Abstract
Background
This study compared the best available treatment for bulimia nervosa, cognitive–behavioural therapy (CBT) augmented by fluoxetine if indicated, with a stepped-care treatment approach in order to enhance treatment effectiveness.
Aims
To establish the relative effectiveness of these two approaches.
Method
This was a randomised trial conducted at four clinical centres (Clinicaltrials.gov registration number: NCT00733525). A total of 293 participants with bulimia nervosa were randomised to one of two treatment conditions: manual-based CBT delivered in an individual therapy format involving 20 sessions over 18 weeks and participants who were predicted to be non-responders after 6 sessions of CBT had fluoxetine added to treatment; or a stepped-care approach that began with supervised self-help, with the addition of fluoxetine in participants who were predicted to be non-responders after six sessions, followed by CBT for those who failed to achieve abstinence with self-help and medication management.
Results
Both in the intent-to-treat and completer samples, there were no differences between the two treatment conditions in inducing recovery (no binge eating or purging behaviours for 28 days) or remission (no longer meeting DSM–IV criteria). At the end of 1-year follow-up, the stepped-care condition was significantly superior to CBT.
Conclusions
Therapist-assisted self-help was an effective first-level treatment in the stepped-care sequence, and the full sequence was more effective than CBT suggesting that treatment is enhanced with a more individualised approach.
Since the original description of bulimia nervosa by Russell in 19791 a substantial treatment literature has been published on this condition. Both antidepressants and certain structured manual-based psychotherapies have been shown to be effective. In the antidepressant literature much of the research has focused on the use of fluoxetine, which appears most effective at 60 mg/day and in the USA remains the only Food and Drug Administration approved drug for this condition.2 Much of the psychotherapy literature has focused on the use of cognitive–behavioural therapy (CBT), which is widely viewed as the treatment of choice for bulimia nervosa.3 The question as to whether or not a combination of antidepressants and CBT should be used remains open.4–6 In further research, a number of guided self-help studies based on CBT have been published for people with bulimia nervosa in several small-scale trials. This literature has been recently reviewed suggesting that such abbreviated treatments are promising by themselves and may form a useful first step in a stepped-care approach.7–9
Brief therapies provided by less skilled therapists may be useful in that a point of concern that has surfaced in the bulimia nervosa treatment literature is that the majority of patients who are seen by practitioners in the community do not receive manual-based interventions.10 Also, most therapists do not use CBT as their primary treatment technique.11,12 Therefore, efficacious manual-based approaches for bulimia nervosa are not being widely utilised or disseminated. In the current study (Clinicaltrials.gov registration number: NCT00733525) we attempted to synthesise these various observations to undertake a randomised trial comparing CBT, augmented by the use of fluoxetine if indicated, with a stepped-care approach using an adaptive treatment strategy.13 An adaptive treatment strategy is a rule for adapting a treatment plan based on the changing clinical condition of a patient due to response to a previous treatment or treatments. Hence, the stepped-care sequence began with treatment using manualised guided self-help CBT, with the addition of fluoxetine if the response based on an algorithm was not sufficient, and then with a full course of CBT for those who failed to achieve abstinence from binge eating and purging. The algorithm was based on data from an earlier study in which we developed a formula to attempt to predict a high likelihood of non-response to CBT.14 This is discussed in the Method in detail. This treatment sequence was designed to provide treatment wherein the first two steps would not require specialist care, with only those who were still symptomatic after these steps receiving specialist care (CBT). The use of shorter initial treatment was based on the findings of a previous multisite trial in which a full course of CBT was followed by interpersonal therapy.15 The combination and sequencing of two lengthy therapies led to a high drop-out rate with little evidence of additional benefit. Our primary hypothesis was that the stepped-care sequence would be more effective than the CBT/fluoxetine one as it allowed for more individualised treatment. This additional treatment could be added based on the needs of the individual participants. Therefore, participants who could benefit from more modest, less intensive intervention could be treated without exposure to a full course of CBT. We also hypothesised that it would be more cost-effective (the cost-efficacy analysis will be published as a separate paper).
A previous randomised trial of the treatment of bulimia nervosa with CBT13 found that a high-risk stratum with low scores on the Social Adjustment Scale16 and high scores on the Shape Concerns Scale from the Eating Disorder Examination17 moderated outcome such that those in the high-risk stratum did better if they received CBT. Hence in the present study we hypothesised that participants with poorer social adjustment and higher shape concerns (high-risk stratum) would show greater improvement in the CBT/fluoxetine sequence.
Method
Participants
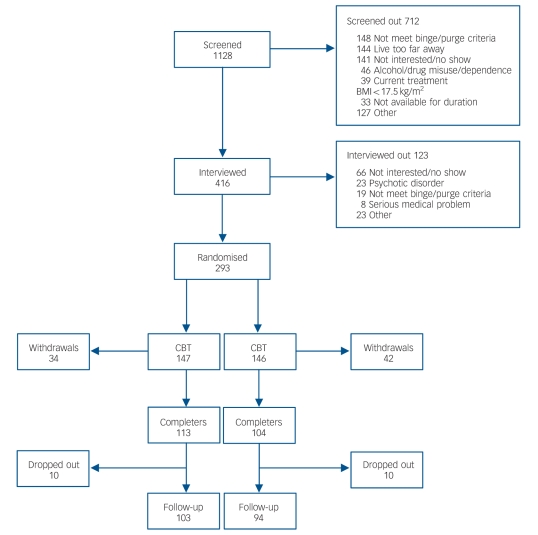
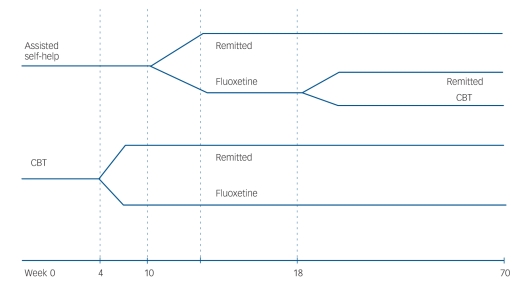
The participants for this study met criteria for either purging or non-purging bulimia nervosa as defined by DSM–IV.18 Both males and females were included. Participants were 18 or older. Potential participants were excluded for the following reasons: current active psychotherapy for their eating disorder; alcohol or drug misuse or dependence in the previous 6 months; acute suicidal risk; a medical illness that would preclude safe study participation; and a history of psychotic disorder. Participants were recruited by referral from clinicians at the clinical sites involved and through mailings to clinicians in the areas in which the study was conducted, as well as by advertisements in the media. A total of 1128 potential participants were screened by telephone (Fig. 1). Of these, 416 were assessed at the four clinical sites, and 293 were randomised into the study, 147 to the CBT group and 146 to the stepped-care group. The overall design is shown in Fig. 2. Written informed consent was obtained from all participants after the procedures had been fully explained.
Fig. 1.
Screening, interviews, enrolment and withdrawals.
BMI, body mass index; CBT, cognitive–behavioural therapy.
Fig. 2.
Study design.
CBT, cognitive–behavioural therapy.
Cognitive–behavioural therapy
The manualised CBT used in this trial in both treatment arms was also used in two recent large multicentre trials of bulimia nervosa.14,19 This treatment was first described in 1981 and subsequently studied in numerous randomised protocols.20,21 In the present study participants received 20 sessions of 50 min with 4 sessions in the first 2 weeks of treatment for a total treatment duration of 18 weeks.
Assisted self-help therapy
The self-help book Overcoming Binge Eating was developed by Fairburn22 to parallel the CBT manual. Participants were seen for approximately 20 min on eight occasions with weekly sessions for 4 weeks, diminishing to bi-weekly and then to monthly, for a treatment duration of 18 weeks. The focus of treatment was on the participant using the book as the main source of information regarding behaviour change. Participants were told that if they were unable to gain sufficient control over their bulimic behaviour with the use of the self-help manual they would be offered medication and, if abstinence was still not achieved, full CBT would also be offered.
Medication management
Fluoxetine was offered to supervised self-help participants and to those CBT participants who were predicted to be non-responders using the algorithm described below. The drug was initiated at a dose of 20 mg. The patients were seen for medication management for 20 min visits at 2-week intervals for five visits and then monthly. During the medication management visits, which were also manual driven, the time was devoted to a discussion of medication side-effects, toxicity and response. After 2 weeks on medication if the participant remained symptomatic, the dosage was increased to 40 mg, and after 2 additional weeks, if the participant continued to have bulimic symptoms, the dosage was increased to 60 mg.
Rationale for early triage of non-responders to medication
In a prior study conducted by this group, we determined whether it was possible to detect clinically useful outcome predictors using signal detection analysis.14 The best predictor of successful outcome was a 70% or more reduction in frequency of purging by the end of session six. We believed that this was a clinically useful finding and incorporated it into the present protocol. Those in the stepped-care group and those in the CBT/fluoxetine group were offered fluoxetine after session six (week 10 of treatment for the stepped-care group and week 4 for the other group). It should be noted that participants could refuse the medication offer and still continue in the study given the effectiveness design.
Stepped-care sequence
Stepped-care began with therapist-assisted self-help followed by fluoxetine if the participant was predicted to be a non-responder. At the end of self-help treatment (18 weeks) participants who had not achieved abstinence were offered full CBT for a further 6 months. Medication, if utilised, was continued until the 1-year follow-up assessment.
Therapy training and supervision
Therapists for self-help were Masters-level or PhD-level clinical psychologists or clinical psychiatric nurse specialists who did not specialise in eating disorders and had not been formally trained in CBT beyond a 1-day training session. Therapists for CBT were PhD-level clinical psychologists with experience in treating people with bulimia nervosa with CBT. The number of therapists varied from two to four across the sites.
The overall treatment supervisor was S.A. He, along with C.G.F., oversaw training in both the CBT and the self-help condition. Training in medication management was overseen by J.E.M. Each therapist treated two individuals with bulimia nervosa with weekly supervision before being certified. All sessions were audiotaped. After the initiation of the study the CBT and self-help therapists met separately with their site supervisors each week. Audiotapes were used in supervision.
Assessors who had been trained in the administration of the Structured Clinical Interview for DSM–IV I and II (SCID–I, SCID–II)23,24 and the Eating Disorder Examination (EDE)17 were available at all treatment sites. Assessors met at the start up meeting and reviewed the EDE protocol in detail. They each rated two EDE tapes, discussing differences in interpretation. At 3-month intervals the data centre sent a tape selected from one of the sites to the other sites for each assessor to rate. These ratings were examined for differences and feedback returned to each rater. The overall interrater agreement ranged from 0.91 to 0.99. Assessors were masked to treatment assignment. However, the mask was not systematically examined, and unmasking may have occurred unintentionally during the interviews due to participants’ reports.
The following instruments were used.
-
Eating disorder symptoms:
- Eating Disorder Examination (EDE):17 the EDE was used as the primary measure of treatment outcome. Because the diagnostic version generates DSM–IV diagnoses of bulimia nervosa, it was also used to determine participant eligibility for the study. The EDE contains four subscales (dietary restraint, eating concern, shape concern and weight concern) associated with core psychopathology of eating disorders;
- weight: height and weight were measured at baseline by the assessor. Weight was measured by the assessor at post-treatment and follow-up evaluations;
- Yale–Brown–Cornell Eating Disorders Scale (YBC–EDS):25 this is an eight-item scale assessing the severity of preoccupations and rituals common in people with eating disorders.
-
Comorbid psychopathology and personality:
- SCID–I/P: this well-studied and frequently used semi-structured interview was used to assess comorbid Axis I disorders;
- SCID–II: this semi-structured interview was used to assess Axis II personality disorders;
-
Social and interpersonal functioning:
- Social Adjustment Scale (SAS):16 the self-report version assesses social functioning in several areas including work, family relationships, social and leisure time and economic functioning.
Statistical analysis
In a previous study14 post hoc analyses revealed strata in which the outcomes of treatment differed. Hence randomisation for the present study included two strata, the first denoted here as the high stratum: SAS <2.2 (participants having a 53% chance of abstinence with CBT) combined with shape concerns <4.6 (participants having a 48% chance of success) and a second stratum denoted here as the low stratum: with SAS scores >2.2 and shape concerns >4.6 (with a 13% chance of success with CBT). Staff at the data coordinating centre performed the randomisation separately for each site and stratum using Efron’s biased coin design.
Relative to power, with a sample size of n = 259, using a 5% two-tailed test seeking 80% power, we concluded that we could detect an effect size slightly over 0.3 (standardised mean difference between treatment groups). The primary analysis was by intention-to-treat using an ANOVA with the site × treatment to test the primary hypothesis that relates to the main effect of treatment. Main effects of site and risk stratum were expected and were removed to get a valid evaluation of the main effect of treatment. Interactive effects were also included in the model because interactive effects that are present but ignored can bias the estimation of both the main effects and of the variance and thus produce biased tests for the main effect of interest. Interactive effects of treatment with either site or risk stratum or their interaction would indicate that the size of the treatment effect is heterogeneous over site or risk stratum. If the heterogeneity is over risk strata, this might indicate a moderating effect on treatment of the variables used to stratify. It may be that one treatment strategy is more effective in one stratum than the other, and perhaps even that one treatment strategy is more effective in one stratum, but the other is more effective in the other stratum. When site × treatment interactions were found, special effort using exploratory data analysis techniques were used to try to identify the site characteristics that might account for such differential treatment effects.
The primary outcome variables were frequency of objective binge eating episodes and frequency of compensatory behaviours, defined as the total of episodes of vomiting, laxative use, diuretic use, fasting and driven exercise as measured on the EDE. Based on results of previous studies, square root transformations of these measures were used to stabilise the variance and thus to permit use of parametric test and estimation procedures. The frequency of objective binge eating episodes and compensatory behaviours were used in two ways to measure the effects of treatment. The first outcomes measure is abstinence defined as no binge eating episodes and no purging episodes of any kind in the past 28 days. The second outcome measure is a modified application of DSM criteria used to measure remittance. We defined remission to be no longer meeting DSM–IV criteria for binge eating and purging, leading to a binary outcome of success or failure. These were examined at the end of treatment and at the 1-year follow-up, to examine both acute treatment effects and maintenance of treatment effects.
When post-test data were missing, the baseline value was carried forward (BOCF). When missing 1-year follow-up data occurred, the 6-month data were used (last observation carried forward, LOCF). If both follow-up assessments were missing, then the conservative method of BOCF was used.
Results
Baseline characteristics and drop out
Data on baseline characteristics including means and standard deviations (or median and interquartile range for objective binge eating and compensatory behaviours) by site and treatment are shown in Table 1. Thirteen participants (4%) had non-purging bulimia nervosa; the remainder met criteria for the purging type. A history of treatment for anorexia nervosa was more common in those assigned to the stepped-care arm (F(1,276) = 6.3, P = 0.012); otherwise there were no significant differences between groups. About a quarter of these individuals met criteria for current major depression, and 27% had a history of anorexia nervosa.
Table 1.
Baseline characteristics by site and treatment group
| Cornell (n = 81)
|
Minnesota (n = 79)
|
North Dakota (n = 60)
|
Stanford (n = 83)
|
Total (n = 293)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CBT (n = 40) | Stepped-care (n = 41) | CBT (n = 35) | Stepped-care (n = 34) | CBT (n = 30) | Stepped-care (n = 30) | CBT (n = 42) | Stepped-care (n = 41) | CBT (n = 147) | Stepped-care (n = 146) | |
| Age, years: mean (s.d.)
|
30.0 (6.4)
|
30.6 (9.5)
|
28.2 (8.0)
|
25.9 (7.4)
|
28.5 (9.9)
|
25.8 (8.9)
|
30.9 (8.0)
|
35.1 (10.2)
|
29.5 (8.0)
|
29.8 (9.8)
|
| BMI, kg/m2: mean (s.d.)
|
22.2 (3.5)
|
22.2 (4.1)
|
22.6 (3.5)
|
23.4 (5.2)
|
25.4 (6.2)
|
23.9 (5.8)
|
23.4 (4.2)
|
24.7 (6.1)
|
23.4 (4.5)
|
23.5 (5.3)
|
| Current depression, %
|
13
|
22
|
23
|
21
|
40
|
23
|
24
|
24
|
24
|
23
|
| Lifetime depression, %
|
55
|
51
|
63
|
65
|
70
|
57
|
62
|
54
|
62
|
56
|
| History of anorexia nervosa,a %
|
38
|
28
|
14
|
38
|
27
|
37
|
12
|
27
|
22
|
32
|
| Personality disorder, %
|
30
|
44
|
26
|
26
|
47
|
37
|
33
|
37
|
33
|
36
|
| College degree, %
|
70
|
68
|
43
|
47
|
17
|
27
|
67
|
73
|
52
|
56
|
| Minority,b %
|
20
|
15
|
6
|
3
|
7
|
7
|
26
|
20
|
16
|
12
|
| Global EDE, mean (s.d.)
|
3.2 (1.1)
|
3.1 (1.2)
|
3.1 (1.1)
|
3.2 (1.2)
|
3.3 (1.3)
|
3.3 (1.1)
|
3.0 (1.0)
|
3.2 (1.4)
|
3.1 (1.1)
|
3.2 (1.2)
|
| EDE objective binges,c median (IQR)
|
27 (27)
|
27 (32)
|
20 (25)
|
30 (25)
|
30 (31)
|
23 (20)
|
26 (22)
|
27 (24)
|
27 (25)
|
27 (24)
|
| EDE compensatory behaviours,c median (IQR)
|
47 (42)
|
50 (42)
|
42 (29)
|
42 (29)
|
48 (47)
|
34 (42)
|
44 (40)
|
43 (37)
|
44 (37)
|
43 (42)
|
| YBC–EDS total score, mean (s.d.)
|
19.6 (4.4)
|
20.5 (0.54)
|
18.0 (6.2)
|
19.3 (3.8)
|
17.9 (6.6)
|
19.0 (6.3)
|
17.7 (5.4)
|
16.9 (7.8)
|
18.3 (5.6)
|
18.9 (5.9)
|
| Social Adjustment Scale, mean (s.d.)
|
2.26 (0.54)
|
2.27 (0.54)
|
2.14 (0.40)
|
2.11 (0.37)
|
2.24 (0.61)
|
2.04 (0.43)
|
2.21 (0.39)
|
2.21 (0.42)
|
2.21 (0.48)
|
2.17 (0.45)
|
| Previous psychological treatment, %
|
83
|
78
|
74
|
85
|
90
|
67
|
71
|
80
|
79
|
78
|
| Previous psychiatric hospitalisation, % | 35 | 22 | 23 | 24 | 30 | 20 | 12 | 23 | 24 | 22 |
CBT, cognitive–behavioural therapy; BMI, body mass index; EDE, Eating Disorder Examination; IQR, interquartile range; YBC–EDS, Yale–Brown–Cornell Eating Disorders Scale.
a. Statistica l l y significant differences (P<0.05) between treatments.
b. Black, Hispanic, Native American.
c. Square root used in analysis.
Overall, 19% of participants did not complete 18 weeks of treatment in the CBT group compared with 25% in the stepped-care group. This difference was not statistically significant. The site drop-out rates for CBT and stepped care respectively were: Cornell, 20% and 22%; Minnesota, 14% and 27%; North Dakota, 33% and 30%; Stanford, 14% and 22%. There were no significant differences across sites.
For comparative purposes, the data on the participants who completed treatment versus those who did not are shown in Table 2. Participants with previous psychiatric hospitalisations were more likely to drop out at Cornell and Minnesota and less likely to at North Dakota and Stanford (F(1,262) = 3.5, P = 0.02). Those individuals in the CBT group with major depression were more likely to drop out at Cornell, North Dakota and Stanford, and less likely to at Minnesota. Those individuals in the stepped-care group with a history of depression were less likely to drop out at Cornell, and more likely to at Minnesota. There was no relationship between depression and drop out at North Dakota or Stanford (F(3,263) = 2.9, P = 0.04). The CBT participants with lower global EDE scores were more likely to drop out at Cornell and North Dakota; at Minnesota and Stanford, higher global EDE was associated with dropping out. Participants in the stepped-care group with lower global EDE assigned were more likely to drop out at Cornell, North Dakota and Stanford, whereas higher EDE scores were associated with drop-out status at Minnesota (F(3,263) = 3.2, P = 0.02). Finally, those in the CBT group with high purging levels were more likely to drop out at Cornell, those with lower levels of purging had higher drop-out rates at Stanford. Purging level was not related to drop-out status in the CBT group at Minnesota or North Dakota. Participants with lower purge rates who received stepped-care were more likely to drop out in North Dakota; a higher rate of purging was associated with drop-out status in the stepped-care group at Cornell, Minnesota and Stanford (F(3,262) = 3.7, P = 0.01).
Table 2.
Participants who completed v. non-completers by site
| Cornell
|
Minnesota
|
North Dakota
|
Stanford
|
Total
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Completed (n = 64) | Dropped out (n = 17) | Completed (n = 55) | Dropped out (n = 14) | Completed (n = 41) | Dropped out (n = 19) | Completed (n = 68) | Dropped out (n = 15) | Completed (n = 228) | Dropped out (n = 65) | |
| Age, years: mean (s.d.)
|
31.4 (8.4)
|
26.2 (4.6)
|
27.3 (8.0)
|
26.1 (7.8)
|
28.3 (9.6)
|
24.7 (8.7)
|
33.6 (9.4)
|
30.3 (8.9)
|
30.5 (9.1)
|
26.7 (7.6)
|
| BMI, kg/m2: mean (s.d.)
|
22.1 (3.9)
|
21.2 (3.0)
|
23.4 (4.6)
|
21.2 (3.0)
|
24.6 (6.4)
|
24.7 (5.2)
|
23.6 (5.0)
|
25.4 (6.0)
|
23.3 (4.9)
|
23.5 (4.8)
|
| Current depression, %
|
17
|
18
|
18
|
36
|
29
|
37
|
22
|
33
|
21
|
31
|
| Lifetime depression,a %
|
53
|
53
|
62
|
71
|
61
|
68
|
57
|
60
|
58
|
63
|
| History of anorexia nervosa,%
|
32
|
35
|
22
|
43
|
37
|
21
|
19
|
20
|
26
|
29
|
| Personality disorder, %
|
28
|
71
|
25
|
29
|
37
|
53
|
37
|
27
|
32
|
46
|
| College degree, %
|
75
|
47
|
49
|
29
|
24
|
16
|
72
|
60
|
59
|
37
|
| Minority,b %
|
19
|
12
|
5
|
0
|
5
|
11
|
24
|
20
|
14
|
11
|
| Global EDE,a mean (s.d.)
|
3.0 (1.2)
|
3.7 (1.0)
|
3.1 (1.1)
|
3.1 (1.4)
|
3.1 (1.2)
|
3.7 (1.2)
|
3.0 (1.2)
|
3.5 (1.2)
|
3.1 (1.2)
|
3.5 (1.2)
|
| EDE objective binges,c median (IQR)
|
26 (28)
|
32 (38)
|
21 (21)
|
43 (45)
|
23 (22)
|
32 (22)
|
28 (22)
|
25 (25)
|
25 (22)
|
32 (28)
|
| EDE compensatory behaviours,a,c median (IQR)
|
43 (41)
|
62 (42)
|
42 (32)
|
54 (79)
|
42 (44)
|
35 (65)
|
44 (35)
|
36 (93)
|
43 (36)
|
54 (63)
|
| YBC–EDS total score, mean (s.d.)
|
19.4 (4.3)
|
22.6 (3.5)
|
18.3 (5.4)
|
19.8 (4.0)
|
18.1 (6.6)
|
19.2 (6.2)
|
16.9 (6.9)
|
18.9 (5.4)
|
18.2 (5.9)
|
20.1 (5.1)
|
| Social Adjustment Scale, mean (s.d.)
|
2.2 (0.5)
|
2.6 (0.6)
|
2.1 (0.4)
|
2.2 (0.4)
|
2.2 (0.5)
|
2.1 (0.5)
|
2.2 (0.4)
|
2.1 (0.5)
|
2.2 (0.4)
|
2.3 (0.5)
|
| Previous psychological treatment,a %
|
83
|
71
|
75
|
100
|
80
|
74
|
75
|
80
|
78
|
80
|
| Previous psychiatric hospitalisation,d % | 27 | 35 | 16 | 50 | 34 | 5 | 18 | 13 | 23 | 25 |
BMI, body mass index; EDE, Eating Disorder Examination; IQR, interquartile range; YBC–EDS, Yale–Brown–Cornell Eating Disorders Scale.
a. Statistically significant difference (P<0.05) between individuals who dropped out and completers within treatment at the sites.
b. Black, Hispanic, Native American.
c. Square root used in analysis.
d. Statistically significant difference (P<0.05) between individuals who dropped out and completers at the sites.
Treatment outcome
The primary outcomes were abstinence and remission in the two treatment arms at the end of treatment and at the 1-year follow-up. In total 65% (n = 95) of participants randomised to the CBT group were treated with fluoxetine compared with 34% (n = 50) in the stepped-care group, and 40% in the stepped-care group were treated with full CBT. A total of 26% (n = 38) received all three treatments. The acceptance rate for medication was high: 83% in the CBT group and 75% in the stepped-care group. The acceptance rate for CBT in the stepped-care group was 67%. Data from the intent-to-treat analysis means and standard deviations (median and interquartile range for EDE objective binges and EDE compensatory behaviours) by site and treatment are shown in online Table DS1. In the CBT group, 122 (82.3%) participants were assessed at end-of-treatment and 115 (78.8%) in the stepped-care group. Of those randomised to the CBT group, 117 (79.6%) were available for at least one of the follow-up assessments and 116 (79.4%) of those randomised to stepped-care were available for at least one follow-up. The abstinence rates were low; 15% in the CBT group at end-of-treatment and 18% at the 1-year follow-up, compared with 11% and 26% respectively in the stepped-care group. These percentages were not significantly different. Remission rates were 57% at end-of-treatment and 44% at follow-up in the CBT group, and 52% and 32% in the stepped-care group. Examining remission there were no significant treatment differences or site × treatment interactions. There was, however, a significant difference favouring the stepped-care arm for objective binge eating episodes (F(1,276) = 4.87, P = 0.028) and compensatory behaviours (F(1,276) = 4.9, P = 0.027) at 1-year follow-up. Results on the completer analysis on these variables are not different and are not shown. For the secondary measures, the stepped-care arm was more successful in reducing BDI (F(1,275) = 4.9, P = 0.027) and YBC–EDS rituals (F(1,276) = 4.0, P = 0.047) at follow-up.
Risk-strata effects
There were significant risk strata × treatment interactions for abstinence at post-test (F(1,277) = 10.7, P = 0.001). At post-treatment, low-stratum participants (predicted to have a better outcome) in the CBT group arm were more likely to achieve abstinence than those in the stepped-care group (17% compared with 8%.) However, for the high-risk stratum (predicted to have a worse outcome) the pattern was reversed. Only 4% of those participants in the CBT group were abstinent compared with 25% of the stepped-care group. Similar patterns were found for compensatory behaviours (F(1,276) = 6.6, P = 0.010), EDE restraint (F(1,276) = 4.8, P = 0.028), global EDE (F(1,276) = 4.4, P = 0.037) and BDI (F(1,275) = 4.7, P = 0.031). At follow-up, stepped-care was more successful in reducing BDI levels in the high-risk stratum (F(1,275) = 7.0, P = 0.009).
Site effects
There was a site × treatment interaction for BDI at post-test (F(3,275) = 4.1, P = 0.007), indicating heterogeneity of treatment across the various sites. Participants at Cornell and Stanford had lower scores when in the CBT group, whereas participants at Minnesota and North Dakota had lower scores when in the stepped-care group.
Discussion
Main findings
At the end of treatment both in the intent-to-treat and completer samples no differences were found between them in inducing recovery (no binge eating or compensatory behaviours for 28 days) or remission (no longer meeting DSM–IV criteria) in this multicentre out-patient study of participants with bulimia nervosa. However, at the end of the 1-year follow-up the stepped-care arm was significantly superior to the CBT arm in terms of reducing binge eating and all compensatory behaviours (vomiting, laxative abuse, diuretic abuse and excessive exercise). Differences were found between treatments based on the hypothesised moderator derived from a previous study.14
Hence, there are three main findings from this trial. First, at the end of treatment a brief supervised self-help version of CBT delivered by less experienced therapists plus fluoxetine for those who needed it as judged by the algorithm was as effective as standard CBT plus fluoxetine, again based on the algorithm. This suggests that therapist-assisted self-help based on CBT is a possible alternative to immediate CBT if used in a stepped-care sequence. The second finding is that the entire stepped-care sequence (self-help, followed by fluoxetine for those who did not achieve abstinence, followed by full CBT for those not achieving abstinence) was more effective at follow-up than CBT followed by fluoxetine for those who were predicted to be non-responders. This suggests that a more individualised treatment with sequential changes to treatment based on response to treatments is both acceptable and more effective. It is of note that the first steps in the stepped-care sequence possibly could be carried out in non-specialist settings and at less cost than CBT. However, it remains unclear what qualifications are required for someone to be able to deliver self-help appropriately. For example, in a study by Walsh et al, nurses who received minimal training were not able to provide assisted self-help in a form that provided additional benefit to fluoxetine treatment in participants with bulimia nervosa.4 The third finding is that participants in the high-risk stratum showed superior improvement in the stepped-care arm of the study. This is a somewhat puzzling finding and contrary to our hypothesis. One possibility is that guided self-help is better adapted to participants with poorer social adjustment than CBT. For example, fewer and shorter therapy sessions may be easier for such individuals to manage. Moreover, the therapy is primarily delivered via a book, giving the individual more flexibility in carrying out the needed behaviour changes.
Strengths and limitations
In planning this study there was some trepidation about sequencing self-help with CBT in that we thought that participants might consider themselves already familiar with the concepts and be less willing to engage in full CBT. However, anecdotal reports from the therapists in this study suggested the opposite, as does the data.
In considering the results of this study, one must also consider the available research on self-help in bulimia nervosa. In some,29,30 but certainly not all prior work,7–9 self-help has performed as well as CBT, generally in small, short-term trials. This suggests that a pure self-help arm may have been of utility in the current design. One limitation to the current study is the fact that participants were not randomised to treatments but to treatment arms containing different sequences of treatment. Hence the study did not test the effectiveness of individual components within the sequences. However, such a study would have been larger and more expensive. Instead, this was designed as an effectiveness trial to better mimic what happens in clinical practice, wherein individuals can elect or decline additional therapies. Another point to be noted is that fluoxetine was titrated to 60 mg rather than being started at that dose. Titration is often used in clinical practice, although the drug can be safely started at 60 mg.31 Another limitation is that potential participants with certain comorbidities were excluded, resulting in the selection of a patient population with a higher likelihood of response. Finally, the participant sample was restricted to those with bulimia nervosa. Therefore, the results cannot be generalised to those with eating disorders not otherwise specified whose condition resemble that of those with bulimia nervosa.
Among the strengths were the large sample size, the use of four treatment sites, central training and ongoing supervision of assessors and therapists, the use of manual-based approaches to all the therapies involved including medication management, and careful oversight among the different sites in terms of initiating and monitoring the study, as well as central monitoring of assessments and treatment by the data and coordinating centre.
Some aspects of the results were surprising and raise interesting questions. The first is that the abstinence rates in the present study were lower than that of two other large studies in those with bulimia nervosa.11,12 However, it should be noted that at the end of treatment remission rates were similar across these studies. It seems unlikely that the quality of the therapy delivered in the current protocol was less than that in the other two studies since many of the therapists were the same.
Funding
The active drug was provided by Eli Lilly. Support was provided by National Institutes of Health (NIH) RO1 MH59100, RO1 MH59234, KO2 MH65919 and P30 DK30456. C.G.F. is supported by the Wellcome Trust, London (046386).
Supplementary Material
Declaration of interest
C.G.F. authored the self-help manual used in this trial.
References
- 1.Russell G. Bulimia nervosa: an ominous variant of anorexia nervosa. Psychol Med 1979; 9: 429–48. [DOI] [PubMed] [Google Scholar]
- 2.Hay P, Bacaltchuk J. Bulimia nervosa. Clin Evid 2003; 10: 1070–84. [PubMed] [Google Scholar]
- 3.Wilson GT, Shafran R. Eating disorders guidelines from NICE. Lancet 2005; 365: 79–81. [DOI] [PubMed] [Google Scholar]
- 4.Walsh BT, Wilson GT, Loeb KL, Devlin MJ, Pike KM, Roose SP, et al. Medication and psychotherapy in the treatment of bulimia nervosa. Am J Psychiatry 1997; 154: 523–31. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell JE, Pyle RL, Eckert ED, Hatsukami D, Pomeroy C, Zimmerman R. A comparison study of antidepressants and structured intensive group psychotherapy in the treatment of bulimia nervosa. Arch Gen Psychiatry 1990; 47: 149–57. [DOI] [PubMed] [Google Scholar]
- 6.Agras WS, Rossiter EM, Arnow B, Schneider JA, Telch CF, Raeburn SD, et al. Pharmacologic and cognitive–behavioural treatment for bulimia nervosa: a controlled comparison. Am J Psychiatry 1992; 149: 82–7. [DOI] [PubMed] [Google Scholar]
- 7.Sysko R, Walsh BT. A critical evaluation of the efficacy of self-help interventions for the treatment of bulimia nervosa and binge-eating disorder. Int J Eat Disord 2008; 41: 97–112. [DOI] [PubMed] [Google Scholar]
- 8.Stefano SC, Bacaltchuk J, Blay SL, Hay P. Self-help treatments for disorders of recurrent binge eating: a systematic review. Acta Psychiatr Scand 2006; 113: 452–9. [DOI] [PubMed] [Google Scholar]
- 9.Hay PP, Bacaltchuk J, Stefano S, Kashyap P. Psychological treatments for bulimia nervosa and binging. Cochrane Database Syst Rev 2009; 7: CD000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crow S, Mussell MP, Peterson C, Knopke A, Mitchell JE. Prior treatment received by patients with bulimia nervosa. Int J Eat Disord 1999; 25: 39–44. [DOI] [PubMed] [Google Scholar]
- 11.Mussell MP, Crosby RD, Crow SJ, Knopke AJ, Peterson CB, Wonderlich SA, et al. Utilization of empirically supported psychotherapy for individuals with eating disorders: a survey of psychologists. Int J Eat Disord 2000; 27: 230–7. [DOI] [PubMed] [Google Scholar]
- 12.von Ranson KM, Robinson KE. Who is providing what type of psychotherapy to eating disorder clients? A survey. Int J Eat Disord 2006, 39: 27–34. [DOI] [PubMed] [Google Scholar]
- 13.Lavori PW, Dawson R. Adaptive treatment strategies in chronic disease. Ann Rev Med 2007; 59: 443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agras WS, Crow SJ, Halmi KA, Mitchell JE, Wilson GT, Kraemer HC. Outcome predictors for the cognitive behavior treatment of bulimia nervosa: data from a multisite study. Am J Psychiatry 2000; 157: 1302–8. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell JE, Halmi K, Wilson GT, Agras WS, Kraemer H, Crow S. A randomized secondary treatment study of women with bulimia nervosa who fail to respond to CBT. Int J Eat Disord 2002; 32: 271–81. [DOI] [PubMed] [Google Scholar]
- 16.Weissman J, Bothwell S. Assessment of social adjustment by patient self-report. Arch Gen Psychiatry 1976; 33: 1111–5. [DOI] [PubMed] [Google Scholar]
- 17.Fairburn CG, Cooper Z. The Eating Disorders Examination (12th edition). In Binge Eating: Nature, Assessment, and Treatment (eds CG Fairburn, GT Wilson): 317–60. Guilford Press, 1993.
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th edn) (DSM–IV). APA, 1994.
- 19.Agras, WS, Walsh T, Fairburn CG, Wilson GT, Kraemer HC. A multicenter comparison of cognitive-behavioural therapy and interpersonal psychotherapy for bulimia nervosa. Arch Gen Psychiatry 2000; 57: 459–66. [DOI] [PubMed] [Google Scholar]
- 20.Fairburn CG, Kirk J, O’Connor M, Cooper PJ. A comparison of two psychological treatments for bulimia nervosa. Longer-term effects of interpersonal psychotherapy, behaviour therapy, and cognitive behaviour therapy. Behav Res Ther 1985; 24: 629–43. [DOI] [PubMed] [Google Scholar]
- 21.Fairburn CG, Jones R, Peveler RC, Hope RA, O’Connor M. Psychotherapy and bulimia nervosa. Longer-term effects of interpersonal psychotherapy, behaviour therapy, and cognitive behaviour therapy. Arch Gen Psychiatry 1993; 50: 419–28. [DOI] [PubMed] [Google Scholar]
- 22.Fairburn CG. Overcoming Binge Eating. Guilford Press, 1995.
- 23.First MB, Gibbon M, Williams JBW, Spitzer R. Structured Clinical Interview for DSM–IV Personality Disorders. Patient Edition (SCID–II. Version 2.0). Biometrics Research Department, New York State Psychiatric Institute, 1995.
- 24.First MB, Spitzer R, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV Axis I Disorders. Patient Edition (SCID-I/P. Version 2.0). Biometrics Research Department, New York State Psychiatric Institute, 1995.
- 25.Mazure CM, Halmi KA, Sunday SR, Romano SJ, Einhorn AM. The Yale–Brown–Cornell Eating Disorder Scale: development, use, reliability and validity. J Psychiatr Res 1994; 28: 425–45. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–71. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Steer RA, Garbin MG. Psychometric properties of the BDI: twenty-five years of evaluation. Clin Psychol Review 1988; 8: 77–100. [Google Scholar]
- 28.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29: 71–83. [DOI] [PubMed] [Google Scholar]
- 29.Bailer U, de Zwaan M, Leisch F, Strnad A, Lennkh-Wolfsberg C, El-Giamal N, et al. Guided self-help versus cognitive–behavioral group therapy in the treatment of bulimia nervosa. Int J Eat Disord 2004; 35: 522–37. [DOI] [PubMed] [Google Scholar]
- 30.Treasure J, Schmidt U, Troop N, Tiller J, Todd G, Keilen M, et al. First step in managing bulimia nervosa: controlled trial of therapeutic manual. BMJ 1994; 308: 686–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein DJ, Wilson MG, Thompson VL, Potvin JH, Rampey Jr AH. Long-term fluoxetine treatment of bulimia nervosa. Fluoxetine Bulimia Nervosa Research Group. Br J Psychiatry 1995; 166: 660–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.