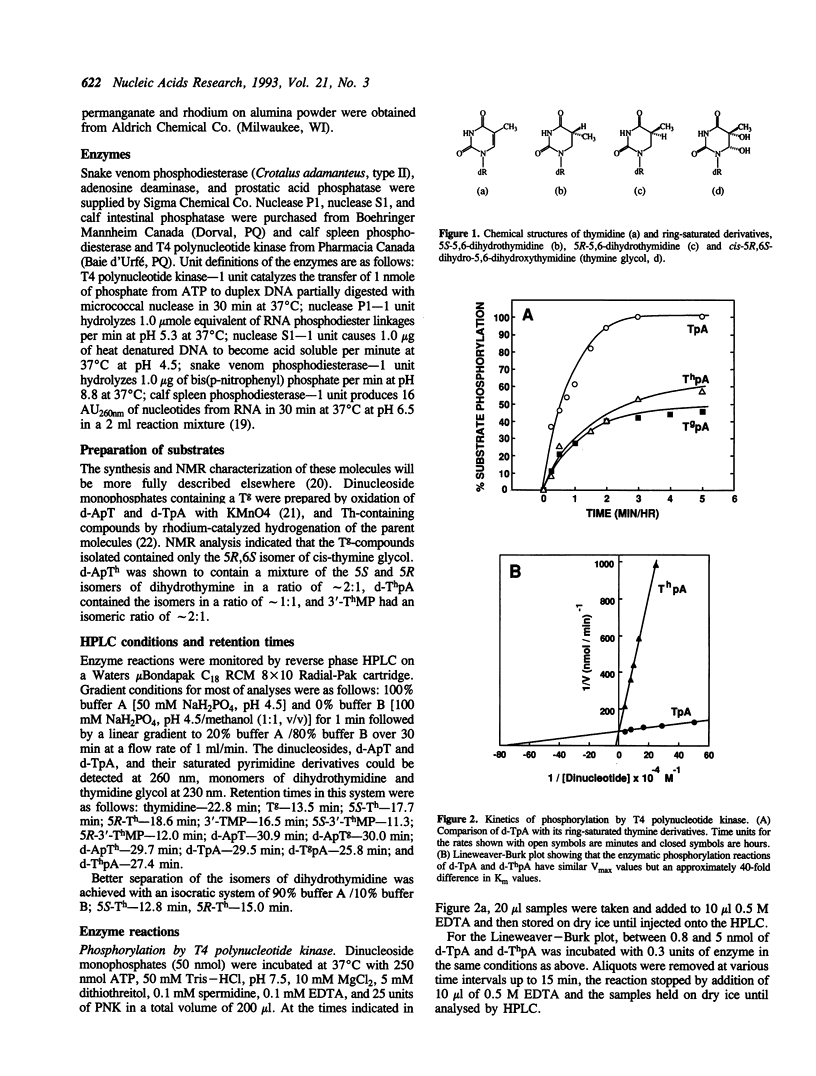
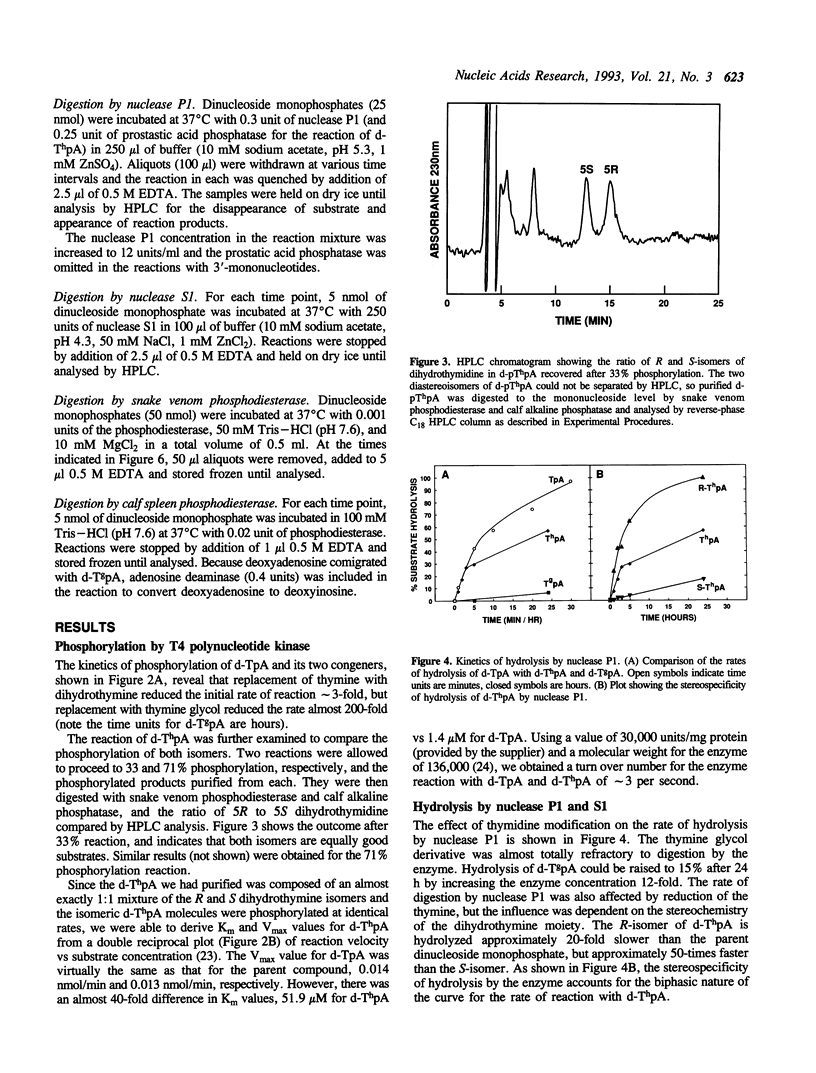
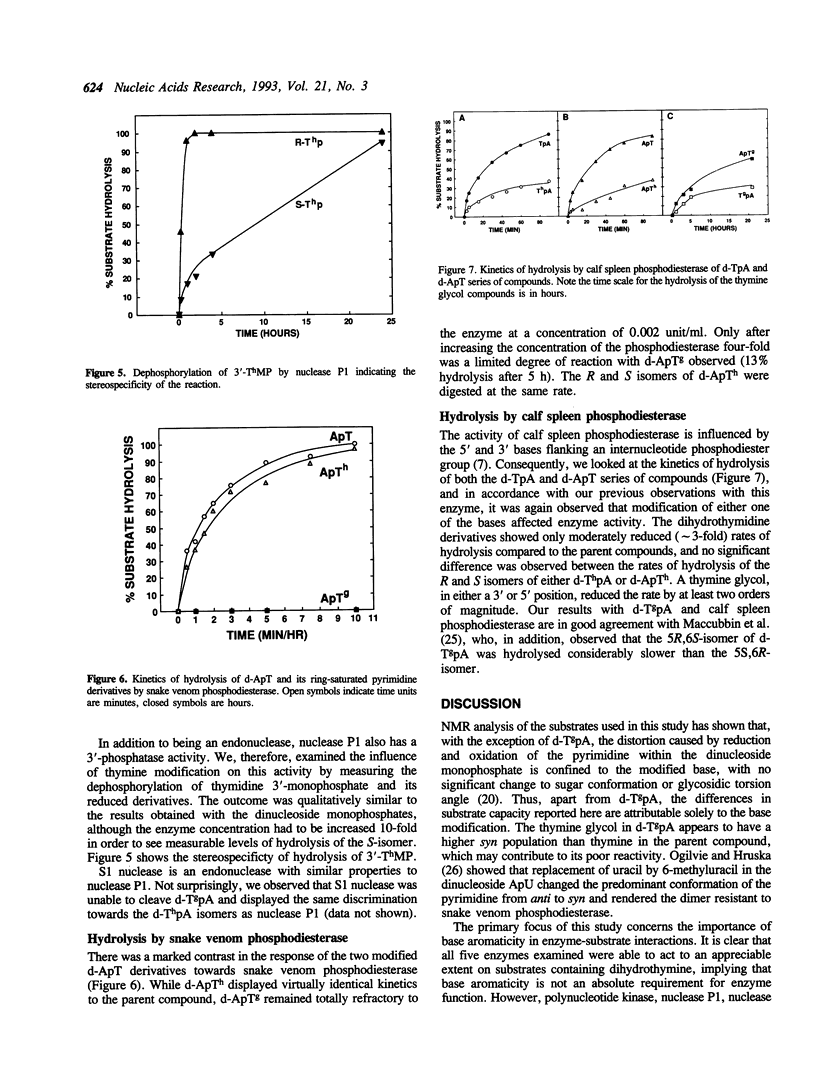
Abstract
Stacking between aromatic amino acids and nucleic acid bases may play an important role in the interaction of enzymes with nucleic acid substrates. In such circumstances, disruption of base aromaticity would be expected to decrease enzyme activity on the modified substrates. We have examined the requirement for DNA base aromaticity of five enzymes that act on single-stranded DNA, T4 polynucleotide kinase, nucleases P1 and S1, and snake venom and calf spleen phosphodiesterases, by comparing their kinetics of reaction with a series of dinucleoside monophosphates containing thymidine or a ring-saturated derivative. The modified substrates contained either cis-5R,6S-di-hydro-5,6-dihydroxythymidine (thymidine glycol) or a mixture of the 5R and 5S isomers of 5,6-dihydrothymidine. It was observed that for all the enzymes, except snake venom phosphodiesterase, the parent molecules were better substrates than the dihydrothymidine derivatives, while the thymidine glycol compounds were significantly poorer substrates. Snake venom phosphodiesterase acted on the unmodified and dihydrothymidine molecules at almost the same rate. These results imply that for all the remaining enzymes base aromaticity is a factor in enzyme-substrate interaction, but that additional factors must contribute to the poorer substrate capacity of the thymidine glycol compounds. The influence of the stereochemistry of the dihydrothymidine derivatives was also investigated. We observed that nuclease P1 and S1 hydrolysed the molecules containing 5R-dihydrothymidine approximately 50-times faster than those containing the S-isomer. The other enzymes displayed no measurable stereospecificity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach A. C., Gupta R. C. Human biomonitoring and the 32P-postlabeling assay. Carcinogenesis. 1992 Jul;13(7):1053–1074. doi: 10.1093/carcin/13.7.1053. [DOI] [PubMed] [Google Scholar]
- Cadet J., Voituriez L., Hruska F. E., Grand A. Crystal structure of the cis-syn photodimer of thymidylyl (3'-5') thymidine cyanoethyl ester. Biopolymers. 1985 May;24(5):897–903. doi: 10.1002/bip.360240512. [DOI] [PubMed] [Google Scholar]
- Dimicoli J. L., Hélène C. Interactions of aromatic residues of proteins with nucleic acids. I. Proton magnetic resonance studies of the binding of tryptophan-containing peptides to poly(adenylic acid) and deoxyribonucleic acid. Biochemistry. 1974 Feb 12;13(4):714–723. doi: 10.1021/bi00701a013. [DOI] [PubMed] [Google Scholar]
- Hegi M. E., Sagelsdorff P., Lutz W. K. Detection by 32P-postlabeling of thymidine glycol in gamma-irradiated DNA. Carcinogenesis. 1989 Jan;10(1):43–47. doi: 10.1093/carcin/10.1.43. [DOI] [PubMed] [Google Scholar]
- Ide H., Wallace S. S. Dihydrothymidine and thymidine glycol triphosphates as substrates for DNA polymerases: differential recognition of thymine C5-C6 bond saturation and sequence specificity of incorporation. Nucleic Acids Res. 1988 Dec 9;16(23):11339–11354. doi: 10.1093/nar/16.23.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan L., Voituriez L., Cadet J. Nuclear magnetic resonance studies of cis-syn, trans-syn, and 6-4 photodimers of thymidylyl(3'-5')thymidine monophosphate and cis-syn photodimers of thymidylyl(3'-5')thymidine cyanoethyl phosphotriester. Biochemistry. 1988 Jul 26;27(15):5796–5803. doi: 10.1021/bi00415a060. [DOI] [PubMed] [Google Scholar]
- Lenz A., Cordes F., Heinemann U., Saenger W. Evidence for a substrate-binding subsite in ribonuclease T1. Crystal structure of the complex with two guanosines, and model building of the complex with the substrate guanylyl-3',5'-guanosine. J Biol Chem. 1991 Apr 25;266(12):7661–7667. [PubMed] [Google Scholar]
- Lillehaug J. R. Physicochemical properties of T4 polynucleotide kinase. Eur J Biochem. 1977 Mar 1;73(2):499–506. doi: 10.1111/j.1432-1033.1977.tb11343.x. [DOI] [PubMed] [Google Scholar]
- Liuzzi M., Weinfeld M., Paterson M. C. Enzymatic analysis of isomeric trithymidylates containing ultraviolet light-induced cyclobutane pyrimidine dimers. I. Nuclease P1-mediated hydrolysis of the intradimer phosphodiester linkage. J Biol Chem. 1989 Apr 15;264(11):6355–6363. [PubMed] [Google Scholar]
- Maccubbin A., Evans M., Paul C. R., Budzinski E. E., Przybyszewski J., Box H. C. Enzymatic excision of radiation-induced lesions from DNA model compounds. Radiat Res. 1991 Apr;126(1):21–26. [PubMed] [Google Scholar]
- Midgley C. A., Murray N. E. T4 polynucleotide kinase; cloning of the gene (pseT) and amplification of its product. EMBO J. 1985 Oct;4(10):2695–2703. doi: 10.1002/j.1460-2075.1985.tb03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K. K., Hruska F. H. Affect of spleen and snake venom phosphodiesterases on nucleotides containing nucleosides in the syn conformation. Biochem Biophys Res Commun. 1976 Jan 26;68(2):375–378. doi: 10.1016/0006-291x(76)91155-4. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E., Danna T. F., van Golen L., Putman K. L. A new sensitive 32P-postlabeling assay based on the specific enzymatic conversion of bulky DNA lesions to radiolabeled dinucleotides and nucleoside 5'-monophosphates. Carcinogenesis. 1989 Jul;10(7):1231–1239. doi: 10.1093/carcin/10.7.1231. [DOI] [PubMed] [Google Scholar]
- Randerath K., Reddy M. V., Gupta R. C. 32P-labeling test for DNA damage. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6126–6129. doi: 10.1073/pnas.78.10.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. V., Bleicher W. T., Blackburn G. R. 32P-postlabeling detection of thymine glycols: evaluation of adduct recoveries after enhancement with affinity chromatography, nuclease P1, nuclease S1, and polynucleotide kinase. Cancer Commun. 1991 Apr;3(4):109–117. doi: 10.3727/095535491820873416. [DOI] [PubMed] [Google Scholar]
- Vaishnav Y., Holwitt E., Swenberg C., Lee H. C., Kan L. S. Synthesis and characterization of stereoisomers of 5,6-dihydro-5,6-dihydroxy-thymidine. J Biomol Struct Dyn. 1991 Apr;8(5):935–951. doi: 10.1080/07391102.1991.10507858. [DOI] [PubMed] [Google Scholar]
- Volbeda A., Lahm A., Sakiyama F., Suck D. Crystal structure of Penicillium citrinum P1 nuclease at 2.8 A resolution. EMBO J. 1991 Jul;10(7):1607–1618. doi: 10.1002/j.1460-2075.1991.tb07683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinfeld M., Liuzzi M., Paterson M. C. Enzymatic analysis of isomeric trithymidylates containing ultraviolet light-induced cyclobutane pyrimidine dimers. II. Phosphorylation by phage T4 polynucleotide kinase. J Biol Chem. 1989 Apr 15;264(11):6364–6370. [PubMed] [Google Scholar]
- Weinfeld M., Liuzzi M., Paterson M. C. Response of phage T4 polynucleotide kinase toward dinucleotides containing apurinic sites: design of a 32P-postlabeling assay for apurinic sites in DNA. Biochemistry. 1990 Feb 20;29(7):1737–1743. doi: 10.1021/bi00459a011. [DOI] [PubMed] [Google Scholar]
- Weinfeld M., Liuzzi M., Paterson M. C. Selective hydrolysis by exo- and endonucleases of phosphodiester bonds adjacent to an apurinic site. Nucleic Acids Res. 1989 May 25;17(10):3735–3745. doi: 10.1093/nar/17.10.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinfeld M., Livingston D. C. Synthesis and properties of oligodeoxyribonucleotides containing an ethylated internucleotide phosphate. Biochemistry. 1986 Sep 9;25(18):5083–5091. doi: 10.1021/bi00366a016. [DOI] [PubMed] [Google Scholar]
- Weinfeld M., Soderlind K. J. 32P-postlabeling detection of radiation-induced DNA damage: identification and estimation of thymine glycols and phosphoglycolate termini. Biochemistry. 1991 Jan 29;30(4):1091–1097. doi: 10.1021/bi00218a031. [DOI] [PubMed] [Google Scholar]
- van Amerongen H., Kuil M. E., Scheerhagen M. A., van Grondelle R. Structure calculations for single-stranded DNA complexed with the single-stranded DNA binding protein GP32 of bacteriophage T4: a remarkable DNA structure. Biochemistry. 1990 Jun 12;29(23):5619–5625. doi: 10.1021/bi00475a029. [DOI] [PubMed] [Google Scholar]


