Abstract
Background
Calcium carbonate is a commonly used dietary supplement and has been shown to interfere with levothyroxine absorption. However, calcium citrate, which is also used for supplementation purposes, has not been studied previously and calcium acetate, which is used to treat hyperphosphatemia in renal failure, has been reported to show little or no interference with levothyroxine absorption in a retrospective pharmacoepidemiologic study. We aimed to compare the effect of these three calcium formulations on levothyroxine absorption.
Materials and Methods
The study was conducted in eight healthy, euthyroid adults. We performed single-dose pharmacokinetic studies in which we measured levothyroxine absorption when given alone or when coadministered with calcium carbonate, calcium citrate, or calcium acetate in doses containing 500 mg elemental calcium. Serum thyroxine was measured at intervals over a 6-hour period after ingestion of the study drugs.
Results
Coadministration of each of the three calcium preparations significantly reduced levothyroxine absorption by about 20%–25% compared with levothyroxine given alone.
Conclusions
Contrary to a prior report, our data suggest that calcium acetate interferes with levothyroxine absorption in a manner similar to that seen with calcium carbonate and calcium citrate. Although the effect of calcium is modest compared with some other medications previously studied, hypothyroid patients should be cautioned to take their levothyroxine well-separated from all of these calcium formulations.
Introduction
Several studies have shown that certain medications can cause the malabsorption of levothyroxine sodium when they are coadministered. These medications include bile acid-binding resins (1), iron salts (2), sucralfate (3), aluminum-containing antacids (4), raloxifene (5), chromium picolinate (6), sevelamer HCl (6), colesevelam HCl (7), and lanthanum carbonate (7). The mechanism by which this malabsorption occurs is presumed to be a binding of the medication to the thyroid hormone, forming an insoluble or nonabsorbable complex (1,2,4,8,9).
Calcium carbonate, which is commonly used for the prevention and treatment of osteoporosis, has also been shown to reduce levothyroxine sodium absorption (8,10). However, other commonly used calcium supplements, including calcium citrate and calcium acetate, have not been prospectively studied. As the population ages, increasing numbers of patients are taking either calcium carbonate or calcium citrate to prevent or treat osteoporosis. Additionally, many patients with advanced renal disease are treated with calcium acetate to lower serum phosphate. It is therefore important to determine if malabsorption of levothyroxine occurs with the concurrent use of either of these calcium preparations.
To evaluate these potential interactions with thyroid hormone, we conducted single-dose absorption studies of levothyroxine in normal volunteers, with and without simultaneous administration of these agents.
Materials and Methods
Subjects
Eight healthy, euthyroid, normal volunteers (four men and four women, age 21–40) participated in this study. None of the subjects had a history of thyroid, renal, hepatic, intestinal, or cardiac disease. In addition, none was taking medication known to alter the absorption or metabolism of levothyroxine (e.g., iron preparations, aluminum-containing antacids, sucralfate, phenytoin, carbamazepine, or oral contraceptives). None of the subjects were taking calcium supplements or multivitamins in the weeks before the study or throughout the study period. The study was approved by the Institutional Review Board of Stony Brook University, and informed consent was obtained from all subjects before participation in the study. The subjects were paid for their participation.
Study design
We utilized a 6-hour pharmacokinetic study of levothyroxine absorption as described in previous reports (3,5,6,7,10). Each subject was studied on four separate occasions; at least 3 weeks elapsed between study sessions. On each occasion, subjects fasted overnight. For the first study in each subject, 1 mg (given as five 0.2-mg tablets) of levothyroxine sodium (Synthroid®) was administered orally along with 240 mL of water. Blood samples were obtained through an indwelling catheter inserted into an arm vein; samples were collected at −15 and 0 minutes before, and at 30, 60, 120, 240, and 360 minutes after the administration of the levothyroxine dose. Subjects were served a light lunch after the +240 minute specimen was collected. On each of the three subsequent study days, the same protocol was followed, and one of the calcium preparations was coadministered with the 1-mg dose of levothyroxine along with 240 mL water. The order in which the calcium preparations were given was randomized. The calcium preparations were all administered in doses containing 500 mg of elemental calcium: calcium carbonate 1250 mg (one 1250-mg tablet; distributed by Roxane Laboratories, Columbus, OH), calcium citrate 2381 mg (two 1190.5 mg tablets; distributed by Puritan's Pride, Oakdale, NY), and calcium acetate 2001 mg (three 667-mg tablets of PhosLo® brand, Fresenius Medical Care North America, Waltham, MA). The 500-mg dose of elemental calcium was chosen because it represents a typical single dosage for all three preparations.
Assays
Blood samples were allowed to clot at room temperature, and serum was stored at −20°C until assayed. Total serum thyroxine (T4) was measured in the Stony Brook University Hospital clinical laboratory using Siemens Advia Centaur immuno-chemiluminescent assays (Siemens Healthcare Diagnostics, Deerfield, IL). All serum samples from each subject were run in a single assay for serum T4. According to the manufacturer, the analytical sensitivity of the T4 assay is 0.3 μg/dL. The intra-assay coefficient of variation is 1.19% at a serum T4 concentration of 7.70 μg/dL and 1.77% at a serum T4 concentration of 14.82 μg/dL.
Statistics
The hormone concentrations in the sera obtained at −15 and 0 minutes were averaged for a baseline measurement of T4. Mean, standard deviation, and standard error (SE) serum T4 values were calculated for the eight subjects at each time point. The response area (above baseline) under the T4 concentration curve was calculated using trapezoidal integration. An initial search for significant differences between treatments was performed on the area under the curve data using one-way analysis of variance for repeated measures. The significance of differences between individual treatments was then explored using Dunnett's t-test for multiple comparisons with a control, applied to the response area data.
Results
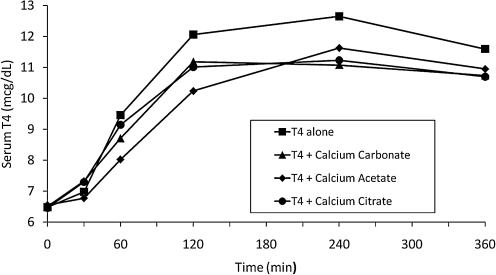
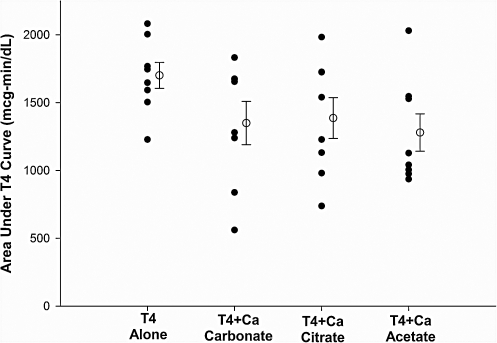
The mean baseline serum T4 concentrations were nearly identical on all four study days (Fig. 1), being 6.49 ± 0.45 (SE) μg/dL on the day levothyroxine was given alone, 6.51 ± 0.44 μg/dL on the day levothyroxine was given with calcium carbonate, 6.46 ± 0.37 μg/dL for the levothyroxine plus calcium citrate test day, and 6.56 ± 0.49 μg/dL on the levothyroxine plus calcium acetate test day. As anticipated, serum T4 rose in all subjects after the administration of 1 mg of levothyroxine alone. This effect was blunted by the coadministration of calcium carbonate, calcium citrate, and calcium acetate (Fig. 1). The mean baseline-subtracted response area for levothyroxine given alone was 1696 ± 96 (SE) μg-min/dL. In comparison, the mean baseline-subtracted response area for levothyroxine plus calcium carbonate was 1344 ± 160 (SE) μg-min/dL (79.2% of the area of levothyroxine alone), for levothyroxine plus calcium citrate was 1381 ± 151 (SE) μg-min/dL (81.4% of the area of levothyroxine alone), and for levothyroxine plus calcium acetate was 1274 ± 137 (SE) μg-min/dL (75.1% of the area of levothyroxine alone). The effect of calcium on levothyroxine absorption varied significantly between subjects; for example, in one subject, none of the calcium preparations decreased the T4 response area, whereas in three other individuals, the T4 response area was decreased by 66% (calcium carbonate), 54% (calcium citrate) and 47% (calcium acetate).
FIG. 1.
Mean serum concentrations of thyroxine (T4) over a 6-hour period after administration of levothyroxine with and without calcium formulations in eight subjects. Standard error bars are omitted for clarity. Standard errors were 0.37–0.49 μg/dL at baseline, 0.43–1.13 μg/dL at 60 minutes, and 0.50–0.76 μg/dL at 240 minutes; the standard errors overlapped at all time points for all tests.
One-way analysis of variance for repeated measures showed that significant differences were present in the areas under the T4 absorption curves (F = 5.37, p < 0.007). Dunnett's multiple comparisons test found that each calcium formulation significantly lowered the T4 absorption area when compared with levothyroxine alone (p < 0.05 for levothyroxine plus calcium carbonate and calcium citrate and p < 0.01 for levothyroxine plus calcium acetate [Fig. 2]).
FIG. 2.
Area (above baseline) under the serum T4 concentration curve in eight normal subjects receiving levothyroxine alone or simultaneously with study medications. Closed circles show values for individual subjects, whereas open circles indicate the mean value for each test; bars indicate standard error of the mean.
Discussion
It is well known that levothyroxine should be taken separately from food, since food adsorbs thyroid hormone and reduces its intestinal absorption (11,12). In addition, several medications are known to interfere with intestinal absorption of levothyroxine when taken simultaneously (1–10). The most commonly used calcium formulation, calcium carbonate, was initially suspected of interacting with levothyroxine following a report of three hypothyroid patients who developed acute increases in serum TSH with the simultaneous administration of levothyroxine and calcium carbonate and prompt improvement after discontinuation of the calcium carbonate (13). Subsequently, two studies have demonstrated calcium carbonate interference with levothyroxine absorption (8,10). However, calcium citrate, which is also used for supplementation purposes, has not been studied previously. Calcium acetate, commonly used in the treatment of hyperphosphatemia in renal failure, was reported to show little or no interference with levothyroxine absorption in a retrospective pharmacoepidemiologic study (14).
The results of our pharmacokinetic study in eight normal subjects indicate that calcium carbonate, calcium citrate, and calcium acetate all lead to a blunted rise in the serum T4 concentration when given concurrently with levothyroxine. This effect was of a similar magnitude with all three agents.
Our results are in contrast to the study by Diskin et al., which concluded that calcium acetate does not appear to interfere with the bioavailability of levothyroxine in patients with hypothyroidism and renal failure (14). This study utilized an observational design in which correlations between the type of phosphate binder used (calcium carbonate, calcium acetate, or sevelamer), serum TSH, and the amount of levothyroxine taken were analyzed over a 2-year period. As an observational study, the reliability is subject to multiple potentially confounding factors, including compliance of the participants as well as variability in the effects of calcium acetate between subjects. In the present study, we utilized a 6-hour pharmacokinetic design, previously validated and described in several reports which demonstrated direct effects of interfering drugs on levothyroxine absorption. An additional factor that may account for the difference between our results and those of Diskin et al. concerns the effects of food; in our study, subjects took levothyroxine and calcium preparations with only water, whereas in the study of Diskin et al., most of the patients took their levothyroxine and phosphate binder together with breakfast (14). It is possible that the presence of food in the stomach may have altered the interaction between levothyroxine and calcium acetate.
Our study also indicates that the effects of all the calcium formulations on levothyroxine absorption are relatively modest when the calcium is administered in usual prescribed doses. Doses of calcium carbonate or calcium citrate that are typically recommended for prevention and treatment of osteoporosis are usually 1200 mg of elemental calcium per day, given in divided doses two to three times daily (15). Similarly, the usual dose of calcium acetate when given for phosphate binding is 2001 mg, which contains 500 mg of elemental calcium (16). In a previous cohort study performed by Singh et al. (8), subjects received 1200 mg of elemental calcium as calcium carbonate taken as a single 3000 mg daily dose with their levothyroxine for a period of 3 months. This resulted in a modest but significant decrease in mean serum free T4 and total T4 levels as well as an increase in serum TSH (the mean serum TSH increased from 1.6 to 2.7 mIU/L). A follow-up single-dose pharmacokinetic study was performed with 2000 mg of elemental calcium (5000 mg of calcium carbonate) given simultaneously with 1 mg of levothyroxine and similarly showed a reduction in the expected rise of serum total T4, free T4, and total triiodothyronine levels (10). However, these effects were relatively modest despite giving large doses of calcium carbonate in amounts much higher than recommended dosing. In our study, the dose of calcium chosen was 500 mg elemental calcium, as recommended for routine use. While our results also indicate a significant effect on levothyroxine absorption with calcium carbonate in concordance with the studies performed by Singh et al. (8,10), the average effect is less pronounced compared with some other medications, in particular with sevelamer hydrochloride and colesevalam which led to 50% and 96% reductions in the baseline-subtracted levothyroxine response area, respectively (6,7). Although not studied, it is possible that the use of lower doses of levothyroxine might result in a greater per cent decrement in T4 absorption when given together with calcium.
One possible limitation of this study is the use of euthyroid subjects; although it is unlikely that different results would be found in patients being treated for hypothyroidism, we did not assess this possibility. A second limitation is the measurement of serum T4 for only 6 hours after drug ingestion. It is possible that a longer period of sampling would alter the magnitude of the changes seen in levothyroxine absorption; however, it would not likely have altered the conclusions. Finally, the interval between study visits was relatively short at 3 weeks. It is possible that a mild levothyroxine-induced hyperthyroidism persisted and influenced the results of subsequent visits; however, our previous studies of the same design have indicated that baseline TSH was similar in subjects on all test days when the interval between tests was 2 or 3 weeks (6,7), and a clinical study of once-weekly administration of levothyroxine reported that serum free T4 and TSH returned to baseline 1 week after ingestion of the weekly dose (17). Additionally, the carry-over effects which might result in subclinical hyperthyroidism would enhance, not diminish, the levothyroxine absorption in the next test (11). Therefore, this would not be expected to account for the decreased absorption seen when levothyroxine was coadministered with the calcium formulations.
In conclusion, the above results show that calcium carbonate, calcium acetate and calcium citrate in the usual prescribed doses all lead to a reduction in levothyroxine absorption when given together with the thyroid hormone. The effect of calcium acetate in the current study was greater than previously reported. Although the average effect on levothyroxine absorption was modest for all three calcium formulations, a 20%–25% decrease in absorption is significant and may be even larger in some individuals. Changes of this magnitude could result in clinically important effects in patients with thyroid cancer, cardiac disorders or depression. It is therefore recommended that patients be cautioned to take their levothyroxine well separated from all of these calcium products.
Footnotes
Presented at the 92nd Annual Meeting of The Endocrine Society, San Diego, California, June 19–22, 2010.
Acknowledgments
We thank the staff of the General Clinical Research Center at Stony Brook University Hospital for their assistance in conducting this study. This work was supported by funds from the General Clinical Research Center, Stony Brook University Hospital, NIH grant M01RR10710.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Northcutt RC. Stiel JN. Hollifield JW. Stant EG., Jr The influence of cholestyramine on thyroxine absorption. JAMA. 1969;208:1857–1861. [PubMed] [Google Scholar]
- 2.Campbell NRC. Hasinoff BB. Stalts H. Rao B. Wong NCW. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010–1013. doi: 10.7326/0003-4819-117-12-1010. [DOI] [PubMed] [Google Scholar]
- 3.Sherman SI. Tielens ET. Ladenson PW. Sucralfate causes malabsorption of L-thyroxine. Am J Med. 1994;96:531–535. doi: 10.1016/0002-9343(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 4.Liel Y. Sperber AD. Shany S. Nonspecific intestinal adsorption of levothyroxine by aluminum hydroxide. Am J Med. 1994;97:363–365. doi: 10.1016/0002-9343(94)90303-4. [DOI] [PubMed] [Google Scholar]
- 5.Siraj ES. Gupta MK. Reddy SSK. Raloxifene causing malabsorption of levothyroxine. Arch Intern Med. 2003;163:1367–1370. doi: 10.1001/archinte.163.11.1367. [DOI] [PubMed] [Google Scholar]
- 6.John-Kalarickal J. Pearlman G. Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763–765. doi: 10.1089/thy.2007.0060. [DOI] [PubMed] [Google Scholar]
- 7.Weitzman SP. Ginsburg KC. Carlson HE. Colesevelam hydrochloride and lanthanum carbonate interfere with the absorption of levothyroxine. Thyroid. 2009;19:77–79. doi: 10.1089/thy.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh N. Singh PN. Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283:2822–2825. doi: 10.1001/jama.283.21.2822. [DOI] [PubMed] [Google Scholar]
- 9.Havrankova J. Lahaie R. Levothyroxine binding by sucralfate. Ann Intern Med. 1992;117:445–446. doi: 10.7326/0003-4819-117-5-445_3. [DOI] [PubMed] [Google Scholar]
- 10.Singh N. Weisler SL. Hershman JM. The acute effect of calcium carbonate on the intestinal absorption of levothyroxine. Thyroid. 2001;11:967–971. doi: 10.1089/105072501753211046. [DOI] [PubMed] [Google Scholar]
- 11.Hays MT. Thyroid hormone and the gut. Endocr Res. 1988;14:203–224. doi: 10.3109/07435808809032986. [DOI] [PubMed] [Google Scholar]
- 12.Lamson MJ. Pamplin CL. Rolleri RL. Klein I. Quantitation of a substantial reduction in levothyroxine (T4) absorption by food. Thyroid. 2004;14:876. (abstract). [Google Scholar]
- 13.Schneyer CR. Calcium carbonate and reduction of levothyroxine efficacy. JAMA. 1998;279:750. doi: 10.1001/jama.279.10.750-b. [DOI] [PubMed] [Google Scholar]
- 14.Diskin CJ. Stokes TJ. Dansby LM. Radcliff L. Carter TB. Effect of phosphate binder upon TSH and L-thyroxine dose in patients on thyroid replacement. Int Urol Nephrol. 2007;39:599–602. doi: 10.1007/s11255-006-9166-6. [DOI] [PubMed] [Google Scholar]
- 15.National Osteoporosis Foundation 2010 Clinician's Guide to Prevention and Treatment of Osteoporosis. National Osteoporosis Foundation; Washington, DC: [Jun 28;2010 ]. [Google Scholar]
- 16.Fresenius Medical Care North America PhosLo® Prescribing Information; Waltham, MA: [Jun 14;2010 ]. [Google Scholar]
- 17.Grebe SKG. Cooke RR. Ford HC. Fagerström JN. Cordwell DP. Lever NA. Purdie GL. Feek CM. Treatment of hypothyroidism with once weekly thyroxine. J Clin Endocrinol Metab. 1997;82:870–875. doi: 10.1210/jcem.82.3.3830. [DOI] [PubMed] [Google Scholar]