SUMMARY
Homeostasis of intracellular pH is a trait critical for survival of Mycobacterium tuberculosis (Mtb) in macrophages. However, mechanisms by which Mtb adapts to acidic environments are poorly understood. In this study, we analyzed the physiological functions of OmpATb, a surface-accessible protein of Mtb. OmpATb did not complement the permeability defects of an M. smegmatis porin mutant to glucose, serine and glycerol, in contrast to the porin MspA. Uptake rates of these solutes were unchanged in an ompATb operon mutant of Mtb indicating that OmpATb is not a general porin. Chemical analysis of low pH culture filtrates showed that the proteins encoded by the ompATb operon are involved in generating a rapid ammonia burst, which neutralized medium pH and preceded exponential growth of Mtb. Addition of ammonia accelerated growth of the ompATb operon mutant demonstrating that ammonia secretion is indeed a mechanism by which Mtb neutralizes acidic environments. Infection experiments revealed that the ompATb operon was not required for full virulence in mice suggesting that Mtb has multiple mechanisms of resisting phagosomal acidification. Taken together, these results show that the ompATb operon is necessary for rapid ammonia secretion and adaptation of Mtb to acidic environments in vitro but not in mice.
Keywords: outer membrane, permeability, porin, virulence, cell wall
INTRODUCTION
Intracellular pH homeostasis is critical for unicellular microorganisms when they face rapid and drastic changes in the environmental pH (Slonczewski et al., 2009). For instance, ingested bacteria encounter the strong acid barrier in the human stomach which has a median pH of 1.4 (Teyssen et al., 1995). Bacteria which enter human hosts by other routes often encounter professional phagocytes such as macrophages which engulf bacteria in a membrane vesicle that ultimately fuses with acidic lysosomes. This process delivers not only bactericidal compounds such as reactive nitrogen and oxygen intermediates and defensins (Rohde et al., 2007), but also reduces the pH inside the bacteria-containing phagosome to less than 5.5 (Lukacs et al., 1991). Not surprisingly, pH adaptation is an evolutionary trait of pathogenic bacteria (Foster, 1999).
Mycobacterium tuberculosis is an intracellular pathogen which inhibits the fusion of phagosomes with lysosomes and resides in a mildly acidic environment of pH 6.2. However, macrophages can overcome this fusion block by activation with interferon-γ and acidify the M. tuberculosis-containing phagosome to pH 4.5 - 5.0 (MacMicking et al., 2003). Increased expression of acid-responsive genes during macrophage infection (Rohde et al., 2007) and attenuation of acid-sensitive mutants in vivo further indicate that M. tuberculosis encounters and responds to acidity in the host (Vandal et al., 2009a). Mycobacteria including M. tuberculosis maintain a neutral internal pH in an acidic environment (Zhang et al., 1999, Rao et al., 2001, Vandal et al., 2008), but the mechanisms utilized by M. tuberculosis in adaptation to acidic environments are poorly understood.
An M. tuberculosis mutant lacking Rv3671c, a membrane-associated protease, was sensitive to acid and failed to maintain intrabacterial pH in vitro and in activated macrophages (Vandal et al., 2008). Growth of the rv3671c deletion mutant was severely attenuated in mice. It was concluded that the ability of M. tuberculosis to resist acid is in large part due to Rv3671c, and that this resistance is essential for virulence. Recently, four additional acid-sensitive mutants of M. tuberculosis lacking proteins implicated in cell wall biosynthesis were shown to be hypersensitive to lipophilic antibiotics and SDS, underlining the importance of an intact cell wall for protection against acid stress (Vandal et al., 2009b).
The primary permeability barrier of mycobacteria is established by an unusual outer membrane (Hoffmann et al., 2007, Hoffmann et al., 2008). Channel-forming proteins such as the porin MspA enable transport of small and hydrophilic solutes across the outer membrane of M. smegmatis (Niederweis et al., 1999, Stahl et al., 2001, Faller et al., 2004, Stephan et al., 2005). MspA-like porins do not exist in M. tuberculosis (Niederweis, 2008a); however, Rv0899 (OmpATb) of M. tuberculosis shares some similarity to the channel-forming OmpA proteins of Gram-negative bacteria. OmpA of E. coli appears to exist in two distinct conformations, only one of which forms pores (Sugawara & Nikaido, 1994). However, the closed conformation seems to be predominant, a finding consistent with the low channel activity of purified OmpA (Sugawara & Nikaido, 1992) and the lack of a permeability defect of ompA mutants (Nikaido et al., 1977). Homologues of OmpATb are only present in pathogenic mycobacteria, but not in saprophytic mycobacteria such as M. smegmatis. Similar to OmpA proteins from Gram-negative bacteria, OmpATb of M. tuberculosis forms channels in vitro (Senaratne et al., 1998, Alahari et al., 2007, Molle et al., 2006). Uptake experiments indicated that OmpATb may have porin function in M. tuberculosis (Raynaud et al., 2002). Growth experiments in vitro and mouse infection experiments indicated that OmpATb plays a role in acid resistance and virulence (Raynaud et al., 2002). Consistent with the role of OmpATb in acid adaptation of M. tuberculosis was the observation that ompATb transcription is strongly induced at acidic pH and in macrophages (Raynaud et al., 2002). However, these two functions appear to be antagonistic because a general response by bacteria to acid stress is to reduce porin expression and thereby decrease the permeability of their outer membrane to protons (Begic & Worobec, 2006, Samartzidou et al., 2003, Thomas & Booth, 1992). Experiments with truncated OmpATb proteins revealed that the N-terminal domain (residues 1-73) is required for export in mycobacteria (Alahari et al., 2007), while the central domain (residues 73-200) is sufficient to form channels in lipid bilayers (Molle et al., 2006). However, the NMR structure showed that the central OmpATb domain does not form any transmembrane or pore structure (Teriete et al., 2010).
In the light of these contradictory findings, we examined whether OmpATb has porin function in both M. smegmatis and M. tuberculosis by uptake experiments. We did not detect any porin activity of OmpATb in mycobacteria. However, we provide evidence that ompATb is part of an operon of three genes, which is required for fast ammonia secretion, rapid pH neutralization and growth of M. tuberculosis in acidic environments.
RESULTS
Expression and localization of OmpATb in the outer membrane of M. smegmatis
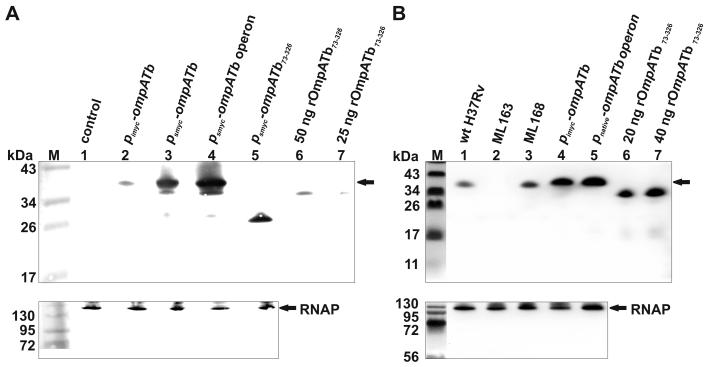
To examine whether OmpATb of M. tuberculosis has porin function, we exploited the pronounced permeability defects of the porin mutant M. smegmatis ML16 (Table 1) which lacks three out of four known porins (MspA, MspC, MspD). For example, the permeability of ML16 for glucose is 50-fold lower than that of wt M. smegmatis (Stephan et al., 2005), enabling the detection of low-activity porins. To examine whether OmpATb has porin function in mycobacteria, we expressed ompATb in M. smegmatis ML16 to determine if the permeability defects of this strain could be complemented. OmpATb levels were higher when expressed under the control of the psmyc promoter of M. smegmatis in the plasmid pML003 (Table 2) in comparison to the pimyc promoter construct in pML588 (Fig. 1A, lanes 2, 3). By contrast, no background was detected in M. smegmatis containing the empty plasmid pMS2 (Fig. 1A, lane 1).
Table 1. Strains used in this work.
The annotations CmR, SmR, and HygR indicate that the strain is resistant to the antibiotics chloramphenicol, streptomycin, and hygromycin, respectively.
| Strain | Parent strain and relevant genotype | Source or reference |
|---|---|---|
| E. coli DH5α | recA1, endA1, gyrA96, thi; re/A1,hsdR17(rK−,mK+), supE44, ϕ80ΔlacZΔM15, ΔlacZ(YA-argF)UE169 | (Hanahan, 1983) |
| E. coli BL21 Rosetta | F− ompT hsdSB(RB− mB−) gal dcm λ(DE3) pLysSRARE; CmR | Novagen |
| M. smegmatis SMR5 | M. smegmatis mc2155; SmR | (Sander et al., 1995) |
| ML16 |
M. smegmatis SMR5, ΔmspA::FRT, ΔmspC::FRT, ΔmspD::FRT, attB::loxP, FRT; SmR |
(Stephan et al., 2005) |
| M. tuberculosis H37Rv | Laboratory strain | ATCC 25618 |
| ML157 | M. tuberculosis, ompATb::pML564 | this study |
| ML160 | M. tuberculosis, ΔompATb::loxP-hyg-loxP; HygR | this study |
| ML163 | M. tuberculosis, ΔompATb::loxP | this study |
| ML168 | ML163, attB::pML759 | this study |
Table 2. Plasmids used in this work.
Up- and downstream homologous sequences of genes are subscripted as up and down. “Origin” denotes origin of replication. The annotations HygR and KmR indicate that the plasmid confers resistance to hygromycin and kanamycin, respectively.
| Plasmid | Components and properties | Source or reference |
|---|---|---|
| pMS2 | ColE1 origin; PAL5000 origin; HygR; 5229 bp | (Kaps et al., 2001) |
| pMN016 | psmyc-mspA; ColE1 origin; PAL5000 origin; HygR; 6164 bp | (Stephan et al., 2005) |
| pMN013 | pimyc-mspA; ColE1 origin; PAL5000 origin; HygR; 6000 bp | (Mailaender et al., 2004) |
| pCV125 | ColE1 origin; KmR; L5 attP; L5 integrase; 8261 bp | (Steyn et al., 2003) |
| pML003 | psmyc-ompATb; ColE1 origin; PAL5000 origin; HygR; 6534 bp | (Song et al., 2008) |
| pML588 | pimyc-ompATb; ColE1 origin; PAL5000 origin; HygR; 6357 bp | (Song et al., 2008) |
| pML763 | pnative-ompATb-rv0900-rv0901; ColE1 origin; PAL5000 origin; HygR; 8023 bp | this study |
| pML1450 | psmyc-ompATb-rv0900-rv0901; ColE1 origin; PAL5000 origin; HygR; 7308 bp | this study |
| pML2334 | psmyc-ompATb73-326; ColE1 origin; PAL5000 origin; HygR; 6292 bp | this study |
| pML759 | ColE1 origin; pimyc-ompATb; KmR; L5 attP; L5 integrase; 9457 bp | this study |
| pML564 | ColE1 origin; PAL5000 origin; HygR; sacB; ompATbup, ompATbdown; 10630 bp | this study |
| pML591 | f1 origin; pT7-ompATbHis; KmR; 6129 bp | this study |
Fig. 1. Expression of ompATb genes in mycobacteria.
A: Western blot analysis of ompATb expression in the porin mutant M. smegmatis ML16 (ΔmspA ΔmspC ΔmspD). Proteins of M. smegmatis ML16 were extracted with 1% SDS, separated on a 10% SDS-polyacrylamide gel, and transferred to a PVDF membrane. Upper panel: OmpATb was detected with an OmpATb-specific antiserum (Senaratne et al., 1998). Lanes: M, Pre-Stained Protein Ladder; 1, ML16/pMS2 (empty vector); 2, ML16/pML588; 3, ML16/pML003; 4, ML16/pML1450; 5, ML16/pML2334; 6, 50 ng rOmpATb73-326 purified from E. coli; 7, 25 ng rOmpATb73-326 purified from E. coli. Arrow denotes full-length OmpATb. Lower panel: M. smegmatis RNA polymerase (RNAP) was detected on the same blot with a monoclonal antibody.
B: Western blot analysis of ompATb expression in M. tuberculosis strains. Proteins were extracted, separated, blotted, and detected as described above. Lanes: M, Pre-Stained Protein Ladder; 1, wt M. tuberculosis H37Rv; 2, ?ompATb strain ML163 (?ompATb::loxP); 3, ML168 (ML163::ompATb); 4, ML163/pML588 (pimyc-ompATb); 5, ML163/pML763 (ompATb operon); 6, 20 ng rOmpATb73-326 purified from E. coli; 7, 40 ng rOmpATb73-326 purified from E. coli. Arrow denotes full-length OmpATb. Lower panel: M. tuberculosis RNAP was detected on the same blot with a monoclonal antibody.
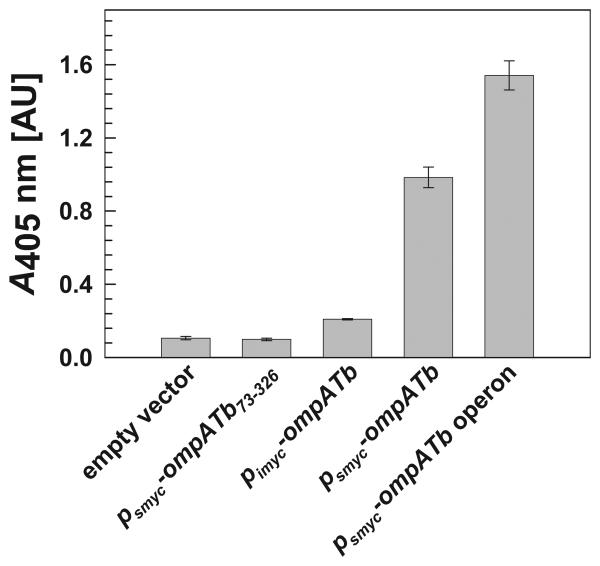
OmpATb is accessible at the cell surface of M. tuberculosis and M. bovis BCG (Song et al., 2008). However, it was unknown whether heterologous ompATb expression in M. smegmatis would result in correctly localized protein, which may require other proteins of M. tuberculosis. To determine whether OmpATb correctly inserted in the outer membrane of the triple porin mutant M. smegmatis ML16, an ELISA with whole cells was employed as recently described (Huff et al., 2009). OmpATb was detected on the cell surface of M. smegmatis ML16 by OmpATb antibodies (Fig. 2). Truncated OmpATb73-326, which lacks the N-terminal domain required for export (Alahari et al., 2007), was expressed in ML16 (Fig. 1A, lane 5), but was not detected on the surface of whole cells (Fig. 2), demonstrating that cells in the ELISA were intact. It is concluded that full-length OmpATb is surface-accessible in M. smegmatis. Further, surface detection of OmpATb (Fig. 2) mirrored expression levels determined in Western blots of cell extracts (Fig. 1A). More OmpATb was detected on the surface of whole cells when ompATb was expressed under the control of the psmyc promoter (pML003) in comparison to the pimyc promoter (pML588), indicating that higher expression levels equate to higher levels of correctly localized, surface-accessible OmpATb in M. smegmatis. For this reason, further experiments in M. smegmatis were performed using the psmyc promoter to drive expression of ompATb.
Fig. 2. Surface accessibility of OmpATb in M. smegmatis by whole cell ELISA.
Surface-accessible OmpATb was probed for on whole cells of M. smegmatis ML16 using a rabbit OmpATb-specific antiserum. Binding of a secondary alkaline phosphatase conjugate antibody was followed by addition of pNPP and subsequent absorbance readings at 405 nm. From left to right, 1st bar: M. smegmatis ML16 (ΔmspA ΔmspC ΔmspD) with the empty vector control pMS2; 2nd bar: ML16/pML2334; 3rd bar: ML16/pML588; 4th bar: ML16/pML003; 5th bar: ML16/pML1450. Data were obtained from four replicates and error bars represent standard errors.
OmpATb does not enhance growth or nutrient uptake of M. smegmatis ML16
Lack of the porins MspA, MspC and MspD and the concomitantly much slower uptake of hydrophilic nutrients results in slower growth of M. smegmatis ML16 on agar plates and in liquid culture (Stephan et al., 2005). This phenotype is complemented by expression of mspA (Fig. S1) and can be used to detect porin activity in vivo. In contrast, expression of ompATb did not complement the slow growth defect of M. smegmatis ML16 on agar plates (Fig. S1).
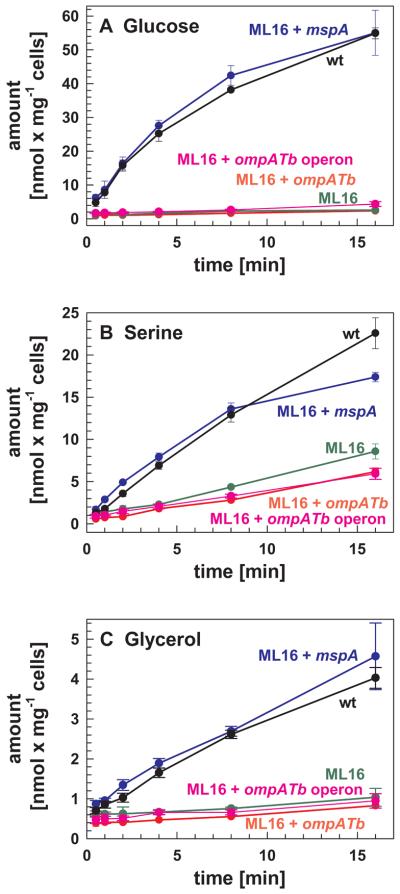
Porin activity can be quantified by measuring the intracellular accumulation of small, hydrophilic solutes if diffusion across the outer membrane constitutes the rate-limiting step in uptake of a particular solute. Glucose has been used as a model solute for these purposes in mycobacteria (Stahl et al., 2001, Mailaender et al., 2004, Yuan et al., 1998, Stephan et al., 2005). As the OmpATb pore closes at low pH (Molle et al., 2006), uptake experiments were performed near a neutral pH. Uptake of glucose by the porin mutant ML16 was reduced 25-fold compared to wt M. smegmatis (Fig. 3A and Table 3), in good agreement with previous results (Stephan et al., 2005, Huff et al., 2009). Expression of mspA in ML16 increased the rate of glucose uptake to wt levels, confirming that reduced uptake was a result of a reduced number of pores in the outer membrane (Stephan et al., 2005). Thus, measuring uptake of radiolabelled glucose by M. smegmatis ML16 is a sensitive method to quantify porin activity in vivo. However, in contrast to mspA, expression of ompATb did not increase the rate of glucose uptake in the ML16 porin mutant.
Fig. 3. OmpATb does not contribute to uptake of hydrophilic solutes across the outer membrane of M. smegmatis.
Accumulation of [14C]glucose (A), [14C]serine (B) and [14C]glycerol (C) by M. smegmatis SMR5/pMS2 (wt, black), ML16 with the empty vector pMS2 (ML16, green), ML16/pMN016 (ML16+mspA, blue), ML16/pML003 (ML16+ompATb, red) and ML16/pML1450 (ML16+ompATb operon, pink) was measured. All assays were performed at 37 °C at final glucose, serine or glycerol concentrations of 20 μM. Uptake experiments were performed in triplicate and are shown with standard deviations.
Table 3. Uptake rates for M. smegmatis and M. tuberculosis towards hydrophilic solutes.
Quantification of uptake rates for glucose, serine and glycerol by the wt strains M. smegmatis SMR5 and M. tuberculosis H37Rv as well as the porin mutant M. smegmatis ML16. All assays were performed at 37°C at a final solute concentration of 20 μM. The M. smegmatis strains contained the empty plasmid pMS2. Uptake rates were determined from the kinetics shown in Figs. 3 and 4 for M. smegmatis and M. tuberculosis, respectively, and are expressed in nmol/min/mg cells.
| SMR5 | ML16 | H37Rv | |
|---|---|---|---|
| glucose | 3.43 | 0.14 | 0.04 |
| serine | 1.41 | 0.53 | 0.13 |
| glycerol | 0.25 | 0.06 | 0.19 |
While these experiments indicated that OmpATb is not permeable to glucose in M. smegmatis, it cannot be excluded that OmpATb is a solute-specific pore. For instance, LamB of E. coli enables transport of maltodextrines but not of other sugars or solutes (Charbit, 2003). Previously, it was reported that OmpATb enhanced uptake of serine but not of the smaller amino acid glycine (Raynaud et al., 2002). Thus, we sought to determine serine permeability of OmpATb in the porin mutant ML16. As shown in Fig. 3B, the porin MspA complemented uptake of serine whereas OmpATb did not increase serine uptake above the background observed in ML16 carrying the empty vector.
It is conceivable that OmpATb may permeate smaller compounds or substrates unrelated to carbohydrates and amino acids. Thus, we measured the uptake of glycerol, which can be used as a carbon source by both M. smegmatis and M. tuberculosis (Winder & Brennan, 1966). Here, we show that MspA complemented the uptake defect of ML16 for this substrate to wt levels (Fig. 3C). However, expression of ompATb did not complement the uptake rate of the small glycerol molecule. Taken together, it is concluded that OmpATb alone does not enable the diffusion of the solutes glycerol, serine and glucose across the outer membrane of M. smegmatis and does not function as a general porin in this organism.
Construction of an ompATb gene deletion mutant in M. tuberculosis
The results obtained with a previously published ompATb mutant of M. tuberculosis (Raynaud et al., 2002) were difficult to interpret due to lack of a complemented strain, as noted before (Smith, 2003). To resolve this issue and to further examine the physiological functions of OmpATb, an M. tuberculosis deletion mutant lacking the ompATb gene was constructed using a two-step allelic exchange approach in combination with sequence-specific recombination to unmark the mutant as recently described (Song et al., 2008). First, the single cross over strain ML157 (Table 1) was obtained after incubation at 41°C, a temperature non-permissive for replication of the plasmid pML564 (Table 2). In the second step, the double cross over strain ML160 (ΔompATb::loxP-hyg-loxP) was obtained after counter-selection of ML157 against the sacB-containing pML564 deletion vector on 2% sucrose plates. Then, expression of the Cre recombinase from the plasmid pCreSacB1 was used to excise the hyg gene from the chromosome to generate the out-of-frame, marker-free ompATb deletion mutant ML163 (ΔompATb::loxP). Southern blot analysis of the ompATb loci of these strains demonstrated that the allelic exchange in the strain ML160 and excision of the hyg gene in ML163 were specific (Fig. S2). The replacement of a 689 bp DNA fragment of the ompATb gene by a loxP site in the chromosome of M. tuberculosis ML163 was confirmed by DNA sequencing. For complementation experiments, the ompATb expression vector pML759 was integrated at the mycobacteriophage L5 attachment site in the chromosome of the ompATb deletion mutant M. tuberculosis ML163. This complemented strain was named M. tuberculosis ML168 (ΔompATb::loxP; attB::ompATb).
Extraction of membrane proteins with SDS and detection with an OmpATb antiserum showed that wt M. tuberculosis produced OmpATb, while no protein was detected in the ΔompATb mutant M. tuberculosis ML163 (Fig. 1B). Expression of ompATb was fully restored in the complemented strain M. tuberculosis ML168 (Fig. 1B).
OmpATb has no detectable porin activity in M. tuberculosis
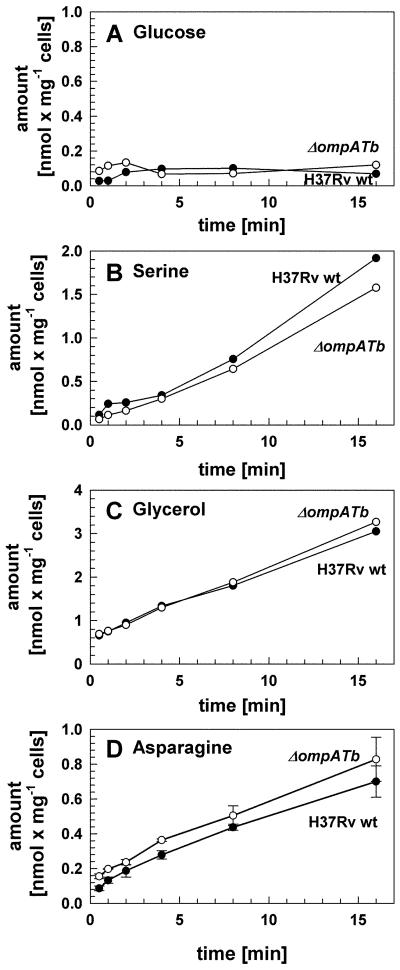
As the lack of porin activity by OmpATb in M. smegmatis may have been due to the absence of other M. tuberculosis proteins required for function, the effect of ompATb deletion on outer membrane permeability was examined in M. tuberculosis. No differences in colony morphology or size were noted for the ΔompATb mutant ML163 either on 7H10/OADC or Dubos agar plates (not shown). Permeability defects of the ΔompATb mutant ML163 were examined by uptake of glucose, serine and glycerol as performed for M. smegmatis. Uptake rates for these solutes were similar for both wt M. tuberculosis H37Rv and the ΔompATb mutant at pH 6.9 (Fig. 4). It is noteworthy that uptake of glucose by M. tuberculosis was very slow compared to M. smegmatis (Table 3). These findings are consistent with the results obtained in M. smegmatis and demonstrate that OmpATb does not play any role in outer membrane permeability of M. tuberculosis for these solutes under the conditions investigated.
Fig. 4. OmpATb does not have porin activity in M. tuberculosis and is not involved in uptake of asparagine.
Accumulation of [14C]glucose (A), [14C]serine (B), [14C]glycerol (C) and [3H]asparagine (D) by M. tuberculosis wt (closed circles) and ML163 (ΔompATb, open circles) was measured. All assays were performed at 37 °C at final glucose, serine, glycerol and asparagine concentrations of 20 μM.
The ompATb gene is encoded in an operon
The ompATb mutant CK69 exhibited delayed growth compared to wt M. tuberculosis at pH 5.5 but not at pH 7.2 (Raynaud et al., 2002). Exponential growth of our ompATb mutant ML163 at pH 5.5 was also delayed by three days in Dubos medium (not shown) and by approximately ten days in Hartmans de Bont (HdB) minimal medium (Fig. 5A). However, expression of an integrated ompATb gene from three different promoters including the native promoter (not shown) and overexpression of ompATb using the plasmid pML588 (Fig. 1B, lane 4) did not complement the growth defect of the ΔompATb mutant ML163 at pH 5.5 (Fig. 5A). This finding suggested that deletion of ompATb may have impaired expression of the downstream genes rv0900 and rv0901 (Fig. S3A). Reverse transcription PCR showed that ompATb, rv0900 and rv0901 are indeed transcribed in an operon (Fig. S3B). No amplification was obtained with the same primer pairs in samples without reverse transcriptase, demonstrating the absence of DNA contamination in RNA preparations. Therefore, the entire operon was cloned into expression plasmids (Table 2) and used to repeat the uptake experiments in M. smegmatis and for further characterization of the functions of OmpATb in M. tuberculosis.
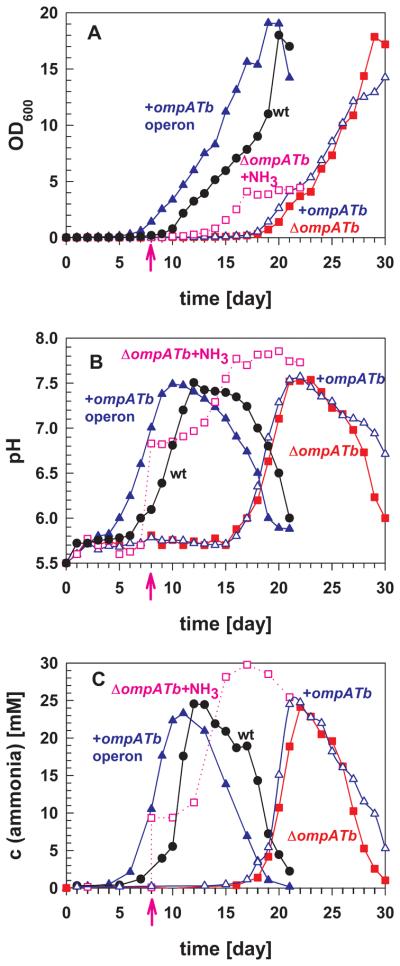
Fig. 5. OmpATb-dependent growth, pH neutralization and ammonia secretion by M. tuberculosis.
M. tuberculosis H37Rv (wt, black circles), the mutant ML163 (ΔompATb, closed red squares), the pML588 complemented mutant (+ompATb, open blue triangles) and the pML763 complemented mutant (+ompATb operon, closed blue triangles) were grown in HdB medium (pH 5.5). In parallel, 10 mM exogenous ammonia was added (pink arrows) to the mutant ML163 culture on day 8 (ΔompATb+NH3, open pink squares). The cell density (OD600) of the culture (A), pH (B) and ammonia concentration (C) were measured each day for 30 days.
OmpATb, Rv0900 and Rv0901 do not have porin activity in M. smegmatis
It is conceivable that channel activity was not detected in the M. smegmatis porin mutant ML16 containing the ompATb expression vector because the entire operon might be required for putative pore formation. Thus, the growth and uptake experiments described above were repeated in M. smegmatis using the psmyc-driven ompATb operon expression plasmid pML1450. Importantly, expression of ompATb from this construct was higher (Fig. 1A) and subsequently resulted in more surface-exposed OmpATb, as determined by whole cell ELISA (Fig. 2). This indicated that Rv0900 and/or Rv0901 contribute to functional expression of ompATb by increasing expression, localization, and/or stability of OmpATb. However, even expression of the ompATb operon did not complement the growth defect of the triple porin mutant M. smegmatis ML16 on agar plates (Fig. S1) nor the uptake defects of ML16 for glucose, serine and glycerol (Fig. 3). Thus, it can be concluded that neither OmpATb alone nor in concert with Rv0900 and Rv0901 has any detectable porin activity in M. smegmatis.
The ompATb operon is required for neutralization of acidic pH in culture
The OmpATb channel closed more frequently in vitro at pH 6 and lower (Molle et al., 2006), yet it was found that transcription of ompATb was upregulated 30-fold at pH 5.5 compared to pH 7.2 (Raynaud et al., 2002). Fifteen-fold higher OmpATb protein levels were indeed obtained when M. tuberculosis was grown at pH 5.5 compared to pH 7.2 (Fig. S4). Based on these results and the finding that overall outer membrane permeability of M. tuberculosis was reduced at pH 5.5 (Raynaud et al., 2002), it was suggested that OmpATb functions not as a porin but rather in acid adaptation (Niederweis, 2003). The lack of identifiable porin activity along with the growth defect of the ompATb mutant at low pH indicates this assumption may be true. However, the molecular mechanisms underlying the contribution of OmpATb, Rv0900 and Rv0901 to adaptation of M. tuberculosis to acidic environments remain unknown. Considering that M. tuberculosis does not grow at pH ≤ 5.5 (Vandal et al., 2009a), we assumed that acidic medium must be neutralized prior to logarithmic phase growth. Thus, growth experiments were performed in which the medium pH was monitored along with the optical density of the cultures. Prior to the onset of and throughout exponential phase growth of wt M. tuberculosis (Fig. 5A), the pH of the medium increased from pH 5.5 to approximately 7.5 (Fig. 5B). The delay in the onset of exponential phase growth of the ompATb mutant ML163 also coincided with a delay in pH neutralization. Expression of the ompATb operon from the native ompATb promoter in pML763 produced high levels of OmpATb (Fig. 1B) and fully complemented both phenotypes (Fig. 5A, B) suggesting a mechanism by which proteins encoded by the ompATb operon are involved in pH neutralization by M. tuberculosis. This result also showed that the M. tuberculosis ML163 strain is an ompATb operon mutant.
The ompATb operon is required for efficient efflux of ammonia under acidic conditions
Considering the channel activity of OmpATb in vitro (Senaratne et al., 1998, Alahari et al., 2007), its surface accessibility (Fig 2) (Song et al., 2008), cell wall-association (Rezwan et al., 2007), and its requirement for acid adaptation (Fig. 5) (Raynaud et al., 2002), we hypothesized that OmpATb might be involved in secretion of a basic compound that neutralizes protons in an acidic medium. To test this hypothesis, we chemically analyzed the supernatants of a culture of wt M. tuberculosis and of the ompATb operon mutant ML163 in acidified Dubos medium (pH 5.5) after 14 days of incubation at 37°C. Gas chromatography combined with mass spectroscopy (GS/MS) demonstrated the presence of ammonia while aliphatic and aromatic amines were not detected (not shown). The concentration of ammonia in the culture supernatant of wt M. tuberculosis was 5.2 ± 0.1 mM, which was determined photometrically using Nessler's reagent, a colorimetric reagent used for detection of ammonia (London et al., 1974). This is consistent with previous findings of large amounts of ammonia in cultures of M. tuberculosis (Long, 1958, Gordon et al., 1980). Importantly, the amount of ammonia in the supernatant of the ompATb operon mutant ML163 was significantly reduced to 4.1 ± 0.2 mM at the same cell density. Some amino acids, amines and hydrazones are known to interfere with the Nessler's reagent. However, similar photometric readings were observed when culture filtrates of both strains were distilled and the trapped liquid was analyzed using Nessler's reagent (not shown). This proves that a volatile species, probably ammonia, is indeed produced by wt M. tuberculosis and at a reduced level by the ompATb operon mutant ML163.
To examine the role of the ompATb operon in ammonia release by M. tuberculosis, we measured ammonia production using an enzymatic assay based on the synthesis of glutamate from 2-oxoglutarate and ammonia by glutamate dehydrogenase. While M. tuberculosis grew in Hartmans de Bont (HdB) minimal medium with nitrate as a single nitrogen source at pH 7.0, it did not grow at pH 5.5 (will be published elsewhere). However, when the sole nitrogen source was substituted in HdB medium for that of Dubos medium, asparagine, ammonia was rapidly detected in the supernatant of M. tuberculosis cultures. Within the first seven days, the concentration of ammonia in the supernatant of wt M. tuberculosis increased to only 1.2 mM (Fig. 5C). This was accompanied by minor increases in pH from 5.5 to 6.0 (Fig. 5B) and in growth (Fig. 5A). Over the next five days, the ammonia concentration increased by more than 10-fold to a final concentration of 25 mM. This “ammonia burst” was associated with a rise in pH from 5.5 to 7.5 and preceded exponential growth of wt M. tuberculosis. It should be noted that the pH subsequently dropped dramatically after 12 days of incubation, but this did not initially affect growth of wt M. tuberculosis (Fig. 5).
Interestingly, the ammonia burst by the ompATb operon mutant ML163 was similar in magnitude as wt M. tuberculosis, but was delayed by about ten days. The delayed ammonia burst accompanied a delayed increase in pH, which both preceded exponential growth of the mutant. Thus, the phenotype of the ompATb operon mutant consists of severe delays in ammonia secretion, medium pH neutralization, and growth compared to wt M. tuberculosis. However, all changes in these parameters after onset were similar in magnitude and kinetics. Expression of the ompATb operon but not ompATb alone fully reverted the delayed onset of ammonia secretion, pH neutralization and growth to wt levels (Fig. 5).
To determine whether secretion of ammonia alone rescued the growth defect of ompATb operon mutant, 10 mM exogenous ammonia was added to the ML163 culture on day eight (Fig. 5B). This “artificial ammonia burst” instantaneously increased the medium pH to 6.8 as expected according to a titration curve of HdB medium (Fig. S5). Interestingly, it did not trigger immediate growth of M. tuberculosis indicating that another factor in addition to pH increase is required for growth of M. tuberculosis. Approximately three more days, a subsequent increase in ammonia, and a further rise in pH were required before growth of the ompATb operon mutant was observed.
Asparagine is the ammonia source for M. tuberculosis at acidic pH in vitro
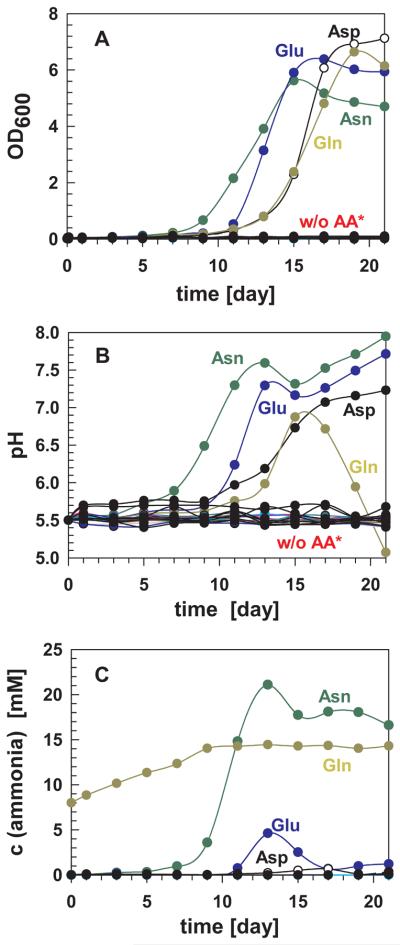
To identify whether M. tuberculosis is also capable of utilizing amino acids other than asparagine for ammonia production and growth under acidic conditions, M. tuberculosis was grown in HdB minimal medium at pH 5.5 supplemented with only one of the twenty natural amino acids as the sole nitrogen source. M. tuberculosis only grew in the presence of asparagine (Asn), aspartic acid (Asp), glutamine (Gln) or glutamic acid (Glu) in acidic medium (Fig. 6A). Growth of M. tuberculosis coincided with an increase in the pH of the medium of these cultures (Fig. 6B). This finding confirmed previous observations that M. tuberculosis cannot grow at pH lower than 5.5 (Vandal et al., 2009a) and needs to increase the pH to above 5.5 before growth is initiated. Interestingly, ammonia was detected only in significant amounts in the culture containing asparagine (Fig. 6C). The small spike of ammonia detected with glutamate as the sole nitrogen source occurred after an increase in the medium pH and subsequent growth of M. tuberculosis (Fig. 6). This experiment also indicated M. tuberculosis releases other alkaline compound(s) accounting for pH neutralization in the presence of aspartic or glutamic acid (Fig. 6B). The pH also increased and growth occurred in acidified M. tuberculosis cultures containing glutamine as a sole nitrogen source indicating that either ammonia or another alkaline compound is produced. However, this cannot be distinguished using the enzymatic ammonia detection (Fig. 6C). Taken together, these results suggest that, among all naturally occurring amino acids, M. tuberculosis can only utilize asparagine and possibly glutamine for ammonia production and growth under acidic conditions in vitro.
Fig. 6. Asparagine is the primary ammonia source for M. tuberculosis H37Rv at acidic pH.
M. tuberculosis H37Rv wt was grown in HdB medium (pH 5.5) supplemented with one of the 20 natural amino acids as the sole nitrogen source. The cell density (OD600) of the culture (A), the pH (B) and the ammonia concentration (C) were measured every two days. w/o AA* indicates that M. tuberculosis in all cultures except those containing asparagine (Asn), glutamine (Gln), glutamic acid (Glu) and aspartic acid (Asp) gave similar growth curves and pH change to the culture without any amino acids supplemented. Cysteine was removed from graph B as it interfered with the assay. For clarity, cultures with amino acid supplements that did not give rise to detectable changes in optical density, pH, or ammonia levels were omitted from graph C except for the culture with no amino acid supplements (filled black circles).
The ompATb operon is not involved in asparagine uptake
An alternative explanation for the delayed ammonia secretion by M. tuberculosis ML163 is that the ompATb operon might be involved in uptake of asparagine which is required for ammonia production in our growth experiments. However, uptake experiments using [3H]asparagine did not show any difference between wt M. tuberculosis and ML163 (Fig. 4D) demonstrating that the ompATb operon is not required for asparagine uptake under these conditions.
The ompATb operon is not required for full virulence of M. tuberculosis in mice
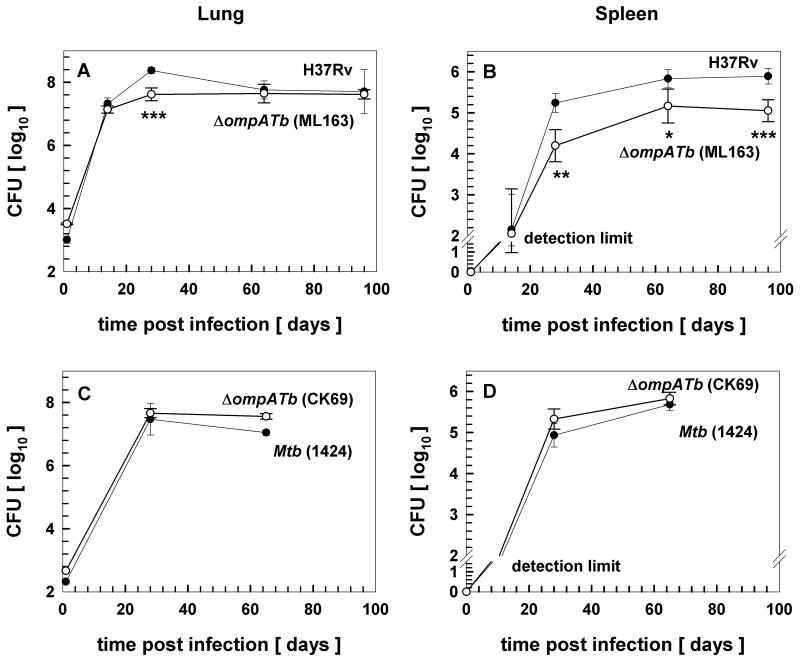
M. tuberculosis infects alveolar macrophages where it resides in slightly acidified vacuoles (Ehrt & Schnappinger, 2009). Thus, the growth defect of the ompATb operon mutant in acidic medium (Fig. 5A) indicates that OmpATb may be required for in vivo survival. The finding that the ompATb mutant CK69 was attenuated in both macrophages and in mice (Raynaud et al., 2002) appeared to support a critical role of OmpATb in virulence of M. tuberculosis. However, screens with high-density transposon libraries did not identify ompATb as a gene required for survival in mice (Sassetti & Rubin, 2003), in macrophages (Rengarajan et al., 2005) or for adaptation to low pH (Vandal et al., 2008). Therefore, we sought to examine whether the ompATb operon is required for virulence of M. tuberculosis using the mutant strain ML163. The wt strain M. tuberculosis H37Rv and the ompATb operon mutant were administered to BALB/c mice by low-dose aerosol infection and bacterial burden was determined over time. While the infectious dose determined on day 1 was higher for the ompATb operon mutant, a minor reduction in CFUs in the lung compared to wt was observed on day 28 post-infection (Fig. 7A). However, the mutant recovered to wt levels in lungs by day 64 and beyond. Bacterial burden in the spleens of infected mice was also reduced for the mutant by day 28 (Fig. 7B). Conversely to lungs, the mutant remained at significantly reduced levels in the spleen throughout the duration of infection experiments, suggesting impaired dissemination of the M. tuberculosis ompATb operon mutant. These experiments indicated that OmpATb, Rv0900 and Rv0901 play no or only a minor role in virulence of M. tuberculosis, in contrast to the major growth defect previously observed in mice lungs with the ompATb mutant CK69 (Raynaud et al., 2002).
Fig. 7. Survival of ompATb mutants of M. tuberculosis in mice.
Wt M. tuberculosis H37Rv and the isogenic ompATb operon mutant ML163 (A, B), or wt M. tuberculosis 1424 and the isogenic ompATb mutant CK69 (Raynaud et al., 2002) (C, D), were administered to BALB/c mice by aerosol infection. At indicated time points, lungs (A, C) and spleens (B, D) were removed from infected mice, homogenized, and the suspensions were plated on solid media. Colony-forming units (CFU) of M. tuberculosis wt (closed circles) and the ompATb mutants (open circles) were counted and plotted. Two-tailed and paired Student's t-tests were performed between wt and the ompATb mutant. * indicates p value <0.1; ** indicates p value <0.05; *** indicates p value <0.01. The detection limits were 30 and 100 CFU's in the lung and in the spleen, respectively.
In an effort to resolve this discrepancy, we obtained the ompATb mutant strain CK69 described by Raynaud et al. (2002) and performed additional infection experiments in mice. Low-dose aerosol infection in BALB/c mice revealed that both at an early time point of infection (day 28) and during the chronic stage of infection (day 65), no attenuation of the mutant (CK69) for growth in lungs or spleens compared to the parent M. tuberculosis strain (1424) was observed (Fig. 7C, D). As the original publication had only assessed virulence following intravenous infection (Raynaud et al., 2002), we also determined growth of the ompATb mutant strain CK69 and its parent strain 1424 after intravenous inoculation of BALB/c mice. No reduced replication of the ompATb mutant CK69 was observed in either lungs or spleens (Fig. S6). Taken together, our in vivo experiments with two ompATb mutants show that neither OmpATb nor Rv0900 and Rv0901 are required for full virulence of M. tuberculosis in a mouse infection model.
DISCUSSION
OmpATb lacks porin activity in vivo
In this study we did not detect any porin activity of OmpATb in either M. smegmatis or in M. tuberculosis. An explanation for the apparent absence of porin activity of OmpATb in mycobacteria despite its in vitro channel activity (Alahari et al., 2007, Molle et al., 2006) might be that OmpATb forms channels of very low activity, as has been described for OmpA of E. coli and OprF of Pseudomonas aeruginosa (Sugawara & Nikaido, 1994, Sugawara et al., 2006). Detection of low porin activity requires the absence of other general porins in the bacterial strain of interest. Since the porin mutant M. smegmatis ML16 still expresses the porin gene mspB, albeit at very low amounts (Stephan et al., 2005), it might be difficult to detect very low activity porins in this strain. Another explanation might be that ompATb is not functionally expressed in the heterologous host M. smegmatis. Indeed, the proteins Rv0900 and Rv0901, which are encoded in the ompATb operon, increase expression levels of OmpATb by an unknown mechanism (Fig. 1A). However, these proteins are not required for correct localization of OmpATb (Fig. 2) and they also do not confer any channel activity to OmpATb in mycobacteria (Fig. 3 and 4). Further, ELISA experiments showed that OmpATb is surface-accessible in M. smegmatis demonstrating correct localization and indicating proper folding of the protein (Fig. 2). Importantly, the ompATb operon mutant M. tuberculosis ML163 lacked any detectable uptake defect indicating that OmpATb does not have porin activity in its native environment (Fig. 4). In particular, we did not find significant changes in serine uptake either in the recombinant M. smegmatis strain or in the ompATb operon mutant of M. tuberculosis, in contradiction to earlier results (Raynaud et al., 2002).
The recently solved NMR structure revealed that OmpATb does not form a transmembrane β-barrel or any pore-forming domain (Teriete et al., 2010), supporting our in vivo results. This structure does not exclude that OmpATb might be permeable to small ions as observed in vitro (Alahari et al., 2007, Molle et al., 2006, Senaratne et al., 1998) based on dynamic conformational changes, as postulated for OmpA of E. coli (Hong et al., 2006, Khalid et al., 2008). The recently published model of a heptameric OmpATb pore (Yang et al., 2011) has no continuous hydrophobic outer surface as observed in all outer membrane proteins including MspA. In addition, the height of this OmpATb heptamer was 3 nm, which may be sufficient to span a planar lipid membrane in vitro, but is too short to span the ~8 nm thick mycobacterial outer membrane (Hoffmann et al., 2008). Taken together, a critical evaluation of all experiments strongly indicates that OmpATb does not function as a general porin in mycobacteria in contrast to previous findings for another ompATb mutant CK69 (Raynaud et al., 2002). However, the apparent permeability defect of the ompATb mutant CK69 was not complemented raising the suspicion that it might be due to secondary mutations. The lack of detectable porin activity for OmpATb is also in contrast to MspA, the only well characterized mycobacterial porin, which forms a stable channel (Faller et al., 2004) and enables the diffusion of diverse small molecules including glucose (Stephan et al., 2005), glycerol (Fig. 3), amino acids (Stephan et al., 2005), phosphate (Wolschendorf et al., 2007), antibiotics (Danilchanka et al., 2008) and ferric ions (Jones & Niederweis, 2010). It should be noted that the absence of detectable porin activity in our experiments does not constitute a proof that OmpATb does not have some channel activity which is not detectable under our experimental conditions. Such an activity could be masked by other, yet unidentified pore proteins in M. tuberculosis, but would probably not contribute significantly to its outer membrane permeability.
Proteins encoded by the ompATb operon are involved in ammonia release by M. tuberculosis to adapt to acidic environments
In the absence of any detectable porin activity, what then might be the real function of OmpATb? It was found previously that ompATb expression is strongly induced at low pH (Raynaud et al., 2002) indicating that OmpATb might play a role in the adaptation of M. tuberculosis to acidic conditions. Here, we show that OmpATb accelerates the secretion of ammonia to neutralize the medium and ultimately enables growth of M. tuberculosis under acidic conditions. M. tuberculosis does not grow at pH 5.5 (Vandal et al., 2009a), and releases large amounts of ammonia in excess of 5 mM to increase the pH to 6.5 before it enters the exponential growth phase (Fig. 5). This is within the physiologically relevant pH range as the pH of M. tuberculosis-containing phagosomes ranges from pH 4.5 to 6.2, depending on the activation state of the macrophage (Vandal et al., 2009a). Ammonia secretion by M. tuberculosis has been known for a long time (Long, 1958, Gordon et al., 1980) and was proposed to play a role in neutralizing acidic pH, in preventing phagosome-lysosome fusion in macrophages (Hart et al., 1987), and diminishing cell-surface expression of MHC class II molecules in macrophages infected with M. tuberculosis (Hmama et al., 1998). In this study, we showed that the growth delay of the ompATb operon mutant under acidic conditions is shortened by addition of exogenous ammonia (Fig. 5). This partial biochemical complementation provides the first direct evidence that ammonia is indeed required by M. tuberculosis to achieve a pH permissive for growth. However, other secreted yet unidentified factors also appear to contribute to growth of M. tuberculosis under acidic conditions (Fig. 6).
The importance of acid resistance mechanisms for bacterial pathogens is not surprising given that excess internal protons cause intracellular damage to molecules such as proteins and DNA (Foster, 1999). Many enteric bacteria induce expression of genes involved in DNA repair and acid shock response when exposed to acidic conditions (Foster, 1995) and have evolved mechanisms to maintain favorable pH levels to promote growth and survival inside the host. For instance, the F1F0-ATPase expels excess protons to maintain intracellular pH and is required for acid-tolerance of Salmonella typhimurium (Foster & Hall, 1991). Neutralization of extracellular pH by ammonia is also a common feature in the bacterial response to acid stress. A classic example is Helicobacter pylori which produces and transports ammonia to the periplasm to neutralize influxing protons in the very acidic environment of the human stomach (Sachs et al., 2006). In acidic conditions of the oral cavity, Streptococcus sanguis secretes an arginine aminopeptidase to release ammonia, while Streptococcus salivarious secretes ammonia produced by high levels of urease expression (Quivey et al., 2001). While this study shows that secretion of ammonia plays an important role in adaptation of M. tuberculosis to acidic conditions in vitro, it is only one of several mechanisms by which M. tuberculosis maintains permissive pH levels such as blocking phagosomal fusion with lysosomes and preventing acquisition of the V-ATPase (Vandal et al., 2009a).
How does the ompATb operon contribute to ammonia release by M. tuberculosis?
Ammonia is a small gas molecule which is thought to be membrane permeable (Antonenko et al., 1997). Thus, protein-mediated transport mechanisms across membranes may not be required. However, highly specific ammonia transporters such as AmtB and some aquaporins (Zheng et al., 2004, Kruse et al., 2006) exist in all three kingdoms of life, indicating that ammonia transport by unmediated membrane diffusion might not be fast enough to support optimal bacterial growth (Soupene et al., 1998). The low fluidity of the mycobacterial outer membrane (Liu et al., 1996) contributes to its extraordinary effectiveness as a permeability barrier (Brennan & Nikaido, 1995). Thus, proteins may be required to accelerate ammonia diffusion across the outer membrane of mycobacteria. However, the apparent lack of porin activity of OmpATb in mycobacteria and identical ammonia release kinetics in wt M. tuberculosis and the ompATb operon mutant (Fig. 5C) do not support a role of OmpATb in direct ammonia transport. Our experiments rather suggest that M. tuberculosis has a mechanism of ammonia release that does not involve OmpATb. Proteins encoded by the ompATb operon might be involved in triggering early ammonia secretion by M. tuberculosis in acidic environments.
An alternative explanation for the role of OmpATb in acid resistance would be that it is involved in the uptake of a nitrogen source used by M. tuberculosis to generate ammonia. In this study, we identified asparagine as the only amino acid efficiently used by M. tuberculosis to produce ammonia (Fig. 6). This finding is consistent with the observation that asparagine is rapidly taken up by M. tuberculosis (Lyon et al., 1969) and its particular role as a nitrogen source (Lyon et al., 1974). However, uptake experiments demonstrated that OmpATb does not play any role in uptake of asparagine (Fig. 4D).
The outer membrane permeability of M. tuberculosis for glucose and serine is extremely low compared to M. smegmatis
The permeability barrier established by the outer membrane is a hallmark of mycobacteria and is crucial for survival of M. tuberculosis in the host (Barry, 2001). However, a systematic analysis of the permeability properties of the outer membrane of M. tuberculosis is lacking, as is our understanding of which proteins are required for uptake of nutrients and drugs by M. tuberculosis (Niederweis, 2008b). Here, we show for the first time a direct comparison of the permeability of M. tuberculosis and M. smegmatis to several nutrients. Uptake rates of glucose and serine by M. tuberculosis were 86- and 11-fold slower compared to wt M. smegmatis and were similar to that of the triple porin mutant M. smegmatis ML16 (Table 3). These results are consistent with previous observations that the porin activity in detergent extracts of M. tuberculosis is much lower than that of M. smegmatis (Kartmann et al., 1999). Our experiments confirm that uptake of these solutes is porin-dependent in M. smegmatis as shown previously (Stephan et al., 2005). It is unclear whether this is the case for M. tuberculosis. Uptake rates for glycerol were similar for M. tuberculosis and M. smegmatis, although we showed that the highly efficient MspA pore accounts for the vast majority of glycerol uptake in M. smegmatis (Fig. 3C). This result combined with the observation that mycobacterial outer membranes have an extremely low fluidity (Liu et al., 1995, Liu et al., 1996) indicate that M. tuberculosis probably has a porin that enables rapid influx of glycerol, because the rate of glycerol diffusion through membranes decreases with reduced membrane fluidity (Eze & McElhaney, 1981).
The ompATb operon is not required for full virulence in mice
In this study, we show that OmpATb is not required for full virulence of M. tuberculosis in mice (Fig. 7 and Fig. S6). Our conclusion is based on assessing replication and persistence of two different ompATb mutants, the one described in this report and the one published by Raynaud et al. (2002). It is unclear why the previously published ompATb mutant did not exhibit, in our hands, the severely attenuated phenotype described earlier (Raynaud et al., 2002). However, after either aerosol or intravenous infection, and both at early and late time points post infection, the ompATb mutant M. tuberculosis CK69 described by Raynaud et al. (2002) did not differ from wild-type M. tuberculosis 1424 in terms of initial growth and chronic persistence, thereby corroborating our findings with our ompATb operon mutant ML163.
Previous screens did not identify ompATb as required for survival in macrophages (Rengarajan et al., 2005), in mice (Sassetti & Rubin, 2003), or under acidic conditions in vitro (Vandal et al., 2008). Microarray-based screens of transposon libraries indicated that rv0900 is required for growth of M. tuberculosis in vitro (Sassetti et al., 2003) and in macrophages (Stewart et al., 2005). However, this study shows the the ompATb operon mutant does not show a growth defect in vitro at neutral pH (not shown) or in mice. These results suggest that rv0900 does not play a critical role for M. tuberculosis growth or viability under those conditions.
Conclusions
The findings of this study indicate that OmpATb is not a general porin, but rather is involved in adaptation of M. tuberculosis to acidic environments by mediating ammonia secretion. Clearly, more work is warranted to understand this function of OmpATb and how the other proteins encoded by the ompATb operon contribute to this function. However, ammonia secretion as mediated by the ompATb operon does not appear to be required for virulence of M. tuberculosis in mice, perhaps because M. tuberculosis employs multiple, possibly redundant, mechanisms to resist phagosomal acidification (Stewart et al., 2005, Vandal et al., 2008) or because alternative, albeit delayed mechanisms for ammonia secretion exist in M. tuberculosis.
Materials and Methods
Chemicals, enzymes and DNA
Hygromycin B was purchased from Calbiochem. All other chemicals were purchased from Merck, Roche or Sigma at the highest purity available. Enzymes for DNA restriction and modification were purchased from New England Biolabs. Isolation and modification of DNA was performed as described (Ausubel et al., 1990). Oligonucleotides were obtained from Integrated DNA Technologies (Table S1).
Bacterial strains and growth conditions
All bacterial strains used in this study are listed in Table 1. Mycobacterial strains were grown at 37°C in Middlebrook 7H9 liquid medium (Difco Laboratories) supplemented with 0.2% glycerol, 0.025% Tyloxapol or on Middlebrook 7H10 agar (Difco Laboratories) supplemented with 0.2% glycerol, unless indicated otherwise. Escherichia coli DH5α was used for all cloning experiments and was routinely grown in LB medium at 37°C. M. tuberculosis strains were grown in Dubos broth or Hartmans de Bont (HdB) minimal medium (Smeulders et al., 1999) or on 7H10 agar plates supplemented with 0.2% glycerol and 10% Middlebrook OADC enrichment (BBL) at 37°C. In HdB medium, (NH4)2SO4 was replaced by Na2SO4 and 15 mM asparagine was used as a nitrogen source. Antibiotics were used when required at the following concentrations: hygromycin (200 μg ml−1 for E. coli; 50 μg ml−1 for mycobacteria) and kanamycin (50 μg ml−1 for E. coli; 30 μg ml−1 for mycobacteria).
Construction of ompATb expression vectors for mycobacteria and E. coli
To construct the ompATb operon (ompATb-rv0900-rv0901) expression plasmid pML763 driven by the native promoter of ompATb, the ompATb-rv0900-rv0901 operon and the adjacent upstream 1.0 kb promoter region was amplified from M. tuberculosis genomic DNA using the primer pair 1091/1149 and ligated into pML003 (Song et al., 2008), which was digested by XbaI and SwaI. The psmyc-ompATb operon expression plasmid pML1450 was constructed by removal of the ompATb-rv0900-rv0901 operon via digestion of pML763 using HpaI and HindIII, and ligation into similarly digested pML003. The N-terminally truncated ompATb73-326 expression plasmid was constructed by PCR amplification of ompATb73-326 from pML752 with the primer pair 2277/2184. A second PCR amplification of the product using the primer pairs 2276/2184 allowed for addition of a shine-delgarno sequence, which was followed by digestion with PacI and HindIII, and ligation into similarly digested pML003.
To construct an integrative expression vector for ompATb, the plasmid pCV125 (Steyn et al., 2003) containing the integrase gene and attachment site (attP) of the mycobacteriophage L5 and a kanamycin resistant gene was used. The pimyc-ompATb fragment was removed from pML588 by XbaI and ClaI digestion, and ligated into similarly digested pCV125. The resulting plasmid was named pML759 (Table 2) and integrated into the L5 attB site of M. tuberculosis ML163 (ΔompATb::loxP). The complemented strain was named ML168 (ΔompATb::loxP, attB::pML759). All plasmid constructions were verified by restriction enzyme digestion and sequencing and are listed in Table 2, while all strains are listed in Table 1.
To construct a C-terminally his-tagged ompATb overexpression vector for E. coli, the ompATb gene was amplified from pML588 by PCR using the primers 960 and 962 (Song et al., 2008). This fragment was digested with NcoI and SwaI and ligated into similarly digested pET28b+ (Novagen), resulting in pML591.
Overexpression of ompATb in E. coli
For protein expression and purification, a 1 L culture was grown to OD600 of 0.6-1.0, and induced with 0.5 mM isopropyl-beta-D-thiogalactopyranoside (IPTG) for 2 h at 37°C. Bacteria were harvested by centrifugation, resuspended in 20 ml lysis buffer (50 mM Tris-HCl, 100 mM NaCl, pH 8.0) and sonicated on ice for 15 min. Then, the cell suspension was centrifuged at 12,000 × g for 15 min at 4°C. The supernatant was removed, filtrated through a 0.22 μm filter, and loaded onto a Ni2+ charged resin column (HIS-Select™ Spin Columns, Sigma) according to the manufacturer's protocol. Bound proteins were washed (50 mM Tris-HCl, 100 mM NaCl, 5 mM imidazole, pH 8.0) and then eluted from the column using elution buffer (50 mM Tris-HCl, 100 mM NaCl, 250 mM imidazole, pH 8.0).
Construction of an unmarked ompATb deletion mutant of M. tuberculosis
To construct a mutant of M. tuberculosis lacking ompATb, fragments of approximately 1,000 bp of DNA up- and downstream of ompATb were amplified by PCR from chromosomal DNA with the oligonucleotide pairs 779/780 and 982/983, respectively. The restriction sites for AscI and SwaI were introduced into the upstream fragment while PacI and BfrBI sites were introduced into the downstream fragments. The individual sequences were digested and ligated into the similarly digested temperature sensitive replication vector pML563 (Song et al., 2008) flanking the loxP-hyg-loxP cassette, and the resulting plasmid was named pML564. The cloned fragments were sequenced to ensure the absence of PCR errors.
The plasmid pML564 was transformed into M. tuberculosis H37Rv and selected on 7H10/OADC/Hyg plates at 37°C. After three weeks, a single colony was picked and inoculated into 10 ml of 7H9/OADC/Hyg medium and incubated at 37°C on a shaker to an OD600 of 1.0. Dilutions from 1 × 103 to 1 × 106 were plated on 7H10/OADC/Hyg plates and incubated at 41°C. After three weeks, 8 colonies were picked and separately transferred into 30 ml of 7H9/OADC/Hyg medium and cultured at 41°C to prepare chromosomal DNA. All eight clones were correct single cross-over candidates (SCO) and confirmed by southern blot analysis. One SCO candidate was named M. tuberculosis ML157. The SCO ML157 was grown in 7H9/OADC/Hyg medium to an OD600 of 1.0. A series of 10-fold dilutions were plated on 7H10/OADC/Hyg medium supplement with 2% sucrose and incubated at 41°C. After four weeks, 8 single colonies were picked and cultured in 7H9/OADC/Hyg media at 41°C to prepare chromosomal DNA. All eight clones were correct double cross-over candidates (DCO) and confirmed by southern blot analysis. One DCO was named M. tuberculosis ML160 (ΔompATb::loxP-hyg-loxP). The Cre recombinase expression vector pCreSacB1 (a kind gift from Dr. Adrie Steyn, University of Alabama at Birmingham) was used to excise the loxP-flanked hygromycin cassette from the chromosome of M. tuberculosis ML160. The plasmid pCreSacB1 was transformed into M. tuberculosis ML160 and selected on 7H10/OADC/Kan plates. After three weeks, 8 single colonies were transferred into 10 ml of 7H9/OADC/Kan medium and cultured at 37°C to an OD600 of 1.0. Whole cell PCR analysis was performed to confirm that the loxP-hyg-loxP cassette was removed from the genome using the primer pair 976/961. Three out of these 8 colonies were confirmed as having lost the loxP-hyg-loxP cassette. Then, a series of 10-fold dilutions from a single colony having lost the loxP-hyg-loxP cassette was plated on 7H10/OADC plates containing 2% sucrose and incubated at 37°C to counter-select against pCreSacB1. After three weeks, 24 single colonies were streaked in parallel on 7H10-OADC, 7H10/OADC/Kan and 7H10/OADC/Hyg plates to confirm the loss of the hyg cassette and pCreSacB1. Twenty out of these 24 candidates failed to grow on plates containing kanamycin, confirming the loss of pCreSacB1 in these candidates. Colony PCR was performed using the primer pair 976/961 and one amplified fragment was submitted for sequencing. Sequencing results confirmed that this colony was an unmarked ΔompATb mutant and was subsequently named M. tuberculosis ML163 (ΔompATb::loxP). In this mutant, 689 bp within the 981 bp ompATb gene were replaced by 41 bp representing the loxP site.
A specific probe (1128 bp) for ompATb was amplified by PCR from chromosomal DNA of M. tuberculosis using the primer pair 781/782. The genomic DNAs of the deletion candidates were digested with PstI, and Southern blot hybridization was performed as previously described (Stephan et al., 2004).
Preparation of detergent extracts from M. smegmatis and M. tuberculosis and analysis by Western blot
10 ml cultures of M. smegmatis or M. tuberculosis expressing ompATb genes were grown to high density, normalized to an OD600 of 3.0, washed in TBS containing 1% SDS (pH 7.2), and concentrated in 1 ml TBS containing 1% SDS lysis buffer. Cells in lysis buffer were transferred to glass bead Lysing Matrix Tubes (MP Biomedicals) and disrupted using a FastPrep FP120 (BIO101/Savant) at 6,000 × g for 90 sec. Whole extracts were incubated at 40°C for 2 h at 800 rpm shaking in a Thermomixer R (Eppendorf). Cell debris was removed by centrifugation at 4°C for 10 min at 13,000 × g and the supernatant was used for subsequent Western blot analysis.
Proteins were separated in a 10% SDS-polyacrylamide gel and transferred to a PVDF membrane using a standard protocol (Ausubel et al., 1987). OmpATb was detected with a rabbit antiserum against OmpATb ((Senaratne et al., 1998); gift from Dr. Philip Draper) while RNA polymerase was detected with the mouse anti-E. coli RNAP mAb 8RB13 (Neoclone). A horseradish peroxidase-conjugated goat-anti-rabbit antibody (Sigma) or goat-anti-mouse antibody (Sigma) served as the secondary antibody for OmpATb and RNAP, respectively. The blot was developed using luminol substrates (ECL, Pierce) and detected in an EpiChemi3 Darkroom (UVP BioImaging system). Purified recombinant rOmpATbHis from E. coli served as a standard reference.
Growth of M. smegmatis strains on agar plates
Cultures of M. smegmatis SMR5/pMS2, ML16/pMN016, ML16/pMS2, ML16/pML003 or ML16/pML1450 were grown to an OD600 of about 1.0, filtered through a 5 μm filter, and dilutions were plated on minimal HdB medium supplemented with 0.2% glycerol. After 4 days of incubation at 37°C, pictures of colonies were taken under white light at 10 × magnification using a Stemi 2000-C stereomicroscope (Zeiss) and an AxioCam MRc camera (Zeiss).
Uptake experiments
Glucose, glycerol and serine uptake measurements were carried out as described before for glucose (Stephan et al., 2005) with minor modifications. The cells were grown on Middlebrook 7H10 plates containing 0.1% Tween 80 at 37°C. The cells were scraped off the plates, suspended in Middlebrook 7H9 medium and filtered through a 5.0 μm filter (Sartorius). The cell suspension was used to inoculate 100 ml of Middlebrook 7H9 medium for M. smegmatis or Dubos medium for M. tuberculosis containing 1 mM of either glucose, glycerol, serine, or asparagine. The cells were harvested at an OD600 of 0.6 by centrifugation (3750 × g at 4°C for 10 min), washed twice in uptake buffer (50 mM Tris-HCl pH 6.9, 15 mM KCl, 10 mM (NH4)2SO4, 0.05% Tween 80) and resuspended in the same buffer. Radio-labelled [14C]glucose (GE healthcare) and non-labelled glucose, [14C]glycerol (GE healthcare) and non-labelled glycerol, [14C]serine (GE healthcare) and non-labelled serine, or [3H]asparagine (Moravek Biochemicals and Radiochemicals) and non-labelled asparagine, were mixed and added to cell suspensions to obtain final concentrations of 20 μM accordingly. The mixtures were incubated at 37°C and 1 ml samples were removed at the indicated times. The cells were collected on a 0.45 μm Spin-X centrifuge tube filter (Costar) by mixing with an equal volume of 10% buffered formalin phosphate (Fisher) containing 0.1 M LiCl, and counted in a liquid scintillation counter (Beckman). The uptake rate was expressed as nmol/mg cells (dry weight).
Whole cell enzyme-linked immunosorbent assay (ELISA)
To examine the cellular localization of OmpATb, an enzyme-linked immunosorbent assay (ELISA) using whole cells (Huff et al., 2009) was employed with modifications. 10 ml cultures of M. smegmatis expressing ompATb were diluted to an OD600 of 1.0, harvested by centrifugation, and washed in 10 ml TBST (50 mM Tris-HCl pH 8, 150 mM NaCl, 1 mM MgCl2, 0.025% tyloxapol). Cells were concentrated in 1 ml TBST and 200 μl were transferred in 4 replicates to a U-bottom 96-well microtiter plate (Becton Dickinson). The plate was centrifuged at 3200 × g for 5 min at 4°C and cells were blocked with 2.5% skim milk (Difco) in TBST for 1 hour at 37°C. Cells were then washed twice with TBST and incubated for 1 hour at 37°C with an anti-OmpATb antiserum (Raynaud et al., 2002). Following three washes with 200 μl TBST (3200 × g, 5 min, 4°C), cells were then incubated with an anti-rabbit IgG secondary antibody conjugated to alkaline phosphatase and incubated for 1 hour at 37°C. Cells were then washed three times in TBST (3200 × g, 5 min, 4°C) and resuspended in 100 μl substrate buffer (0.1 M glycine, 1 mM ZnCl2, 1 mM MgCl2, 1% 4-nitrophenyl phosphate (pNPP)). After incubation for 2 hours at 37°C, the reaction was stopped with the addition of 100 μl 2 M NaOH. The plate was centrifuged and 100 μl of the supernatant was transferred to a flat-bottom 96-well microtiter plate (Nunc). Phosphatase activity was quantified by reading the absorption at 405 nm using a microplate reader (Synergy HT, Bio-TEK Instrument Inc, USA).
pH-dependent growth of M. tuberculosis at pH 7.2 and pH 5.5
M. tuberculosis ML163 (ΔompATb::loxP) was transformed with either the integrative ompATb expression vector pML759 (pimyc-ompATb), replicative vector pML588 (pimyc-ompATb) or pML763 (pnative-ompATb operon). The pML759 plasmid was integrated into the genome after plating on 7H10/OADC/Kan plates and confirmed by Western blot. This resulting strain was named M. tuberculosis ML168 (ΔompATb::loxP; attB::ompATb). M. tuberculosis H37Rv wt, ML163, ML168 and the strains complemented with replicative vectors were inoculated into 10 ml 7H9 Middlebrook medium and grown to an OD600 of 1.0. Cells were then harvested, washed twice with sterilized Millipore water, and transferred into 200 ml Hartmans de Bont (HdB) minimal medium (Smeulders et al., 1999) (pH 5.5, without OADC) supplemented with 15 mM asparagine as the sole nitrogen source. The initial OD600 for all cultures was 0.01. Samples of 3 ml were taken every day from each culture for 20 days and filtrated through 0.22 μm filters before measuring the pH using a pH meter (Symphony, VWR) and the ammonia concentration as described below. Samples of 1 ml were taken from the cultures every day and mixed with equal volume of formalin for 30 min. The OD600 of these samples was measured using a spectrophotometer (SmartSpec™ Plus, Biorad).
Chemical analysis of the culture medium of M. tuberculosis
To identify chemical differences in the supernatants, wt M. tuberculosis and the ΔompATb mutant ML163 were grown in Dubos medium for 14 days. Samples of 5 ml were removed every day from each culture and filtrated through two polyvinylidene fluoride-membrane (PVDF) membrane filters (0.22 μm, Millipore) to remove live cells and kept at −20°C before analysis. These samples were filtered again through Nylon Luer-Lock membrane filters (0.22 μm, Roth) and then injected into a gas chromatograph with a mass selective detector (HP 5971A MSD). Ammonia was separated from other compounds using a capillary column containing a cross-linked polyethyleneglycol matrix (HP Innowax) and identified by comparison with analytical GC/MS data available from a mass spectral data base (NIST, 2008).
To quantitatively determine ammonia concentrations, we used a spectroscopic method based on the specific formation of the yellow/brownish adduct HgO•Hg(NH2)I upon addition of Nessler's reagent to an ammonia-containing solution. Formation of the adduct in the supernatants of M. tuberculosis cultures was determined directly by measuring the absorbance at 520 nm (London et al., 1974). The analytical procedure was done as described earlier (Yuen & Polland, 1954). A linear calibration curve was obtained using bis-ammonium salt of citric acid.
As a control experiment to determine whether the compound which reacted with Nessler's reagent was volatile, the supernatants of M. tuberculosis were adjusted to pH >12 using NaOH and distilled by high-vacuum or increased temperature using a Schlenk apparatus in combination with a trap cooled with liquid nitrogen. Then, the ammonia content of the distillate was determined photometrically using Nessler's reagent as described above.
Enzymatic determination of ammonia in culture supernatants of M. tuberculosis
To determine the ammonia concentration in culture supernatants, wt M. tuberculosis, the ΔompATb mutant ML163, and the ML163 complemented strains with pML588 or pML763 were grown in HdB medium for 30 days. Samples of 3 ml were removed every day from each culture and filtrated through two PVDF membrane filters (0.22 μm, Millipore) to remove cells. These samples were frozen at −20°C before analysis. The ammonia concentration in these samples was determined by using an enzymatic method based on the synthesis of glutamate from 2-oxoglutarate and ammonia by glutamate dehydrogenase. The oxidation of nicotinamide-adenine dinucleotide (NADH) during this reaction is stoichiometric to the amount of ammonia and is measured by the loss of absorbance of NADH at 340 nm. The method was used as described in the manufacturer's protocol (Roche).
pH-dependent expression of ompATb in M. tuberculosis
Two cultures of wt M. tuberculosis H37Rv were grown in Dubos medium at pH 7.2 and 5.5 at 37°C. The pH of the medium was adjusted to pH 7.2 or 5.5 using 1 M NaOH or 1 M HCl before autoclaving. Samples of 20 ml were harvested at an OD600 of approximately 3.0 by centrifugation and resuspended in 2 ml PEN buffer (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, 0.1 mM EDTA, pH 7.2) containing 1% SDS, and mixed with 1 ml glass beads (Q-Bio Gene). The cells were disrupted using the Fastprep FP120 beater (BIO101) twice at 6,000 × g for a total of 90 seconds, and incubated for 2 h at 40°C while shaking. The cell debris was removed by centrifugation at 16,000 × g for 20 min and the detergent extracts were analyzed by gel electrophoresis. The proteins were then blotted on a polyvinylidene fluoride-membrane, and detected by a rabbit anti-OmpATb antiserum as described previously (Song et al., 2008).
Utilization of amino acids as sole nitrogen sources by M. tuberculosis
To test which amino acids are utilized as nitrogen sources by M. tuberculosis under acidic conditions, wild-type H37Rv was grown in 200 ml HdB media (pH5.5, with OADC) supplemented with or without asparagine, aspartic acid, glutamine, glutamic acid, serine, arginine, methionine, proline, alanine, histidine, glycine, phenylalanine, leucine, valine, threonine, tyrosine, tryptophan, cysteine, isoleucine or lysine in parallel. The amino acid was added as the sole nitrogen source in HdB media. The optical density, pH and ammonia concentration were measured every two days as described above. For ammonia measurements, only the cultures supplemented with asparagine, glutamine, aspartic acid and glutamic acid were measured because no visible growth was found in the cultures supplemented with the other 16 amino acids.
Transcription of the ompATb-rv0900-rv0901 operon
M. tuberculosis RNA extraction and cDNA preparation were performed as described previously (Hillmann et al., 2007). The primer pairs 976/977, 959/782, and 959/983 (Table S1) were used to amplify the ompATb-rv0900-rv0901 fragments as indicated in Fig. S3. RNA and chromosomal DNA as controls were used as PCR templates to amplify fragments of the ompATb-rv0900-rv0901 operon.
Mice infection experiments
Balb/c mice (female, 4-5 weeks old) were infected by aerosols with M. tuberculosis H37Rv wt and the isogenic ompATb operon mutant ML163. The cells were resuspended in sterile phosphate-buffered saline (PBS) at an OD600 of 0.2. Ten ml cell suspensions were loaded into a nebulizer of an aerosol chamber containing 40 mice. This provided a 1000 CFU/ml inoculum in lungs for both strains. After 0, 2, 4, 8 and 12 weeks of infection, the mice were sacrificed. Lungs and spleens were homogenized in 0.8 ml PBS by bead-beating, diluted 100x and 0.1 ml suspensions were plated on 7H10/OADC plates. The colonies on agar plates were counted after 1 month of incubation at 37°C.
Female, 6 to 8 week-old, specific pathogen-free BALB/c mice were purchased from Charles River Laboratories (Sülzfeld, Germany) and maintained in individually ventilated cages (IVC, Ebeco, Castrop-Rauxel, Germany) under biosafety level III conditions. All animal experiments performed were in accordance with the German Animal Protection Law and were approved by the Animal Research Ethics Board of the Ministry of Environment, Nature Protection and Agriculture (Kiel, Germany).
For infection experiments using the previously published wt and ompATb mutant strains, cultures of wt M. tuberculosis (strain 1424) or the ompATb deletion mutant CK69 (Raynaud et al., 2002) were grown to mid-log phase, harvested, aliquoted, and frozen at −80°C. After thawing, viable cell counts were determined by plating serial dilutions on Middlebrook 7H10 agar supplemented with 10% bovine serum (Biowest, Nuaillé, France). Aerogenic infections with approximately 200 CFU of the wild type or the mutant strains were carried out in a Glas-Col aerosol infection device (Glas-Col, Terre-Haute, IN). Actual inoculum size was confirmed 24 h after infection by determining the bacterial burden in undiluted lung homogenates of three infected mice per strain. For intravenous infection, 0.2 ml of a bacterial suspension containing approximately 1x105 CFU in PBS were injected into a lateral tail vein. The inocula were confirmed 24 h post infection by determining the bacterial load in undiluted lung homogenates and in serial ten-fold dilutions of spleen and liver homogenates (three infected mice per strain). To follow the course of infection, the bacterial load in lungs and spleens was determined at days 28 and 65 after infection. Organs from five animals per time point and strain were aseptically removed, weighed and homogenized in distilled sterile water containing 0.05% Tween 80. Ten-fold serial dilutions of organ homogenates were plated on Middlebrook 7H10 agar supplemented with 10% bovine serum and incubated at 37°C for 21 days. Colonies on plates were enumerated and results are expressed as log10 CFU per organ.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Peter Sander for providing the M. tuberculosis ompATb mutant CK69, Dr. Philip Draper for the OmpATb antiserum, Dr. Adrie Steyn for the pCreSacB1 plasmid and Dr. Sabine Ehrt for critically reading the manuscript. JH was supported by a fellowship from the NIH training grant “Basic Mechanisms of Lung Diseases” (T32 HL07553). The TARGET program was supported by the National Institutes of Health (NIH) contract N01 AI30036. This work was funded by grants to MN from the National Institutes of Health (AI063432, AI074805), the Potts Memorial Foundation and the Center for AIDS Research of the University of Alabama at Birmingham.
Abbreviations
- OPOE
n-octylpolyethylene oxide
- wt
wild-type
- ELISA
enzyme-linked immunosorbent assay
REFERENCES
- Alahari A, Saint N, Campagna S, Molle V, Molle G, Kremer L. The N-terminal domain of OmpATb is required for membrane translocation and pore-forming activity in mycobacteria. J Bacteriol. 2007;189:6351–6358. doi: 10.1128/JB.00509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko YN, Pohl P, Denisov GA. Permeation of ammonia across bilayer lipid membranes studied by ammonium ion selective microelectrodes. Biophys J. 1997;72:2187–2195. doi: 10.1016/S0006-3495(97)78862-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FA, Brent R, Kingston RE, Moore DD, Seidmann JG, Smith JA, Struhl K. Current protocols in molecular biology. Greene Publishing and Wiley-Interscience; New York: 1990. [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidmann JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. John Wiley & Sons; New York: 1987. [Google Scholar]
- Barry CE. Interpreting cell wall ‘virulence factors’ of Mycobacterium tuberculosis. Trends Microbiol. 2001;9:237–241. doi: 10.1016/s0966-842x(01)02018-2. [DOI] [PubMed] [Google Scholar]
- Begic S, Worobec EA. Regulation of Serratia marcescens ompF and ompC porin genes in response to osmotic stress, salicylate, temperature and pH. Microbiology. 2006;152:485–491. doi: 10.1099/mic.0.28428-0. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu. Rev. Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- Charbit A. Maltodextrin transport through LamB. Front Biosci. 2003;8:s265–274. doi: 10.2741/1046. [DOI] [PubMed] [Google Scholar]
- Danilchanka O, Pavlenok M, Niederweis M. Role of porins for uptake of antibiotics by Mycobacterium smegmatis. Antimicrob Agents Chemother. 2008;52:3127–3134. doi: 10.1128/AAC.00239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrt S, Schnappinger D. Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze MO, McElhaney RN. The effect of alterations in the fluidity and phase state of the membrane lipids on the passive permeation and facilitated diffusion of glycerol in Escherichia coli. J Gen Microbiol. 1981;124:299–307. doi: 10.1099/00221287-124-2-299. [DOI] [PubMed] [Google Scholar]
- Faller M, Niederweis M, Schulz GE. The structure of a mycobacterial outer-membrane channel. Science. 2004;303:1189–1192. doi: 10.1126/science.1094114. [DOI] [PubMed] [Google Scholar]
- Foster JW. Low pH adaptation and the acid tolerance response of Salmonella typhimurium. Crit Rev Microbiol. 1995;21:215–237. doi: 10.3109/10408419509113541. [DOI] [PubMed] [Google Scholar]
- Foster JW. When protons attack: microbial strategies of acid adaptation. Curr Opin Microbiol. 1999;2:170–174. doi: 10.1016/S1369-5274(99)80030-7. [DOI] [PubMed] [Google Scholar]
- Foster JW, Hall HK. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J Bacteriol. 1991;173:5129–5135. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AH, Hart PD, Young MR. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. 1980;286:79–80. doi: 10.1038/286079a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hart PD, Young MR, Gordon AH, Sullivan KH. Inhibition of phagosome-lysosome fusion in macrophages by certain mycobacteria can be explained by inhibition of lysosomal movements observed after phagocytosis. J Exp Med. 1987;166:933–946. doi: 10.1084/jem.166.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmann D, Eschenbacher I, Thiel A, Niederweis M. Expression of the major porin gene mspA is regulated in Mycobacterium smegmatis. J Bacteriol. 2007;189:958–967. doi: 10.1128/JB.01474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmama Z, Gabathuler R, Jefferies WA, de Jong G, Reiner NE. Attenuation of HLA-DR expression by mononuclear phagocytes infected with Mycobacterium tuberculosis is related to intracellular sequestration of immature class II heterodimers. J Immunol. 1998;161:4882–4893. [PubMed] [Google Scholar]
- Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. Cryo-electron tomography and vitreous sections reveal the outer membrane of mycobacteria. Int J Med Microbiol. 2007;297:138–139. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Leis A, Niederweis M, Plitzko JM, Engelhardt H. Disclosure of the mycobacterial outer membrane: Cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl. Acad. Sci. U S A. 2008;105:3963–3967. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Szabo G, Tamm LK. Electrostatic couplings in OmpA ion-channel gating suggest a mechanism for pore opening. Nat Chem Biol. 2006;2:627–635. doi: 10.1038/nchembio827. [DOI] [PubMed] [Google Scholar]
- Huff J, Pavlenok M, Sukumaran S, Niederweis M. Functions of the periplasmic loop of the porin MspA from Mycobacterium smegmatis. J Biol Chem. 2009;284:10223–10231. doi: 10.1074/jbc.M808599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Niederweis M. Role of porins in iron uptake by Mycobacterium smegmatis. J Bacteriol. 2010;192:6411–6417. doi: 10.1128/JB.00986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaps I, Ehrt S, Seeber S, Schnappinger D, Martin C, Riley LW, Niederweis M. Energy transfer between fluorescent proteins using a co-expression system in Mycobacterium smegmatis. Gene. 2001;278:115–124. doi: 10.1016/s0378-1119(01)00712-0. [DOI] [PubMed] [Google Scholar]
- Kartmann B, Stenger S, Niederweis M. Porins in the cell wall of Mycobacterium tuberculosis. J. Bacteriol. 1999;181:6543–6546. doi: 10.1128/jb.181.20.6543-6546.1999. Authors' correction appeared in J. Bacteriol. 6181, 7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid S, Bond PJ, Carpenter T, Sansom MS. OmpA: gating and dynamics via molecular dynamics simulations. Biochim Biophys Acta. 2008;1778:1871–1880. doi: 10.1016/j.bbamem.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Kruse E, Uehlein N, Kaldenhoff R. The aquaporins. Genome Biol. 2006;7:206. doi: 10.1186/gb-2006-7-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Barry CE, 3rd, Besra GS, Nikaido H. Mycolic acid structure determines the fluidity of the mycobacterial cell wall. J. Biol. Chem. 1996;271:29545–29551. doi: 10.1074/jbc.271.47.29545. [DOI] [PubMed] [Google Scholar]
- Liu J, Rosenberg EY, Nikaido H. Fluidity of the lipid domain of cell wall from Mycobacterium chelonae. Proc. Natl. Acad. Sci. U S A. 1995;92:11254–11258. doi: 10.1073/pnas.92.24.11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J, Skrzynia C, Goldberg ME. Renaturation of Escherichia coli tryptophanase after exposure to 8 M urea. Evidence for the existence of nucleation centers. Eur J Biochem. 1974;47:409–415. doi: 10.1111/j.1432-1033.1974.tb03707.x. [DOI] [PubMed] [Google Scholar]
- Long ER. Utilization of nitrogen for growth. In: Long ER, editor. The chemistry and chemotherapy of tuberculosis. The Williams & Wilkins Company; London: 1958. pp. 80–85. [Google Scholar]
- Lukacs GL, Rotstein OD, Grinstein S. Determinants of the phagosomal pH in macrophages. In situ assessment of vacuolar H(+)-ATPase activity, counterion conductance, and H+ “leak”. J Biol Chem. 1991;266:24540–24548. [PubMed] [Google Scholar]
- Lyon RH, Hall WH, Costas-Martinez C. Uptake and distribution of labeled carbon from 14C-asparagine by Mycobacterium tuberculosis. J Bacteriol. 1969;98:317–318. doi: 10.1128/jb.98.1.317-318.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon RH, Hall WH, Costas-Martinez C. Effect of L-asparagine on growth of Mycobacterium tuberculosis and on utilization of other amino acids. J Bacteriol. 1974;117:151–156. doi: 10.1128/jb.117.1.151-156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 2003;302:654–659. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- Mailaender C, Reiling N, Engelhardt H, Bossmann S, Ehlers S, Niederweis M. The MspA porin promotes growth and increases antibiotic susceptibility of both Mycobacterium bovis BCG and Mycobacterium tuberculosis. Microbiology. 2004;150:853–864. doi: 10.1099/mic.0.26902-0. [DOI] [PubMed] [Google Scholar]
- Molle V, Saint N, Campagna S, Kremer L, Lea E, Draper P, Molle G. pH-dependent pore-forming activity of OmpATb from Mycobacterium tuberculosis and characterization of the channel by peptidic dissection. Mol Microbiol. 2006;61:826–837. doi: 10.1111/j.1365-2958.2006.05277.x. [DOI] [PubMed] [Google Scholar]
- Niederweis M. Mycobacterial porins - new channel proteins in unique outer membranes. Mol. Microbiol. 2003;49:1167–1177. doi: 10.1046/j.1365-2958.2003.03662.x. [DOI] [PubMed] [Google Scholar]
- Niederweis M. Mycobacterial porins. In: Daffe M, Reyrat J-M, editors. The mycobacterial cell envelope. ASM Press; Washington, DC: 2008a. pp. 153–165. [Google Scholar]
- Niederweis M. Nutrient acquisition by mycobacteria. Microbiology. 2008b;154:679–692. doi: 10.1099/mic.0.2007/012872-0. [DOI] [PubMed] [Google Scholar]
- Niederweis M, Ehrt S, Heinz C, Klöcker U, Karosi S, Swiderek KM, Riley LW, Benz R. Cloning of the mspA gene encoding a porin from Mycobacterium smegmatis. Mol. Microbiol. 1999;33:933–945. doi: 10.1046/j.1365-2958.1999.01472.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H, Song SA, Shaltiel L, Nurminen M. Outer membrane of Salmonella. XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem. Biophys. Res. Commun. 1977;76:324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- NIST MSDC. Wiley Registry of Mass Spectral Data, with NIST 2008. 8th Edition John Wiley & Sons, Inc.; 2008. [Google Scholar]
- Quivey RG, Kuhnert WL, Hahn K. Genetics of acid adaptation in oral streptococci. Crit Rev Oral Biol Med. 2001;12:301–314. doi: 10.1177/10454411010120040201. [DOI] [PubMed] [Google Scholar]
- Rao M, Streur TL, Aldwell FE, Cook GM. Intracellular pH regulation by Mycobacterium smegmatis and Mycobacterium bovis BCG. Microbiology. 2001;147:1017–1024. doi: 10.1099/00221287-147-4-1017. [DOI] [PubMed] [Google Scholar]
- Raynaud C, Papavinasasundaram KG, Speight RA, Springer B, Sander P, Böttger EC, Colston MJ, Draper P. The functions of OmpATb, a pore-forming protein of Mycobacterium tuberculosis. Mol. Microbiol. 2002;46:191–201. doi: 10.1046/j.1365-2958.2002.03152.x. [DOI] [PubMed] [Google Scholar]
- Rengarajan J, Bloom BR, Rubin EJ. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci USA. 2005;102:8327–8332. doi: 10.1073/pnas.0503272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezwan M, Laneelle MA, Sander P, Daffe M. Breaking down the wall: fractionation of mycobacteria. J Microbiol Methods. 2007;68:32–39. doi: 10.1016/j.mimet.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Rohde K, Yates RM, Purdy GE, Russell DG. Mycobacterium tuberculosis and the environment within the phagosome. Immunol Rev. 2007;219:37–54. doi: 10.1111/j.1600-065X.2007.00547.x. [DOI] [PubMed] [Google Scholar]
- Sachs G, Kraut JA, Wen Y, Feng J, Scott DR. Urea transport in bacteria: acid acclimation by gastric Helicobacter spp. J Membr Biol. 2006;212:71–82. doi: 10.1007/s00232-006-0867-7. [DOI] [PubMed] [Google Scholar]
- Samartzidou H, Mehrazin M, Xu Z, Benedik MJ, Delcour AH. Cadaverine inhibition of porin plays a role in cell survival at acidic pH. J Bacteriol. 2003;185:13–19. doi: 10.1128/JB.185.1.13-19.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander P, Meier A, Boettger EC. rpsL+: a dominant selectable marker for gene replacement in mycobacteria. Mol. Microbiol. 1995;16:991–1000. doi: 10.1111/j.1365-2958.1995.tb02324.x. [DOI] [PubMed] [Google Scholar]
- Sassetti CM, Boyd DH, Rubin EJ. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol. 2003;48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci USA. 2003;100:12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaratne RH, Mobasheri H, Papavinasasundaram KG, Jenner P, Lea EJ, Draper P. Expression of a gene for a porin-like protein of the OmpA family from Mycobacterium tuberculosis H37Rv. J. Bacteriol. 1998;180:3541–3547. doi: 10.1128/jb.180.14.3541-3547.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonczewski JL, Fujisawa M, Dopson M, Krulwich TA. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv Microb Physiol. 2009;55:1–79. doi: 10.1016/S0065-2911(09)05501-5. 317. [DOI] [PubMed] [Google Scholar]
- Smeulders MJ, Keer J, Speight RA, Williams HD. Adaptation of Mycobacterium smegmatis to stationary phase. J. Bacteriol. 1999;181:270–283. doi: 10.1128/jb.181.1.270-283.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev. 2003;16:463–496. doi: 10.1128/CMR.16.3.463-496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Sandie R, Wang Y, Andrade-Navarro MA, Niederweis M. Identification of outer membrane proteins of Mycobacterium tuberculosis. Tuberculosis. 2008;88:526–544. doi: 10.1016/j.tube.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E, He L, Yan D, Kustu S. Ammonia acquisition in enteric bacteria: physiological role of the ammonium/methylammonium transport B (AmtB) protein. Proc Natl Acad Sci U S A. 1998;95:7030–7034. doi: 10.1073/pnas.95.12.7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl C, Kubetzko S, Kaps I, Seeber S, Engelhardt H, Niederweis M. MspA provides the main hydrophilic pathway through the cell wall of Mycobacterium smegmatis. Mol. Microbiol. 2001;40:451–464. doi: 10.1046/j.1365-2958.2001.02394.x. Authors' correction appeared in Mol. Microbiol. 457, 1509. [DOI] [PubMed] [Google Scholar]
- Stephan J, Bender J, Wolschendorf F, Hoffmann C, Roth E, Mailänder C, Engelhardt H, Niederweis M. The growth rate of Mycobacterium smegmatis depends on sufficient porin-mediated influx of nutrients. Mol. Microbiol. 2005;58:714–730. doi: 10.1111/j.1365-2958.2005.04878.x. [DOI] [PubMed] [Google Scholar]
- Stephan J, Stemmer V, Niederweis M. Consecutive gene deletions in Mycobacterium smegmatis using the yeast FLP recombinase. Gene. 2004;343:181–190. doi: 10.1016/j.gene.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Stewart GR, Patel J, Robertson BD, Rae A, Young DB. Mycobacterial mutants with defective control of phagosomal acidification. PLoS Pathog. 2005;1:269–278. doi: 10.1371/journal.ppat.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn AJ, Joseph J, Bloom BR. Interaction of the sensor module of Mycobacterium tuberculosis H37Rv KdpD with members of the Lpr family. Mol Microbiol. 2003;47:1075–1089. doi: 10.1046/j.1365-2958.2003.03356.x. [DOI] [PubMed] [Google Scholar]
- Sugawara E, Nestorovich EM, Bezrukov SM, Nikaido H. Pseudomonas aeruginosa porin OprF exists in two different conformations. J Biol Chem. 2006;281:16220–16229. doi: 10.1074/jbc.M600680200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara E, Nikaido H. Pore-forming activity of OmpA protein of Escherichia coli. J. Biol. Chem. 1992;267:2507–2511. [PubMed] [Google Scholar]
- Sugawara E, Nikaido H. OmpA protein of Escherichia coli outer membrane occurs in open and closed channel forms. J. Biol. Chem. 1994;269:17981–17987. [PubMed] [Google Scholar]
- Teriete P, Yao Y, Kolodzik A, Yu J, Song H, Niederweis M, Marassi FM. Mycobacterium tuberculosis Rv0899 adopts a mixed alpha/beta-structure and does not form a transmembrane beta-barrel. Biochemistry. 2010;49:2768–2777. doi: 10.1021/bi100158s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssen S, Chari ST, Scheid J, Singer MV. Effect of repeated boluses of intravenous omeprazole and primed infusions of ranitidine on 24-hour intragastric pH in healthy human subjects. Dig Dis Sci. 1995;40:247–255. doi: 10.1007/BF02065405. [DOI] [PubMed] [Google Scholar]
- Thomas AD, Booth IR. The regulation of expression of the porin gene ompC by acid pH. J. Gen. Microbiol. 1992;138:1829–1835. doi: 10.1099/00221287-138-9-1829. [DOI] [PubMed] [Google Scholar]
- Vandal OH, Nathan CF, Ehrt S. Acid resistance in Mycobacterium tuberculosis. J Bacteriol. 2009a;191:4714–4721. doi: 10.1128/JB.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Ehrt S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med. 2008;14:849–854. doi: 10.1038/nmXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandal OH, Roberts JA, Odaira T, Schnappinger D, Nathan CF, Ehrt S. Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J Bacteriol. 2009b;191:625–631. doi: 10.1128/JB.00932-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder FG, Brennan PJ. Initial steps in the metabolism of glycerol by Mycobacterium tuberculosis. J Bacteriol. 1966;92:1846–1847. doi: 10.1128/jb.92.6.1846-1847.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolschendorf F, Mahfoud M, Niederweis M. Porins are required for uptake of phosphates by Mycobacterium smegmatis. J Bacteriol. 2007;189:2435–2442. doi: 10.1128/JB.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Auguin D, Delbecq S, Dumas E, Molle G, Molle V, Roumestand C, Saint N. Structure of the Mycobacterium tuberculosis OmpATb protein: A model of an oligomeric channel in the mycobacterial cell wall. Proteins. 2011;79:645–661. doi: 10.1002/prot.22912. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zhu Y, Crane DD, Barry CE., 3rd The effect of oxygenated mycolic acid composition on cell wall function and macrophage growth in Mycobacterium tuberculosis. Mol. Microbiol. 1998;29:1449–1458. doi: 10.1046/j.1365-2958.1998.01026.x. [DOI] [PubMed] [Google Scholar]
- Yuen SH, Polland AG. Determination of nitrogen in agricultural materials by the Nessler reagent. II. - Micro-determinations in plant tissue and in soil extracts. J. Sci. Food Agric. 1954;5:364–369. [Google Scholar]
- Zhang Y, Scorpio A, Nikaido H, Sun Z. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Bacteriol. 1999;181:2044–2049. doi: 10.1128/jb.181.7.2044-2049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Kostrewa D, Berneche S, Winkler FK, Li XD. The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc Natl Acad Sci U S A. 2004;101:17090–17095. doi: 10.1073/pnas.0406475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.