Abstract
Recent studies have begun to scrutinize the presynaptic machinery and vesicle populations that give rise to action potential evoked and spontaneous forms of neurotransmitter release. In several cases this work produced unexpected results which lend support to the notion that regulation, mechanisms, postsynaptic targets and possibly presynaptic origins of evoked and spontaneous neurotransmitter release differ. Furthermore, the list of regulatory pathways that impact spontaneous and evoked release in a divergent manner is rapidly growing. These findings challenge our classical views on the relationship between evoked and spontaneous neurotransmission. In contrast to the well-characterized neuromodulatory pathways that equally suppress or augment all forms of neurotransmitter release, molecular substrates specifically controlling spontaneous release remain unclear. In this review, we outline possible mechanisms that may underlie the differential regulation of distinct forms of neurotransmission and help demultiplex complex neuronal signals and generate parallel signaling events at their postsynaptic targets.
Introduction
During activity, synaptic nerve terminals release neurotransmitter in response to presynaptic action potential firing and ensuing Ca2+ influx. In addition, several substances, often called secretagogues, including α-latrotoxin, lanthanides, and certain neuromodulators can trigger release [1, 2, 3]. However, since the early work of Bernard Katz and colleagues, it has been wellknown that neurotransmitter release can also occur spontaneously in the absence of presynaptic action potentials or secretagogues, albeit with a low probability [4].
Recent studies have suggested several physiological roles for this stimulus-independent form of neurotransmitter release [5, 6]. Spontaneous neurotransmitter release, which typically corresponds to fusion of a single synaptic vesicle, can be sufficient to trigger postsynaptic action potential firing or regulate postsynaptic excitability and spike timing [3, 7, 8]. In addition, several forms of homeostatic plasticity seen after cessation of neuronal activity can be strongly modulated in duration and magnitude by spontaneous release events [9, 10, 11, 12, 13]. Furthermore, these low probability stochastic fusion events have been shown to suppress dendritic local protein translation and help maintain the stability of synaptic responses [10]. In agreement with these observations, recent studies have suggested a spatial segregation for the two forms of release [14, 15], supporting the premise that they activate parallel signal transduction pathways with minimum overlap [6].
Differential regulation of spontaneous and evoked release
In contrast to the emerging consensus on the distinct functional role of spontaneous neurotransmission in neuronal signaling [6, 16, 17], the presynaptic mechanisms that underlie spontaneous release and the degree of its segregation from the machinery giving rise to action potential evoked neurotransmission has been under intense debate [18, 19, 20, 21, 22, 23, 24, 25]. Nevertheless, several studies have provided strong evidence for specific regulation of spontaneous neurotransmitter release by certain signaling pathways and, in some cases, actions of these pathways have been shown to alter spontaneous and evoked release in the opposite direction [26, 27].
Mechanistically, it is straightforward to envision how neuromodulators may selectively target evoked release or modify both evoked and spontaneous release in the same direction. This regulation can be achieved by inhibition of voltage-gated Ca2+ channel function or block of presynaptic excitability leading to impaired evoked release [28, 29]. Some neuromodulators such as adenosine [30], serotonin [31] or GABA, acting via GABAB receptors [32], target the release machinery as well as Ca2+ channels, thus suppressing both evoked and spontaneous release. Exogenous application of phorbol esters or diacylglycerol generation leads to augmentation of both evoked and spontaneous release [33, 34, 35, 36, 37]. In contrast to these well-characterized neuromodulatory pathways, mechanisms controlling selective regulation of spontaneous release but not evoked release or opposite regulation of spontaneous and evoked release remain unclear.
Examples of this anomalous regulation include selective inhibition of spontaneous but not Ca2+-dependent evoked release by activation of presynaptic group II metabotropic glutamate receptors in rat cerebellar slices [38], specific enhancement of spontaneous miniature excitatory postsynaptic currents (mEPSCs) but not evoked EPSCs in response to BDNF application in immature visual cortical neurons [39], and selective activity-dependent decrease in the frequency of miniature EPSCs triggered by inhibition of DNA methyltransferases, key enzymes that methylate DNA and regulate gene expression [40]. Induction of endoplasmic reticulum stress also causes a dramatic four-fold increase in spontaneous excitatory transmission coupled with only a small increase in evoked neurotransmitter release probability [41]. Finally, loss of presenilins, integral components of γ-secretase, a key enzyme involved in the etiology of Alzheimer's disease, or treatment with a γ-secretase inhibitor augments the rate of spontaneous release without altering evoked synaptic transmission [42]. This regulation appears to be achieved by modulation of tonic calcium influx into presynaptic terminals, consistent with the proposed role of presenilins in regulation of neuronal Ca2+ homeostasis [43, 44]. With regard to opposing regulation of spontaneous and evoked release, certain nitric oxide-related species inhibit evoked neurotransmission but enhance spontaneous mEPSCs [45]. Additionally, cholesterol depletion or inhibition of cholesterol synthesis in neurons causes an increase in the rate of spontaneous transmission but impairs evoked neurotransmission [26, 27].
This growing list of examples challenges our classical views on the relationship between evoked and spontaneous neurotransmitter release. Several recent studies of the presynaptic machinery and the vesicle populations have produced unexpected results which support specific regulation of spontaneous neurotransmitter release. In the following sections, we outline four possible mechanisms that emerged from recent work and may underlie the differential regulation of distinct forms of neurotransmission.
Mechanisms underlying differential regulation of spontaneous and evoked release
1. Segregation of evoked and spontaneous release at the level of individual presynaptic terminals
Numerous fluorescent imaging studies have documented substantial co-localization of spontaneous and evoked synaptic vesicle recycling in individual synaptic boutons [18, 19, 20, 22, 46, 47]. These findings come with the caveat that typical fluorescent identification criteria used in imaging experiments select for synapses with robust recycling properties, over ones that may manifest a more inefficient release property [18, 22, 47]. Indeed, immature synaptic boutons typically favor spontaneous release and fail to respond to action potential stimulation [48, 49, 50], which raises the possibility that a population of nascent synapses in an otherwise mature synaptic network may selectively sustain spontaneous release. Interestingly, a recent study from our group also revealed a set of presynaptic terminals that support action potential driven release with negligible concurrent spontaneous vesicle exocytosis [14]. The prevalence of these types of synapses is hard to ascertain due to the inherent bias associated with optical analysis in identification of functional synaptic boutons. In this study, majority of synapses (~80%) were capable of both evoked and spontaneous release, although the kinetics of the two forms of release did not show correlation in a given synapse [14]. These findings suggest that spontaneous and evoked release have substantial overlap in their sites of origin, but they may not be linked with respect to their relative activities. Taken together, although complete segregation of spontaneous and evoked neurotransmitter release into different synapses remains an unlikely possibility, there is evidence supporting existence of synaptic niches dominated by one form of release versus another.
2. Differential Ca2+-dependence of spontaneous versus evoked neurotransmitter release
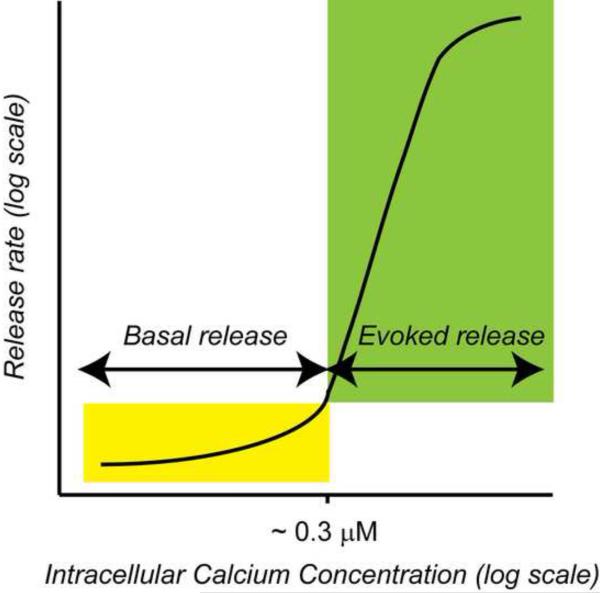
Tonic levels of Ca2+ in the presynaptic milieu or pulsatile release of Ca2+ from internal stores, called Ca2+ sparks, can potently regulate neurotransmitter release under resting conditions [51]. Even in the absence of presynaptic action potentials, nerve terminals manifest brief bursts of high fusion activity, clearly deviating from the low frequency, random nature of spontaneous release [52, 53]. These presynaptic bursts are typically triggered by fluctuations in intracellular Ca2+ [54], although some studies suggest that resting neurotransmitter release also strictly relies on intracellular Ca2+ without these large Ca2+ fluctuations [55]. In contrast to the steep Ca2+ dependence of evoked transmission, spontaneous neurotransmission displays close to linear Ca2+ dependence [34, 55, 56, 57]. This suggests that at low Ca2+ concentrations, Ca2+ signaling may selectively impact spontaneous release but not evoked release [Figure 1]. This phenomenon may underlie the role of presenilins in regulation of spontaneous release. A further implication of this idea suggests that limited Ca2+ influx may impact spontaneous but not evoked release, depending perhaps on the type of channel being activated or on the distance of the channel from the presynaptic release machinery. Calcium entry through non-voltage-gated channels has been shown to affect spontaneous glutamate burst activity (via nicotinic acetylcholine receptor activation and subsequent calcium-induced calcium release) [3] and both evoked and spontaneous asynchronous release (via capsaicin receptor TRPV1 activation) [58]. Finally, in some preparations, spontaneous release is sensitive to blockade of voltage-gated Ca2+ channels. In mouse brainstem, both excitatory and inhibitory spontaneous release are substantially decreased when N-type channel function is perturbed either by deletion of the neurexins, a class of trans-synaptic cell adhesion molecules, or by application of a specific channel blocker [29]. Furthermore, phorbol ester treatment of hippocampal neurons potentiates spontaneous release through L-type channel activation, though evoked release was not similarly potentiated [37]. These examples suggest differential calcium signaling contributes substantially to the regulation of spontaneous versus evoked release.
Figure 1. Calcium-dependence of spontaneous and evoked neurotransmitter release.
Diagram depicts the calcium-dependent increase in the rate of vesicle fusion [see 34, 57]. At low intracellular Ca2+ concentrations indicated by the yellow zone, small changes in Ca2+ signaling may selectively alter spontaneous release without significantly modifying evoked release properties.
Ca2+-dependent regulation of the spontaneous release rate may also require divergent molecular players compared to evoked release. At least two calcium sensors operate during neurotransmitter release at central synapses. Spontaneously recycling synaptic vesicles possess synaptotagmin1, the canonical Ca2+ sensor for synchronous evoked release [18]. Although evoked asynchronous release may utilize an additional sensor or sensors with decreased calcium cooperativity, the majority of spontaneous release appears to be mediated by synaptotagmin1 [55]. However, specific Ca2+ binding residues within synaptotagmin1 that support the Ca2+-dependence of spontaneous release differ from the key residues that determine cooperativity of evoked release [55]. In addition, synaptotagmin1 appears to function as a fusion clamp for the second, more sensitive sensor exclusively driving spontaneous release, as loss of this protein produced a paradoxical increase in mIPSC frequency [55]. Double C2 domain 2b (doc2b) has recently been identified as an alternate Ca2+ sensor specifically mediating spontaneous release [56]. The Ca2+-dependence and fusion propensity of spontaneous release can also be modified by other synaptic vesicle proteins such as synaptotagmin12 [59].
3. Differences between presynaptic fusion machineries giving rise to spontaneous and evoked release
Several recent findings indicate that although both forms of fusion largely utilize the same molecular machinery, they rely on distinct molecular interactions of the same components for normal function. Structure-function analyses of neuronal SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) including plasma membrane-associated SNAP-25 and vesicular synaptobrevin2 (also called VAMP2), which together with syntaxin1 comprise the core synaptic vesicle fusion machinery [60, 61], revealed three key differences between molecular interactions that give rise to spontaneous and evoked fusion. First, loss of SNAP-25 or synaptobrevin2 in central neurons largely abolishes Ca2+-dependent evoked release but leaves some spontaneous release intact [62, 63, 64] suggesting a role for alternate SNAREs in mediating low levels of spontaneous release [65]. Several additional SNAREs which exhibit a domain structure similar to that of synaptobrevin2 are expressed at low levels on synaptic vesicles, including VAMP4, VAMP7 and Vps10p tail interactor 1 a (Vti1a) [66, 67, 68, 69].
Non-canonical SNAREs represent an attractive possibility to mediate specific forms of neurotransmission; indeed, recent studies implicate VAMP7 in the regulation of asynchronous and spontaneous release at the mossy fiber terminals [69]. Additionally, the secretagogue α-latrotoxin can augment resting levels of release without relying on the canonical SNARE machinery components, implicating that a separate complement of molecules may support spontaneous transmission [1].
Second, in synaptobrevin2-deficient synapses, spontaneous release could be rescued by expression of a synaptobrevin2 construct with an insertion of 12 residues between the SNARE motif and transmembrane region, whereas the same construct was not able to restore action potential evoked release [70], indicating the physical constraints on SNARE complex assembly are less stringent for spontaneous release. Finally, expression of SNAP-25 mutants destabilizing the C-terminal end of the SNARE-bundle in neurons obtained from SNAP-25 null mice abolished spontaneous neurotransmitter release but caused only a small reduction in evoked release probability. In contrast, destabilizing the middle or deleting the N-terminal end of the SNARE-bundle potentiated the propensities of both spontaneous and evoked fusion. Interestingly, both manipulations caused a more dramatic change in spontaneous release compared to evoked neurotransmission [71]. These biophysical differences in SNARE complex formation between spontaneous and evoked vesicle fusion may depend on the interactions of specific auxiliary molecules, such as complexin. Indeed, complexin knockdown produces reciprocal effects on spontaneous and evoked release in cultured cortical neurons, where the frequency of excitatory mEPSCs is increased fourfold whereas evoked EPSC amplitudes are decreased fourfold [72]. This growing body of evidence describes clear distinctions between SNARE complexes driving spontaneous and evoked vesicle fusion, which may be regulated by a specific molecule or molecules at the level of SNARE complex formation.
4. Diversity of synaptic vesicle populations giving rise to spontaneous and evoked neurotransmission
Synaptic vesicles are classically assigned to one of three pools based primarily on their latency to release upon stimulation: the readily releaseable pool (RRP), the recycling pool, and the reserve pool [73]. Although synaptic vesicle pools are indistinguishable at the ultrastructural level (apart from the RRP vesicles docked at the active zone) and no molecular basis for separation of the recycling and reserve pools has been described, several studies have suggested that the two vesicle populations do not overlap and function to maintain evoked and spontaneous transmission, respectively [18, 19, 20], supporting distinctions not only in fusion mechanisms but also in the overall identity of vesicles that sustain the two forms of release [Figure 2]. Key evidence supporting the “two pools” hypothesis comes from the finding that spontaneously recycled vesicles preferentially exocytose spontaneously rather than with activity, and that blocking vesicle refilling at rest by preventing vesicle re-acidification selectively decreased spontaneous transmission without significantly affecting evoked transmission [18]. This idea is controversial, as some studies have described contradictory findings whereby vesicles from the same population are released spontaneously and with activity [22, 23, 24]. While the majority of studies addressing this issue utilize a number of classical and novel fluorescent imaging methodologies to monitor synaptic vesicle release and recycling [18, 19, 20, 21, 22, 23, 24], new evidence from the postsynaptic side points to a model in which distinct receptor populations are activated by spontaneous or evoked glutamate release in hippocampal neurons [14]. This observation is also consistent with the total internal reflection microcopy based observations of spontaneous and evoked vesicle fusion in goldfish retinal bipolar cells [15]. The notion that postsynaptic receptors detecting evoked or spontaneous transmitter release are spatially segregated on the same synapse was convincingly demonstrated with optical imaging and modeling approaches [14, 15], and has precedence in the finding that low (40 action potentials at 2 Hz) frequency stimulation recycles vesicles to a specific pool that is kinetically faster than those vesicles mobilized by high frequency stimulation, and is organized closer to the active zone [75]. Moreover, acute application of dynasore, a reversible inhibitor of the essential endocytic protein dynamin, showed that evoked synchronous and asynchronous release originate from the same vesicle pool that recycles rapidly in a dynamin-dependent manner, while a distinct vesicle pool sustains spontaneous release independent of dynamin activation [2]. These findings imply that the distinct identities of spontaneous and evoked recycling vesicles are not perturbed upon exocytosis-endocytosis. The premise of two separate pools for spontaneous and evoked release is also consistent with the prevalence of synapses that only support spontaneous neurotransmission and spontaneous synaptic vesicle recycling at early stages of synapse maturation [48, 49, 50]. Interestingly, purified synaptic vesicles show an intrinsic tendency for unregulated constitutive fusion [76], suggesting that evoked regulated fusion may constitute a gain-of-function which is attained gradually during synapse maturation. Accordingly, mature synapses may also contain a population of these “immature” vesicles that are unable to respond to brief action potential stimulation but fuse and recycle constitutively [14, 48]. Ultimately, in light of very recent publications questioning the distinction between vesicles used for spontaneous or evoked transmission [23, 24], controversy still remains as to whether vesicles released spontaneously or with stimulation populate the same or different pools. Future studies with better molecular specificity will help resolve this issue.
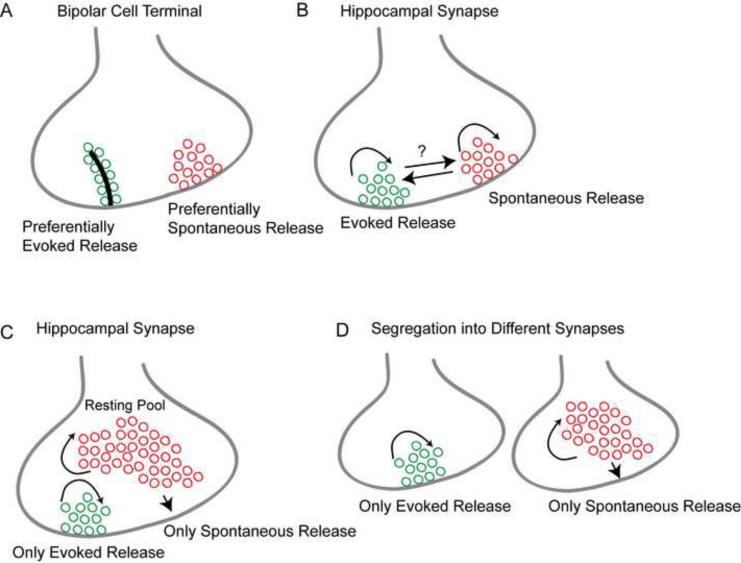
Figure 2. Segregation of evoked and spontaneous neurotransmission.
(A) A recent study using total internal reflection fluorescence microscopy on retinal bipolar cell presynaptic terminals showed that spontaneous fusion events were largely excluded from synaptic ribbons which comprised the preferential site for evoked fusion [15].
(B) Work in hippocampal synapses suggested that spontaneous and evoked fusion may be carried out via separate pools of vesicles, which may recycle independently [18, 19, 20]. Taken together with the evidence from the postsynaptic side [14], these observations points to a model in which distinct receptor populations are activated by spontaneous or evoked glutamate release in hippocampal neurons [14]. This model is also consistent with the total internal reflection microcopy based observations of spontaneous and evoked vesicle fusion in goldfish retinal bipolar cells [15].
(C) A study by Fredj and Burrone (2009) [19] suggested that spontaneous neurotransmitter release is preferentially maintained by the resting pool of vesicles that do not typically contribute to evoked neurotransmission.
(D) Immature synaptic boutons typically favor spontaneous release and fail to respond to action potential stimulation [48, 49, 50], which raises the possibility that a population of nascent synapses in an otherwise mature synaptic network may selectively sustain spontaneous release. In addition, some presynaptic terminals may support action potential driven release with negligible concurrent spontaneous vesicle exocytosis [14]. Therefore, some synapses may have a strong propensity for spontaneous fusion whereas others may preferentially release neurotransmitter in response to action potentials.
Conclusions
Recent studies suggest a framework where certain neuronal signaling pathways specifically regulate spontaneous neurotransmitter release. This release mode selective modulation of neurotransmission supports the premise that spontaneous and evoked neurotransmission comprise independent neuronal signal transduction pathways that may operate in a spatially segregated manner [6, 77]. Elucidation of molecular substrates that underlie this regulatory selectivity will allow dissection of neurotransmitter signaling with respect to its origins, downstream targets as well as behavioral consequences. This approach can open new avenues in neuronal signaling by enabling manipulation of neurotransmission in a release mode specific manner.
Acknowledgements
The work in our laboratory is supported by grants from the National Institute of Mental Health (R01MH066198) to E.T.K and (F32MH093109) to D.M.O.R. E.T.K. is an Established Investigator of the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Deák F, Liu X, Khvotchev M, Li G, Kavalali ET, Sugita S, Südhof TC. Alpha-latrotoxin stimulates a novel pathway of Ca2+-dependent synaptic exocytosis independent of the classical synaptic fusion machinery. J Neurosci. 2009;29:8639–48. doi: 10.1523/JNEUROSCI.0898-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung C, Deák F, Kavalali ET. Molecular substrates mediating lanthanide-evoked neurotransmitter release in central synapses. J Neurophysiol. 2008;100:2089–100. doi: 10.1152/jn.90404.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma G, Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–939. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- 4.Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 5.Wasser CR, Kavalali ET. Leaky synapses: Regulation of spontaneous neurotransmission in central synapses. Neuroscience. 2009;158:177–188. doi: 10.1016/j.neuroscience.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kavalali ET, Chung C, Khvotchev M, Leitz J, Nosyreva E, Raingo J, Ramirez DMO. Spontaneous Neurotransmission: An Independent Pathway for Neuronal Signaling? Physiology (Bethesda) 2011 doi: 10.1152/physiol.00040.2010. in press. [DOI] [PubMed] [Google Scholar]
- 7.Carter AG, Regehr WG. Quantal events shape cerebellar interneuron firing. Nat Neurosci. 2002;5:1309–1318. doi: 10.1038/nn970. [DOI] [PubMed] [Google Scholar]
- 8.Otmakhov N, Shirke AM, Malinow R. Measuring the impact of probabilistic transmission on neuronal output. Neuron. 1993;10:1101–1111. doi: 10.1016/0896-6273(93)90058-y. [DOI] [PubMed] [Google Scholar]
- 9•.Sutton MA, Wall NR, Aakalu GN, Schuman EM. Regulation of dendritic protein synthesis by miniature synaptic events. Science. 2004;304:1979–1983. doi: 10.1126/science.1096202. [DOI] [PubMed] [Google Scholar]; See annotation to [11••].
- 10•.Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]; See annotation to [11••].
- 11••.Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]; This series of elegant studies provides one of the first observations of a functional basis for miniature neurotransmission, and extends the original finding that miniature release events bidirectionally control dendritic protein synthesis by describing a specific role for spontaneous transmission in homeostatic plasticity. The final paper identifies the enzyme eEF2 kinase, involved in regulation of ribosomal translocation, as the link between mEPSCs and dendritic protein synthesis, and presents strong evidence for differential signaling pathways operating downstream of spontaneous and action potential evoked transmission.
- 12.Aoto J, Nam CI, Poon MM, Ting P, Chen L. Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron. 2008;60:308–320. doi: 10.1016/j.neuron.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung C, Kavalali ET. Seeking a function for spontaneous neurotransmission. Nat Neurosci. 2006;9:989–990. doi: 10.1038/nn0806-989. [DOI] [PubMed] [Google Scholar]
- 14••.Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, Kavalali ET. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci. 2008;28:10151–10166. doi: 10.1523/JNEUROSCI.2432-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using the use-dependent NMDAR antagonist MK-801, the authors demonstrate electrophysiologically the existence of different populations of NMDARs that are responsive to glutamate released either spontaneously or as a result of action potential stimulation, observations which were substantiated by imaging of both spontaneous and evoked vesicle fusion in the same boutons and modeling studies consistent with two separate receptor populations.
- 15•.Zenisek D. Vesicle association and exocytosis at ribbon and extraribbon sites in retinal bipolar cell presynaptic terminals. Proc Natl Acad Sci USA. 2008;105:4922–4927. doi: 10.1073/pnas.0709067105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with [14••], this paper provides further evidence for the spatial segregation of different types of neurotransmission using single-vesicle imaging of goldfish bipolar cell ribbon synapses. Vesicles released as a result of stimulation are predominantly localized to the ribbon, whereas spontaneous release often occurs at extraribbon sites.
- 16.Lee MC, Yasuda R, Ehlers MD. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N, Carruthers CJ, Sutton MA. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68:1143–1158. doi: 10.1016/j.neuron.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Sara Y, Virmani T, Deak F, Liu X, Kavalali ET. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron. 2005;45:563–573. doi: 10.1016/j.neuron.2004.12.056. [DOI] [PubMed] [Google Scholar]; The results of experiments using a variety of classical imaging methodologies and electrophysiology presented in this paper demonstrate that the vesicle pools underlying spontaneous and evoked release are different and recycle independently.
- 19••.Fredj NB, Burrone J. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat Neurosci. 2009;12:751–758. doi: 10.1038/nn.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]; Another demonstration of release-mode specific vesicle pools, this report utilizes a novel imaging technique that exploits the high-affinity avidin-biotin interaction to detect spontaneous and activity-dependent release and provides a quantitative estimate of the numbers of vesicles populating the resting and recycling pools.
- 20••.Chung C, Barylko B, Leitz J, Liu X, Kavalali ET. Acute dynamin inhibition dissects synaptic vesicle recycling pathways that drive spontaneous and evoked neurotransmission. J Neurosci. 2010;30:1363–1376. doi: 10.1523/JNEUROSCI.3427-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper identifies a specific molecular basis for independently recycling pools of synaptic vesicles that maintain different forms of neurotransmission. Both evoked synchronous and asynchronous release originate from a pool that recycles rapidly in a dynamin-dependent manner, whereas vesicles released spontaneously are derived from a separate pool which does not require dynamin for recycling. The results are consistent with an earlier study in Drosophila [74•].
- 21•.Mathew SS, Pozzo-Miller L, Hablitz JJ. Kainate modulates presynaptic GABA release from two vesicle pools. J Neurosci. 2008;28:725–731. doi: 10.1523/JNEUROSCI.3625-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides additional support for the presence of two separate vesicle pools maintaining spontaneous and evoked transmission in inhibitory neurons of the prefrontal cortex. The results obtained were similar to those found in glutamatergic synapses [18••].
- 22•.Groemer TW, Klingauf J. Synaptic vesicles recycling spontaneously and during activity belong to the same vesicle pool. Nat Neurosci. 2007;10:145–147. doi: 10.1038/nn1831. [DOI] [PubMed] [Google Scholar]; This report presents evidence that the synaptic vesicle populations driving spontaneous and evoked release are the same, using optical imaging and electrophysiological techniques similar to those used in [18••].
- 23.Hua Y, Sinha R, Martineau M, Kahms M, Klingauf J. A common origin of synaptic vesicles undergoing evoked and spontaneous fusion. Nat Neurosci. 2010;13:1451–3. doi: 10.1038/nn.2695. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm BG, Groemer TW, Rizzoli SO. The same synaptic vesicles drive active and spontaneous release. Nat Neurosci. 2010;13:1454–6. doi: 10.1038/nn.2690. [DOI] [PubMed] [Google Scholar]
- 25.Hablitz JJ, Mathew SS, Pozzo-Miller L. GABA vesicles at synapses: are there 2 distinct pools? Neuroscientist. 2009;15:218–224. doi: 10.1177/1073858408326431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wasser CR, Ertunc M, Liu X, Kavalali ET. Cholesterol-dependent balance between evoked and spontaneous synaptic vesicle recycling. J Physiol. 2007;579:413–429. doi: 10.1113/jphysiol.2006.123133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamir O, Charlton MP. Cholesterol and synaptic transmitter release at crayfish neuromuscular junctions. J Physiol. 2006;571:83–99. doi: 10.1113/jphysiol.2005.098319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipscombe D, Kongsamut S, Tsien RW. Alpha-adrenergic inhibition of sympathetic neurotransmitter release mediated by modulation of N-type calcium-channel gating. Nature. 1989;340:639–642. doi: 10.1038/340639a0. [DOI] [PubMed] [Google Scholar]
- 29.Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Südhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–48. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 30.Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–33. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–7. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- 32.Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature. 2003;424:775–8. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- 33.Malenka RC, Madison DV, Nicoll RA. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986;321:175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- 34••.Lou X, Scheuss V, Schneggenburger R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 2005;435:497–501. doi: 10.1038/nature03568. [DOI] [PubMed] [Google Scholar]; A quantitative description of Ca2+-dependence of neurotransmitter release in calyx of Held synapses. Using an elegant allosteric model the authors can account for all forms of release starting at very low Ca2+ concentrations supporting basal release up to high Ca2+ concentrations required to trigger evoked release [compare to 57•].
- 35.Virmani T, Ertunc M, Sara Y, Mozhayeva M, Kavalali ET. Phorbol esters target the activity-dependent recycling pool and spare spontaneous vesicle recycling. J Neurosci. 2005;25:10922–10929. doi: 10.1523/JNEUROSCI.3766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swartz KJ, Merritt A, Bean BP, Lovinger DM. Protein kinase C modulates glutamate receptor inhibition of Ca2+ channels and synaptic transmission. Nature. 1993;361:165–8. doi: 10.1038/361165a0. [DOI] [PubMed] [Google Scholar]
- 37.Waters J, Smith SJ. Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms. J Neurosci. 2000;20:7863–70. doi: 10.1523/JNEUROSCI.20-21-07863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glitsch M. Selective inhibition of spontaneous but not Ca2+ -dependent release machinery by presynaptic group II mGluRs in rat cerebellar slices. J Neurophysiol. 2006;96:86–96. doi: 10.1152/jn.01282.2005. [DOI] [PubMed] [Google Scholar]
- 39.Taniguchi N, Takada N, Kimura F, Tsumoto T. Actions of brain-derived neurotrophic factor on evoked and spontaneous EPSCs dissociate with maturation of neurones cultured from rat visual cortex. J Physiol. 2000;527:579–592. doi: 10.1111/j.1469-7793.2000.t01-1-00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson ED, Kavalali ET, Monteggia LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28:395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nosyreva E, Kavalali ET. Activity-dependent augmentation of spontaneous neurotransmission during endoplasmic reticulum stress. J Neurosci. 2010;30:7358–7368. doi: 10.1523/JNEUROSCI.5358-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pratt KG, Zhu P, Watari H, Cook DG, Sullivan JM. A novel role for γ-Secretase: selective regulation of spontaneous neurotransmitter release from hippocampal neurons. J Neurosci. 2011;31:899–906. doi: 10.1523/JNEUROSCI.4625-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Südhof TC, Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan ZH, Segal MM, Lipton SA. Nitric oxide-related species inhibit evoked neurotransmission but enhance spontaneous miniature synaptic currents in central neuronal cultures. Proc Natl Acad Sci U S A. 1996;93:15423–15428. doi: 10.1073/pnas.93.26.15423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murthy VN, Stevens CF. Reversal of synaptic vesicle docking at central synapses. Nat Neurosci. 1999;2:503–507. doi: 10.1038/9149. [DOI] [PubMed] [Google Scholar]
- 47.Prange O, Murphy TH. Correlation of miniature synaptic activity and evoked release probability in cultures of cortical neurons. J Neurosci. 1999;19:6427–6438. doi: 10.1523/JNEUROSCI.19-15-06427.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mozhayeva MG, Sara Y, Liu X, Kavalali ET. Development of vesicle pools during maturation of hippocampal synapses. J Neurosci. 2002;22:654–665. doi: 10.1523/JNEUROSCI.22-03-00654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen W, Wu B, Zhang Z, Dou Y, Rao ZR, Chen YR, Duan S. Activity-induced rapid synaptic maturation mediated by presynaptic cdc42 signaling. Neuron. 2006;50:401–414. doi: 10.1016/j.neuron.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Wittenmayer N, Körber C, Liu H, Kremer T, Varoqueaux F, Chapman ER, Brose N, Kuner T, Dresbach T. Postsynaptic Neuroligin1 regulates presynaptic maturation. Proc Natl Acad Sci USA. 2009;106:13564–13569. doi: 10.1073/pnas.0905819106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- 52.Abenavoli A, Forti L, Bossi M, Bergamaschi A, Villa A, Malgaroli A. Multimodal quantal release at individual hippocampal synapses: evidence for no lateral inhibition. J Neurosci. 2002;22:6336–46. doi: 10.1523/JNEUROSCI.22-15-06336.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Popescu IR, Morton LA, Franco A, Di S, Ueta Y, Tasker JG. Synchronized bursts of miniature inhibitory postsynaptic currents. J Physiol. 2010;588:939–51. doi: 10.1113/jphysiol.2009.181461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glitsch MD. Spontaneous neurotransmitter release and Ca(2+)-How spontaneous is spontaneous neurotransmitter release? Cell Calcium. 2008;43:9–15. doi: 10.1016/j.ceca.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 55••.Xu J, Pang ZP, Shin OH, Sudhof TC. Synaptotagmin-1 functions as a Ca(2+) sensor for spontaneous release. Nat Neurosci. 2009;12:759–766. doi: 10.1038/nn.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]; A thorough investigation of the role of synaptotagmin-1 in calcium sensing during both spontaneous and evoked release produced the paradoxical finding that, although the majority of spontaneous release uses the same calcium sensor as stimulation-evoked release (Syt1), this protein also functions to clamp a second, more sensitive calcium sensor operating exclusively during spontaneous or asynchronous release.
- 56••.Groffen AJ, Martens S, Diez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]; Double C2 domain B (doc2B) is identified as an alternate calcium sensor driving spontaneous release. This protein has numerous characteristics expected of a putative spontaneous calcium sensor, such as increased calcium sensitivity and the ability to compete with Syt1 for SNARE complex binding (see also [55•• and 57•]).
- 57•.Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Südhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]; A quantitative description of asynchronous neurotransmitter release in calyx of Held synapses, this study supports a release mode-specific model of neurotransmitter release driven by two separate calcium sensors of differing calcium cooperativity. A more sensitive calcium sensor allows asynchronous release to predominate at low physiological calcium concentrations.
- 58•.Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron. 2010;65:657–69. doi: 10.1016/j.neuron.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; The capsaicin receptor TRPV1 facilitates glutamatergic transmission in solitary tract afferents in an activity-dependent manner by increasing asynchronous glutamate release. This paper provides compelling evidence for the presence of different signaling pathways downstream of different forms of neurotransmitter release.
- 59•.Maximov A, Shin OH, Liu X, Sudhof TC. Synaptotagmin-12, a synaptic vesicle phosphoprotein that modulates spontaneous neurotransmitter release. J Cell Biol. 2007;176:113–124. doi: 10.1083/jcb.200607021. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report identifies synaptotagmin-12, a non-canonical isoform that does not bind calcium, as a specific regulator of spontaneous release rate. Syt12 competes with Syt1 for SNARE complex binding, specifically increasing mini frequency when overexpressed in neurons, and is regulated by PKA phosphorylation.
- 60.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 61.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 63.Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Benditó G, Molnár Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- 64.Bronk P, Deak F, Wilson MC, Liu X, Sudhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+ -dependent and Ca2+ -independent neurotransmission. J Neurophysiol. 98:794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- 65.Hua SY, Raciborska DA, Trimble WS, Charlton MP. Different VAMP/synaptobrevin complexes for spontaneous and evoked transmitter release at the crayfish neuromuscular junction. J Neurophysiol. 1998;80:3233–3246. doi: 10.1152/jn.1998.80.6.3233. [DOI] [PubMed] [Google Scholar]
- 66.Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–46. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 67.Antonin W, Riedel D, von Mollard GF. The SNARE Vti1a-beta is localized to small synaptic vesicles and participates in a novel SNARE complex. J Neurosci. 2000;20:5724–32. doi: 10.1523/JNEUROSCI.20-15-05724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muzerelle A, Alberts P, Martinez-Arca S, Jeannequin O, Lafaye P, Mazié JC, Galli T, Gaspar P. Tetanus neurotoxin-insensitive vesicle-associated membrane protein localizes to a presynaptic membrane compartment in selected terminal subsets of the rat brain. Neuroscience. 2003;122:59–75. doi: 10.1016/s0306-4522(03)00567-0. [DOI] [PubMed] [Google Scholar]
- 69.Scheuber A, Rudge R, Danglot L, Raposo G, Binz T, Poncer JC, Galli T. Loss of AP-3 function affects spontaneous and evoked release at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2006;103:16562–7. doi: 10.1073/pnas.0603511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Deak F, Shin OH, Kavalali ET, Sudhof TC. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26:6668–6676. doi: 10.1523/JNEUROSCI.5272-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report describes the structural requirements for synaptobrevin-2 in synaptic vesicle exocytosis. The abilities of alterations in the SNARE motif composition or position to affect vesicle exocytosis were measured. Surprisingly, extending the SNARE motif further from the vesicle membrane could still support spontaneous release but not evoked release, indicating the SNARE complex formed during spontaneous release is relatively looser than that driving evoked release.
- 71.Weber JP, Reim K, Sørensen JB. Opposing functions of two sub-domains of the SNARE-complex in neurotransmission. EMBO J. 2010;29:2477–2490. doi: 10.1038/emboj.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maximov A, Tang J, Yang X, Pang ZP, Südhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–21. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- 74•.Koenig JH, Ikeda K. Contribution of active zone subpopulation of vesicles to evoked and spontaneous release. J Neurophysiol. 1999;81:1495–1505. doi: 10.1152/jn.1999.81.4.1495. [DOI] [PubMed] [Google Scholar]; A convincing demonstration of separately recycling vesicle pools in Drosophila neuromuscular junction synapses using the shibire temperature-sensitive dynamin mutant. Specific depletion of quickly and slowly recycling pools inhibited evoked and spontaneous release, respectively.
- 75.Vanden Berghe P, Klingauf J. Synaptic vesicles in rat hippocampal boutons recycle to different pools in a use-dependent fashion. J Physiol. 2006;572:707–20. doi: 10.1113/jphysiol.2005.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holt M, Riedel D, Stein A, Schuette C, Jahn R. Synaptic vesicles are constitutively active fusion machines that function independently of Ca2+ Curr Biol. 2008;18:715–722. doi: 10.1016/j.cub.2008.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sutton MA, Schuman EM. Partitioning the synaptic landscape: distinct microdomains for spontaneous and spike-triggered neurotransmission. Sci Signal. 2009;2:pe19. doi: 10.1126/scisignal.265pe19. [DOI] [PubMed] [Google Scholar]