Abstract
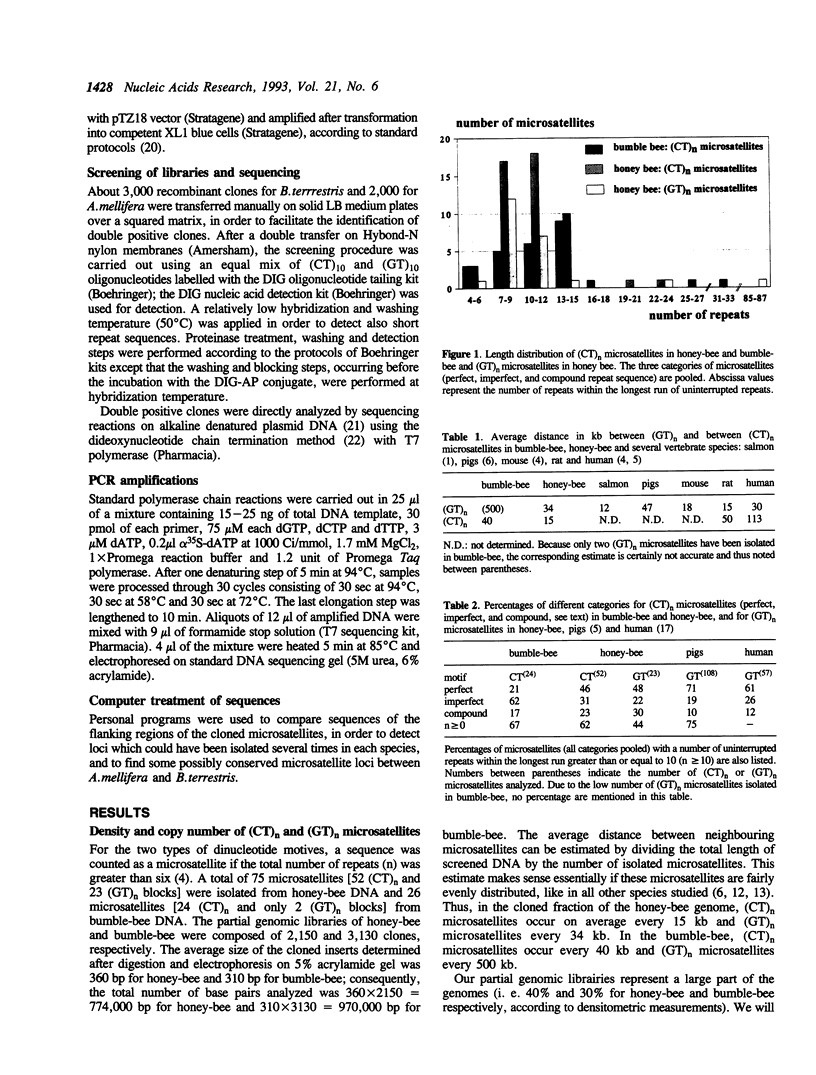
A set of 52 (CT)n and 23 (GT)n microsatellites in honeybee, 24 (CT)n and 2 (GT)n microsatellites in bumble-bee (n > 6) have been isolated from partial genomic libraries and sequenced. On average, (CT)n and (GT)n microsatellites occur every 15 kb and 34 kb in honeybee and every 40 kb and 500 kb in bumble-bee, respectively. The prevailing categories are imperfect repeats for (CT)n microsatellites in bumble-bee, and perfect repeats for both (CT)n and (GT)n microsatellites in honey-bee. Comparisons with data available in vertebrates indicate a lower proportion of perfect repeats in bees but length distributions are very similar regardless the phylum. This result extends to insects the concept of an evolutionary conservation for quantitative and qualitative characteristics of (CT)n and (GT)n microsatellites. Many (CT)n and (GT)n repeats are surrounded with various types of microsatellites, revealing an associative distribution of short repeat sequences. As expected, a high level of intrapopulational polymorphism has been found with one tested honeybee microsatellite. Also, flanking regions of this microsatellite are similar enough to allow PCR amplification in several other species of Apis and Bombus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Weber J. L. Survey of human and rat microsatellites. Genomics. 1992 Apr;12(4):627–631. doi: 10.1016/0888-7543(92)90285-z. [DOI] [PubMed] [Google Scholar]
- Braaten D. C., Thomas J. R., Little R. D., Dickson K. R., Goldberg I., Schlessinger D., Ciccodicola A., D'Urso M. Locations and contexts of sequences that hybridize to poly(dG-dT).(dC-dA) in mammalian ribosomal DNAs and two X-linked genes. Nucleic Acids Res. 1988 Feb 11;16(3):865–881. doi: 10.1093/nar/16.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A., Civitello A., Hammond H. A., Caskey C. T. DNA typing and genetic mapping with trimeric and tetrameric tandem repeats. Am J Hum Genet. 1991 Oct;49(4):746–756. [PMC free article] [PubMed] [Google Scholar]
- Fries R., Eggen A., Stranzinger G. The bovine genome contains polymorphic microsatellites. Genomics. 1990 Oct;8(2):403–406. doi: 10.1016/0888-7543(90)90301-a. [DOI] [PubMed] [Google Scholar]
- Garnery L., Cornuet J. M., Solignac M. Evolutionary history of the honey bee Apis mellifera inferred from mitochondrial DNA analysis. Mol Ecol. 1992 Oct;1(3):145–154. doi: 10.1111/j.1365-294x.1992.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Gross D. S., Garrard W. T. The ubiquitous potential Z-forming sequence of eucaryotes, (dT-dG)n . (dC-dA)n, is not detectable in the genomes of eubacteria, archaebacteria, or mitochondria. Mol Cell Biol. 1986 Aug;6(8):3010–3013. doi: 10.1128/mcb.6.8.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan J., Dubay C., Pankowiak M. P., Becuwe N., Weissenbach J. A genetic linkage map of human chromosome 20 composed entirely of microsatellite markers. Genomics. 1992 Feb;12(2):183–189. doi: 10.1016/0888-7543(92)90364-x. [DOI] [PubMed] [Google Scholar]
- Jordan R. A., Brosemer R. W. Characterization of DNA from three bee species. J Insect Physiol. 1974 Dec;20(12):2513–2520. doi: 10.1016/0022-1910(74)90035-3. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Lowenhaupt K., Rich A., Nordheim A. (dC-dA)n.(dG-dT)n sequences have evolutionarily conserved chromosomal locations in Drosophila with implications for roles in chromosome structure and function. EMBO J. 1987 Jun;6(6):1781–1789. doi: 10.1002/j.1460-2075.1987.tb02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlötterer C., Amos B., Tautz D. Conservation of polymorphic simple sequence loci in cetacean species. Nature. 1991 Nov 7;354(6348):63–65. doi: 10.1038/354063a0. [DOI] [PubMed] [Google Scholar]
- Serikawa T., Kuramoto T., Hilbert P., Mori M., Yamada J., Dubay C. J., Lindpainter K., Ganten D., Guénet J. L., Lathrop G. M. Rat gene mapping using PCR-analyzed microsatellites. Genetics. 1992 Jul;131(3):701–721. doi: 10.1093/genetics/131.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirugo G., Keats B., Fujita R., Duclos F., Purohit K., Koenig M., Mandel J. L. Friedreich ataxia in Louisiana Acadians: demonstration of a founder effect by analysis of microsatellite-generated extended haplotypes. Am J Hum Genet. 1992 Mar;50(3):559–566. [PMC free article] [PubMed] [Google Scholar]
- Stallings R. L., Ford A. F., Nelson D., Torney D. C., Hildebrand C. E., Moyzis R. K. Evolution and distribution of (GT)n repetitive sequences in mammalian genomes. Genomics. 1991 Jul;10(3):807–815. doi: 10.1016/0888-7543(91)90467-s. [DOI] [PubMed] [Google Scholar]
- Stephen D., Jones C., Schofield J. P. A rapid method for isolating high quality plasmid DNA suitable for DNA sequencing. Nucleic Acids Res. 1990 Dec 25;18(24):7463–7464. doi: 10.1093/nar/18.24.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D. Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res. 1989 Aug 25;17(16):6463–6471. doi: 10.1093/nar/17.16.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D., Renz M. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984 May 25;12(10):4127–4138. doi: 10.1093/nar/12.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. L. Informativeness of human (dC-dA)n.(dG-dT)n polymorphisms. Genomics. 1990 Aug;7(4):524–530. doi: 10.1016/0888-7543(90)90195-z. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Winterø A. K., Fredholm M., Thomsen P. D. Variable (dG-dT)n.(dC-dA)n sequences in the porcine genome. Genomics. 1992 Feb;12(2):281–288. doi: 10.1016/0888-7543(92)90375-3. [DOI] [PubMed] [Google Scholar]
- Wong A. K., Yee H. A., van de Sande J. H., Rattner J. B. Distribution of CT-rich tracts is conserved in vertebrate chromosomes. Chromosoma. 1990 Sep;99(5):344–351. doi: 10.1007/BF01731722. [DOI] [PubMed] [Google Scholar]



