Abstract
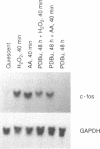
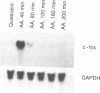
We found previously that stimulation of c-fos and c-myc mRNA expression are early events in hydrogen peroxide-induced growth in rat aortic smooth muscle (RASM) cells. In the present study, we investigated the role of phospholipase A2 (PLA2) and protein kinase C (PKC) in mediating hydrogen peroxide-induced c-fos mRNA expression in RASM cells. Mepacrine and p-bromophenacylbromide, potent inhibitors of PLA2 activity, blocked hydrogen peroxide-induced c-fos mRNA expression. Arachidonic acid, a product of PLA2 activity, stimulated the expression of c-fos mRNA with a time course similar to that of hydrogen peroxide. PKC down-regulation attenuated both hydrogen peroxide and arachidonic acid-induced c-fos mRNA expression by 50%. Nordihydroguaiaretic acid (a lipoxygenase-cytochrome P450 monooxygenase inhibitor) significantly inhibited both hydrogen peroxide and arachidonic acid-induced c-fos mRNA expression, whereas indomethacin (a cyclooxygenase inhibitor) had no effect. Together, these findings indicate that 1) hydrogen peroxide-induced c-fos mRNA expression is mediated by PLA2-dependent arachidonic acid release, 2) both PKC-dependent and independent mechanisms are involved in hydrogen peroxide-induced expression of c-fos mRNA and 3) arachidonic acid metabolism via the lipoxygenase-cytochrome P450 monooxygenase pathway appears to be required for hydrogen peroxide-induced expression of c-fos mRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Curran T. Transcriptional regulation by Fos and Jun in vitro: interaction among multiple activator and regulatory domains. Mol Cell Biol. 1991 Jul;11(7):3624–3632. doi: 10.1128/mcb.11.7.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Auwerx J., Sassone-Corsi P. AP-1 (Fos-Jun) regulation by IP-1: effect of signal transduction pathways and cell growth. Oncogene. 1992 Nov;7(11):2271–2280. [PubMed] [Google Scholar]
- Bauskin A. R., Alkalay I., Ben-Neriah Y. Redox regulation of a protein tyrosine kinase in the endoplasmic reticulum. Cell. 1991 Aug 23;66(4):685–696. doi: 10.1016/0092-8674(91)90114-e. [DOI] [PubMed] [Google Scholar]
- Brinkman H. J., van Buul-Wortelboer M. F., van Mourik J. A. Involvement of cyclooxygenase- and lipoxygenase-mediated conversion of arachidonic acid in controlling human vascular smooth muscle cell proliferation. Thromb Haemost. 1990 Apr 12;63(2):291–297. [PubMed] [Google Scholar]
- Burkhard S. J., Traugh J. A. Changes in ribosome function by cAMP-dependent and cAMP-independent phosphorylation of ribosomal protein S6. J Biol Chem. 1983 Nov 25;258(22):14003–14008. [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chakraborti S., Gurtner G. H., Michael J. R. Oxidant-mediated activation of phospholipase A2 in pulmonary endothelium. Am J Physiol. 1989 Dec;257(6 Pt 1):L430–L437. doi: 10.1152/ajplung.1989.257.6.L430. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooper D. R., Watson J. E., Acevedo-Duncan M., Pollet R. J., Standaert M. L., Farese R. V. Retention of specific protein kinase C isozymes following chronic phorbol ester treatment in BC3H-1 myocytes. Biochem Biophys Res Commun. 1989 May 30;161(1):327–334. doi: 10.1016/0006-291x(89)91600-8. [DOI] [PubMed] [Google Scholar]
- Crane F. L., Sun I. L., Clark M. G., Grebing C., Löw H. Transplasma-membrane redox systems in growth and development. Biochim Biophys Acta. 1985 Aug 1;811(3):233–264. doi: 10.1016/0304-4173(85)90013-8. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Dell K. R., Severson D. L. Effect of cis-unsaturated fatty acids on aortic protein kinase C activity. Biochem J. 1989 Feb 15;258(1):171–175. doi: 10.1042/bj2580171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellem K. A., Kay G. F. Ferricyanide can replace pyruvate to stimulate growth and attachment of serum restricted human melanoma cells. Biochem Biophys Res Commun. 1983 Apr 15;112(1):183–190. doi: 10.1016/0006-291x(83)91814-4. [DOI] [PubMed] [Google Scholar]
- Erikson E., Maller J. L. A protein kinase from Xenopus eggs specific for ribosomal protein S6. Proc Natl Acad Sci U S A. 1985 Feb;82(3):742–746. doi: 10.1073/pnas.82.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafeur V., Jiang Z. P., Böhlen P. Signal transduction by bFGF, but not TGF beta 1, involves arachidonic acid metabolism in endothelial cells. J Cell Physiol. 1991 Nov;149(2):277–283. doi: 10.1002/jcp.1041490214. [DOI] [PubMed] [Google Scholar]
- Force T., Kyriakis J. M., Avruch J., Bonventre J. V. Endothelin, vasopressin, and angiotensin II enhance tyrosine phosphorylation by protein kinase C-dependent and -independent pathways in glomerular mesangial cells. J Biol Chem. 1991 Apr 5;266(10):6650–6656. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Glasgow W. C., Afshari C. A., Barrett J. C., Eling T. E. Modulation of the epidermal growth factor mitogenic response by metabolites of linoleic and arachidonic acid in Syrian hamster embryo fibroblasts. Differential effects in tumor suppressor gene (+) and (-) phenotypes. J Biol Chem. 1992 May 25;267(15):10771–10779. [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. N., Höllwarth M. E., Parks D. A. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Haliday E. M., Ramesha C. S., Ringold G. TNF induces c-fos via a novel pathway requiring conversion of arachidonic acid to a lipoxygenase metabolite. EMBO J. 1991 Jan;10(1):109–115. doi: 10.1002/j.1460-2075.1991.tb07926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Free radicals, reactive oxygen species and human disease: a critical evaluation with special reference to atherosclerosis. Br J Exp Pathol. 1989 Dec;70(6):737–757. [PMC free article] [PubMed] [Google Scholar]
- Kontos H. A. George E. Brown memorial lecture. Oxygen radicals in cerebral vascular injury. Circ Res. 1985 Oct;57(4):508–516. doi: 10.1161/01.res.57.4.508. [DOI] [PubMed] [Google Scholar]
- Kovary K., Bravo R. Expression of different Jun and Fos proteins during the G0-to-G1 transition in mouse fibroblasts: in vitro and in vivo associations. Mol Cell Biol. 1991 May;11(5):2451–2459. doi: 10.1128/mcb.11.5.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Larsson R., Cerutti P. Oxidants induce phosphorylation of ribosomal protein S6. J Biol Chem. 1988 Nov 25;263(33):17452–17458. [PubMed] [Google Scholar]
- Larsson R., Cerutti P. Translocation and enhancement of phosphotransferase activity of protein kinase C following exposure in mouse epidermal cells to oxidants. Cancer Res. 1989 Oct 15;49(20):5627–5632. [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki A., Berezesky I. K., Fargnoli J., Holbrook N. J., Trump B. F. Role of [Ca2+]i in induction of c-fos, c-jun, and c-myc mRNA in rat PTE after oxidative stress. FASEB J. 1992 Feb 1;6(3):919–924. doi: 10.1096/fasebj.6.3.1740241. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- Messina J. L., Standaert M. L., Ishizuka T., Weinstock R. S., Farese R. V. Role of protein kinase C in insulin's regulation of c-fos transcription. J Biol Chem. 1992 May 5;267(13):9223–9228. [PubMed] [Google Scholar]
- Miller A. M., Cullen M. K., Kobb S. M., Weiner R. S. Effects of lipoxygenase and glutathione pathway inhibitors on leukemic cell line growth. J Lab Clin Med. 1989 Mar;113(3):355–361. [PubMed] [Google Scholar]
- Murakami K., Routtenberg A. Direct activation of purified protein kinase C by unsaturated fatty acids (oleate and arachidonate) in the absence of phospholipids and Ca2+. FEBS Lett. 1985 Nov 18;192(2):189–193. doi: 10.1016/0014-5793(85)80105-8. [DOI] [PubMed] [Google Scholar]
- Murrell G. A., Francis M. J., Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990 Feb 1;265(3):659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nose K., Shibanuma M., Kikuchi K., Kageyama H., Sakiyama S., Kuroki T. Transcriptional activation of early-response genes by hydrogen peroxide in a mouse osteoblastic cell line. Eur J Biochem. 1991 Oct 1;201(1):99–106. doi: 10.1111/j.1432-1033.1991.tb16261.x. [DOI] [PubMed] [Google Scholar]
- Palumbo E. J., Sweatt J. D., Chen S. J., Klann E. Oxidation-induced persistent activation of protein kinase C in hippocampal homogenates. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1439–1445. doi: 10.1016/0006-291x(92)90463-u. [DOI] [PubMed] [Google Scholar]
- Ralph R. K., Wojcik S. Inhibitors of lipoxygenase have antiproliferative effects on P815 murine mastocytoma cells. Cancer Lett. 1990 Mar;49(3):181–185. doi: 10.1016/0304-3835(90)90156-r. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Berk B. C. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992 Mar;70(3):593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Durbin H., Clingan D., de Asua L. J. Iron salts and transferrin are specifically required for cell division of cultured 3T6 cells. Biochem Biophys Res Commun. 1977 Apr 11;75(3):556–562. doi: 10.1016/0006-291x(77)91508-x. [DOI] [PubMed] [Google Scholar]
- Ruppersberg J. P., Stocker M., Pongs O., Heinemann S. H., Frank R., Koenen M. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature. 1991 Aug 22;352(6337):711–714. doi: 10.1038/352711a0. [DOI] [PubMed] [Google Scholar]
- Sakata A., Ida E., Tominaga M., Onoue K. Arachidonic acid acts as an intracellular activator of NADPH-oxidase in Fc gamma receptor-mediated superoxide generation in macrophages. J Immunol. 1987 Jun 15;138(12):4353–4359. [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellmayer A., Uedelhoven W. M., Weber P. C., Bonventre J. V. Endogenous non-cyclooxygenase metabolites of arachidonic acid modulate growth and mRNA levels of immediate-early response genes in rat mesangial cells. J Biol Chem. 1991 Feb 25;266(6):3800–3807. [PubMed] [Google Scholar]
- Setty B. N., Graeber J. E., Stuart M. J. The mitogenic effect of 15- and 12-hydroxyeicosatetraenoic acid on endothelial cells may be mediated via diacylglycerol kinase inhibition. J Biol Chem. 1987 Dec 25;262(36):17613–17622. [PubMed] [Google Scholar]
- Shearman M. S., Shinomura T., Oda T., Nishizuka Y. Protein kinase C subspecies in adult rat hippocampal synaptosomes. Activation by diacylglycerol and arachidonic acid. FEBS Lett. 1991 Feb 25;279(2):261–264. doi: 10.1016/0014-5793(91)80163-w. [DOI] [PubMed] [Google Scholar]
- Shibanuma M., Kuroki T., Nose K. Stimulation by hydrogen peroxide of DNA synthesis, competence family gene expression and phosphorylation of a specific protein in quiescent Balb/3T3 cells. Oncogene. 1990 Jul;5(7):1025–1032. [PubMed] [Google Scholar]
- Shibanuma M., Kuroki T., Nose K. Superoxide as a signal for increase in intracellular pH. J Cell Physiol. 1988 Aug;136(2):379–383. doi: 10.1002/jcp.1041360224. [DOI] [PubMed] [Google Scholar]
- Slivka S. R., Insel P. A. Alpha 1-adrenergic receptor-mediated phosphoinositide hydrolysis and prostaglandin E2 formation in Madin-Darby canine kidney cells. Possible parallel activation of phospholipase C and phospholipase A2. J Biol Chem. 1987 Mar 25;262(9):4200–4207. [PubMed] [Google Scholar]
- Staal F. J., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun I. L., Crane F. L. Bleomycin control of transplasma membrane redox activity and proton movement in HeLa cells. Biochem Pharmacol. 1985 Mar 1;34(5):617–622. doi: 10.1016/0006-2952(85)90254-0. [DOI] [PubMed] [Google Scholar]
- Sun I. L., Crane F. L. Transplasmalemma NADH dehydrogenase is inhibited by actinomycin D. Biochem Biophys Res Commun. 1981 Jul 16;101(1):68–75. doi: 10.1016/s0006-291x(81)80011-3. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Berk B. C., Izumo S., Tsuda T., Alexander R. W., Nadal-Ginard B. Angiotensin II induces c-fos mRNA in aortic smooth muscle. Role of Ca2+ mobilization and protein kinase C activation. J Biol Chem. 1989 Jan 5;264(1):526–530. [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledano M. B., Leonard W. J. Modulation of transcription factor NF-kappa B binding activity by oxidation-reduction in vitro. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4328–4332. doi: 10.1073/pnas.88.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travo P., Barrett G., Burnstock G. Differences in proliferation of primary cultures of vascular smooth muscle cells taken from male and female rats. Blood Vessels. 1980;17(2):110–116. doi: 10.1159/000158240. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S., Nathan C. F. Release of arachidonate and reduction of oxygen. Independent metabolic bursts of the mouse peritoneal macrophage. J Biol Chem. 1986 Sep 5;261(25):11563–11570. [PubMed] [Google Scholar]