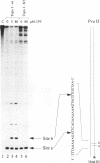
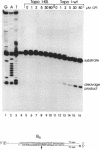
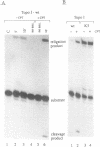
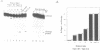Abstract
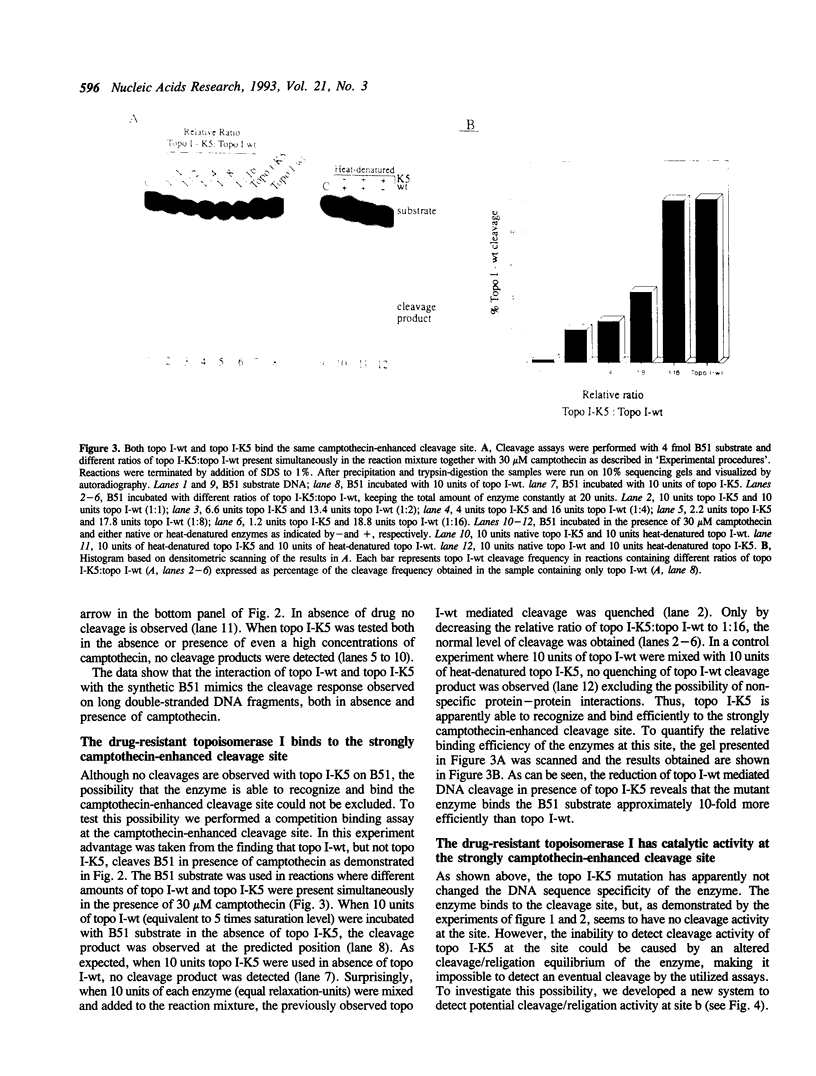
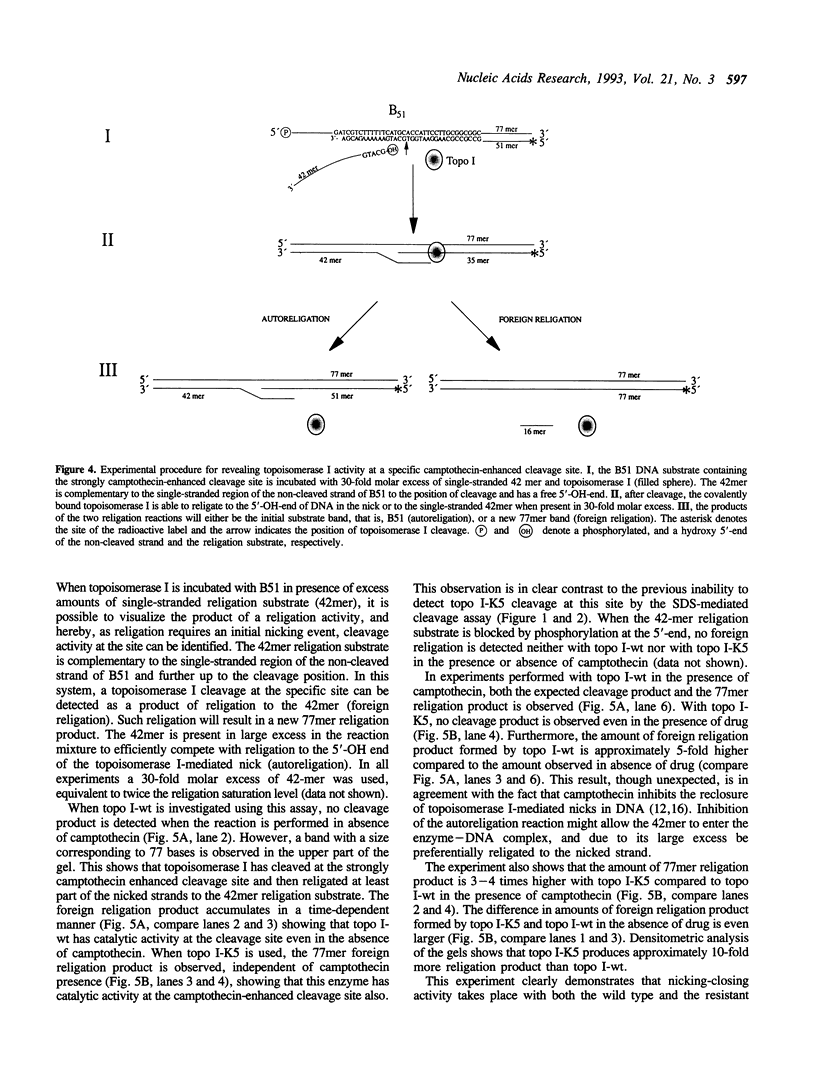
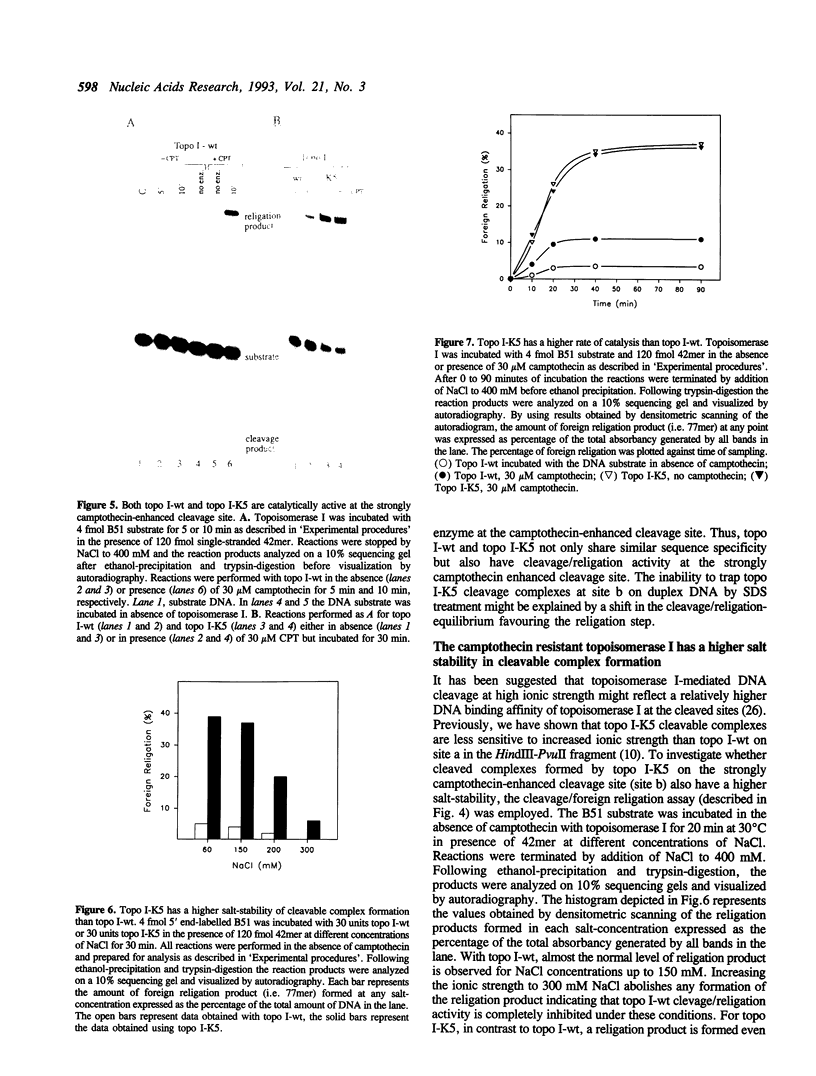
We investigated topoisomerase I activity at a specific camptothecin-enhanced cleavage site by use of a partly double-stranded DNA substrate. The cleavage site belongs to a group of DNA topoisomerase I sites which is only efficiently cleaved by wild-type topoisomerase I (topo I-wt) in the presence of camptothecin. With a mutated camptothecin-resistant form of topoisomerase I (topo I-K5) previous attempts to reveal cleavage activity at this site have failed. On this basis it was questioned whether the mutant enzyme has an altered DNA sequence recognition or a changed rate of catalysis at the site. Utilizing a newly developed assay system we demonstrate that topo I-K5 not only recognizes and binds to the strongly camptothecin-enhanced cleavage site but also has considerable cleavage/religation activity at this particular DNA site. Thus, topo I-K5 has a 10-fold higher rate of catalysis and a 10-fold higher affinity for DNA relative to topo I-wt. Our data indicate that the higher cleavage/religation activity of topo I-K5 is a result of improved DNA binding and a concomitant shift in the equilibrium between cleavage and religation towards the religation step. Thus, a recently identified point mutation which characterizes the camptothecin-resistant topo I-K5 has altered the enzymatic catalysis without disturbing the DNA sequence specificity of the enzyme.
Full text
PDF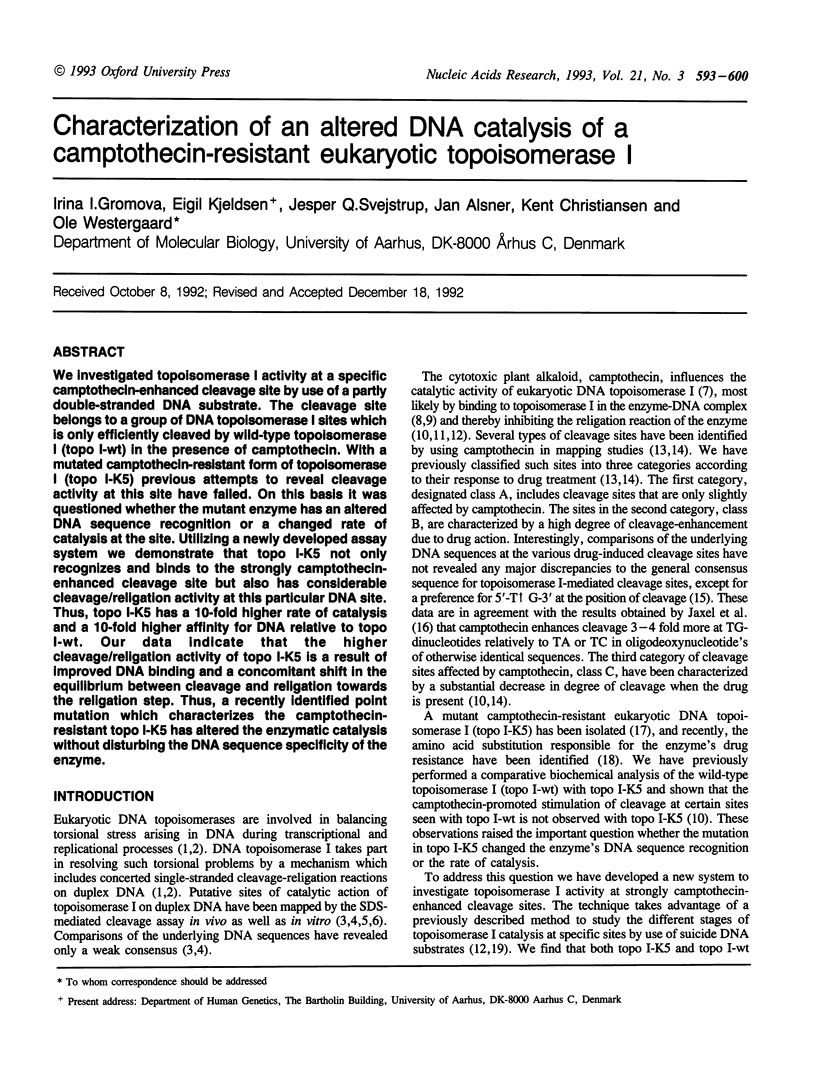




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. H., Christiansen K., Zechiedrich E. L., Jensen P. S., Osheroff N., Westergaard O. Strand specificity of the topoisomerase II mediated double-stranded DNA cleavage reaction. Biochemistry. 1989 Jul 25;28(15):6237–6244. doi: 10.1021/bi00441a015. [DOI] [PubMed] [Google Scholar]
- Andersen A. H., Gocke E., Bonven B. J., Nielsen O. F., Westergaard O. Topoisomerase I has a strong binding preference for a conserved hexadecameric sequence in the promoter region of the rRNA gene from Tetrahymena pyriformis. Nucleic Acids Res. 1985 Mar 11;13(5):1543–1557. doi: 10.1093/nar/13.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoh T., Ishii K., Suzuki Y., Ikegami Y., Kusunoki Y., Takemoto Y., Okada K. Characterization of a mammalian mutant with a camptothecin-resistant DNA topoisomerase I. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5565–5569. doi: 10.1073/pnas.84.16.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been M. D., Burgess R. R., Champoux J. J. Nucleotide sequence preference at rat liver and wheat germ type 1 DNA topoisomerase breakage sites in duplex SV40 DNA. Nucleic Acids Res. 1984 Apr 11;12(7):3097–3114. doi: 10.1093/nar/12.7.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonven B. J., Gocke E., Westergaard O. A high affinity topoisomerase I binding sequence is clustered at DNAase I hypersensitive sites in Tetrahymena R-chromatin. Cell. 1985 Jun;41(2):541–551. doi: 10.1016/s0092-8674(85)80027-1. [DOI] [PubMed] [Google Scholar]
- Champoux J. J., Aronoff R. The effects of camptothecin on the reaction and the specificity of the wheat germ type I topoisomerase. J Biol Chem. 1989 Jan 15;264(2):1010–1015. [PubMed] [Google Scholar]
- D'Arpa P., Machlin P. S., Ratrie H., 3rd, Rothfield N. F., Cleveland D. W., Earnshaw W. C. cDNA cloning of human DNA topoisomerase I: catalytic activity of a 67.7-kDa carboxyl-terminal fragment. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2543–2547. doi: 10.1073/pnas.85.8.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrassi F., De Salvia R., Tanzarella C., Palitti F. Induction of chromosomal aberrations and SCE by camptothecin, an inhibitor of mammalian topoisomerase I. Mutat Res. 1989 Mar;211(1):125–130. doi: 10.1016/0027-5107(89)90112-7. [DOI] [PubMed] [Google Scholar]
- Edwards K. A., Halligan B. D., Davis J. L., Nivera N. L., Liu L. F. Recognition sites of eukaryotic DNA topoisomerase I: DNA nucleotide sequencing analysis of topo I cleavage sites on SV40 DNA. Nucleic Acids Res. 1982 Apr 24;10(8):2565–2576. doi: 10.1093/nar/10.8.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromova I. I., Buchman V. L., Abagyan R. A., Ulyanov A. V., Bronstein I. B. Sequence dependent modulating effect of camptothecin on the DNA-cleaving activity of the calf thymus type I topoisomerase. Nucleic Acids Res. 1990 Feb 11;18(3):637–645. doi: 10.1093/nar/18.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg R. P., Busby R. W., Caranfa M. J., Holden K. G., Johnson R. K., Hecht S. M., Kingsbury W. D. Irreversible trapping of the DNA-topoisomerase I covalent complex. Affinity labeling of the camptothecin binding site. J Biol Chem. 1990 Nov 5;265(31):19287–19295. [PubMed] [Google Scholar]
- Hertzberg R. P., Caranfa M. J., Hecht S. M. On the mechanism of topoisomerase I inhibition by camptothecin: evidence for binding to an enzyme-DNA complex. Biochemistry. 1989 May 30;28(11):4629–4638. doi: 10.1021/bi00437a018. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Huff A. C., Leatherwood J. K., Kreuzer K. N. Bacteriophage T4 DNA topoisomerase is the target of antitumor agent 4'-(9-acridinylamino)methanesulfon-m-anisidide (m-AMSA) in T4-infected Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1307–1311. doi: 10.1073/pnas.86.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K., Hasegawa T., Fujisawa K., Andoh T. Rapid purification and characterization of DNA topoisomerase I from cultured mouse mammary carcinoma FM3A cells. J Biol Chem. 1983 Oct 25;258(20):12728–12732. [PubMed] [Google Scholar]
- Jaxel C., Capranico G., Kerrigan D., Kohn K. W., Pommier Y. Effect of local DNA sequence on topoisomerase I cleavage in the presence or absence of camptothecin. J Biol Chem. 1991 Oct 25;266(30):20418–20423. [PubMed] [Google Scholar]
- Kjeldsen E., Bonven B. J., Andoh T., Ishii K., Okada K., Bolund L., Westergaard O. Characterization of a camptothecin-resistant human DNA topoisomerase I. J Biol Chem. 1988 Mar 15;263(8):3912–3916. [PubMed] [Google Scholar]
- Kjeldsen E., Mollerup S., Thomsen B., Bonven B. J., Bolund L., Westergaard O. Sequence-dependent effect of camptothecin on human topoisomerase I DNA cleavage. J Mol Biol. 1988 Jul 20;202(2):333–342. doi: 10.1016/0022-2836(88)90462-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. C., Shen C. K. Specificity and flexibility of the recognition of DNA helical structure by eukaryotic topoisomerase I. J Mol Biol. 1990 Mar 5;212(1):67–78. doi: 10.1016/0022-2836(90)90305-6. [DOI] [PubMed] [Google Scholar]
- Shuman S., Prescott J. Specific DNA cleavage and binding by vaccinia virus DNA topoisomerase I. J Biol Chem. 1990 Oct 15;265(29):17826–17836. [PubMed] [Google Scholar]
- Svejstrup J. Q., Christiansen K., Andersen A. H., Lund K., Westergaard O. Minimal DNA duplex requirements for topoisomerase I-mediated cleavage in vitro. J Biol Chem. 1990 Jul 25;265(21):12529–12535. [PubMed] [Google Scholar]
- Svejstrup J. Q., Christiansen K., Gromova I. I., Andersen A. H., Westergaard O. New technique for uncoupling the cleavage and religation reactions of eukaryotic topoisomerase I. The mode of action of camptothecin at a specific recognition site. J Mol Biol. 1991 Dec 5;222(3):669–678. doi: 10.1016/0022-2836(91)90503-x. [DOI] [PubMed] [Google Scholar]
- Tamura H., Kohchi C., Yamada R., Ikeda T., Koiwai O., Patterson E., Keene J. D., Okada K., Kjeldsen E., Nishikawa K. Molecular cloning of a cDNA of a camptothecin-resistant human DNA topoisomerase I and identification of mutation sites. Nucleic Acids Res. 1991 Jan 11;19(1):69–75. doi: 10.1093/nar/19.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen B., Mollerup S., Bonven B. J., Frank R., Blöcker H., Nielsen O. F., Westergaard O. Sequence specificity of DNA topoisomerase I in the presence and absence of camptothecin. EMBO J. 1987 Jun;6(6):1817–1823. doi: 10.1002/j.1460-2075.1987.tb02436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosberg H. P. DNA topoisomerases: enzymes that control DNA conformation. Curr Top Microbiol Immunol. 1985;114:19–102. doi: 10.1007/978-3-642-70227-3_2. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]