Abstract
Hypoadiponectinemia and decreased adiponectin gene expression in white adipose tissue (WAT) have been well observed in obese subjects and animal models. However, the mechanism for obesity-associated hypoadiponectinemia is still largely unknown. To investigate the regulatory role of energy intake, dietary fat, and adiposity in adiponectin gene expression and blood adiponectin level, a series of feeding regimens was employed to manipulate energy intake and dietary fat in obese-prone C57BL/6, genetically obese ob/ob, obese-resistant A/J and peroxisome proliferator-activated receptor-α gene knockout (PPARα KO) mice. Adiponectin gene expression in WAT and circulating adiponectin levels were studied in these dietary intervention-treated mice. Our study showed that calorie restriction (CR) robustly increased adiponectin gene expression in epididymal fat and blood adiponectin levels in both low-fat (LF) and high-fat (HF) diet-fed C57BL/6 mice. Although HF pair-fed C57BL/6 mice received the same amount of calories as LF ad libitum-fed mice, HF diet clearly increased adiposity but showed no significant effects on adiponectin gene expression and blood adiponectin level. CR also significantly increased blood adiponectin levels in ob/ob and A/J mice. Neither CR nor HF feeding displayed any significant effect on blood adiponectin half-life in C57BL/6 mice. Interestingly, CR increased PPARα expression in epididymal fat of C57BL/6 mice. Low levels of blood adiponectin and adiponectin gene expression in WAT were observed in PPARα KO mice. PPARα agonist treatment increased adiponectin mRNA levels in 3T3-L1 adipocytes. Furthermore, CR failed to increase adiponectin gene expression and blood adiponectin levels in PPARα KO mice. Therefore, our study demonstrated that energy intake, not dietary fat, plays an important role in regulating adiponectin gene expression and blood adiponectin level. PPARα mediates CR-enhanced adiponectin gene expression in WAT.
Keywords: obesity, adipocyte, calorie restriction
adiponectin is an adipocyte-derived hormone with insulin-sensitizing function and plays an important role in maintaining energy homeostasis. Adiponectin gene expression in white adipose tissue (WAT) and blood concentrations are inversely associated with body mass index in humans (1, 8, 44). However, despite the close association of adiposity and hypoadiponectinemia, it is still not certain whether WAT expansion itself reduces adiponectin gene expression and adiponectin levels in circulation.
The primary function of WAT is energy storage. Prolonged positive energy imbalance or excessive energy intake increases triglyceride accumulation in adipocytes and enlarges WAT mass. Therefore, calorie-enriched foods are generally blamed as the main cause of the obesity epidemic. In addition, high-fat (HF) diet has been widely used for inducing obesity in animal models that exhibit reduced adiponectin gene expression in WAT (3, 10, 25). This raises the question whether energy intake or dietary fat content controls adiponectin gene expression in WAT.
Calorie restriction (CR) is known for counteracting the deleterious aspects of positive energy imbalance and obesity-related metabolic diseases. A decrease in daily calorie intake by 20–40% of baseline requirement improves insulin sensitivity as well as reducing body weight and WAT mass (11, 36, 39). CR also alters adipocytokine expression in WAT (39). Among these adipocytokines, CR dramatically increases the gene expression and blood levels of adiponectin, particularly in rodents (9, 30, 35, 42, 43, 48).
Transcriptional regulation of adiponectin gene expression plays an important role in maintaining blood adiponectin concentration. Several transcription factors, including PPARγ, CCAAT enhancer-binding protein-α (C/EBPα), and FoxO1 induce adiponectin gene transcription (24, 27, 32, 33, 46). Within the PPAR family, PPARγ is probably best known for increasing circulating adiponectin levels by upregulating adiponectin expression at the transcriptional level (24). Interestingly, significantly increased blood adiponectin concentrations were also observed in PPARα agonist-treated human subjects and animal models (16, 21, 22, 26, 38). In addition, an in vitro experiment has demonstrated that PPARα directly increases adiponectin gene transcription through the peroxisome proliferator response elements (PPRE) at the adiponectin promoter (16).
In this study, feeding regimens and mouse models were used to discriminate the regulatory effects of energy intake, dietary fat, and adiposity on adiponectin gene expression. We report that total energy intake, but not dietary fat, plays an important role in controlling adiponectin gene expression and blood level. Compared with energy intake, adiposity exhibits a minor effect on circulating adiponectin levels. Our study also indicates that PPARα mediates CR-induced adiponectin gene expression in WAT.
RESEARCH DESIGN AND METHODS
Materials.
Insulin, 3-isobutyl-1-methylxanthine (IBMX), and dexamethasone were purchased from Sigma (St. Louis, MO). Anti-adiponectin antibody was from R&D Systems (Minneapolis, MN). Anti-GAPDH and -C/EBPα antibodies were from Cell Signaling (Danvers, MA) and Santa Cruz Biotechnology (Santa Cruz, CA). Antibody against PPARα was ordered from Invitrogen. HF diet (60 kcal% fat, D12492) and LF diet (10 kcal% fat, D12450B) were produced by Research Diet (New Brunswick, NJ). SYBR Green, penicillin-streptomycin, and Dulbecco's modified Eagle's medium (DMEM) were from Invitrogen. Fenofibrate, WY-14643, and rosiglitazone were from Cayman Chemical (Ann Arbor, MI). 125I-labeled mouse adiponectin was from Phoenix Pharmaceuticals (Gurlingame, CA).
Experimental animals.
Male C57BL/6, ob/ob, PPARα KO (stock number 008154), and A/J mice were from Jackson Laboratory (Bar Harbor, ME). Feeding regimens were started at 6 wk of age for all mice except PPARα KO mice, which were started at 3 mo of age. Body sizes of CR or pair-fed mice were similar to controls. All mice were maintained under standardized conditions with 12:12-h light-dark cycle. The experiments using mouse models were carried out under the Association for Assessment and Accreditation of Laboratory Animal Care guidelines with approval of the University of Kentucky and University of California San Diego Animal Care and Use Committees. The mice were randomly grouped. Food intake of LF or HF ad libitum groups (free access to food, LF-AL, HF-AL) was monitored daily. Calorie intakes of LF-AL mice were used for calculating food supply to CR or pair-fed mice. Energy intake was calculated on the basis of 3.85 kcal/g for the LF diet and 5.24 kcal/g for the HF diet. LF-CR and HF-CR mice received the same amount of energy intake, but dietary fat contents were different. Pair-fed mice received the same amount of calories as LF-AL. CR was gradually applied to the mice over a 2-wk lead period until energy intake reached 60% of that of the LF-AL mice. For PPARα KO mice, CR was 30% reduction of energy intake of LF-AL. Food was provided to mice daily around noon. Body weight was measured every week at the same time. Blood samples were collected every other week in the fed state. At the end of the study, blood, epididymal fat, and other tissue samples were collected after overnight fasting. Tissue samples were snap-frozen using liquid nitrogen and stored at −80°C for later protein and RNA preparation.
Adiponectin half-life assay.
Blood adiponectin half-life was measured in C57BL/6 mice after the 24-wk feeding regimens. The assay was carried out under fasting condition. 125I-labeled adiponectin (0.1 μCi per mouse) was injected into mice through the tail veins. Blood samples were collected 15, 30, 45, 60, 120, and 180 min after injection. Radioactivities of TCA-precipitated serum proteins were measured. The clearance rate of labeled adiponectin in blood was calculated and half-life determined using Prism software (San Diego, CA).
Cell culture.
3T3-L1 cell culture and induction of adipocyte differentiation were described in a previous publication (33). Two days after completion of adipocyte differentiation, 3T3-L1 adipocytes were treated overnight with indicated chemicals and control reagents in DMEM. RNA was extracted from adipocytes for real-time PCR assays.
Western blot and real-time PCR assays.
Total serum adiponectin levels were measured using Western blotting, and multimeric adiponectin was assayed under nonreducing conditions (34). Protein samples were resolved using NuPAGE gels (Invitrogen). Protein was blotted with indicated antibodies (see details in figure legends). Protein levels were semiquantified by image density scanning using Quantity One software with an internal reference for assays of multiple gels.
Total RNA was prepared from epididymal fat and 3T3-L1 adipocytes with TRIzol (Invitrogen). cDNA was synthesized using SuperScript III Reverse Transcriptase and oligo(dT)12–18 primer (Invitrogen). Real-time PCR was performed using the mx3000p Real-Time PCR system (Stratagene) and SYBR Green dye (Molecular Probes, Eugene, OR). The sequences for PCR primers are in table 1.
Table 1.
Sequences for real-time primers
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) | Accession No. |
|---|---|---|---|
| 18S rRNA | CGAAAGCATTTGCCAAGAAT | AGTCGGCATCGTTTATGGTC | X00685 |
| Adipoq | AAAGGAGAGCCTGGAGAAGC | AAAGGAGAGCCTGGAGAAGC | NM_009605 |
| Acox1 | GCCCAACTGTGACTTCCATC | GCCAGGACTATCGCATGATT | NM_015729 |
| Ehhadh | CCGGTCAATGCCATCAGT | CTAACCGTATGGTCCAAACTAGC | NM_023737 |
| Fabp4 (ap2) | TTGTGGAAGTCACGCCTTT | GAAAACGAGATGGTGACAAGC | NM_024406 |
| Fasn | ACTCCACAGGTGGGAACAAG | CCCTTGATGAAGAGGGATCA | NM_007988 |
Statistical analysis.
Data are expressed as means ± standard error of the mean (SE). Statistical analysis was performed using Student's t-test or ANOVA, followed by Bonferroni posttests using Prism software. Differences were considered significant at P < 0.05.
RESULTS
Energy intake, but not dietary fat, controls blood adiponectin concentration in mice.
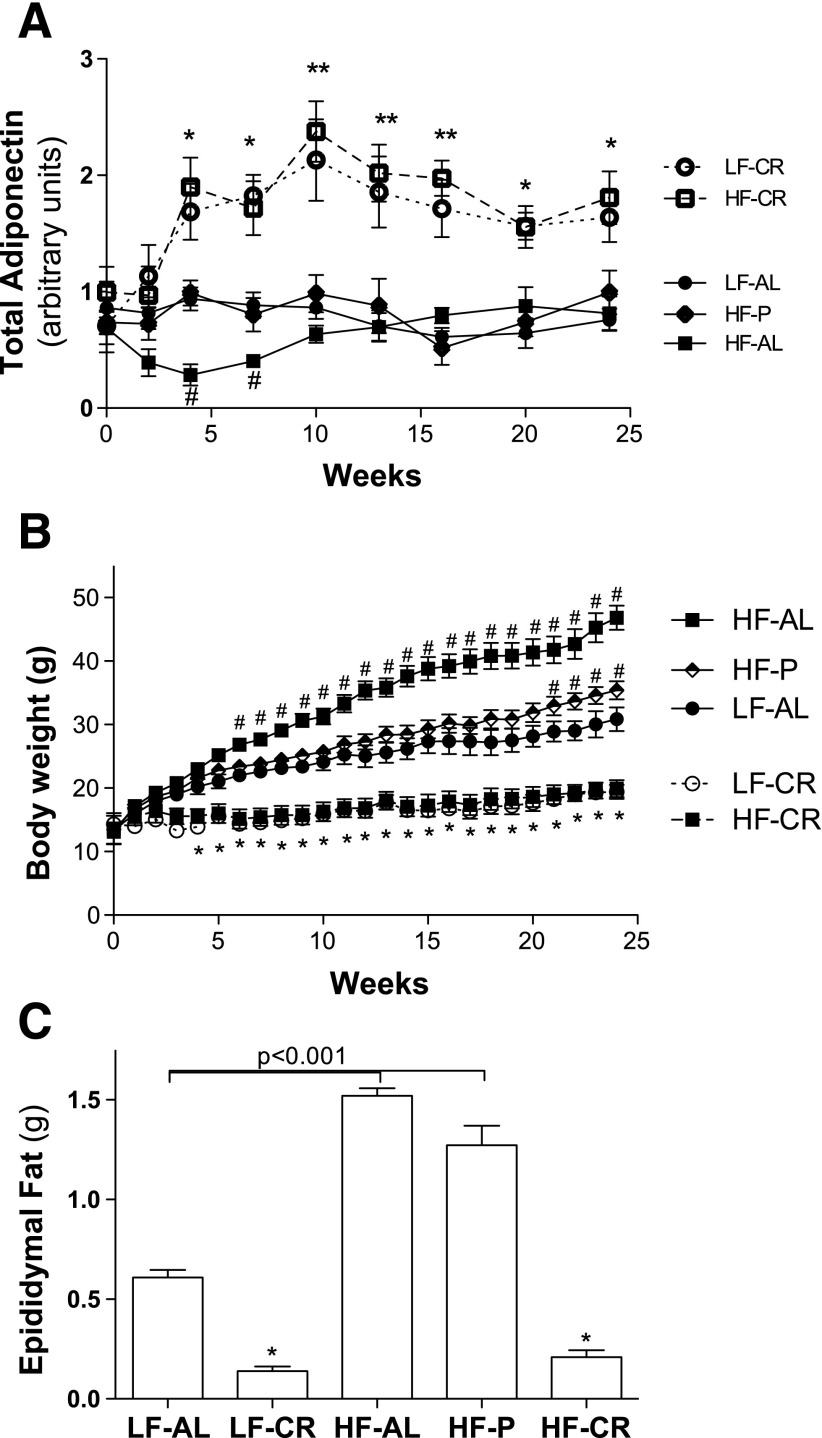
To determine the role of energy intake and dietary fat in controlling adiponectin gene expression and blood adiponectin level, C57BL/6 male mice were fed the HF or LF diet with controlled levels of calories. Total energy intakes (calories) were kept the same between LF-CR and HF-CR mice, HF-P (high-fat diet pair-fed) and LF-AL mice but different in dietary fat content. As shown in Fig. 1A, total serum adiponectin levels were significantly increased in LF-CR and HF-CR mice after 4 wk of treatment. There were no differences in serum adiponectin levels between LF-CR and HF-CR mice during the whole study. Serum adiponectin levels of HF-AL mice (free access to food) were significantly reduced at 4 wk compared with the other groups of mice (Fig. 1A). Interestingly, circulating adiponectin levels of HF-AL mice recovered at 10 wk of feeding and stayed at levels similar to those of LF-AL and HF-P (received same calories as LF-AL) mice during the rest of the study. These results are consistent with previous studies that reported that prolonged HF feeding does not reduce blood adiponectin levels in mice (3, 6, 12). Serum multimeric adiponectin levels were also measured, and results showed that multimeric adiponectin increased or reduced proportionally with total adiponectin levels in LF-CR, HF-CR, or HF-AL mice at each time point (data not shown). Since energy intakes and blood adiponectin were same between LF-CR and HF-CR mice and between LF-AL and HF-P mice, these studies indicate that energy intake, but not dietary fat, plays an important role in maintaining blood adiponectin levels in mice. Due to the proportional changes of multimeric and total adiponectin in these mice, total adiponectin levels are presented in the rest of this study.
Fig. 1.
CR increases blood adiponectin levels in both LF and HF-fed C57BL/6 mice. HF, high fat; LF, low fat; AL, ad libitum fed; CR, calorie restricted; P, pair fed. Energy intake of LF-AL mice was calculated based on daily food intake. HF-P or HF-CR mice received HF diet with calories equal to those of LF-AL and LF-CR mice, respectively. Restriction of energy intake for LF-CR and HF-CR mice was gradually applied: 20% in week 1, 30% in week 2, and then 40% of the value for LF-AL mice. A: blood samples were collected at indicated time points and stored for analysis. Serum adiponectin was measured using Western blotting with an internal reference sample between gels. Relative adiponectin levels were quantified by scanning band densities using Quantity One software. B: body weights were measured every week. C: epididymal fat was collected and weighed at the end of the study after overnight fasting. Data are presented as mean ± SE. *P < 0.05, **P < 0.001, LF-CR or HF-CR vs. LF-AL;#P < 0.05 HF-AL or HF-P vs. LF-AL at the same time point; n = 10 per group.
CR increases blood adiponectin levels in genetic obese ob/ob and obese-resistant A/J mice.
By comparing epididymal fat mass and body weight between HF-P and LF-AL mice (Fig. 1, B and C), our study showed that high-level dietary fat increases mouse WAT mass. However, serum adiponectin of HF-P and LF-AL mice were at the same levels during the entire study (Fig. 1A), which suggests that adiposity may play a minor role in controlling blood adiponectin levels in mice. Therefore, genetically obese ob/ob mice and obese-resistant A/J mice were employed to further investigate the effects of energy intake and adiposity on serum adiponectin levels.
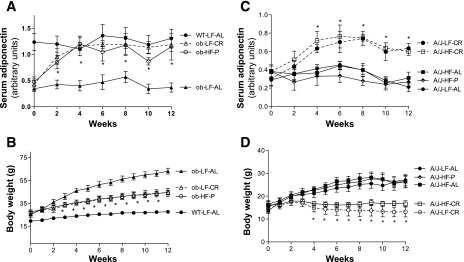
By measuring food intake, we found that the average daily energy intake of ob/ob mice was ∼62% higher than that of C57BL/6 (WT) control mice [Supplemental Fig. S1 (supplementary materials are found in the online version of this paper at the Journal website)]. Due to the 40% restriction of energy intake in CR mice, energy intake of calorie-restricted ob/ob mice (ob-LF-CR) was almost identical to that of WT LF ad libitum-fed (WT-LF-AL) mice. To avoid further worsening of energy homeostasis in ob/ob mice, the HF diet was given only to pair-fed mice, while normal chow was provided to the other groups. Serum adiponectin levels of ob-LF-AL mice were significantly lower than that of WT-LF-AL mice at every time point (Fig. 2A). Two weeks after initiation of CR and pair-feeding, serum adiponectin levels increased in both ob-LF-CR and ob-HF-P mice and stayed at similar levels to those of WT-LF-AL mice during the rest of the study (Fig. 2A). Three weeks after diet intervention, body weights of ob-LF-CR and ob-HF-P mice were significantly reduced compared with those of ob-LF-AL mice, but were still remarkably higher than those of WT-LF-AL mice (Fig. 2B). These results show that restriction of energy intake increases and even restores blood adiponectin levels in ob/ob mice despite the fact that they remain still obese.
Fig. 2.
CR increases circulating adiponectin levels in genetic obese ob/ob and obese-resistant A/J mice. The duration of this study was 12 wk. Energy intake and CR strategy were the same as for C57BL/6 mice, as described in research design and methods and Fig. 1 legend. A and C: serum adiponectin levels were measured using Western blotting and quantified by density scanning. *P < 0.05 ob-LF-CR or ob-HF-P vs. ob-LF-AL; A/J-LF-CR or A/J-HF-CR vs. A/J-LF-AL, A/J-HF-P, and A/J-HF-AL at the same time point. B and D: body weights were measured weekly. *P < 0.05, ob-LF-CR and ob-HF-P vs. ob-LF-AL, A/J-LF-CR, or A/J-HF-CR vs. A/J-LF-AL at the same time point; n = 8 per group. Data are presented as means ± SE.
In contrast to C57BL/6 mice, A/J mice are well known for resistance to HF diet-induced obesity. The same feeding regimens of C57BL/6 mice were applied to A/J mice. As expected, HF diet feeding did not induce significant increase of body weight of A/J mice (Fig. 2D). Similar to the results of C57BL/6 mice, serum adiponectin levels were significantly increased in both A/J-LF-CR and A/J-HF-CR mice (Fig. 2C). For unknown reasons, elevated energy intake in A/J-HF-AL mice lasted for the initial 5 wk of the study (Supplemental Fig. S1B). Consistent with a previous study (6), blood adiponectin levels were similar between HF and LF fed A/J mice (Fig. 2C). Together, these results indicate that CR is the most effective feeding regimen to increase blood adiponectin levels in mice. These studies also suggest that WAT mass may play a minor role in adiponectin gene expression in the context of calorie restriction.
CR increases adiponectin gene expression and has no effect on blood adiponectin half-life.
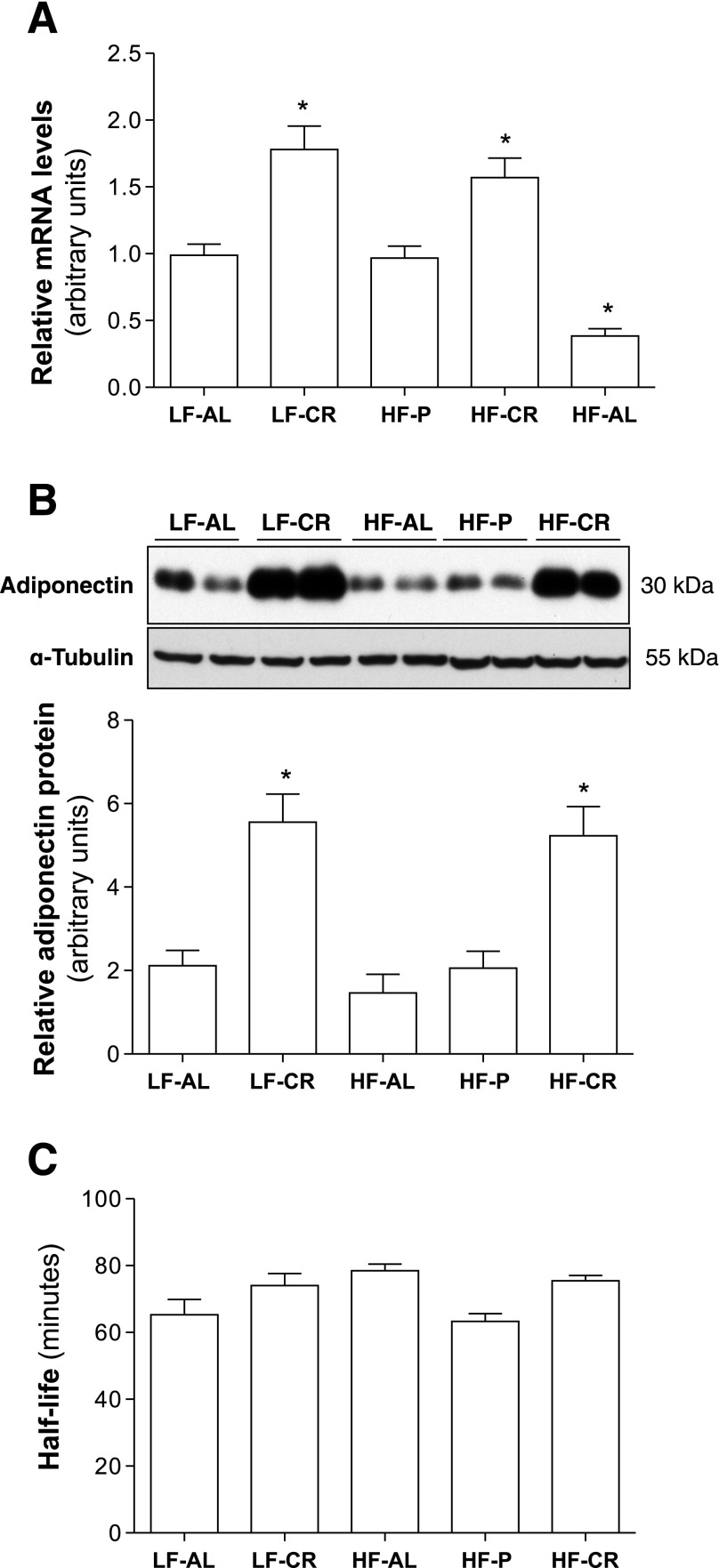
The levels of adiponectin mRNA and protein in epididymal fat were measured at the end of diet intervention. As shown in Fig. 3, A and B, adiponectin mRNA and protein levels were remarkably increased in epididymal fat of both LF-CR and HF-CR mice. These results indicate that CR increases adiponectin gene expression in WAT of mice.
Fig. 3.
CR increases adiponectin gene expression but has no effect on blood adiponectin clearance rate. After 24-wk feeding regimens in C57BL/6 mice, as described in Fig. 1, epididymal fat tissues were collected. Levels of adiponectin mRNA (A) and protein (B) were measured using real-time PCR or Western blot. C: 125I-labeled adiponectin (0.1 μCi) was injected into mice through the tail vein. Blood samples were collected, and radioactivities of precipitated serum proteins were measured. Circulating adiponectin half-life was calculated. Data are presented as means ± SE. *P < 0.05, LF-CR, HF-CR, or HF-AL vs. LF-AL; n = 10.
To determine whether energy intake and dietary fat alter the blood adiponectin clearance rate, 125I-labeled mouse adiponectin was employed to measure blood adiponectin half-life after 24 wk of feeding regimens of C57BL/6 mice. The half-life of adiponectin in LF-CR, HF-AL, and HF-CR mice was slightly greater than that in LF-AL mice but without statistical significance (Fig. 3C). These results suggest that energy intake and dietary fat do not have any significant effect on blood adiponectin clearance.
CR increases PPARα, PPARγ, and Sirt1 gene expression in epididymal fat.
To further investigate the underlying mechanisms through which CR increases adiponectin gene expression, the expression levels of a series of transcription factors and genes that might mediate CR-induced metabolic adaptation were measured using real-time PCR and Western blotting.
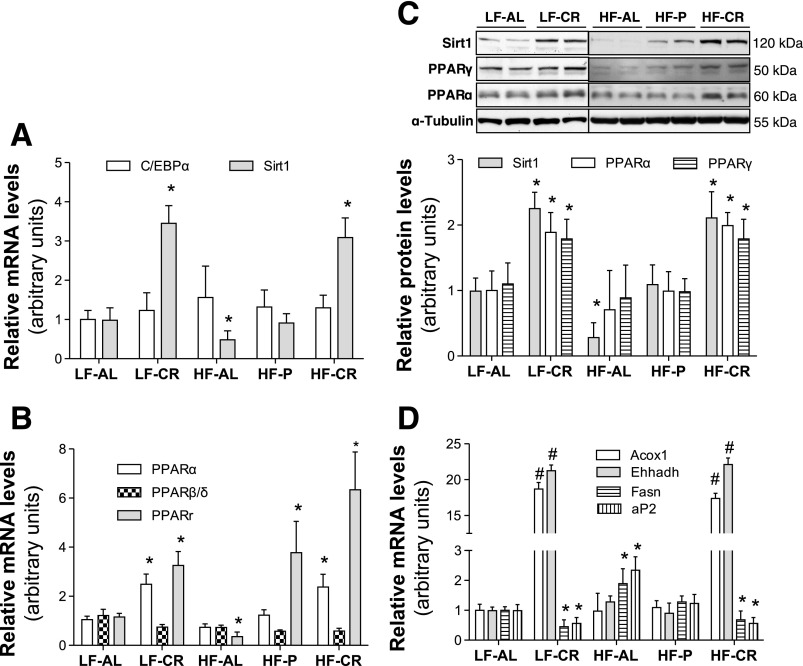
Sirt1 is a member of the silent information regulator 2 family, which are well-conserved nicotinamide adenine dinucleotide (NAD)+-dependent protein deacetylases. Despite the fact that the role of Sirt1 in regulating longevity in mammals is uncertain, it has been well documented that Sirt1 is important in mediating CR-induced metabolic adaptation. Consistent with the others' and our previous studies, Sirt1 mRNA (Fig. 4A) and protein (Fig. 4C) were significantly increased in epididymal fat of both LF-CR and HF-CR mice and reduced in HF-AL mice. Previous studies, including our in vitro study, have demonstrated that Sirt1 increases adiponectin gene expression (2, 33). Therefore, elevated Sirt1 in WAT of CR-treated mice seems to be responsible for enhanced adiponectin gene expression and increased blood adiponectin levels. However, the inhibitory effects of Sirt1 on adiponectin gene expression and secretion have been reported (4, 31). Unfortunately, most of these in vivo studies were carried out in transgenic mice. Due to ubiquitous expression and profound effects of Sirt1 on glucose and lipid metabolism, indirect regulatory effects of Sirt1 on adiponectin gene expression in these transgenic mice cannot be ruled out. Therefore, further in vivo studies are warranted to confirm the role of Sirt1 in CR-induced adiponectin gene expression.
Fig. 4.
CR increases PPARα gene expression in WAT. Total RNA samples were prepared from epididymal fat of 24-wk feeding regimen-treated C57BL/6 mice. mRNA levels of indicated genes were measured using real-time PCR with a relative quantification setting (A, B, and D, n = 10). Protein levels of indicated genes were measured using Western blotting (C, n = 6). Data are presented as means ± SE. *P < 0.05 vs. LF-AL;#P < 0.01 vs. LF-AL.
PPARγ and C/EBPα are two adipogenic master transcription factors that play important roles in adiponectin gene transcription. Our results show that C/EBPα mRNA (Fig. 4A) and protein levels (data not shown) in epididymal fat of LF-CR and HF-CR mice were similar to those of LF-AL and HF-P mice. Interestingly, expression of PPARγ was robustly increased in epididymal fat of both LF-CR and HF-CR mice (Fig. 4, B and C). There was an increase of PPARγ mRNA in epididymal fat of HF-P mice (Fig. 4B), but we could not detect any change of PPARγ at protein level (Fig. 4C). The increase of adiponectin and PPARγ gene expression in epididymal fat of CR-treated mice seems to suggest that PPARγ mediates CR-induced adiponectin gene transcription. However, the available information and data from the current study do not completely support this notion. It has been demonstrated that CR inhibits PPARγ transactivity and PPARγ regulated lipid metabolism in adipocytes through Sirt1 (29). Therefore, despite the increase of PPARγ gene expression in epididymal fat of CR-treated mice, PPARγ activity may be suppressed. Our study indeed showed a decrease of PPARγ target genes aP2 and fatty acid synthase (Fasn) in the epididymal fat of CR-treated mice (Fig. 4D).
PPARα and PPARδ are two other members of the PPAR family. Despite low abundance of PPARα and PPARδ in adipocytes compared with PPARγ, they are also important in regulating lipid metabolism in adipocytes. We measured expression levels of PPARα and PPARδ in epididymal fat from these mice. To our surprise, expression of PPARα, but not PPARδ, was significantly increased in epididymal fat of both LF-CR and HF-CR mice (Fig. 4, B and C). Interestingly, mRNA levels of PPARα target gene Acox1 and Ehhadh were also robustly elevated in CR-treated mice (Fig. 4D).
PPARα agonist increases adiponectin gene expression in 3T3-L1 adipocytes.
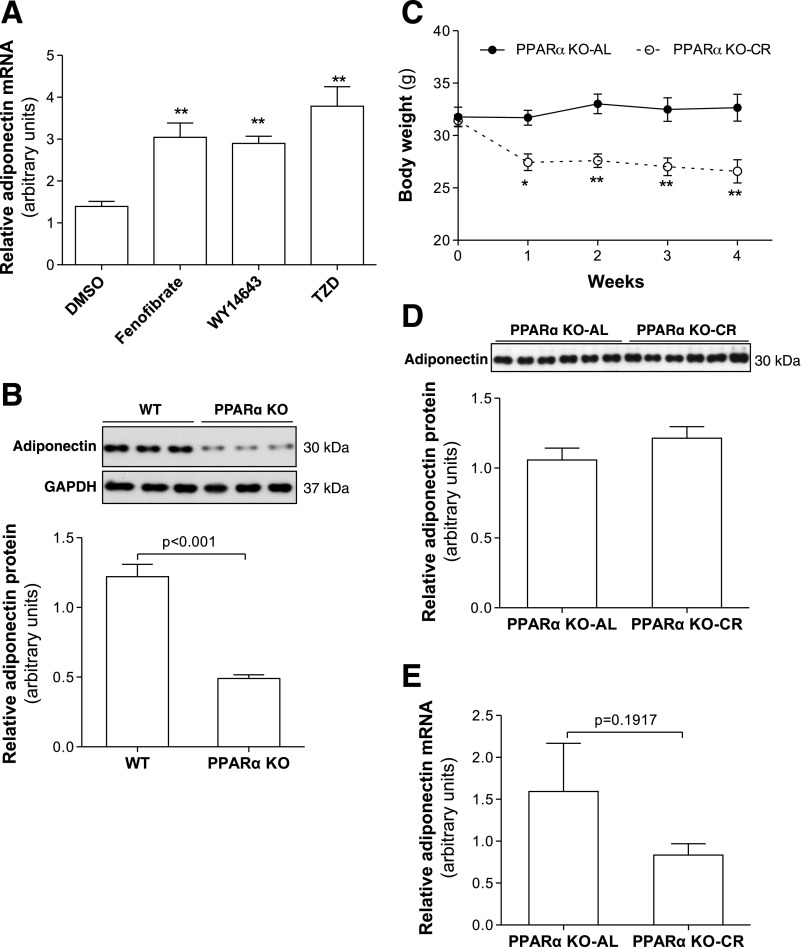
Previous studies have reported that PPARα upregulates adiponectin gene transcription through PPRE at the adiponectin promoter (16). To verify the effects of PPARα on adiponectin gene expression, PPARα activators fenofibrate and WY-14643 and PPARγ agonist rosiglitazone (TZD) were used to treat differentiated 3T3-L1 adipocytes. In line with previous reports and similar to the effects of TZD, both fenofibrate and WY-14643 treatment robustly increased adiponectin mRNA levels (Fig. 5A). In addition, adiponectin mRNA in epididymal fat (Fig. 5B) and blood adiponectin levels (Supplemental Fig. S3, A and B) were remarkably lower in PPARα KO mice than in WT control mice. These results further confirm that PPARα upregulates adiponectin gene expression.
Fig. 5.
PPARα upregulates adiponectin gene expression in adipocytes. A: fully differentiated mature 3T3-L1 adipocytes were treated with fenofibrate (5 μM), WY-14643 (5 μM), or TZD (1 μM) overnight. Adiponectin mRNA levels were measured by real-time PCR. B: proteins were extracted from epididymal fat of 8-wk-old male PPARα KO or C57BL/6 control (WT) mice. Adiponectin levels were measured by Western blotting. C: food intake of PPARα KO-CR mice was gradually reduced to 70% of that of PPARα KO-AL mice. Body weights were measured every week. Four weeks after CR treatment, serum adiponectin (D) and adiponectin mRNA levels (E) in epididymal fat were measured by Western blotting or real-time PCR. Data are presented as means ± SE. *P < 0.05, **P < 0.01 vs. PPARα KO-AL; n = 6.
CR failed to increase blood adiponectin levels in PPARα KO mice.
To determine the role PPARα in CR-induced adiponectin gene expression, PPARα KO male mice were subjected to CR treatment. Due to the increased sensitivity of PPARα KO mice to fasting and to avoid hypoglycemia and severe alteration in lipid metabolism, 30% (instead of 40%) restriction of energy intake was used for PPARα KO mice. In a separate study, we found that 30% CR was sufficient to increase blood adiponectin levels in C57BL/6 mice (data not shown). CR treatment significantly reduced body weight and epididymal fat tissue mass in PPARα KO-CR mice (Fig. 5C). There was a slight increase of serum total adiponectin in PPARα KO-CR mice, however, without statistical significance (Fig. 5D). In line with serum adiponectin, expression levels of adiponectin in epididymal fat were similar between PPARα KO-CR and PPARα KO-AL mice (Fig. 5E and Supplemental Fig. S3C). These results indicate that CR fails to increase adiponectin gene expression and blood adiponectin levels in PPARα KO mice.
DISCUSSION
Although adiponectin transcript and protein have been found in some nonfat tissues, WAT plays a predominant role in maintaining relatively high levels of adiponectin in circulation. Paradoxically, reduced adiponectin gene expression in WAT and hypoadiponectinemia are well documented in obese human subjects and some animal models. Calorie- or fat-enriched foods are the common causes of obesity, which is characterized by excessive WAT mass. Therefore, it was generally postulated that dietary fat and enlarged WAT mass directly suppress adiponectin gene expression resulting in hypoadiponectinemia. By using various mouse models and feeding regimens, our current study provides clear evidence indicating that energy intake, particularly restricted energy intake, plays a key role in controlling adiponectin gene expression and circulating adiponectin levels in mice. Our study also reveals that, within normal energy intake ranges, high-level dietary fat does not impair adiponectin gene expression and blood adiponectin levels despite the increased WAT mass in some strains of HF-fed mice. Although further studies are required, our study suggests that WAT mass itself may play a minor role in maintaining blood adiponectin concentrations in mice. In supporting this notion, several human studies have reported that hypoadiponectinemia is not closely associated with body fat content (17, 28, 40, 44).
Multiple transcription factors and signaling pathways are involved in regulation of adiponectin gene expression. It is known that obesity not only alters lipid metabolism but also impairs insulin signaling and other cellular functions. Therefore, decreased adiponectin gene expression in obesity may be attributed to multiple factors such as increased inflammatory cyctokines and hypoxia in WAT (45). Limited information was generated in this study regarding how energy intake regulates adiponectin gene expression in WAT and blood adiponectin concentration. Similar to other hormones, expression, secretion, and clearance in circulation should be important in maintaining blood adiponectin levels. Our study shows that the blood adiponectin half-life in C57BL/6 mouse is ∼65 min, which is close to the value in FVB mice (∼75 min) but much shorter than the 2.5-h adiponectin half-life in human subjects (14, 17). It has been reported that the plasma adiponectin clearance rate is prolonged in HF-fed mice (14). Our study showed that the serum adiponectin half-life in HF fed mice was slightly longer but without statistical significance. This may be caused by the difference in the strain of mice and duration of HF feeding. Importantly, although blood adiponectin levels were robustly increased in LF-CR or HF-CR mice, serum adiponectin half-lives were similar to that of LF-AL mice (Fig. 3C). These results indicate that CR-induced increase of circulating adiponectin does not occur through altering the blood adiponectin clearance rate. Therefore, we propose that energy intake controls circulating adiponectin levels through a mechanism(s) at adiponectin gene expression and/or protein secretion.
CR is by far the most effective physical intervention that increases adiponectin gene expression and circulating levels in rodents (9, 42, 48). Consistent with these reports, our study suggests that CR increases blood adiponectin concentration by increasing adiponectin gene expression. Therefore, studying the mechanisms through which CR increases adiponectin gene expression will enrich our understanding of how energy intake regulates adiponectin gene expression in WAT. CR also provides a unique in vivo system to identify the key regulator(s) of adiponectin gene expression.
Strictly, CR is prolonged starvation with marginal energy deficiency. Due to the energy storage nature of WAT, the impacts of CR on WAT metabolism and adipogenic gene expression have been well documented (11). Interestingly, our study reveals that CR increases both PPARγ and PPARα expression but has no effect on C/EBPα in mouse WAT (Fig. 4). A previous study reported that prolonged fasting increased PPARγ mRNA in visceral fat of rats (23). However, further studies are required to verify the involvement of PPARγ in CR-induced adiponectin gene expression in WAT with the reasons discussed in the result section.
PPARα is well known for improving blood lipid profiles and enhancing fatty acid oxidation (19, 47). CR induces a dramatic shift of fuel selection from glycolysis to fatty acid oxidation (5, 7, 13). Increased PPARα gene expression may mediate the CR-induced oxidative metabolic shift. Although there is a relatively low abundance of PPARα in WAT compared with brown adipose and liver in mice, the direct regulatory roles of PPARα in gene expression and energy metabolism have been clearly identified in WAT (18, 20, 37, 41). Consistent with previous studies (16, 21, 22, 26, 38), our study reveals that PPARα agonist stimulates adiponectin gene expression in cultured adipocytes (Fig. 5A). Significantly decreased adiponectin gene expression and hypoadiponectinemia were observed in PPARα KO mice (Fig. 5B). These results are inconsistent with a previous study, which did not observe any change in serum adiponectin in PPARα KO mice (15). The discrepancy is most likely caused by the difference in genetic background. The PPARα KO mice we used were in C57BL/6 background, whereas the mice used in the other study were in 129S1/SvImJ background. Our study also revealed that CR enhances expression of both PPARα and its target gene Acox1 and Ehhadh in WAT, whereas PPARγ target gene aP2 and Fasn were suppressed (Fig. 4, B–D). Most importantly, CR failed to increase adiponectin gene expression in WAT and circulating adiponectin levels in PPARα KO mice. Therefore we conclude that PPARα plays a key role in mediating CR-induced adiponectin gene expression in WAT.
In summary, by using feeding regimens with controlled energy intake and dietary fat content and mouse models, our study demonstrates that the amount of energy intake, but not dietary fat, is important in controlling adiponectin gene expression in WAT. The critical role of PPARα in CR-induced adiponectin gene expression was also identified.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-080418 (J. H. Shao) and DK-077643 (J. H. Shao), and American Diabetes Association Grant 7-07-CD23 (J. H. Shao).
DISCLOSURES
No conflicts of interest are reported by these authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Jerome Schaack (University of Colorado at Denver and Health Sciences Center) for reading and discussion of the manuscript.
REFERENCES
- 1. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83, 1999. [DOI] [PubMed] [Google Scholar]
- 2. Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab 8: 333–341, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnea M, Shamay A, Stark AH, Madar Z. A high-fat diet has a tissue-specific effect on adiponectin and related enzyme expression. Obesity (Silver Spring) 14: 2145–2153, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell 6: 759–767, 2007. [DOI] [PubMed] [Google Scholar]
- 5. Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab 298: E108–E116, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bullen JW, Jr, Bluher S, Kelesidis T, Mantzoros CS. Regulation of adiponectin and its receptors in response to development of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 292: E1079–E1086, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Cao SX, Dhahbi JM, Mote PL, Spindler SR. Genomic profiling of short- and long-term caloric restriction effects in the liver of aging mice. Proc Natl Acad Sci USA 98: 10630–10635, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46: 459–469, 2003. [DOI] [PubMed] [Google Scholar]
- 9. Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes 52: 268–276, 2003. [DOI] [PubMed] [Google Scholar]
- 10. Duval C, Thissen U, Keshtkar S, Accart B, Stienstra R, Boekschoten MV, Roskams T, Kersten S, Muller M. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57Bl/6 mice. Diabetes 59: 3181–3191, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA 297: 986–994, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Griffin TM, Fermor B, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, Cao L, Setton LA, Guilak F. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther 12: R130, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guarente L. Mitochondria—a nexus for aging, calorie restriction, and sirtuins? Cell 132: 171–176, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halberg N, Schraw TD, Wang ZV, Kim JY, Yi J, Hamilton MP, Luby-Phelps K, Scherer PE. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes 58: 1961–1970, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haluzik M, Gavrilova O, LeRoith D. Peroxisome proliferator-activated receptor-alpha deficiency does not alter insulin sensitivity in mice maintained on regular or high-fat diet: hyperinsulinemic-euglycemic clamp studies. Endocrinology 145: 1662–1667, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Hiuge A, Tenenbaum A, Maeda N, Benderly M, Kumada M, Fisman EZ, Tanne D, Matas Z, Hibuse T, Fujita K, Nishizawa H, Adler Y, Motro M, Kihara S, Shimomura I, Behar S, Funahashi T. Effects of peroxisome proliferator-activated receptor ligands, bezafibrate and fenofibrate, on adiponectin level. Arterioscler Thromb Vasc Biol 27: 635–641, 2007. [DOI] [PubMed] [Google Scholar]
- 17. Hoffstedt J, Arvidsson E, Sjolin E, Wahlen K, Arner P. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J Clin Endocrinol Metab 89: 1391–1396, 2004. [DOI] [PubMed] [Google Scholar]
- 18. Islam KK, Knight BL, Frayn KN, Patel DD, Gibbons GF. Deficiency of PPARalpha disturbs the response of lipogenic flux and of lipogenic and cholesterogenic gene expression to dietary cholesterol in mouse white adipose tissue. Biochim Biophys Acta 1734: 259–268, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Jay MA, Ren J. Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr Diabetes Rev 3: 33–39, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Knauf C, Rieusset J, Foretz M, Cani PD, Uldry M, Hosokawa M, Martinez E, Bringart M, Waget A, Kersten S, Desvergne B, Gremlich S, Wahli W, Seydoux J, Delzenne NM, Thorens B, Burcelin R. Peroxisome proliferator-activated receptor-alpha-null mice have increased white adipose tissue glucose utilization, GLUT4, and fat mass: role in liver and brain. Endocrinology 147: 4067–4078, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Koh KK, Han SH, Quon MJ, Yeal Ahn J, Shin EK. Beneficial effects of fenofibrate to improve endothelial dysfunction and raise adiponectin levels in patients with primary hypertriglyceridemia. Diabetes Care 28: 1419–1424, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Li P, Shibata R, Maruyama S, Kondo M, Ohashi K, Ouchi N, Murohara T. Fenofibrate promotes ischemia-induced revascularization through the adiponectin-dependent pathway. Am J Physiol Endocrinol Metab 299: E560–E566, 2010. [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Bujo H, Takahashi K, Shibasaki M, Zhu Y, Yoshida Y, Otsuka Y, Hashimoto N, Saito Y. Visceral fat: higher responsiveness of fat mass and gene expression to calorie restriction than subcutaneous fat. Exp Biol Med (Maywood) 228: 1118–1123, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50: 2094–2099, 2001. [DOI] [PubMed] [Google Scholar]
- 25. Naderali EK, Estadella D, Rocha M, Pickavance LC, Fatani S, Denis RG, Williams G. A fat-enriched, glucose-enriched diet markedly attenuates adiponectin mRNA levels in rat epididymal adipose tissue. Clin Sci (Lond) 105: 403–408, 2003. [DOI] [PubMed] [Google Scholar]
- 26. Oki K, Koide J, Nakanishi S, Nakashima R, Yamane K. Fenofibrate increases high molecular weight adiponectin in subjects with hypertriglyceridemia. Endocr J 54: 431–435, 2007. [DOI] [PubMed] [Google Scholar]
- 27. Park BH, Qiang L, Farmer SR. Phosphorylation of C/EBPbeta at a consensus extracellular signalregulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol Cell Biol 24: 8671–8680, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pellme F, Smith U, Funahashi T, Matsuzawa Y, Brekke H, Wiklund O, Taskinen MR, Jansson PA. Circulating adiponectin levels are reduced in nonobese but insulin-resistant first-degree relatives of type 2 diabetic patients. Diabetes 52: 1182–1186, 2003. [DOI] [PubMed] [Google Scholar]
- 29. Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429: 771–776, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Piccio L, Stark JL, Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol 84: 940–948, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol 27: 4698–4707, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiao L, Maclean PS, Schaack J, Orlicky DJ, Darimont C, Pagliassotti M, Friedman JE, Shao J. C/EBPalpha regulates human adiponectin gene transcription through an intronic enhancer. Diabetes 54: 1744–1754, 2005. [DOI] [PubMed] [Google Scholar]
- 33. Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem 281: 39915–39924, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes 57: 1824–1833, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raitakari M, Ilvonen T, Ahotupa M, Lehtimaki T, Harmoinen A, Suominen P, Elo J, Hartiala J, Raitakari OT. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol 24: 124–128, 2004. [DOI] [PubMed] [Google Scholar]
- 36. Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab 92: 865–872, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ribet C, Montastier E, Valle C, Bezaire V, Mazzucotelli A, Mairal A, Viguerie N, Langin D. Peroxisome proliferator-activated receptor-alpha control of lipid and glucose metabolism in human white adipocytes. Endocrinology 151: 123–133, 2010. [DOI] [PubMed] [Google Scholar]
- 38. Rosenson RS. Effect of fenofibrate on adiponectin and inflammatory biomarkers in metabolic syndrome patients. Obesity (Silver Spring) 17: 504–509, 2009. [DOI] [PubMed] [Google Scholar]
- 39. Varady KA, Hellerstein MK. Do calorie restriction or alternate-day fasting regimens modulate adipose tissue physiology in a way that reduces chronic disease risk? Nutr Rev 66: 333–342, 2008. [DOI] [PubMed] [Google Scholar]
- 40. Vozarova B, Stefan N, Lindsay RS, Krakoff J, Knowler WC, Funahashi T, Matsuzawa Y, Stumvoll M, Weyer C, Tataranni PA. Low plasma adiponectin concentrations do not predict weight gain in humans. Diabetes 51: 2964–2967, 2002. [DOI] [PubMed] [Google Scholar]
- 41. Walker CG, Sugden MC, Gibbons GF, Holness MJ. Peroxisome proliferator-activated receptor alpha deficiency modifies glucose handling by isolated mouse adipocytes. J Endocrinol 193: 39–43, 2007. [DOI] [PubMed] [Google Scholar]
- 42. Wang Z, Masternak MM, Al-Regaiey KA, Bartke A. Adipocytokines and the regulation of lipid metabolism in growth hormone transgenic and calorie-restricted mice. Endocrinology 148: 2845–2853, 2007. [DOI] [PubMed] [Google Scholar]
- 43. Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr 84: 1033–1042, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86: 1930–1935, 2001. [DOI] [PubMed] [Google Scholar]
- 45. Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 33: 54–66, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med 351: 1106–1118, 2004. [DOI] [PubMed] [Google Scholar]
- 47. Zandbergen F, Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochim Biophys Acta 1771: 972–982, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu M, Miura J, Lu LX, Bernier M, DeCabo R, Lane MA, Roth GS, Ingram DK. Circulating adiponectin levels increase in rats on caloric restriction: the potential for insulin sensitization. Exp Gerontol 39: 1049–1059, 2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.