Abstract
A highly efficient and step-economical synthesis of zincophorin methyl ester has been achieved. The unprecedented step-economy of this zincophorin synthesis is principally due to an application of the tandem silylformylation-crotylsilylation/Tamao oxidation-diastereoselective tautomerization reaction that achieves in a single step what would typically require a significant multi-step sequence.
Polyketide natural products continue to influence small molecule drug development efforts. Both natural products (e.g. discodermolide1) and designed analogs thereof (e.g. fludelone2) have progressed into clinical trials, and it seems reasonable to anticipate that it might only be a matter of time before approved drugs begin to emerge from such medicinal chemistry programs. It is equally reasonable to anticipate that most such compounds will have to be synthesized (as will, of course, most analogs), as was certainly the case for both discodermolide3 and fludelone. It is for this reason that, despite decades of beautiful, powerful, and profoundly influential chemistry devoted to the synthesis of such structures, there remains a great need for creative new approaches that achieve significantly greater levels of “ideality.”4 Progress in this regard would be expected to have an impact on every aspect of polyketide natural product-based synthesis and drug development efforts.
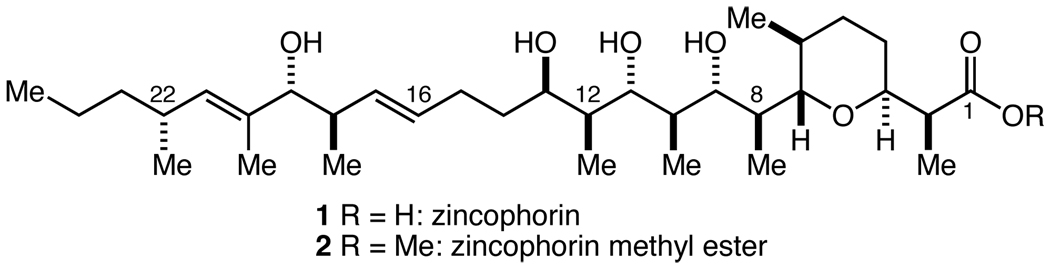
Zincophorin (1) and its methyl ester (2)5 have been popular targets for synthetic chemists ever since the groundbreaking synthesis by Danishefsky in 1987 (Figure 1).6,7 Two additional total syntheses have been reported since then by Meyer and Cossy8 and by Miyashita9 (in addition to numerous reports of fragment syntheses10), and, interestingly, all three syntheses (of 2) required ~47/~52 total steps11 with longest linear sequences of 36, 28, and 37 steps resepectively. As part of a broad program devoted to the development of highly efficient and step-economical syntheses of polyketide structures of this type,12 we decided to undertake a new synthesis of zincophorin methyl ester. Our primary motivation was to set for ourselves the goal of completing the synthesis in about half the number of total steps as the three previous syntheses, because we felt that achieving this would require a fresh approach and true methodological innovation (i.e. greater ideality). We report here the results of these efforts that have culminated in a 27/31 total step synthesis of 2.
Figure 1.
Zincophorin and zincophorin methyl ester.
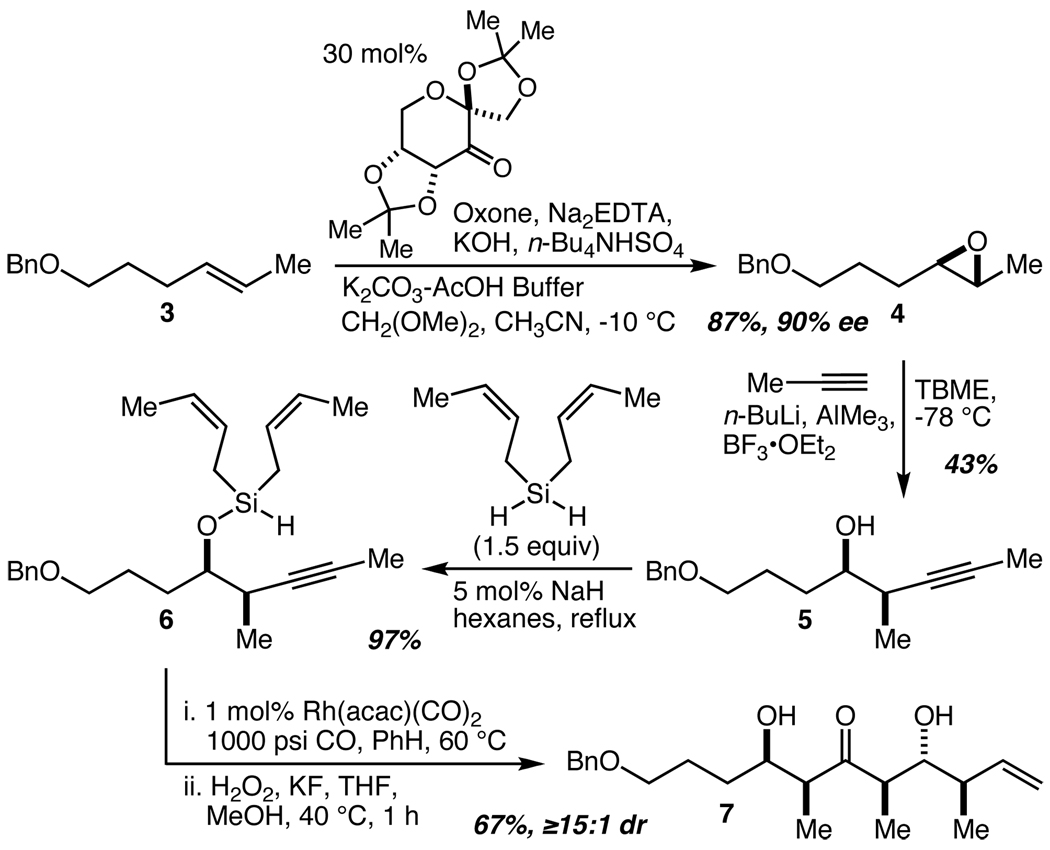
The synthesis commenced with an asymmetric epoxidation of alkene 313 using Shi’s catalyst14 to provide 4 in 87% yield and 90% ee (Scheme 1). Epoxide opening using Pagenkopf’s procedure15 gave 5 in 43% yield.16 NaH-catalyzed silane alcoholysis with di-cis-crotylsilane12d then provided 6 in 97% yield and set the stage for an application of the tandem silylformylation-crotylsilylation/Tamao oxidation-diastereoselective tautomerization reaction.12k Applied to 6, this complex series of chemical events produced 7 in 67% yield with ≥15:1 overall diastereoselectivity. The transformation of 6 into 7 (which we have carried out on multi-gram scale) is remarkable not only for the direct installation of a ketone, three stereocenters, and an alkene, but also for the simplicity of the starting materials (a crotyl-SiH fragment, a propynyl fragment, CO, and H2O2). Overall, 7, which contains five of the ten stereocenters of the C(1)–C(16) fragment, is accessed in just four steps from 3, and this sequence is further noteworthy for what is not employed: protecting groups, non-strategic redox reactions, and chiral auxiliaries, controllers, and/or reagents. Using Baran’s algorithm, this adds up to a four step sequence that delivers five stereocenters with 100% ideality.4
Scheme 1.
A four-step synthesis of 7 from 3.
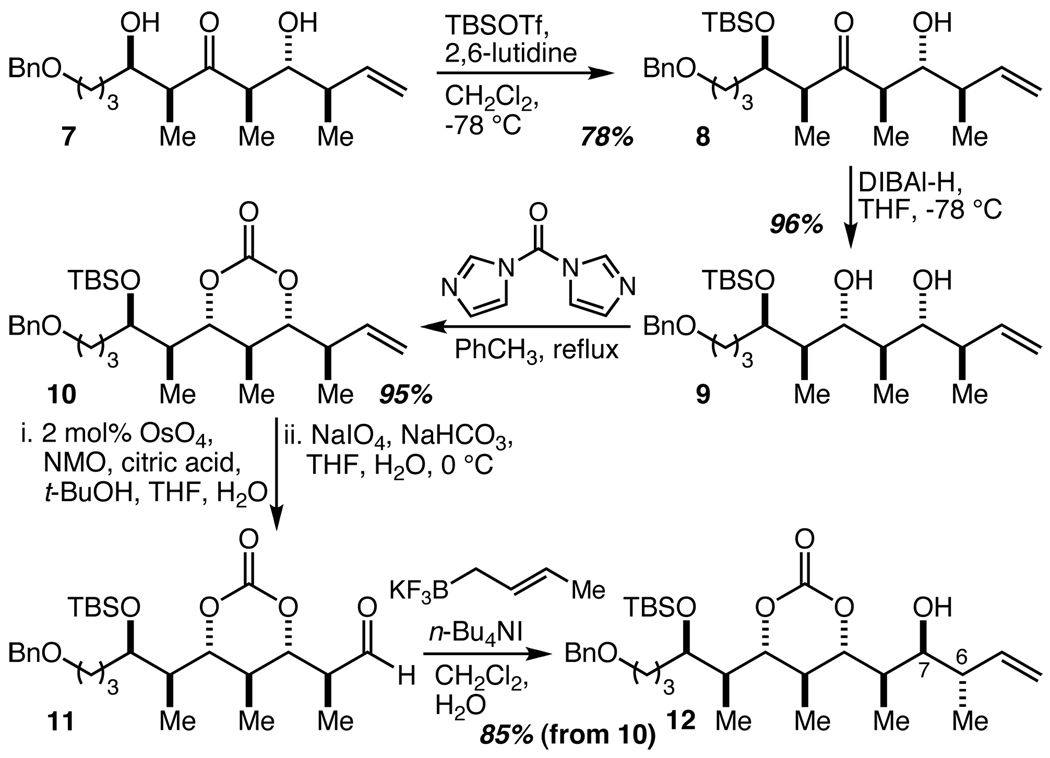
A series of straightforward steps (selective protection to give 8, syn-selective β-hydroxy ketone reduction17 to give 9, diol protection to give 10, and alkene oxidative cleavage) converted ketone 7 into aldehyde 11 and set up a crotylation reaction to establish the C(6) and C(7) stereocenters (Scheme 2). The desired product, 12, is the result of Felkin addition of a Type I trans-crotylmetal reagent to aldehyde 11, and this is a fully matched case18 that should not require external asymmetric induction for very high levels of diastereoselectivity.19 A survey of various Type I trans-crotylmetal reagents revealed that the potassium trans-crotyltrifluoroborate reagent introduced by Batey20 was possessed of superior characteristics from the perspective of both efficiency and practicality/ease of use. In the present case its use led to the isolation of 12 in 85% yield (from 10) with ≥20:1 diastereoselectivity.
Scheme 2.
Synthesis of the C(11), C(7), and C(6) stereocenters.
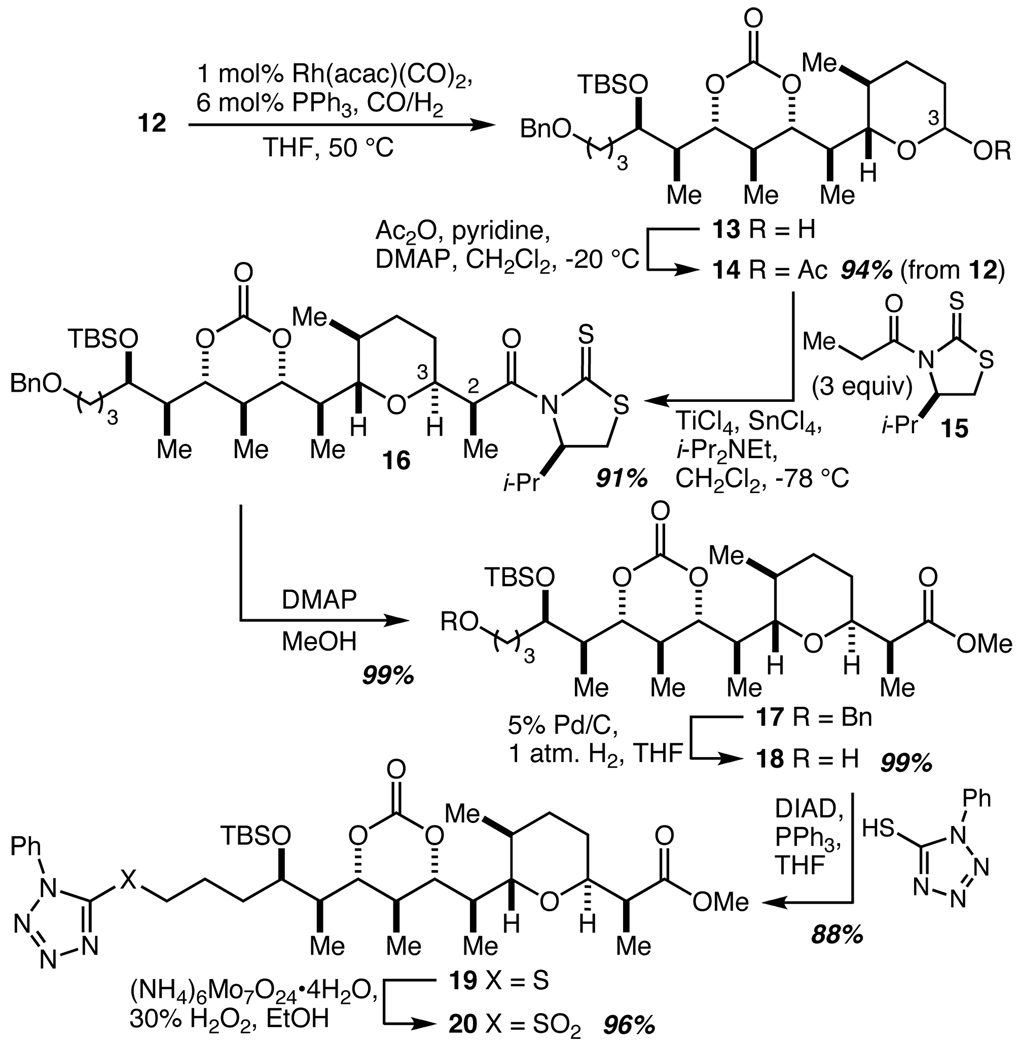
The final stereochemical challenges in the synthesis of the C(1)–C(16) fragment were the C(2) and C(3) stereocenters that would accompany tetrahydropyran ring synthesis. It was clear that the most direct way to accomplish those goals in a single step would be the addition of a propionate enolate to an oxocarbenium ion at C(3). To set up such a reaction, 12 was subjected to hydroformylation to give hemiacetal 13 which was acetylated to give 14 in 94% overall yield (Scheme 3). While the well-established preference for axial attack on the oxocarbenium ion generated from 14 would give the desired outcome at C(3), control of the C(2) center was much more speculative. Extensive experimentation with various achiral propionate enolate species failed to reveal an adequate solution, and we therefore turned to the use of chiral enolates that would allow for control of enolate face selectivity. Romea and Urpí have developed a protocol for the highly stereoselective addition of the titanium enolate derived from 15 to oxocarbenium ions derived from acetals, glycals, and pseudoglycals,21 and this appeared to be a highly relevant precedent. Indeed, the titanium enolate derived from 15 was treated with 14 and SnCl4 to produce 16 in 91% yield as a single diastereomer. Methanolysis proceeded exceptionally smoothly to give 17 and this was followed by a three-step conversion of the benzyl ether into the N-phenyltetrazolylsulfone 20.
Scheme 3.
Completion of the C(1)–C(16) fragment.
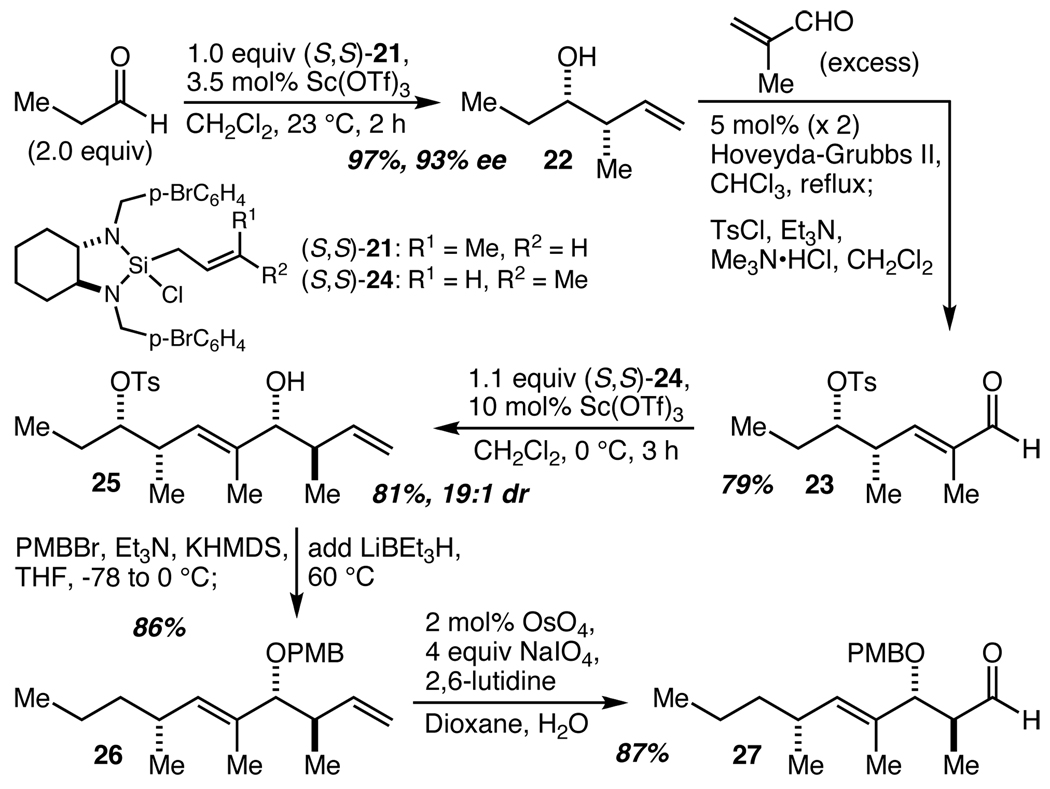
The synthesis of the (17)-C(25) fragment commenced with a Sc(OTf)3-catalyzed crotylation of propionaldehyde using cis-crotylsilane 2122 (Scheme 4).23 This reaction proceeded smoothly at ambient temperature to provide 22 in 97% yield (based on the use of 21 as the limiting reagent) and 93% ee. Highly trans-selective (>20:1) cross-metathesis with excess methacrolein and the second generation Hoveyda-Grubbs catalyst24 was followed without purification by alcohol tosylation using the Tanabe protocol25 to provide 23 in 79% yield. A second application of the Sc(OTf)3-catalyzed crotylation reaction with trans-crotylsilane 24 then gave 25 in 81% yield with excellent (19:1) diastereoselectivity. Protection of the alcohol as its para-methoxy benzyl (PMB) ether was followed in the same pot by tosylate reduction with LiBEt3H to give 26 in 86% yield. Finally, one pot oxidative cleavage produced aldehyde 27 in 87% yield. The synthesis of 27 thus proceeded in just five steps and 46% overall yield from 21 and relied on two applications of the operationally attractive Sc(OTf)3-catalyzed crotylation methodology.
Scheme 4.
Synthesis of the C(17)–C(25) fragment.
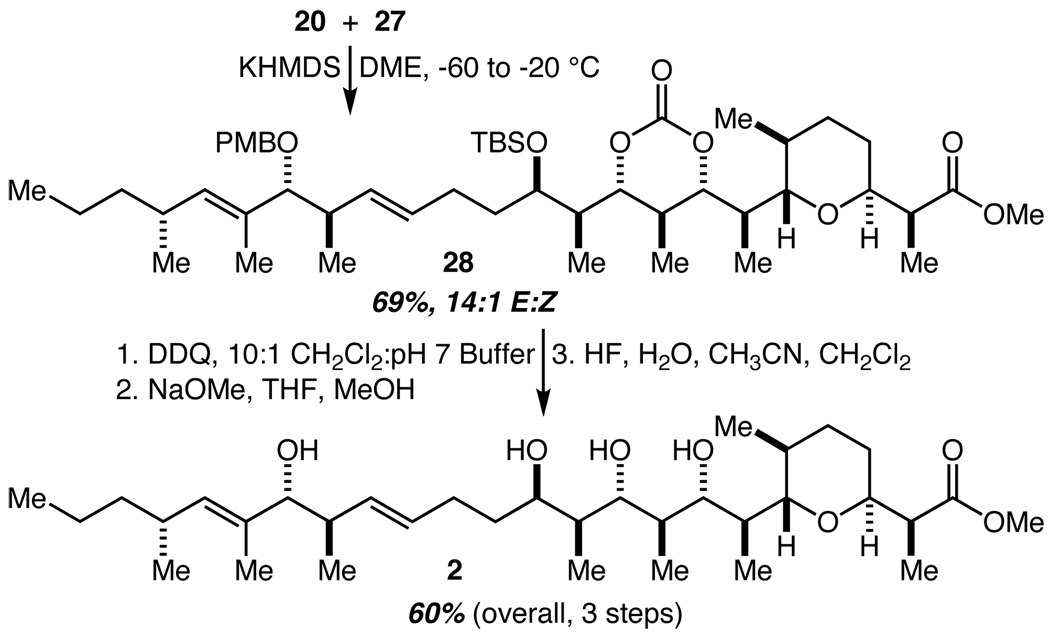
Julia-Kociensky olefination26 with sulfone 20 and aldehyde 27 proceeded smoothly and with excellent trans selectivity to provide 28 in 69% yield (Scheme 5).27 Three sequential deprotection steps (oxidative PMB removal, basic carbonate methanolysis, and TBS deprotection) then completed the synthesis of zincophorin methyl ester 2 in 60% overall yield. Full spectral comparison to the data provided by Cossy8b and Miyashita9 confirmed the identity of our synthetic material.
Scheme 5.
Completion of the synthesis.
This synthesis of zincophorin methyl ester proceeds in 27/31 total steps,11 with a longest linear sequence of 22 steps from (E)-4-hexen-1-ol in 4.2% overall yield.28 Another useful measure of efficiency is steps/stereocenter,29 and in this regard it is noteworthy that the synthesis of the C(1)–C(16) fragment 20 – which contains 10 stereocenters – required just 1.8 steps/stereocenter. Regardless of the metrics used to guage efficiency, it is clear that much of the effciency and step-economy of the route derives from the four-step transformation of 3 to 7. The “ideality” of that sequence is without precedent and we remain committed to the further development of these and related transformations for application to the synthesis of important and complex polyketide natural products and analogs thereof.
Supplementary Material
Acknowledgment
This work was supported by a grant form the National Institute of General Medical Sciences (GM58133). T.H. was supported by an NSERC postdoctoral fellowship. We thank the National Science Foundation (CRIF-0840451) for acquisition of a 400 MHz NMR spectrometer.
Footnotes
Supporting Information Available: Experimental procedures, characterization data, and complete refs. 3d and 3e. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Discodermolide’s journey from discovery to clinical trials has been briefly reviewed. See: Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Nat. Rev. Drug Discov. 2009;8:69. doi: 10.1038/nrd2487.
- 2.Rivkin A, Chou T-C, Danishefsky SJ. Angew. Chem. Int. Ed. 2005;44:2838. doi: 10.1002/anie.200461751. [DOI] [PubMed] [Google Scholar]
- 3.(a) Mickel SJ, Sedelmeier GH, Niederer D, Daeffler R, Osmani A, Schreiner K, Seeger-Weibel M, Berod B, Schaer K, Gamboni R. Org. Process Res. Dev. 2004;8:92. [Google Scholar]; (b) Mickel SJ, Sedelmeier GH, Niederer D, Schuerch F, Grimler D, Koch G, Daeffler R, Osmani A, Hirni A, Schaer K, Gamboni R. Org. Process Res. Dev. 2004;8:101. [Google Scholar]; (c) Mickel SJ, Sedelmeier GH, Niederer D, Schuerch F, Koch G, Kuesters E, Daeffler R, Osmani A, Seeger-Weibel M, Schmid E, Hirni A, Schaer K, Gamboni R. Org. Process Res. Dev. 2004;8:107. [Google Scholar]; (d) Mickel SJ, et al. Org. Process Res. Dev. 2004;8:113. [Google Scholar]; (e) Mickel SJ, et al. Org. Process Res. Dev. 2004;8:122. [Google Scholar]
- 4.Gaich T, Baran PS. J. Org. Chem. 2010;75:4657. doi: 10.1021/jo1006812. [DOI] [PubMed] [Google Scholar]
- 5.(a) Gräfe U, Schade W, Roth M, Radics L, Incze M, Ujszaszy K. J. Antibiot. 1984;37:836. doi: 10.7164/antibiotics.37.836. [DOI] [PubMed] [Google Scholar]; (b) Brooks HA, Gardner D, Poyser JP, King TJ. J. Antibiot. 1984;37:1501. doi: 10.7164/antibiotics.37.1501. [DOI] [PubMed] [Google Scholar]
- 6.(a) Danishefsky SJ, Selnick HG, DeNinno MP, Zelle RE. J. Am. Chem. Soc. 1987;109:1572. [Google Scholar]; (b) Danishefsky SJ, Selnick HG, Zelle RE, DeNinno MP. J. Am. Chem. Soc. 1988;110:4368. [Google Scholar]
- 7.Synthetic efforts towards Zincophorin have been recently reviewed. See: Song Z, Lohse AG, Hsung RP. Nat. Prod. Rep. 2009;26:560. doi: 10.1039/b821450f.
- 8.(a) Defosseux M, Blanchard N, Meyer C, Cossy J. Org. Lett. 2003;5:4037. doi: 10.1021/ol035177n. [DOI] [PubMed] [Google Scholar]; (b) Defosseux M, Blanchard N, Meyer C, Cossy J. J. Org. Chem. 2004;69:4626. doi: 10.1021/jo0496042. [DOI] [PubMed] [Google Scholar]
- 9.Komatsu K, Tanino K, Miyashita M. Angew. Chem. Int. Ed. 2004;43:4341. doi: 10.1002/anie.200460434. [DOI] [PubMed] [Google Scholar]
- 10.(a) Cywin CL, Kallmerten J. Tetrahedron Lett. 1993;34:1103. [Google Scholar]; (b) Marshall JA, Palovich MR. J. Org. Chem. 1998;63:3701. [Google Scholar]; (c) Chemler SR, Roush WR. J. Org. Chem. 1998;63:3800. doi: 10.1021/jo0267908. [DOI] [PubMed] [Google Scholar]; (d) Guindon Y, Murtagh L, Caron V, Landry SR, Jung G, Bencheqroun M, Faucher A-M, Guérin B. J. Org. Chem. 2001;66:5427. doi: 10.1021/jo010310f. [DOI] [PubMed] [Google Scholar]; (e) Mulzer J, Sieg A, Brücher C, Müller D, Martin HJ. Synlett. 2005:685. [Google Scholar]; (f) Song Z, Hsung RP. Org. Lett. 2007;9:2199. doi: 10.1021/ol070791a. [DOI] [PubMed] [Google Scholar]; (g) Song Z, Hsung RP, Lu T, Lohse AG. J. Org. Chem. 2007;72:9722. doi: 10.1021/jo7017922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.In all of these syntheses, as well as our own, reagents that had to be synthesized are employed (see 15 in Scheme 3, e.g.). These syntheses can often be performed once on a large scale, and the reagents are typically reacted with vastly more precious material. In a very real sense, therefore, these steps have essentially no impact on the overall efficiency of the synthesis. The first number given for total steps does not count these steps, whereas the second number counts every step from commercially available materials.
- 12.Zacuto MJ, Leighton JL. J. Am. Chem. Soc. 2000;122:8587. [Google Scholar]; (b) Dreher SD, Leighton JL. J. Am. Chem. Soc. 2001;123:341. doi: 10.1021/ja0035102. [DOI] [PubMed] [Google Scholar]; (c) O’Malley SJ, Leighton JL. Angew. Chem. Int. Ed. 2001;40:2915. [PubMed] [Google Scholar]; (d) Zacuto MJ, O’Malley SJ, Leighton JL. J. Am. Chem. Soc. 2002;124:7890. doi: 10.1021/ja026511y. [DOI] [PubMed] [Google Scholar]; (e) Schmidt DR, O’Malley SJ, Leighton JL. J. Am. Chem. Soc. 2003;125:1190. doi: 10.1021/ja0283201. [DOI] [PubMed] [Google Scholar]; (f) Zacuto MJ, O’Malley SJ, Leighton JL. Tetrahedron. 2003;59:8889. [Google Scholar]; (g) Schmidt DR, Park PK, Leighton JL. Org. Lett. 2003;5:3535. doi: 10.1021/ol035431b. [DOI] [PubMed] [Google Scholar]; (h) Bolshakov S, Leighton JL. Org. Lett. 2005;7:3809. doi: 10.1021/ol0515006. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Zacuto MJ, Leighton JL. Org. Lett. 2005;7:5525. doi: 10.1021/ol052373g. [DOI] [PubMed] [Google Scholar]; (j) Park PK, O’Malley SJ, Schmidt DR, Leighton JL. J. Am. Chem. Soc. 2006;128:2796. doi: 10.1021/ja058692k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Spletstoser JT, Zacuto MJ, Leighton JL. Org. Lett. 2008;10:5593. doi: 10.1021/ol802489w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nogawa M, Sugawara S, Iizuka R, Shimojo M, Ohta H, Hatanaka M, Matsumoto K. Tetrahedron. 2006;62:12071. [Google Scholar]
- 14.(a) Wang Z-X, Tu Y, Frohn M, Zhnag J-R, Shi Y. J. Am. Chem. Soc. 1997;119:11224. [Google Scholar]; (b) Shi Y. Acc. Chem. Res. 2004;37:488. doi: 10.1021/ar030063x. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H, Engers DW, Morales CL, Pagenkopf BL. Tetrahedron. 2007;63:8774. [Google Scholar]
- 16.The moderate yield of this reaction is due not to the formation of a regioisomer, but rather due to competitive methylalumination of the alkyne that leads to alkene products that we have been unable to supress.
- 17.Kiyooka S-I, Kuroda H, Shimasaki Y. Tetrahedron Lett. 1986;27:3009. [Google Scholar]
- 18.(a) Roush WR. J. Org. Chem. 1991;56:4151. [Google Scholar]; (b) Evans DA, Dart MJ, Duffy JL, Yang MG, Livingston AB. J. Am. Chem. Soc. 1995;117:6619. [Google Scholar]
- 19.(a) Lewis MD, Kishi Y. Tetrahedron Lett. 1982;23:2343. [Google Scholar]; (b) Hoffmann RW, Weidmann U. Chem. Ber. 1985;118:3966. [Google Scholar]
- 20.Thadani AN, Batey RA. Org. Lett. 2002;4:3827. doi: 10.1021/ol026619i. [DOI] [PubMed] [Google Scholar]
- 21.(a) Cosp A, Romea P, Talavera P, Urpí F, Vilarrasa J, Font-Bardia M, Solans X. Org. Lett. 2001;3:615. doi: 10.1021/ol0070177. [DOI] [PubMed] [Google Scholar]; (b) Larrosa I, Romea P, Urpí F, Balsells D, Vilarrasa J, Font-Bardia M, Solans X. Org. Lett. 2002;4:4651. doi: 10.1021/ol0270226. [DOI] [PubMed] [Google Scholar]; (c) Larrosa I, Romea P, Urpí F. Org. Lett. 2006;8:527. doi: 10.1021/ol052900w. [DOI] [PubMed] [Google Scholar]
- 22.Hackman BM, Lombardi PJ, Leighton JL. Org. Lett. 2004;6:4375. doi: 10.1021/ol0480731. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Ho S, Leighton JL. J. Am. Chem. Soc. 2011;133 doi: 10.1021/ja200712f. ASAP, April 12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J. Am. Chem. Soc. 2000;122:8168. [Google Scholar]
- 25.Yoshida Y, Sakakura Y, Aso N, Okada S, Tanabe Y. Tetrahedron. 1999;55:2183. [Google Scholar]
- 26. Blakemore PR, Cole WJ, Kocienski PJ, Morley A. Synlett. 1998:26. For a review, see: Blakemore PR. J. Chem. Soc., Perkin Trans. 2002;1:2563.
- 27.Attempts to perform the olefination reaction with a TBS protecting group for the C(19) alcohol were unsuccessful, and this necessitated the switch to a PMB protecting group. The side-reactivity of a β-OTBS group on the aldehyde coupling partner in Julia-Kocienski olefination reactions has been explained in detail by Evans. See: Evans DA, Nagorny P, McRae KJ, Sonntag L-S, Reynolds DJ, Vounatsos F. Angew. Chem. Int. Ed. 2007;46:545. doi: 10.1002/anie.200603652.
- 28.The 43% yield for the conversion of 4 to 5 has, of course, a large impact on the overall yield. Because it comes very near the beginning of the sequence, however, it has less of an impact in terms of the loss of valuable material. In that regard, it is worth noting that the overall yield of the 19-step sequence from 5 to 2 is 12%
- 29.Yeung K-S, Paterson I. Chem. Rev. 2005;105:4237. doi: 10.1021/cr040614c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.