Abstract
Experience-dependent modifications of neural circuits and function are believed to heavily depend on changes in synaptic efficacy such as LTP/LTD. Hence, much effort has been devoted to elucidating the mechanisms underlying these forms of synaptic plasticity. While most of this work has focused on excitatory synapses, it is now clear that diverse mechanisms of long-term inhibitory plasticity have evolved to provide additional flexibility to neural circuits. By changing the excitatory/inhibitory balance, GABAergic plasticity can regulate excitability, neural circuit function and ultimately, contribute to learning and memory, and neural circuit refinement. Here we discuss recent advancements in our understanding of the mechanisms and functional relevance of GABAergic inhibitory synaptic plasticity.
Keywords: GABA, GABA receptor, LTP, LTD, metaplasticity, heterosynaptic plasticity, homeostatic plasticity, epilepsy, autism, schizophrenia
Introduction
Traditionally, changes in synaptic strength at excitatory synapses have been postulated to underlie learning and memory. Studies of activity-dependent regulation of inhibitory synapses have been hampered by the plethora of GABAergic cell types and the difficulty of isolating specific inhibitory inputs. However, it is becoming increasingly clear that GABAergic synapses are also plastic, exhibiting activity-dependent, long-term bi-directional changes in strength of connectivity throughout the brain [1]. Depending on the inhibitory interneuron cell type and brain region, diverse forms of inhibitory plasticity have been reported (Table 1), manifesting as changes in either presynaptic GABA release or the number/sensitivity/responsiveness of postsynaptic GABAA receptors (GABAARs) [2-3]. Because of the crucial role of inhibitory synapses in regulating both neuronal excitability and impact of excitatory synapses, including the induction of excitatory synaptic plasticity, changes in GABAergic synaptic efficacy can have important functional consequences. Growing experimental evidence shows that inhibitory synaptic plasticity, by altering the excitatory/inhibitory balance, plays important roles in neural circuit refinement and in most forms of experience-dependent learning. Pathological levels of neural activity can also induce long-term changes in GABAergic synaptic efficacy, and dysregulations of inhibitory drive have been implicated in several neuropsychiatric conditions [1]. Here we focus on long-term activity-dependent changes of fast GABAergic transmission. We first describe some of the cellular mechanisms involved in the different forms of inhibitory plasticity (i.e. long-term potentiation and long-term depression, I-LTP and I-LTD , respectively). We then identify emerging seminal characteristics of I-LTP/I-LTD and the directions to which our current understanding of these forms of plasticity point future studies.
Table1.
Long-term inhibitory synaptic plasticity: examples and mechanisms
| Synapse, Brain Area | Induction Requirements | Expression Mechanism |
Reference(s) |
|---|---|---|---|
| I-LTP | |||
| Visual Cortex (L5) | GABABR-dependent, BDNF-TrkB signaling | Presynaptic | [23,40,88] |
| Developing Xenopus retinotectal system |
Ca2+ influx through postsynaptic NMDARs, strong excitatory inputs, BDNF-TrkB retrograde signaling |
Presynaptic | [24] |
| Neonatal Hippocampus (CA1 area) |
BDNF-TrkB signaling, repetitive depolarizing pulses, postsynaptic calcium influx through VGCCs |
Presynaptic | [22,89,90] |
| Neonatal Hippocampus (mossy fiber-CA3) |
BDNF-TrkB signaling, postsynaptic PKA, L-type VGCC-dependent |
Presynaptic | [25] |
| Ventral Tegmental Area | NO retrograde signaling, NMDAR activation, postsynaptic calcium |
Presynaptic | [42,43] |
| Cerebellar cortex (stellate interneurons) |
Activation of presynaptic NMDARs, presynaptic calcium, cAMP/PKA, Rim1α |
Presynaptic | [51,52] |
| Cerebellar Purkinje neurons | Postsynaptic depolarization, CaMKII activation | Postsynaptic | [67–69] |
| Deep Cerebellar Nuclei | NMDAR activation, postsynaptic calcium | Postsynaptic | [59] |
| Hippocampus (CA1 area) | Group I mGluR and GABABR activation, postsynaptic calcium |
Postsynaptic | [91] |
| Developing Visual Cortex (L4 FSN-pyr) |
Pairing of presynaptic activity with sub-threshold postsynaptic depolarization |
Postsynaptic | [77] |
| Lateral Amygdala | NMDAR-independent | Postsynaptic? | [92] |
| Developing Auditory Cortex | BDNF-TrkB signaling | Postsynaptic? | [41] |
| I-LTD | |||
| Hippocampus (CA1) | eCB signaling, presynaptic activity, Rim1α | Presynaptic | [9,15*,93] |
| Lateral Amygdala | eCB signaling, postsynaptic PKA, Rim1α | Presynaptic | [10,11,93] |
| Prefrontal cortex (L2/3, L5) | eCB signaling, D2R activation | Presynaptic | [16] |
| Developing Visual Cortex (L2/3) | eCB signaling | Presynaptic | [14*] |
| Dorsal striatum | eCB signaling | Presynaptic | [12] |
| Superior colliculus | eCB signaling | Presynaptic | [13] |
| Neonatal Hippocampus (CA1 area) | NMDAR-dependent, postsynaptic calcium | Presynaptic | [90,94] |
| Hippocampus (CA1) | NMDAR-dependent, calcineurin | Postsynaptic | [62] |
| Deep Cerebellar Nuclei | Postsynaptic calcium, protein phosphatses | Postsynaptic | [65] |
| Developing Xenopus retinotectal system |
Activation of presynaptic NMDAR, relatively weak excitatory inputs |
Presynaptic | [24,49] |
| Ventral Tegmental Area | eCB signaling, D2R activation | Pre and postsynaptic |
[17,19] |
| Developing auditory brainstem | Low frequency stimulation, postsynaptic calcium | Postsynaptic | [95] |
| Bi-Directional plasticity | |||
| Deep Cerebellar Nuclei | Postsynaptic rebound firing determines polarity of plasticity |
Undetermined | [64] |
| Neocortex (FSN-pyr) | Spike-timing dependent, postsynaptic calcium | Postsynaptic | [96] |
| Entorhinal Cortex (L2 neurons) | Spike-timing dependent, postsynaptic calcium | Postsynaptic? | [97] |
| Visual Cortex (L5 pyr) | Repetitive postsynaptic firing, calcium influx through voltage-dependent calcium channels |
Postsynaptic | [74] |
| Subthalamic Nucleus | Rebound burst firing, calcium influx through voltage-dependent calcium channels |
Postsynaptic | [85] |
Notes: The table focuses on recent publications providing some mechanistic insight on I-LTP/I-LTD (i.e., not all publications reporting some form of long-term inhibitory plasticity are included).
“Presynaptic” and “Postsynaptic” refers to expression mechanisms supported by experimental evidence. In some cases, such mechanisms do exclude the untested possibility that another mechanism (i.e. postsynaptic or presynaptic, respectively) may also participate.
Unless otherwise stated, studies were done in rodents (typically rats and mice).
Presynaptic Forms of inhibitory synaptic plasticity
Some of the best characterized forms of GABAergic plasticity involve regulated changes in the release of GABA from presynaptic terminals. As in most cases of GABAergic plasticity, the induction of presynaptic forms are heterosynaptic requiring non-GABAergic stimuli, often from nearby excitatory synapses (Fig. 1). This requires that some signal be communicated to the GABAergic presynaptic terminal to trigger the plasticity. Several presynaptic forms of long-term plasticity that we will discuss here do so via retrograde messengers. These molecules are so called because of the unconventional way by which they signal, being produced in the postsynaptic cell in an activity-dependent manner and traveling back across the synapse to modulate presynaptic neurotransmitter release [4]. In an additional case glutamate itself serves to directly induce plasticity at GABAergic terminals.
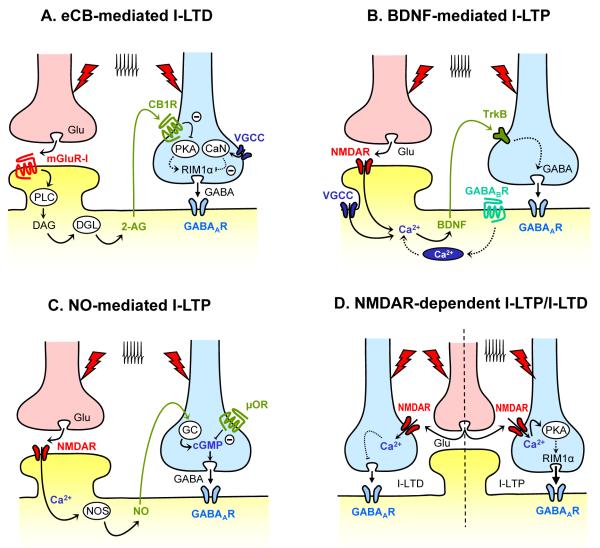
Figure 1. Molecular mechanisms underlying presynaptic forms of GABAergic plasticity following repetitive afferent activity.
A. eCB-mediated I-LTD mediated is triggered by postsynaptic activation of group I metabotropic glutamate receptors (mGluR-I), leading to the production of diacylglyercol (DAG) by phospholipase C (PLC). Diacylglycerol lipase (DGL) converts DAG to the major eCB, 2-AG, which is released from the postsynaptic cell and travels back across the synapse to activate type 1 cannabinoid receptors (CB1Rs) on the GABAergic terminal. CB1R activation subsequently reduces protein kinase A activity. Coincident interneuronal activity increases intracellular calcium via voltage-gated calcium channels (VGCCs) and enhances calcineurin (CaN) activity. Combined reduction and enhancement of PKA and CaN activity, respectively, may lower the phosphorylation status of an unidentified substrate in the release machinery to persistently depress GABA release in a Rim1α-dependent manner. B. I-LTP mediated by brain-derived neurotrophic factor (BDNF) is initiated by intracellular calcium rise due to opening of either NMDARs or VGCCs, or calcium release from intracellular stores, depending on the synapse and brain region. Postsynaptic GABABR activation reportedly can lead to calcium release from stores via an unknown mechanism. BDNF acts as a retrograde messenger to activate TrkB receptor tyrosine kinases on the inhibitory terminal to potentiate GABA release through an unclear pathway. C. I-LTP mediated by nitric oxide (NO) is induced by intracellular calcium rise in the postsynaptic cell via opening of NMDARs to activate nitric oxide synthase (NOS). NO readily permeates through the membrane and stimulates presynaptic guanylate cyclase (GC), augmenting cGMP levels to enhance GABA release. Mu-opioid receptors (μORs) antagonize the ability of NO to increase cGMP signaling. D. Glutamate (Glu) can also directly activate presynaptic NMDARs on the GABAergic interneuron, without the need for retrograde messengers. Depending on the synapse, calcium increase at the terminals can depress GABA release through uncharacterized mechanisms or potentiate GABA release in a PKA- and RIM1α-dependent manner.
Endocannabinoid-mediated I-LTD
One of the best characterized retrograde messengers is a group of lipophilic molecules collectively called endocannabinoids (eCBs). In many brain regions, repetitive afferent stimulation triggers eCB mobilization from the postsynaptic cell to the presynaptic terminal, where they bind to type 1 cannabinoid receptors (CB1Rs) to suppress neurotransmitter release either in a short-term or long-term manner (eCB-LTD) [5-8]. eCB-LTD is widely expressed in the brain and has been found at both glutamatergic and GABAergic synapses [6]. Although the pattern of afferent activity may differ between the different brain regions, a common theme of eCB-LTD at GABAergic synapses (I-LTD) is the initial requirement for glutamate from neighboring excitatory synapses to activate postsynaptic group I metabotropic glutamate receptors (I-mGluRs) (Fig. 1A). eCB-mediated I-LTD has been identified in the hippocampus [9], amygdala [10-11], dorsal striatum [12], brainstem [13] and visual cortex [14]. Importantly, at least in the hippocampus, CB1Rs are expressed by cholecystokinin-positive interneurons [8] and thus, hippocampal I-LTD is selectively induced at inhibitory synapses from these cells.
Although I-LTD is a heterosynaptic form of plasticity, this does not preclude a necessity for presynaptic GABAergic interneuronal activity. Indeed, it was recently shown that CB1R activation is not sufficient to induce I-LTD, and that this form of plasticity can be blocked by hyperpolarizing the GABAergic interneuron and preventing it from firing during induction [15]. Together, these findings show that hippocampal I-LTD is associative, requiring the pairing of presynaptic interneuronal activity with eCB signaling to convert transient changes into long-lasting ones. Similar requirement has been recently reported in the visual cortex [14]. Interneuronal activity likely brings in calcium, which in turn activates the calcium-sensitive phosphatase, calcineurin [15]. CB1Rs are Gi/o-coupled receptors, whose activation results in a decrease in the adenylyl cyclase-protein kinase A (PKA) transduction cascade. The simultaneous enhancement of calcineurin activity and reduction in PKA activity may shift the phosphorylation status of some as yet unidentified substrate in the release machinery towards a dephosphorylated state to cause a long-term decrease in GABA release.
Other factors may also modulate eCB signaling in the presynaptic terminal to induce I-LTD. The type 2 dopamine receptor (D2R) shares common signaling pathways with CB1Rs, ultimately reducing PKA activity. In the prefrontal cortex, activation of either receptor suppresses GABAergic transmission [16]. Furthermore, co-activation of D2R and CB1R by low concentrations of receptor agonists supralinearly depresses inhibitory transmission. Augmenting endogenous dopamine levels facilitates I-LTD induction, which requires both D2R and CB1R activation. Notably, dopaminergic modulation of eCB signaling is not restricted to the prefrontal cortex but may be a general mechanism by which brain regions richly innervated by dopaminergic afferents function. A similar requirement for D2Rs in I-LTD has been shown in the VTA [17].
Several physiological functions of eCB-mediated I-LTD have been proposed. eCB-mediated I-LTD enhances the excitatory influence of glutamatergic inputs on action potential generation [9,12] and modulates the modifiability of excitatory synapses [10,18]. Thus, by mediating long-term disinhibition of neurons, eCB signaling can shift the excitatory/inhibitory balance towards excitation and consequently promote signal propagation within and across neural networks. In the intact animal, amygdalar I-LTD has been associated with extinction of learned fear [11] and I-LTD in the VTA has been implicated in cocaine-induced synaptic changes that may underlie drug addiction [19]. Furthermore, eCB-mediated depression of inhibition is instrumental for the maturation of visual cortical circuits during the critical period [14].
BDNF-TrkB mediated I-LTP
Neurotrophins such as brain-derived neurotrophic factor (BDNF) can be secreted from either axon terminals or dendritic compartments in response to neuronal activity and have been shown to modulate both excitatory and inhibitory transmission [20-21]. Several recent electrophysiological studies have provided evidence for a retrograde action of dendritically released BDNF to potentiate inhibitory synaptic efficacy in the hippocampus, visual cortex and optic tectum (Fig. 1B) [22-25]. Potentiation of GABA release is blocked by bath application, but not by intracellular loading of an inhibitor of TrkB receptor signaling (K252a), supporting a mechanism by which postsynaptic release of BDNF retrogradely modulates presynaptic function. Although the precise stimulation pattern for induction varies from brain region to brain region, a postsynaptic calcium rise is invariably required. The source of the calcium may be NMDARs [24], L-type voltage-gated calcium channels [25-26] or calcium release from internal stores [23]. Intracellular calcium presumably functions to enable BDNF secretion [20]. Interestingly, exogenous BDNF when co-delivered with synaptic stimulation can rescue LTP of GABA transmission of cells which were loaded with the fast calcium chelator BAPTA, but only at activated synapses [23-24]. As in eCB-mediated I-LTD [15], input-specificity of BDNF-mediated plasticity may derive from a requirement for coincident presynaptic interneuron activity to enhance TrkB signaling [21,24].
Some considerations should be given when deciphering the effects of BDNF-TrkB signaling on GABAergic transmission. For example, acute application of BDNF, depending on brain structure, cell type, and developmental status of the brain, can suppress GABA release [27-28] possibly via eCB mobilization [29], or modulate GABAergic transmission in a postsynaptic manner [27,30-33]. An additional level of complexity results from the fact that BDNF can also increase the number of GABAergic terminals [34-36] and regulate the expression of the chloride transporter KCC2 (see below) [37-39]. BDNF-mediated forms of I-LTP reportedly occur in a restricted time window when GABAergic circuits are maturing [22,24,40-41]. Consistent with this observation the direction of BDNF effects is likely to be developmentally regulated.
Nitric oxide-mediated I-LTP
For many years, nitric oxide (NO) has been reported to modulate the strength of excitatory synapses in the CNS. Recently, it was found that NO acts as a key retrograde signal to persistently potentiate GABA release onto dopaminergic neurons in the VTA (Fig. 1C) [42], a brain area essential for reward processing and drug addiction. This form of I-LTP (a.k.a. LTPGABA) is also heterosynaptic, i.e. it is triggered by high frequency stimulation of glutamatergic fibers and its induction requires NMDAR activation, postsynaptic calcium rise, and NO-cGMP signaling. Thus, inhibition of NO synthase, guanylate cyclase or cGMP-dependent protein kinase (PKG), or application of NO scavengers abolished I-LTP in the VTA, whereas application of an NO donor or cGMP analog mimicked this form of plasticity [42-43]. The precise mechanism by which cGMP elevation persistently increases GABA release remains unknown.
Consistent with the notion that exposure to addictive drugs can alter synaptic plasticity in the brain reward pathway, a single exposure to morphine in vivo abolishes I-LTP in VTA slices 24 h later [42]. This effect is reminiscent of the observation that a single in-vivo exposure to Δ9THC, the psychoactive component in marijuana, blocks eCB-mediated synaptic plasticity, including hippocampal I-LTD [44]. In both cases, the injected agonist induces a long-term adaptation in the circuit that disappears few days post injection. Morphine likely targets opioid receptors at GABAergic terminals and, by interfering with guanylate cyclase function, abolishes LTPGABA induction [42]. Likewise, Δ9THC, by triggering functional tolerance of presynaptic CB1Rs, abolishes hippocampal I-LTD [44]. In addition to morphine, it has been recently reported that a single exposure to cocaine and nicotine, as well as acute stress, also blocks NO-mediated I-LTP in the VTA [45]. Intriguingly, a single injection of ethanol reportedly induces a long-lasting increase of GABA release onto VTA DA neurons [46], as well as a μ-opioid receptor-dependent blockade of I-LTP in the VTA [47]. Together, these observations underscore GABAergic synaptic plasticity as an important target of addictive drugs. Neural adaptations in the reward/learning circuit occurring as a result of changes in inhibitory plasticity could contribute to early stages of addiction.
It is interesting that both potentiation and depression of inhibitory synapses may be induced in the VTA. Several pieces of experimental evidence highlight potential similarities and differences between eCB and NO signaling in the VTA. Like eCB-mediated I-LTD in the VTA (Pan et al., 2008), NO-mediated I-LTP is triggered by postsynaptic glutamatergic receptors (I-mGluR and NMDAR, respectively). However, blocking postsynaptic calcium rise with the calcium chelator BAPTA abolishes I-LTP but not I-LTD in the VTA [19,42]. Furthermore, eCB-dependent I-LTD in the VTA is triggered by a longer induction protocol and requires the additional activation of D2Rs [17,19]. Future studies are needed to decipher the physiological conditions under which inhibitory synapses are potentiated or depressed in the VTA. Finally, it is worth noting that activity-dependent, long-lasting increase of GABA release via NO retrograde signaling has been recently reported in the thalamus [48], raising the possibility that NO-mediated I-LTP may occur in other brain regions beyond the VTA.
Presynaptic NMDAR-dependent I-LTD/I-LTP
Not all presynaptic forms of GABAergic plasticity seem to require retrograde signaling (Fig. 1D). In the developing Xenopus retinotectal system, glutamate release from excitatory synapses occurring as a result of high-frequency visual stimulation or repetitive stimulation (e.g. TBS) may directly activate NMDARs on the nerve terminals of adjacent coactive GABAergic neurons, leading to I- LTD [24,49]. Activation of presynaptic NMDARs is necessary but may not sufficient for induction. As for eCB-mediated I-LTD [15], coincident GABAergic interneuron activity, most likely by promoting Ca2+ raise at the GABAergic terminal, thereby boosting the Ca2+ influx through presynaptic NMDARs, also seems to be required. As previously shown at excitatory synapses [50], NMDARs via glutamate may act as presynaptic coincidence detectors for synchronous activities of nearby glutamatergic and GABAergic nerve terminals and is responsible for coordinated induction of I-LTD.
In the rodent cerebellar cortex, recent studies have identified another heterosynaptic form of inhibitory plasticity that also requires activation of NMDARs on GABAergic terminals [51-52]. However, Ca2+ influx through presynaptic NMDARs in this case triggers a long-lasting increase of GABA release from stellate cells (SCs). The glutamate required for induction arises from the repetitive activation of neighboring parallel fibers. This form of I-LTP identified at SC-SC inhibitory synapses, like other presynaptic forms of LTP at excitatory synapses, such as hippocampal mossy fiber LTP, parallel fiber LTP, and cortico-lateral amygdala LTP (for a recent review, see [53]), requires cAMP/PKA signaling and the active zone protein Rim1α [51]. Whether induction of presynaptic NMDAR-dependent I-LTP at SC-SC synapses also requires presynaptic activity, as for I-LTD in the hippocampus and optic tectum [15,49], remains to be tested. As illustrated in Fig. 1D, these presynaptic NMDAR-dependent but retrograde signaling-independent forms of plasticity clearly highlight the heterosynaptic nature of all presynaptic forms of I-LTP and I-LTD, regardless of the underlying molecular mechanisms.
Postsynaptic Forms of inhibitory synaptic plasticity
Multiple forms of inhibitory plasticity have been attributed to postsynaptic changes in GABAergic transmission [2] (Table 1). GABAergic synaptic strength can be altered postsynaptically through several mechanisms (Fig. 2). For example, increases or decreases in channel function can occur as a result of GABAAR phosphorylation by multiple kinases, including PKC, CaMKII, Src and PKA [54]. The function of GABAARs is further controlled by constitutive cycling, regulated insertion and removal, as well as lateral diffusion at the synaptic membrane surface [55-57]. Additionally, postsynaptic GABAergic signaling can be modulated by altering the intracellular concentration of the permeable ions [58]. Each of these mechanisms now has been implicated in the expression for some of the many forms of inhibitory plasticity being steadily identified, a few of which are discussed below.
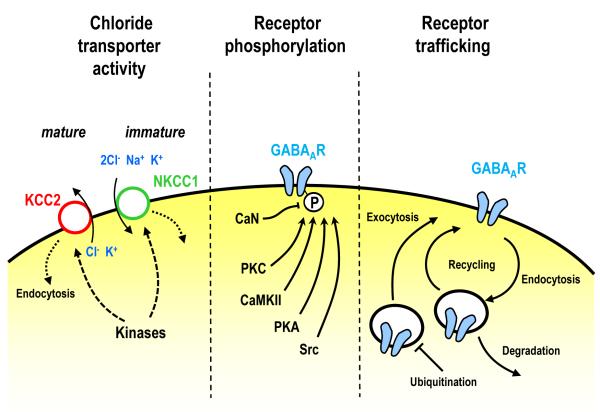
Figure 2. Cellular expression mechanisms underlying postsynaptic forms of GABAergic plasticity.
Left, the concentration of ions inside the cell may change as a result in an enhancement in transporter activity following induction of plasticity. Intracellular chloride is high due to import activity of NKCC1 in the immature brain and is low due to the extrusion activity of KCC2 in the mature brain [82]. Activity can couple to changes in chloride transporter function, possibly through direct phosphorylation of the chloride transporters or by inducing trafficking-mediated changes in surface transporter levels. The altered chloride transported function results in a change in the driving force for chloride and consequently amplitude of GABAAR-mediated responses. Middle, changes in receptor function may occur as a result of direct phosphorylation by kinases including protein kinase C (PKC), calmodulin-kinase II (CaMKII), PKA or Src, or dephosphorylation by CaN [54,70]. Right, the number of GABAARs may change due to receptor trafficking regulation. Inhibitory responses can decrease as a result of enhanced endocytosis, or increase due to enhanced exocytosis [87]. Ubiquitination of the receptor can reduce the stability of receptors in the ER resulting in a decrease in receptor insertion [79].
GABAAR modulation as a mechanism of inhibitory synaptic plasticity
GABAAR trafficking as a mechanism of rapid inhibitory synaptic plasticity is suggested by studies of I-LTP in the deep cerebellar nuclei (DCN), which are associated with motor learning. This form of plasticity is blocked by postsynaptic introduction of the exocytosis inhibitor tetanus toxin supporting a role for receptor insertion [59]. The loss of surface GABAARs also has been observed with inhibitory plasticity. In the hippocampus, CaMKII activation [60] as well as neuronal activity linked to experimental epilepsy can elevate GABAAR surface levels [61]. In contrast, synaptic NMDAR activation elicits a calcineurin-dependent decrease in GABAergic transmission and mIPSC amplitude, consistent with a removal of surface GABAARs [62-63]. Inhibitory synapses on DCN neurons also exhibit I-LTD [64-65], which in young rats is similarly mediated by calcineurin [64-65]. Recently, it has been reported that a different phosphatase, PP2A, appears to mediate BDNF-induced reductions in surface GABAAR s occurring in medial prefrontal cortex during cocaine withdrawal [66].
A rapid potentiation of inhibition, known as rebound potentiation (RP), has been observed in cerebellar Purkinje neurons (PNs) [67]. Postsynaptic depolarization through the activation of excitatory inputs induces CaMKII dependent increases in the responsiveness of postsynaptic GABAAR s [68] requiring the interaction of the β2 GABAAR subunit with the GABAA Receptor Associated Protein (GABARAP) [69]. The exact expression mechanism of RP is not certain, and CaMKII activation can have multiple effects on GABAAR s signaling [70]. In cerebellar granule cells, CaMKII activation was found to increase the decay times of β3-subunit containing receptors while increasing the IPSC amplitude of β2 containing consistent with a possible insertion of receptors [71]. In hippocampal neurons, the CaMKII activation couples to a GABARAP-dependent insertion of synaptic GABAAR s [72-73]. While similarities with the pathway utilized during RP suggest that receptor insertion could be a common expression mechanism for both forms of plasticity, changes in surface expression have not been observed with RP [69], and it remains possible that GABARAP may play multiple roles in modulating inhibitory plasticity.
Recently, repetitive firing in neocortical neurons, a condition associated with arousal, was reported to cause depression of IPSCs that is blocked by inhibitors of endocytosis introduced into the postsynaptic cell [74]. In contrast, cell firing during slow membrane voltage oscillations, mimicking activity during the sleep state, elevates inhibition through an increase in GABAAR function likely mediated by channel insertion [74]. Acquisition of a conditioned fear response is also accompanied by a decrease in surface GABAAR expression [75] and mIPSC amplitude and frequency [76] recorded in the amygdala. Conversely, fear extinction, elevates surface GABAARs and mIPSCs. As in PNs and hippocampal neurons [69,72], disruption of GABARAP blocked the increases in inhibition. Most intriguingly, disruption of GABARAP function in the behaving animal was also able to inhibit the extinction of the conditioned fear response [76]. Together these studies not only emphasize the extent to which GABAAR trafficking may contribute to the modulation of inhibitory transmission throughout the brain, but also highlight the range of behavioral properties which may be strongly influenced by inhibitory plasticity.
The insertion of receptors may also underlie the expression of I-LTP in the V1 region of visual cortex [77]. Pairing activation of fast-spiking basket cells with star pyramidal cell depolarization induces a lasting potentiation of IPSCs resulting from an increase in open channel number, indicative of either modulation of channel function or receptor trafficking. Interestingly, this I-LTP is occluded by and may therefore be the mechanism of potentiated inhibition resulting from early visual deprivation. As such, I-LTP may contribute to altered V1 function linked to the loss of visual acuity observed following visual deprivation during a developmental critical period. Activity deprivation has been found, in contrast, to depress inhibitory transmission in cultured cortical neurons through a loss of surface GABAAR [78-79] attributed in one study to increases in β3 subunit ubiquitination and associated reductions in surface GABAAR stability [79]. Regulation of GABAAR has similarly been found to contribute to a number of homeostatic forms of inhibitory plasticity [78], however the exact mechanisms responsible for many forms remains to be resolved [80].
Altered chloride gradients mediate postsynaptic regulation of inhibition
Postsynaptic GABAergic plasticity also occurs independently of direct receptor regulation. Inhibitory strength is dependent on the cellular chloride gradient which is controlled by the function of chloride transporters. In the mature brain, this function is mainly performed by the neuron-specific K+ - Cl− cotransporter, KCC2 [81]. Chloride transporters and the anion gradient have been found to be regulated by development and activity [58,82]. In hippocampal neurons, temporally coordinated pre- and postsynaptic activity shifted the chloride reversal potential (ECl); an effect that was occluded by blockade of chloride transporters suggesting a down regulation of the activity of those transporters [83-84]. Depending on the developmental age, and consequent differences in transporter expression, this causes either a depolarizing (in mature neurons) or hyperpolarizing (in inmature neurons) shift in ECl, thereby weakening or strengthening inhibition, respectively. Long-lasting bidirectional shifts in ECl induced by rebound burst-firing can be observed in the subthalamic nucleus [85]. The importance of changes in chloride concentration on physiological events is underscored by the observed changes in ECl and transporter activity that have been reported with pain and epilepsy [86].
Emerging properties and future directions
In the last decade, our knowledge on activity-dependent plasticity at inhibitory synapses has expanded dramatically. Below we highlight relevant emerging properties and some of the immediate questions that we understand remain to be elucidated in this field.
Long-term inhibitory synaptic plasticity is widely expressed in the brain. While most studies on I-LTP/I-LTD have focused on mechanistic questions –i.e. induction requirements, expression mechanism– using in vitro preparations, few studies have shown that experience-dependent plasticity involves changes in GABAergic circuitry. Like excitatory synaptic plasticity, inhibitory plasticity may also contribute to learning and memory and the refinement of neural circuits. However, more in vivo studies using natural activation paradigms will be necessary to demonstrate the role of GABAergic synaptic plasticity under physiological conditions.
Inhibitory and excitatory synaptic plasticity can occur simultaneously. Indeed, common induction protocols triggering long-term plasticity at excitatory synapses can also engage heterosynaptic plasticity at inhibitory synapses. In some cases, however, the induction requirements for synaptic plasticity at inhibitory synapses are slightly different than at excitatory synapses and as a result, inhibitory LTP or LTD can occur in the absence of excitatory LTP/LTD. Understanding the precise conditions and synaptic rules that govern the induction of LTP and LTD at both glutamatergic and GABAergic synapses is necessary for a more realistic representation of how experience-dependent synaptic plasticity contributes to brain function and neural circuits refinement.
Whether the expression mechanism is pre or postsynaptic, long-term GABAergic plasticity is largely heterosynaptic in nature; that is, induction depends upon glutamatergic synapses that drive activity, thereby providing a way for excitatory activity to regulate inhibitory synapses. In turn, synaptic plasticity at inhibitory synapses can have important functional consequences at excitatory synapses. For example, long-term changes in GABAergic synaptic efficacy not only regulate the excitatory/inhibitory balance but also contribute to metaplasticity by regulating the inducibility of LTP/LTD at excitatory synapses. Whether metaplasticity is also expressed at inhibitory synapses is unclear.
While the signaling cascades involved in the induction inhibitory synaptic plasticity have been identified in most cases, the precise molecular mechanisms underlying long-lasting changes in GABAergic transmission (i.e. expression mechanisms) are largely unknown. Notably, how I-LTP/I-LTD can be reversed or switched off remains to be elucidated. Also, the role of protein synthesis in inhibitory plasticity is largely unexplored.
Some of the typical properties of LTP/LTD at excitatory synapses, such as associativity, can also be identified at inhibitory synapses. For example, induction of inhibitory plasticity in some cases requires coincident interneuronal activity. Associativity may be a mechanism by which input-specificity is attained, allowing the strength of only the active synapses to be modified.
Maintaining excitatory/inhibitory balance is critical to the stability of neural networks. As a result of activity-driven synaptic plasticity, the excitatory/inhibitory balance can be disrupted. In addition to I-LTP/I-LTD, homeostatic changes in inhibition have been observed in a number of systems. Homeostatic regulation of inhibitory transmission has emerged as an important mechanism for the control of excitability and the maintenance of stability in neural networks. How exactly this stabilizing form of regulation interacts with I-LTP/I-LTD to dynamically control neural activity in vivo remains to be elucidated.
Plasticity of inhibitory synapses may be triggered by excessive neuronal activity as may occur during seizures and other pathologic conditions. Thus, inhibitory plasticity is likely to play a central role in numerous neuropsychiatric conditions. In fact, disruption of inhibition has been implicated in several neuropsychiatric disorders, including epilepsy, anxiety, autism spectrum disorders, Parkinson’s and Huntington’s diseases, sleep disorders and neuronal injury. It appears that either disruption or hyperactivation of inhibitory plasticity may contribute to the dysregulation of inhibition in several diseases; however, understanding of the precise involvement of inhibitory plasticity mechanisms requires further studies.
Acknowledgments
We wish to thank all scientists whose data are reviewed in this article. We apologize to all the investigators whose work could not be cited owing to space constraints. Supported by NIH/NIDA and the Irma T. Hirschl Career Scientist Award (to P.E.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Woodin MA, Maffei A, editors. Inhibitory Synaptic Plasticity. edn 1st Springer; New York Dordrecht Heidelberg London: 2011. (••) This book summarizes most recent advancements on GABAergic plasticity in the mammalian brain. A must read to all those interested in inhibitory synapses and brain function under normal and pathological conditions.
- 2.Gaiarsa J, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci. 2002;25:564–570. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- 3.McBain CJ, Kauer JA. Presynaptic plasticity: targeted control of inhibitory networks. Curr Opin Neurobiol. 2009;19:254–262. doi: 10.1016/j.conb.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–170. doi: 10.1016/j.neuron.2009.06.021. (••) A comprehensive review of the various retrograde signaling mechanisms involved in the regulation of excitatory and inhibitory synaptic transmission.
- 5.Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- 6.Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. (•) A very good summary of eCB-mediated LTD at both excitatory and inhibitory synapses.
- 7.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- 8.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 9.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 10.Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 12.Adermark L, Lovinger DM. Frequency-dependent inversion of net striatal output by endocannabinoid-dependent plasticity at different synaptic inputs. J Neurosci. 2009;29:1375–1380. doi: 10.1523/JNEUROSCI.3842-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henneberger C, Redman SJ, Grantyn R. Cortical efferent control of subcortical sensory neurons by synaptic disinhibition. Cereb Cortex. 2007;17:2039–2049. doi: 10.1093/cercor/bhl112. [DOI] [PubMed] [Google Scholar]
- 14.Jiang B, Huang S, de Pasquale R, Millman D, Song L, Lee HK, Tsumoto T, Kirkwood A. The maturation of GABAergic transmission in visual cortex requires endocannabinoid-mediated LTD of inhibitory inputs during a critical period. Neuron. 2010;66:248–259. doi: 10.1016/j.neuron.2010.03.021. (•) Consistent with a proposed role for eCBs in development, this work demonstrated clearly that these retrograde messengers may sculpt maturation of visual cortical circuits by mediating LTD of inhibitory transmission.
- 15.Heifets BD, Chevaleyre V, Castillo PE. Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc Natl Acad Sci U S A. 2008;105:10250–10255. doi: 10.1073/pnas.0711880105. (•) This paper demonstrates that eCB-mediated I-LTD requires coincident interneuronal activity, providing a mechanism for how eCB signaling during transient suppression is consolidated into a persistent depression in an input-specific manner.
- 16.Chiu CQ, Puente N, Grandes P, Castillo PE. Dopaminergic modulation of endocannabinoid-mediated plasticity at GABAergic synapses in the prefrontal cortex. J Neurosci. 2010;30:7236–7248. doi: 10.1523/JNEUROSCI.0736-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008;28:14018–14030. doi: 10.1523/JNEUROSCI.4035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chevaleyre V, Castillo PE. Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron. 2004;43:871–881. doi: 10.1016/j.neuron.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 19.Pan B, Hillard CJ, Liu QS. Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci. 2008;28:1385–1397. doi: 10.1523/JNEUROSCI.4033-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lessmann V, Gottmann K, Malcangio M. Neurotrophin secretion: current facts and future prospects. Prog Neurobiol. 2003;69:341–374. doi: 10.1016/s0301-0082(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 21.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gubellini P, Ben-Ari Y, Gaiarsa JL. Endogenous neurotrophins are required for the induction of GABAergic long-term potentiation in the neonatal rat hippocampus. J Neurosci. 2005;25:5796–5802. doi: 10.1523/JNEUROSCI.0824-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inagaki T, Begum T, Reza F, Horibe S, Inaba M, Yoshimura Y, Komatsu Y. Brain-derived neurotrophic factor-mediated retrograde signaling required for the induction of long-term potentiation at inhibitory synapses of visual cortical pyramidal neurons. Neurosci Res. 2008;61:192–200. doi: 10.1016/j.neures.2008.02.006. (•) The authors finally uncovered the retrograde messenger for potentiation of GABAergic transmission in the visual cortex, one of the first forms of inhibitory plasticity ever reported in the CNS.
- 24.Liu Y, Zhang LI, Tao HW. Heterosynaptic scaling of developing GABAergic synapses: dependence on glutamatergic input and developmental stage. J Neurosci. 2007;27:5301–5312. doi: 10.1523/JNEUROSCI.0376-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivakumaran S, Mohajerani MH, Cherubini E. At immature mossy-fiber-CA3 synapses, correlated presynaptic and postsynaptic activity persistently enhances GABA release and network excitability via BDNF and cAMP-dependent PKA. J Neurosci. 2009;29:2637–2647. doi: 10.1523/JNEUROSCI.5019-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuczewski N, Langlois A, Fiorentino H, Bonnet S, Marissal T, Diabira D, Ferrand N, Porcher C, Gaiarsa JL. Spontaneous glutamatergic activity induces a BDNF-dependent potentiation of GABAergic synapses in the newborn rat hippocampus. J Physiol. 2008;586:5119–5128. doi: 10.1113/jphysiol.2008.158550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abidin I, Eysel UT, Lessmann V, Mittmann T. Impaired GABAergic inhibition in the visual cortex of brain-derived neurotrophic factor heterozygous knockout mice. J Physiol. 2008;586:1885–1901. doi: 10.1113/jphysiol.2007.148627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- 29.Lemtiri-Chlieh F, Levine ES. BDNF evokes release of endogenous cannabinoids at layer 2/3 inhibitory synapses in the neocortex. J Neurophysiol. 2010;104:1923–1932. doi: 10.1152/jn.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci. 2001;13:1320–1328. doi: 10.1046/j.0953-816x.2001.01506.x. [DOI] [PubMed] [Google Scholar]
- 31.Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-derived neurotrophic factor modulates fast synaptic inhibition by regulating GABA(A) receptor phosphorylation, activity, and cell-surface stability. J Neurosci. 2004;24:522–530. doi: 10.1523/JNEUROSCI.3606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizoguchi Y, Kanematsu T, Hirata M, Nabekura J. A rapid increase in the total number of cell surface functional GABAA receptors induced by brain-derived neurotrophic factor in rat visual cortex. J Biol Chem. 2003;278:44097–44102. doi: 10.1074/jbc.M305872200. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci. 2007;27:7234–7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu B, Wang KH, Nose A. Molecular mechanisms underlying neural circuit formation. Curr Opin Neurobiol. 2009;19:162–167. doi: 10.1016/j.conb.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marty S, Wehrle R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− cotransporter KCC2. Development. 2003;130:1267–1280. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- 38.Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, et al. BDNF-induced TrkB activation down-regulates the K+-Cl−cotransporter KCC2 and impairs neuronal Cl- extrusion. J Cell Biol. 2002;159:747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wardle RA, Poo MM. Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci. 2003;23:8722–8732. doi: 10.1523/JNEUROSCI.23-25-08722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komatsu Y. Age-dependent long-term potentiation of inhibitory synaptic transmission in rat visual cortex. J Neurosci. 1994;14:6488–6499. doi: 10.1523/JNEUROSCI.14-11-06488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Kotak VC, Sanes DH. Normal hearing is required for the emergence of long-lasting inhibitory potentiation in cortex. J Neurosci. 2010;30:331–341. doi: 10.1523/JNEUROSCI.4554-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- 43.Nugent FS, Niehaus JL, Kauer JA. PKG and PKA signaling in LTP at GABAergic synapses. Neuropsychopharmacology. 2009;34:1829–1842. doi: 10.1038/npp.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mato S, Chevaleyre V, Robbe D, Pazos A, Castillo PE, Manzoni OJ. A single in-vivo exposure to delta 9THC blocks endocannabinoid-mediated synaptic plasticity. Nat Neurosci. 2004;7:585–586. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- 45.Niehaus JL, Murali M, Kauer JA. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur J Neurosci. 2010;32:108–117. doi: 10.1111/j.1460-9568.2010.07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guan YZ, Ye JH. Ethanol blocks long-term potentiation of GABAergic synapses in the ventral tegmental area involving mu-opioid receptors. Neuropsychopharmacology. 2010;35:1841–1849. doi: 10.1038/npp.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bright DP, Brickley SG. Acting locally but sensing globally: impact of GABAergic synaptic plasticity on phasic and tonic inhibition in the thalamus. J Physiol. 2008;586:5091–5099. doi: 10.1113/jphysiol.2008.158576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lien CC, Mu Y, Vargas-Caballero M, Poo MM. Visual stimuli-induced LTD of GABAergic synapses mediated by presynaptic NMDA receptors. Nat Neurosci. 2006;9:372–380. doi: 10.1038/nn1649. [DOI] [PubMed] [Google Scholar]
- 50.Duguid I, Sjostrom PJ. Novel presynaptic mechanisms for coincidence detection in synaptic plasticity. Curr Opin Neurobiol. 2006;16:312–322. doi: 10.1016/j.conb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Lachamp PM, Liu Y, Liu SJ. Glutamatergic modulation of cerebellar interneuron activity is mediated by an enhancement of GABA release and requires protein kinase A/RIM1alpha signaling. J Neurosci. 2009;29:381–392. doi: 10.1523/JNEUROSCI.2354-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu SJ, Lachamp P. The activation of excitatory glutamate receptors evokes a long-lasting increase in the release of GABA from cerebellar stellate cells. J Neurosci. 2006;26:9332–9339. doi: 10.1523/JNEUROSCI.2929-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 54.Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13:341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 55.Bannai H, Levi S, Schweizer C, Inoue T, Launey T, Racine V, Sibarita JB, Mikoshiba K, Triller A. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. doi: 10.1016/j.neuron.2009.04.023. (••) This study uses single particle tracking of GABARs labeled with quantum dots to elegantly demonstrate that the diffusion and synaptic localization of GABAARs is controlled by activity-dependent regulation mediated by intracellular calcium levels and calcineurin activty.
- 56.Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tretter V, Moss SJ. GABA(A) Receptor Dynamics and Constructing GABAergic Synapses. Front Mol Neurosci. 2008;1:7. doi: 10.3389/neuro.02.007.2008. (•) Concise review of GABAAR trafficking, its regulation and impact on excitability
- 58.Fiumelli H, Woodin MA. Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr Opin Neurobiol. 2007;17:81–86. doi: 10.1016/j.conb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Ouardouz M, Sastry BR. Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. J Neurophysiol. 2000;84:1414–1421. doi: 10.1152/jn.2000.84.3.1414. [DOI] [PubMed] [Google Scholar]
- 60.Wang RA, Cheng G, Kolaj M, Randic M. Alpha-subunit of calcium/calmodulin-dependent protein kinase II enhances gamma-aminobutyric acid and inhibitory synaptic responses of rat neurons in vitro. J Neurophysiol. 1995;73:2099–2106. doi: 10.1152/jn.1995.73.5.2099. [DOI] [PubMed] [Google Scholar]
- 61.Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- 62.Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- 63.Wang J, Liu S, Haditsch U, Tu W, Cochrane K, Ahmadian G, Tran L, Paw J, Wang Y, Mansuy I, et al. Interaction of calcineurin and type-A GABA receptor gamma 2 subunits produces long-term depression at CA1 inhibitory synapses. J Neurosci. 2003;23:826–836. doi: 10.1523/JNEUROSCI.23-03-00826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21:827–835. doi: 10.1016/s0896-6273(00)80598-x. [DOI] [PubMed] [Google Scholar]
- 65.Morishita W, Sastry BR. Postsynaptic mechanisms underlying long-term depression of GABAergic transmission in neurons of the deep cerebellar nuclei. J Neurophysiol. 1996;76:59–68. doi: 10.1152/jn.1996.76.1.59. [DOI] [PubMed] [Google Scholar]
- 66.Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67:821–833. doi: 10.1016/j.neuron.2010.08.012. (••) This study investigates changes in GABAergic transmission in the medial prefrontal cortex (mPFC) during cocaine withdrawal. Following the termination of repeated cocaine injections in rats, elevated BDNF levels were found to decrease surface GABAARs via TrKB signaling. The consequence of this receptor downregulation is a facilitation of excitatory LTP of synapses onto layer V pyramidal neurons. The physiological impact of these changes is supported by the observation that knock down of TrkB in the mPFC was able to reduce locomotor sensitivity to cocaine normally observed following drug withdrawal.
- 67.Kano M, Rexhausen U, Dreessen J, Konnerth A. Synaptic excitation produces a long-lasting rebound potentiation of inhibitory synaptic signals in cerebellar Purkinje cells. Nature. 1992;356:601–604. doi: 10.1038/356601a0. [DOI] [PubMed] [Google Scholar]
- 68.Kano M, Fukunaga K, Konnerth A. Ca(2+)-induced rebound potentiation of gamma-aminobutyric acid-mediated currents requires activation of Ca2+/calmodulin-dependent kinase II. Proc Natl Acad Sci U S A. 1996;93:13351–13356. doi: 10.1073/pnas.93.23.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawaguchi SY, Hirano T. Sustained structural change of GABA(A) receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. J Neurosci. 2007;27:6788–6799. doi: 10.1523/JNEUROSCI.1981-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Houston C, He Q, Smart TG. CaMKII phosphorylation of the GABA-A receptor: receptor subtype- and synapse-specific modulation. J Physiol. 2009;587:2115–2125. doi: 10.1113/jphysiol.2009.171603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Houston C, Hosie A, Smart TG. Distinct Regulation of beta-2 and beta-3 subunit-containing cerebellar synaptic GABA-A receptors by calcium/calmodulin-dependent protein kinase II. J Neurosci. 2008;28:7574–7584. doi: 10.1523/JNEUROSCI.5531-07.2008. (•) This study uses transgenic animals to establish that CaMKII has receptor subunit selective effects on GABAergic responses in cerebellar granule cells. While mIPSCs mediated by receptors with β2 subunits exhibit increased amplitudes, β3 receptor mediated responses undergo an increase in decay times.
- 72.Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marsden KC, Shemesh A, Bayer KU, Carroll RC. Selective translocation of Ca2+/calmodulin protein kinase II{alpha} (CaMKII{alpha}) to inhibitory synapses. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1010346107. (•) The authors find that regulation of GABAAR levels in hippocampal neurons is modulated by the activity dependent translocation of CaMKII to inhibitory synapses. CaMKII is found to be selectively localized to excitatory or inhibitory synapses depending on the magnitude of the calcium elevating stimulus and the activation state of CaMKII.
- 74.Kurotani T, Yamada K, Yoshimura Y, Crair MC, Komatsu Y. State-dependent bidirectional modification of somatic inhibition in neocortical pyramidal cells. Neuron. 2008;57:905–916. doi: 10.1016/j.neuron.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chhatwal J, Myers K, Ressler K, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin HC, Mao SC, Gean PW. Block of gamma-aminobutyric acid-A receptor insertion in the amygdala impairs extinction of conditioned fear. Biol Psychiatry. 2009;66:665–673. doi: 10.1016/j.biopsych.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- 78.Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saliba RS, Michels G, Jacob TC, Pangalos M, Moss SJ. Activity-dependent ubiquitination of GABA(A) receptors regulates their accumulation at synaptic sites. J Neurosci. 2007;27:13341–13351. doi: 10.1523/JNEUROSCI.3277-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 82.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 83.Balena T, Woodin MA. Coincident pre- and postsynaptic activity downregulates NKCC1 to hyperpolarize E(Cl) during development. Eur J Neurosci. 2008;27:2402–2412. doi: 10.1111/j.1460-9568.2008.06194.x. [DOI] [PubMed] [Google Scholar]
- 84.Woodin MA, Ganguly K, Poo MM. Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl- transporter activity. Neuron. 2003;39:807–820. doi: 10.1016/s0896-6273(03)00507-5. [DOI] [PubMed] [Google Scholar]
- 85.Wang L, Kitai ST, Xiang Z. Activity-dependent bidirectional modification of inhibitory synaptic transmission in rat subthalamic neurons. J Neurosci. 2006;26:7321–7327. doi: 10.1523/JNEUROSCI.4656-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Koninck Y. Altered chloride homeostasis in neurological disorders: a new target. Curr Opin Pharmacol. 2007;7:93–99. doi: 10.1016/j.coph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 87.Arancibia-Carcamo IL, Kittler JT. Regulation of GABA(A) receptor membrane trafficking and synaptic localization. Pharmacol Ther. 2009;123:17–31. doi: 10.1016/j.pharmthera.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 88.Komatsu Y. GABAB receptors, monoamine receptors, and postsynaptic inositol trisphosphate-induced Ca2+ release are involved in the induction of long-term potentiation at visual cortical inhibitory synapses. J Neurosci. 1996;16:6342–6352. doi: 10.1523/JNEUROSCI.16-20-06342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caillard O, Ben-Ari Y, Gaiarsa JL. Long-term potentiation of GABAergic synaptic transmission in neonatal rat hippocampus. J Physiol. 1999;518(Pt 1):109–119. doi: 10.1111/j.1469-7793.1999.0109r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McLean HA, Caillard O, Ben-Ari Y, Gaiarsa JL. Bidirectional plasticity expressed by GABAergic synapses in the neonatal rat hippocampus. J Physiol. 1996;496(Pt 2):471–477. doi: 10.1113/jphysiol.1996.sp021699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patenaude C, Chapman CA, Bertrand S, Congar P, Lacaille JC. GABAB receptor- and metabotropic glutamate receptor-dependent cooperative long-term potentiation of rat hippocampal GABAA synaptic transmission. J Physiol. 2003;553:155–167. doi: 10.1113/jphysiol.2003.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bauer EP, LeDoux JE. Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala. J Neurosci. 2004;24:9507–9512. doi: 10.1523/JNEUROSCI.3567-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caillard O, Ben-Ari Y, Gaiarsa JL. Mechanisms of induction and expression of long-term depression at GABAergic synapses in the neonatal rat hippocampus. J Neurosci. 1999;19:7568–7577. doi: 10.1523/JNEUROSCI.19-17-07568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang EH, Kotak VC, Sanes DH. Long-term depression of synaptic inhibition is expressed postsynaptically in the developing auditory system. J Neurophysiol. 2003;90:1479–1488. doi: 10.1152/jn.00386.2003. [DOI] [PubMed] [Google Scholar]
- 96.Holmgren CD, Zilberter Y. Coincident spiking activity induces long-term changes in inhibition of neocortical pyramidal cells. J Neurosci. 2001;21:8270–8277. doi: 10.1523/JNEUROSCI.21-20-08270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]