcb.10.9.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein S. J., Dasgupta A. Inhibition of rRNA synthesis by poliovirus: specific inactivation of transcription factors. J Virol. 1989 Nov;63(11):4689–4696. doi: 10.1128/jvi.63.11.4689-4696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safrany G., Tanaka N., Kishimoto T., Ishikawa Y., Kato H., Kominami R., Muramatsu M. Structural determinant of the species-specific transcription of the mouse rRNA gene promoter. Mol Cell Biol. 1989 Jan;9(1):349–353. doi: 10.1128/mcb.9.1.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp A., Clos J., Hädelt W., Schreck R., Cvekl A., Grummt I. Isolation and functional characterization of TIF-IB, a factor that confers promoter specificity to mouse RNA polymerase I. Nucleic Acids Res. 1990 Mar 25;18(6):1385–1393. doi: 10.1093/nar/18.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp A., Pfleiderer C., Rosenbauer H., Grummt I. A growth-dependent transcription initiation factor (TIF-IA) interacting with RNA polymerase I regulates mouse ribosomal RNA synthesis. EMBO J. 1990 Sep;9(9):2857–2863. doi: 10.1002/j.1460-2075.1990.tb07475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. L., Nilson L. Multiple mechanisms for the inhibition of rRNA synthesis during HL-60 leukemia cell differentiation. J Cell Physiol. 1988 Sep;136(3):526–530. doi: 10.1002/jcp.1041360319. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tanaka N., Kato H., Ishikawa Y., Hisatake K., Tashiro K., Kominami R., Muramatsu M. Sequence-specific binding of a transcription factor TFID to the promoter region of mouse ribosomal RNA gene. J Biol Chem. 1990 Aug 15;265(23):13836–13842. [PubMed] [Google Scholar]
- Tower J., Sollner-Webb B. Transcription of mouse rDNA is regulated by an activated subform of RNA polymerase I. Cell. 1987 Sep 11;50(6):873–883. doi: 10.1016/0092-8674(87)90514-9. [DOI] [PubMed] [Google Scholar]
- Veinot-Drebot L. M., Singer R. A., Johnston G. C. Heat shock causes transient inhibition of yeast rRNA gene transcription. J Biol Chem. 1989 Nov 25;264(33):19473–19474. [PubMed] [Google Scholar]
- Voit R., Schnapp A., Kuhn A., Rosenbauer H., Hirschmann P., Stunnenberg H. G., Grummt I. The nucleolar transcription factor mUBF is phosphorylated by casein kinase II in the C-terminal hyperacidic tail which is essential for transactivation. EMBO J. 1992 Jun;11(6):2211–2218. doi: 10.1002/j.1460-2075.1992.tb05280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]