Abstract
C-peptide is a 31-amino acid peptide cleaved from proinsulin during insulin synthesis. Initially thought to be inert, C-peptide may modulate the inflammatory response in the setting of endotoxemia and ischemia reperfusion. However, the spectrum of its biological effects is unclear. We hypothesized that exogenous administration of C-peptide would modulate pro- and anti-inflammatory signaling pathways and thereby attenuate lung inflammation in an in vivo model of hemorrhagic shock. Hemorrhagic shock was induced in male Wistar rats (aged 3–4 mo) by withdrawing blood to a mean arterial pressure of 50 mmHg. At 3 h after hemorrhage, rats were rapidly resuscitated by returning their shed blood. At the time of resuscitation and every hour thereafter, animals received C-peptide (280 nmol/kg) or vehicle parenterally. Animals were euthanized at 1 and 3 h after resuscitation. C-peptide administration at resuscitation following hemorrhagic shock ameliorated hypotension and blunted the systemic inflammatory response by reducing plasma levels of IL-1, IL-6, macrophage inflammatory protein-1α, and cytokine-induced neutrophil chemoattractant-1. This was associated with a reduction in lung neutrophil infiltration and plasma levels of receptor for advanced glycation end products. Mechanistically, C-peptide treatment was associated with reduced expression of proinflammatory transcription factors activator protein-1 and NF-κB and activation of the anti-inflammatory transcription factor peroxisome proliferator-activated receptor-γ. Our data suggest that C-peptide ameliorates the inflammatory response and lung inflammation following hemorrhagic shock. These effects may be modulated by altering the balance between pro- and anti-inflammatory signaling in the lung.
Keywords: acute lung injury, peroxisome proliferator-activated receptor-γ
hemorrhagic shock and resuscitation (HS/R) is associated with a development of an inflammatory state that may lead to the development of multiple organ failure and death (14, 15). Lung injury is a common occurrence and may contribute to other distant organ failures (14). Clinically the diagnosis of acute lung injury (ALI) is based on consensus criteria that do not account for the underlying etiology of lung injury (9). Recent studies in rodents and humans have assessed the utility of biomarkers for diagnosing ALI and demonstrated the potential role of plasma levels of the multiligand binding receptor RAGE (receptor for advanced glycation end products) as a marker of lung injury (17, 33). At a molecular level, lung injury and systemic inflammation following HS/R are characterized by a complex process that involves both proinflammatory and anti-inflammatory signaling pathways and is characterized by dysregulated production of proinflammatory enzymes, cytokines, and adhesion molecules. The proinflammatory response is characterized by activation of mitogen-activated protein kinases (MAPK) such as extracellular signal-regulated kinase (ERK 1/2) and c-Jun NH2-terminal kinase (JNK) and IκB kinases (IKK), which mediate the transduction of oxidant stress signals to the nuclear transcription factors activator protein-1 (AP-1) and nuclear factor-κB (NF-κB) (11, 12, 16). The anti-inflammatory response may also be modulated by signaling pathways that involve peroxisome proliferator-activated receptor-γ (PPAR-γ). PPAR-γ is a nuclear transcription factor that regulates expression of inflammatory response genes and has been demonstrated to exert anti-inflammatory properties in models of sepsis (22, 27). Previously, in a rodent model of hemorrhagic shock we have demonstrated that ciglitazone, a PPAR-γ ligand, ameliorated lung inflammation and reduced systemic levels of cytokines and chemokines by modulating NF-κB signaling (12). Hence, using a strategy aimed at downregulating proinflammatory pathways and simultaneously upregulating the anti-inflammatory pathways may attenuate the inflammatory response following hemorrhagic shock.
C-peptide is a 31-amino acid peptide cleaved from proinsulin during insulin synthesis (31). It is stored and released into the circulation from the pancreatic β cells in equimolar concentrations with insulin. C-peptide was thought to have minimal biological activity; however, recent data suggest that this may not be the case (35). Specifically, in diabetic animals it attenuates neural and vascular dysfunction, whereas in nondiabetic animals it may mediate leukocyte endothelial interactions; modulate nitric oxide production and neutrophil infiltration (20, 29, 37). The details of its putative receptor and its cellular mechanisms of action are unclear; however, it appears that C-peptide may exert its biological effects by binding to a G protein-coupled receptor (28, 35). Recently published data indicate that C-peptide may attenuate lung injury in a nondiabetic rodent model of sepsis as a result of activation of PPAR-γ and modulation of MAPK signaling (5, 34). Therefore, C-peptide may act as an endogenous factor modulating both proinflammatory and anti-inflammatory signaling pathways. However, the biological effects of exogenous administration of C-peptide on inflammation following hemorrhagic shock are unknown.
Our present study aimed at exploring biological effects of C-peptide on the systemic inflammatory response and lung inflammation following severe hemorrhage in an in vivo rodent model of hemorrhagic shock. We hypothesized that exogenous administration of C-peptide would be beneficial by modulating pro- and anti-inflammatory signaling pathways.
MATERIAL AND METHODS
Rodent model of HS/R.
Male Wistar rats aged 3–4 mo (Charles River Laboratories, Wilmington, MA) were anesthetized with pentobarbital (80 mg/kg) intraperitoneally. The trachea was cannulated and the animals were placed on a rodent ventilator (Harvard Apparatus, Holliston, MA) with similar settings, tidal volume 2 ml, rate 60 breaths/min, and inspired O2 fraction of 0.4. The right femoral artery and left common carotid artery were cannulated (PE-50 tubing) and used for drawing blood and measuring mean arterial blood pressure (MAP), respectively. Following the surgical procedure rats were allowed to stabilize for 10 min. Hemorrhagic shock was induced by withdrawing blood from the femoral artery over 10 min into a reservoir as previously described (42). The animals were kept at a MAP of 50 mmHg for 3 h by withdrawing or reinjecting blood through the femoral artery. At 3 h rats were rapidly resuscitated over 10 min with their own shed blood supplemented with Ringer Lactate solution to a final volume equal to total shed blood and monitored for another 3 h. Heart rates and MAP were measured via a pressure transducer and digitized by use of a MacLab analog-to-digital converter. This data was analyzed by using Chart 5 software (AD Instruments, Colorado Springs, CO) at 30-min intervals during the entire experiment. The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the institutional Animal Care and Use Committee at Cincinnati Children's Hospital Medical Center.
Experimental groups.
Rats were assigned to six groups: 1) Control (n = 5) rats underwent surgery following which they were euthanized. 2) Sham (n = 6) rats underwent surgery but no HS/R; they were maintained under anesthesia with pentobarbital and kept on the ventilator. 3) Sham+Vehicle (n = 6) rats underwent surgery but no HS/R; they were treated with vehicle (0.1% acetic acid) intra-arterially at 3 h and hourly thereafter and were euthanized at 1 h (n = 3) and 3 h (n = 3) following vehicle treatment. 4) Sham+C-Peptide (n = 6) rats underwent surgery but no HS/R; they were treated with C-peptide (280 nmol/kg or 1 mg/kg) intra-arterially at 3 h and hourly thereafter and were euthanized at 1 h (n = 3) and 3 h (n = 3) following C-peptide treatment. 5) HS/R+Vehicle (n = 10) rats underwent surgery followed by HS/R; after 3 h they were resuscitated and treated with vehicle (0.1% acetic acid) intra-arterially at resuscitation and hourly thereafter. Rats in these groups were euthanized at 1 h (n = 5) and 3 h (n = 5) following resuscitation. 6) HS/R+C-Peptide (n = 10) rats underwent surgery followed by HS/R; after 3 h they were resuscitated and treated with C-peptide (280 nmol/kg or 1 mg/kg) intra-arterially at resuscitation and hourly thereafter. Rats in these groups were euthanized at 1 h (n = 5) and 3 h (n = 5) following resuscitation.
The dose of C-peptide was chosen on the basis of prior work in an in vivo model of endotoxemia (34). Acetic acid was the vehicle used to reconstitute C-peptide and was used as the vehicle. Arterial whole blood (100 μl) was collected and analyzed by using a hand-held analyzer (iStat 1, Abbott Laboratories, Princeton, NJ) for lactate and glucose levels following surgery (baseline), at the end of hemorrhage, and at the end of the experiment. Following euthanasia lungs and plasma samples were collected from each rat at the end of the experiment and stored at −70°C. A part of the lung tissue was collected and stored in formalin for histopathology.
Plasma cytokine and chemokine levels.
Plasma levels of interleukin (IL)-1, IL-6, macrophage inflammatory protein-1 α (MIP-1α) were determined by a multiplex array system (Millipore, Billerica, MA). Cytokine-induced neutrophil chemoattractant 1 (CINC-1) levels were determined by an ELISA technique (R&D Systems, Minneapolis, MN).
Plasma RAGE levels.
Plasma levels of RAGE were quantified by using an ELISA technique as specified by the manufacturer (RayBiotech, Norcross, GA).
MPO activity.
Myeloperoxidase (MPO) activity was determined as an index of lung neutrophil infiltration. It was defined as the quantity of enzyme degrading 1 μmol hydrogen peroxide/min at 37°C and expressed in units per 100 mg tissue (43).
Histopathological analysis.
Lung tissue was fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained with hematoxylin-eosin stain. These sections were evaluated by light microscopy for evidence of lung inflammation and injury.
Subcellular fractionation and nuclear protein extraction.
Lung samples were homogenized with a Polytron homogenizer (Omni International, Kennesaw, GA) in a buffer containing 0.32 M sucrose, 10 mM Tris·HCl (pH 7.4), 1 mM EGTA, 2 mM EDTA, 5 mM NaN3, 10 mM β-mercaptoethanol, 20 μM leupeptin, 0.15 μM pepstatin A, 0.2 mM PMSF, 50 mM NaF, 1 mM sodium orthovanadate, and 0.4 nM microcystin. The homogenates were centrifuged (1,000 g, 10 min), and the supernatant (cytosol plus membrane extract) was collected to evaluate the content of PPAR-γ, pERK1/2, and ERK1/2. The pellets were solubilized in Triton buffer [1% Triton X-100, 150 mM NaCl, 10 mM Tris·HCl (pH 7.4), 1 mM EGTA, 1 mM EDTA, 0.2 mM sodium orthovanadate, 20 μM leupeptin A, and 0.2 mM PMSF]. The lysates were centrifuged (15,000 g, 30 min, 4°C), and the supernatant (nuclear extract) was collected.
Western blot analyses.
The content of PPAR-γ, pERK, and ERK were determined by immunoblot analyses using primary antibody against PPAR-γ, pERK, and ERK. Immunoreaction was visualized by chemiluminescence and on a photographic film. Densitometric analysis of blots was performed by use of ImageQuant (Molecular Dynamics, Sunnyvale, CA).
AP-1 and PPAR-γ activation.
AP-1 and PPAR-γ. Activation was assessed by electrophoretic mobility shift assays (EMSA) as previously described (41). Oligonucleotide probes corresponding to the AP-1 or PPAR-γ consensus sequence were labeled with [γ-32P]ATP by using T4 polynucleotide kinase and were purified in Bio-Spin chromatography columns (Bio-Rad, Hercules, CA). Twenty-five micrograms of nuclear protein were preincubated with EMSA buffer [12 mM HEPES (pH 7.9), 4 mM Tris·HCl (pH 7.9), 25 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1 mM DTT, 50 ng/ml poly(dI-dC), 12% glycerol (vol/vol), and 0.2 mM PMSF] on ice for 10 min before addition of the radiolabeled oligonucleotide for an additional 10 min. Protein-nucleic acid complexes were resolved by using a nondenaturing polyacrylamide gel consisting of 5% acrylamide (29:1 ratio of acrylamide-bisacrylamide) and were run in 0.5 × TBE (45 mM Tris·HCl, 45 mM boric acid, and 1 mM EDTA) for 1 h at constant current (30 mA). Gels were transferred to Whatman 3M paper (Clifton, NJ), dried under a vacuum at 80°C for 1 h, and exposed to photographic film at −70°C with an intensifying screen. Densitometric analysis was performed by using ImageQuant (Molecular Dynamics).
NF-κB (p65) activation.
NF-κB (p65) activation was detected in lung nuclear extracts by using an ELISA-based transcription factor assay kit (TransAM NF-κB p65; Active Motif, Carlsbad, CA) as previously described (6, 26). Briefly 20 μg of lung nuclear extract was added to a 96-well plate coated with the NF-κB consensus site (5′-GGGACTTTCC-3′) and incubated for 1 h at room temperature followed by washes and addition of a primary antibody specific for the p65 subunit. Following this, a horseradish peroxidase-conjugated secondary antibody was added and a colorimetric readout was measured at 450 nM on a spectrophotometer. To monitor the specificity of the assay competition was performed by adding wild type or mutated oligonucleotides at a concentration of 20 pmol per well.
Statistical analysis.
Data were analyzed using SigmaStat for Windows Version 3.10 (SysStat Software, San Jose, CA). MAP values are expressed as means ± SD; the remainder of the values in the figures and text are expressed as means ± SE of n observations, where n represents the number of animals in each group. Specifically, MAP results were analyzed by a two-way analysis of variance (ANOVA) with Bonferroni correction. For the remainder of the data analysis a one way ANOVA or a Kruskal-Wallis one-way ANOVA on ranks was utilized. A value of P < 0.05 was considered significant.
RESULTS
C-peptide ameliorates hypotension following resuscitation.
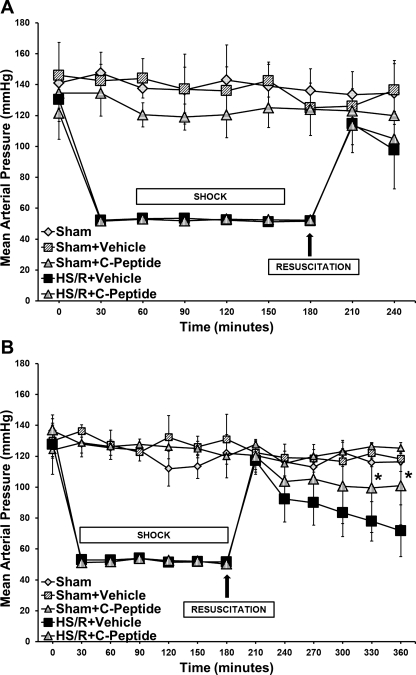
There were no significant differences in MAP at baseline among rats in the sham group; similarly, rats in the HS/R groups had no significant difference in MAP at baseline and during hemorrhage (Fig. 1, A and B). Sham rats had no significant difference in MAP throughout the experiment; additionally, treatment with C-peptide did not have any effect on MAP in this group (Fig. 1, A and B). Following resuscitation at 1 h there were no differences in MAP between vehicle- and C-peptide-treated rats (Fig. 1A). However, at 3 h after resuscitation, C-peptide-treated rats had a significantly higher MAP compared with vehicle-treated rats (101.3 ± 25 vs. 71.8 ± 16.7 mmHg, P < 0.05) (Fig. 1B). Of note, despite the differences in MAP, both groups undergoing HS/R had similar levels of plasma lactate at baseline, during hemorrhage, and following resuscitation. Hence our data suggest that C-peptide administration at the time of resuscitation and thereafter ameliorated hypotension in rats undergoing hemorrhagic shock.
Fig. 1.
Effect of in vivo treatment with vehicle or C-peptide on mean arterial pressure (MAP). A: time course depiction of MAP up to 1 h following resuscitation. B: time course depiction of MAP up to 3 h following resuscitation. Each data point represents mean ± SD of 3 rats in sham groups and 5 rats in hemorrhagic shock and resuscitation (HS/R) groups. *P < 0.05 vs. HS/R+Vehicle group.
C-peptide does not cause hypoglycemia.
C-peptide has been demonstrated to affect glucose utilization in diabetic patients (23). Hence we measured whole blood glucose levels at baseline, during hemorrhage, and following resuscitation. C-peptide-treated rats had no difference in blood glucose levels compared with vehicle-treated rats at baseline and during hemorrhage. At 1 and 3 h after resuscitation blood glucose levels were 107 ± 13 and 95 ± 18 mg/dl and in vehicle-treated and 87 ± 3 and 120 ± 15 mg/dl C-peptide treated rats, respectively.
C-peptide reduces plasma cytokine and chemokine levels.
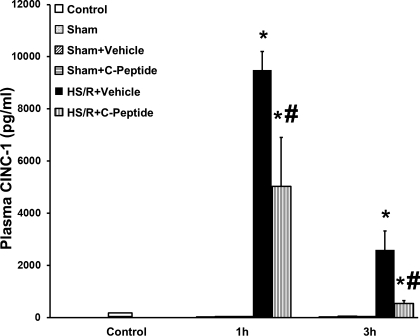
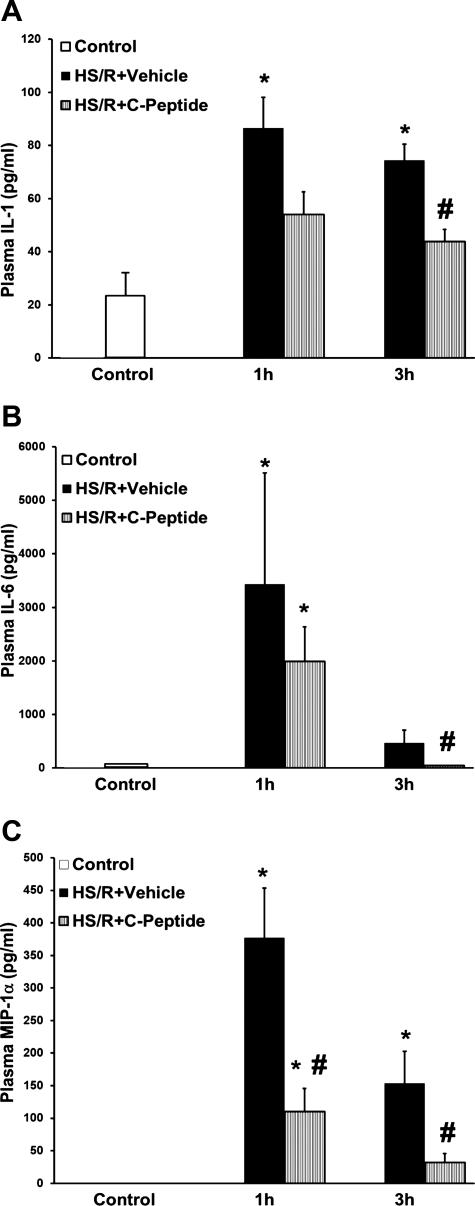
Plasma CINC-1 levels were measured at 1 and 3 h after resuscitation (Fig. 2). Rats undergoing HS/R has a significant increase in CINC-1 level compared with rats in both the control and sham groups at both time points. However, in rats undergoing HS/R, C-peptide treatment significantly reduced CINC-1 levels at 1 and 3 h compared with vehicle-treated rats (Fig. 2). Similarly, C-peptide treatment reduced plasma IL-1, IL-6, and MIP-1α levels at 1 and 3 h after resuscitation in rats undergoing HS/R compared with vehicle-treated rats (Fig. 3, A–C). Taken together, these data demonstrate that C-peptide treatment reduces plasma chemokine and cytokine levels to varying degrees in a time-course manner following HS/R, suggesting that C-peptide may blunt the systemic inflammatory state following HS/R.
Fig. 2.
Effect of in vivo treatment with vehicle or C-peptide on plasma cytokine-induced neutrophil chemoattractant 1 (CINC-1) levels at 1 and 3 h following HS/R. Each data point represents mean ± SE of 3 rats in sham groups, 5 rats in control and HS/R groups. *P < 0.05 vs. Control group; #P < 0.05 vs. HS/R+Vehicle group.
Fig. 3.
Effect of in vivo treatment with vehicle or C-peptide on plasma levels of IL-1 (A), IL-6 (B), and macrophage inflammatory protein-1 α (MIP-1α; C) at 1 and 3 h following HS/R. Each data point represents mean ± SE of 5 rats. *P < 0.05 vs. Control group; #P < 0.05 vs. HS/R+Vehicle group.
C-peptide treatment reduced lung neutrophil infiltration and injury.
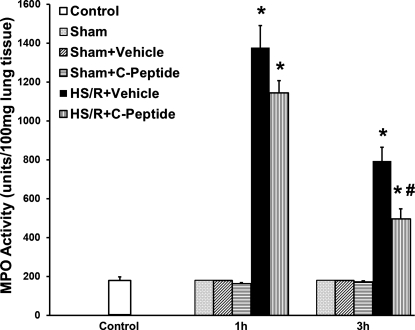
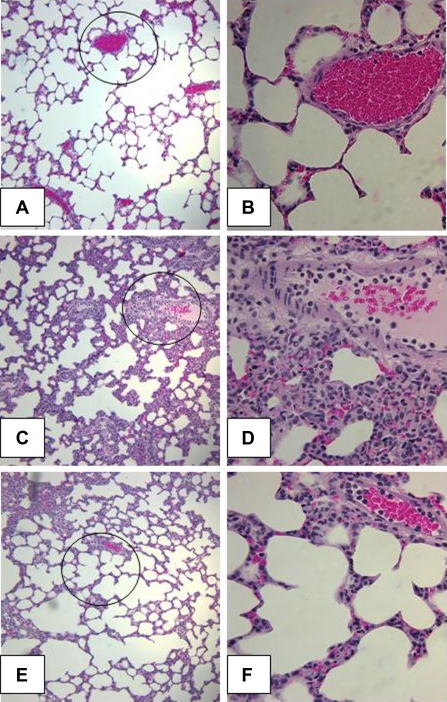
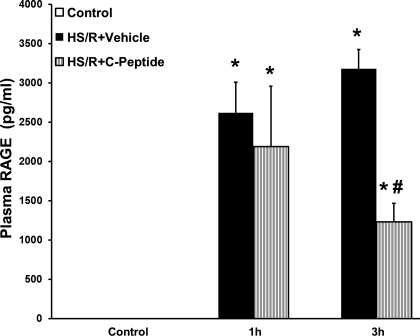
Lung neutrophil infiltration is associated with development of lung injury and is a common occurrence following hemorrhagic shock. We assessed lung neutrophil infiltration at 1 and 3 h following resuscitation using a MPO assay. Compared with levels in control and sham rats, rats undergoing HS/R and vehicle treatment had a significant increase in MPO activity (Fig. 4). However, rats undergoing HS/R who received C-peptide had a significant reduction in MPO activity compared with vehicle-treated rats at 1 and 3 h following resuscitation (Fig. 4). On histological examination HS/R vehicle-treated rats had remarkable alteration in lung architecture specifically increased interstitial edema and inflammatory cell infiltration compared with sham rats (Fig. 5, A–D). These pathological findings were attenuated in C-peptide-treated rats (Fig. 5, E and F). Furthermore, we quantified plasma RAGE levels as an objective surrogate of lung injury in our model. HS/R rats undergoing vehicle treatment had a significant increase in plasma RAGE levels compared with control rats (Fig. 6). In these rats, C-peptide treatment significantly reduced plasma RAGE levels compared with vehicle treated rats (Fig. 6).
Fig. 4.
Effect of in vivo treatment with vehicle or C-peptide on lung MPO activity at 1 and 3 h following HS/R. Each data point represents mean ± SE of 3 rats in sham groups, 5 rats in basal and HS/R groups. *P < 0.05 vs. Control group; #P < 0.05 vs. HS/R+Vehicle group.
Fig. 5.
Effect of in vivo treatment with vehicle or C-peptide on lung histology at 3 h after HS/R. A: representative photomicrograph of histology showing lung architecture from a rat in the Control group (magnification ×100). B: magnified section of prior image (indicated by circle) to show alveolar architecture and blood vessel from a rat in the Control group (magnification ×400). C: representative photomicrograph of histology showing lung architecture from a rat in the HS/R+Vehicle group (magnification ×100). D: magnified section of prior image (indicated by circle) demonstrating interstitial edema and inflammatory cell infiltration from a rat in the HS/R+Vehicle group (magnification ×400). E: representative photomicrograph of histology showing lung architecture from a rat in the HS/R+C-peptide group (magnification ×100). F: magnified section of prior image (indicated by circle) demonstrating a reduction in interstitial edema and inflammatory cell infiltration from a rat in the HS/R+C-peptide group (magnification ×400).
Fig. 6.
Effect of in vivo treatment with vehicle or C-peptide on plasma receptor for advanced glycation end products (RAGE) level at 1 and 3 h following HS/R. Each data point represents mean ± SE of 5 rats. *P < 0.05 vs. Control group; #P < 0.05 vs. HS/R+Vehicle group.
C-peptide increased PPAR-γ activity in the lung.
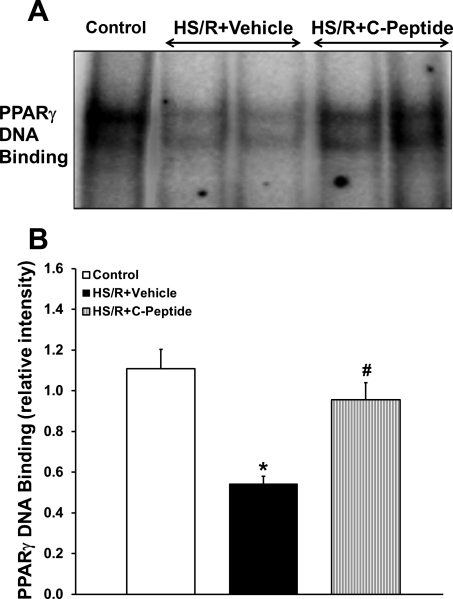
C-peptide has been demonstrated to activate the transcription factor PPAR-γ in opossum kidney cells in vitro and in vivo in the lungs of septic mice (4, 34). To discern the anti-inflammatory effect of C-peptide in our model, we assessed PPAR-γ nuclear expression and DNA binding in rats undergoing HS/R. By Western blot analysis nuclear expression of PPAR-γ was unchanged in C-peptide treated rats compared with vehicle-treated animals. However, vehicle-treated rats that underwent HS/R had a reduction in DNA binding compared with control rats (Fig. 7). C-peptide treatment in the HS/R group significantly increased DNA binding compared with vehicle-treated animals (Fig. 7).
Fig. 7.
Effect of in vivo treatment with vehicle or C-peptide on PPAR-γ DNA binding in lung at 3 h following HS/R. A: representative autoradiograph of EMSA for PPAR-γ. B: image analysis of PPAR-γ DNA binding determined by densitometry. Each data point represents mean ± SE of 5 rats from 2 separate experiments. *P < 0.05 vs. Control group; #P < 0.05 vs. HS/R+Vehicle group.
C-peptide reduced ERK1/2 phosphorylation in the lung.
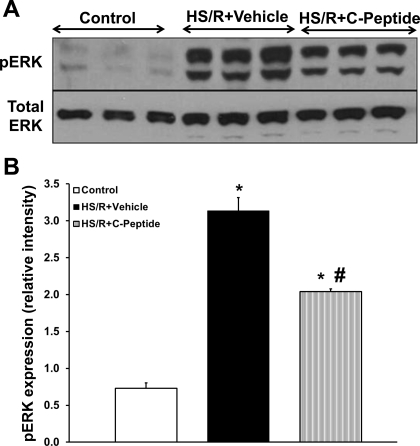
Since C-peptide has been reported to also modulate ERK1/2, we assessed ERK1/2 activation in lung extracts in rats undergoing HS/R (5, 34). There was negligible phosphorylation of ERK1/2 in control rats; 3 h after resuscitation vehicle-treated rats had a significant increase in ERK1/2 phosphorylation (Fig. 8). On the other hand, C-peptide treated rats had a significant reduction in ERK1/2 phosphorylation compared with vehicle-treated rats 3 h after resuscitation (Fig. 8), thus suggesting a reduction in kinase activation.
Fig. 8.
Effect of in vivo treatment with vehicle or C-peptide on phosphorylated form of ERK (pERK) in lung cytosol extracts at 3 h following HS/R. A: representative Western blot of phosphorylated form of ERK and total ERK. B: image analysis of nuclear content of phosphorylated form of ERK as determined by densitometry. Each data point represents mean ± SE of 5 rats from 2 separate experiments. *P < 0.05 vs. Control group; #P < 0.05 vs. HS/R+Vehicle group.
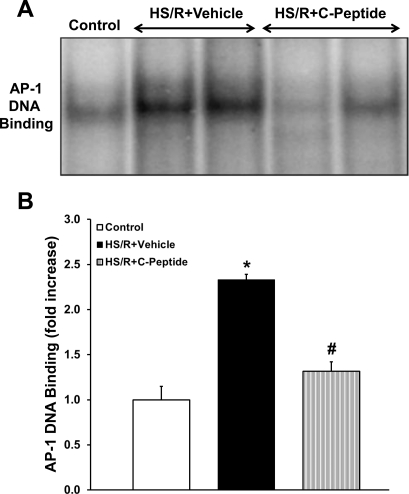
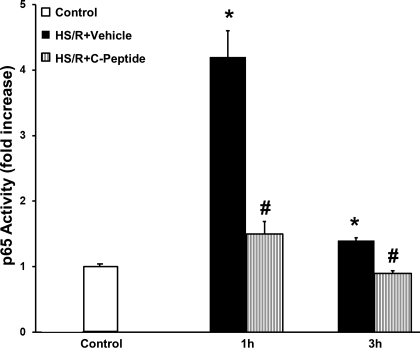
C-peptide reduced activation of AP-1 and NF-κB in the lung.
AP-1 and NF-κB are important transcription factors that modulate the inflammatory response following oxidative stress (2, 24). Hence we investigated the effect of C-peptide administration on AP-1 and NF-κB (p65 subunit) activation in the lung in rats undergoing HS/R. Vehicle-treated rats had a significant increase in AP-1 DNA binding following resuscitation compared with control rats (Fig. 9). C-peptide treatment significantly reduced AP-1 DNA binding following resuscitation compared with vehicle-treated rats (Fig. 9). Similarly, at 1 and 3 h following resuscitation vehicle-treated rats had a 4- and 1.6-fold increase in p65 activation, respectively, compared with control rats (Fig. 10). C-peptide treatment reduced p65 activation compared with vehicle-treated rats at both time points following resuscitation (Fig. 10). Taken together, these results indicate that C-peptide administration during resuscitation blunts activation of important proinflammatory transcription factors in the lung.
Fig. 9.
Effect of in vivo treatment with vehicle or C-peptide on activator protein-1 (AP-1) DNA binding in lung at 3 h following HS/R. A: representative autoradiograph of EMSA for AP-1. B: image analysis of AP-1 DNA binding determined by densitometry. Fold increase was calculated vs. control value, which was set at 1.0. Each data point represents mean ± SE of 5 rats from 2 separate experiments. *P < 0.05 vs. Control group; #P < 0.05 vs. HS/R+Vehicle group.
Fig. 10.
Effect of in vivo treatment with vehicle or C-peptide on activation of p65 subunit of NF-κB in lung nuclear extracts as determined by transcription factor assay at 1 and 3 h following HS/R. Fold increase was calculated vs. control value which was set at 1.0. Each data point represents mean ± SE of 5 rats. *P < 0.05 vs. Control group; #P < 0.05 vs. HS/R+Vehicle group.
DISCUSSION
Our present work represents the first study to explore the effects of C-peptide on the inflammatory response and lung injury in the setting of HS/R. Notably, when administered during resuscitation following hemorrhagic shock, C-peptide reduced the systemic inflammatory response as shown by a reduction in cytokine (IL-1, IL-6) and chemokine (CINC-1, MIP-1α) levels. Furthermore, C-peptide reduced lung damage, neutrophil infiltration, and plasma RAGE levels. In the lung, this beneficial effect was associated with inhibition of proinflammatory transcription factors AP-1 and NF-κB and activation of the anti-inflammatory transcription factor PPAR-γ. These anti-inflammatory effects were notable early after resuscitation and were independent of blood glucose levels.
C-peptide treatment in rats undergoing HS/R was associated with an improvement in blood pressure. These rats had a significantly higher MAP at the 3-h time point following resuscitation compared with vehicle-treated rats. Notably, C-peptide did not increase MAP in the sham group. Hence, it is possible that amelioration of the hypotensive state could be secondary to the blunted systemic inflammatory response seen with C-peptide treatment in rats subjected to HS/R. However, it has also been shown that C-peptide may have cardioprotective effects following ischemia-reperfusion. In this regard Young et al. (37) in an ex vivo model of global myocardial ischemia reperfusion have demonstrated that C-peptide improves left ventricular contractility. Although we did not assess myocardial contractility in our study such an effect may have contributed to our results.
Lung injury is one of the commonest consequences of resuscitated hemorrhagic shock and is characterized by a massive infiltration of neutrophils, which are a major cell type involved in exaggerated lung inflammation seen following resuscitation (1). Previous studies have demonstrated that C-peptide treatment is associated with reduction in lung injury scores in a murine model of endotoxic shock (34). Similarly in our study, we observed that C-peptide-treated rats had a reduction in lung interstitial and alveolar edema, alveolar damage, and infiltration of inflammatory cells compared with vehicle-treated rats. The present study also shows that C-peptide's therapeutic effect on lung damage may be a consequence of a reduction in neutrophil recruitment through inhibition of chemokine release, notably CINC-1 and MIP-1α. Other experimental evidence indicates that C-peptide may interfere with neutrophil adhesion and recruitment (29). Scalia et al. (29) demonstrated a reduction in leukocyte rolling and adherence with reduced endothelial cell surface expression of adhesion molecules (P-selectin and ICAM-1) in rat mesenteric vasculature with C-peptide treatment. More recently, C-peptide has been shown to reduce endothelial cell expression of VCAM-1 and a reduction in hyperglycemia mediated production of chemokines IL-8 and monocyte chemoattractant protein-1 (25). It is unlikely that this mechanism contributed to the anti-inflammatory effects seen with C-peptide in our work as hyperglycemia was not noted in either vehicle or C-peptide treated rats. However, these data do suggest that C-peptide may act as an important modulator of leukocyte endothelial cell interaction in the lung thereby impacting neutrophil trafficking and preventing neutrophil-induced lung damage.
HS/R is associated with alveolar epithelial cell injury and death (7). These cells serve an important barrier function in the alveolus and play an important role in alveolar fluid clearance and are involved in the pathogenesis of ALI (36). Recent work has suggested that the soluble forms of the protein RAGE, which is expressed on alveolar epithelial cells, may serve as a marker of alveolar epithelial cell injury (33). Furthermore, plasma RAGE levels in trauma patients were helpful in discriminating patients with ALI (17). We utilized plasma RAGE a potential marker of lung injury in our rodent model. In our study C-peptide-treated rats undergoing HS/R had a decrease in plasma RAGE levels at 1 and 3 h following resuscitation compared with vehicle-treated rats. This would suggest a reduction in lung injury, possibly alveolar epithelial cell injury. Whether this reduction in plasma RAGE levels is secondary to the effect of C-peptide-mediated reduction in neutrophil infiltration or a direct effect of C-peptide on RAGE signaling and expression cannot be discerned from our work. Taken together with lung histology and neutrophil infiltration, it would appear that C-peptide does reduce lung inflammation and injury following HS/R.
It has been shown that oxidative stress during HS/R may activate several MAPKs such as ERK 1/2, JNK, and IKK, which mediate the transduction of oxidant stress signals to the nuclear transcription factors AP-1 and NF-κB (16). Furthermore, recent studies from our laboratory have demonstrated that, whereas the proinflammatory responses are exaggerated in the lung following hemorrhagic shock, the anti-inflammatory pathway of the nuclear receptor PPAR-γ is dramatically depressed (40).
PPAR-γ is a nuclear transcription factor whose activation is well described to be associated with anti-inflammatory effects in systemic inflammatory processes such as septic shock, myocardial ischemia-reperfusion, and hemorrhagic shock (39). In our present work, C-peptide administration induced a significant increase in PPAR-γ DNA binding in the lung compared with vehicle-treated rats. Interestingly, nuclear expression of PPAR-γ was not different between C-peptide-treated and vehicle-treated rats. The mechanism by which C-peptide activates PPAR-γ in vivo is unclear. In vitro studies in proximal renal tubular cells have shown that C-peptide increases PPAR-γ transcriptional activity in a concentration-dependant manner without altering PPAR-γ protein expression (4). Similarly, in vivo C-peptide administration in endotoxemic mice increased PPAR-γ activation in the lung with a concurrent blunting of the inflammatory response and lung injury (34). Hence, it appears that C-peptide may act as a PPAR-γ activator in the lung and thereby may dampen the inflammatory response.
NF-κB is a major proinflammatory transcription factor that is activated early after hemorrhage and resuscitation and is involved in the pathogenesis of lung inflammation and injury (30). The NF-κB protein consists of two subunits p65 and p50. In the quiescent state these subunits exist complexed with IκB proteins in the cytosol. Classically, the activation sequence for this pathway involves phosphorylation of IκBα by an upstream kinase with subsequent degradation and release of the p65 and p50 subunits (38). These subunits translocate to the nucleus, where they orchestrate the expression of numerous genes involved in the inflammatory process (38). We assessed activation of the p65 subunit of NF-κB by utilizing a transcription factor ELISA. Following hemorrhage and resuscitation vehicle-treated rats had a significant increase in p65 activation compared with sham rats. C-peptide treatment significantly reduced p65 activation to basal levels compared with vehicle-treated rats. Similarly to our findings, in vitro work in human aortic endothelial cells exposed to hyperglycemia demonstrated an increase in nuclear translocation of the p65 subunit; C-peptide treatment significantly reduced translocation of p65 and binding activity of p50 (25). These results suggest that C-peptide has the ability to inhibit activation of NF-κB subunits by inhibiting their nuclear translocation.
AP-1 represents another important transcriptional factor that regulates the synthesis of a multitude of proinflammatory proteins (24). Vehicle-treated rats had a significant increase in AP-1 DNA binding following resuscitation compared with sham rats. C-peptide treatment significantly reduced AP-1 DNA binding following resuscitation compared with vehicle-treated rats. In support of these findings, we have also observed that AP-1 activation is inhibited in the kidney following resuscitation with C-peptide treatment and this event is associated with improved kidney function (13). The mechanism by which C-peptide may effect NF-κB and AP-1 activation is unclear. Our study suggests that C-peptide may modulate the upstream activation of MAPK signaling. C-peptide has been previously described to modulate the activation of ERK1/2 members of the MAPK family that regulates the inflammatory response (18). For example, C-peptide treatment reduced ERK1/2 in the lung in endotoxemic mice (34). On the contrary, in vitro studies have demonstrated that C-peptide may activate ERK1/2 as shown by Al-Rasheed et al. (5) in opossum kidney cells, thereby impacting cell proliferation. It has also been reported that following trauma-hemorrhage ERK1/2 activation is associated with the development of a proinflammatory state that may contribute to organ injury (19). In support of a pathogenic role of these kinases, experimental studies have shown that ERK1/2 inhibition may be beneficial in reducing organ injury following trauma hemorrhage (3, 21). In our study, vehicle-treated rats had a significant activation of ERK1/2 in lung extracts compared with sham rats. C-peptide treatment reduced ERK1/2 phosphorylation compared with vehicle-treated rats. Taken together, these data suggest that in vivo C-peptide may act as a potent inhibitor of ERK1/2 activation. Other studies from our laboratory also demonstrate that C-peptide may inhibit JNK activation in the rodent kidney following hemorrhagic shock (13). Furthermore, in vitro studies suggest the existence of an interesting cross-talk between kinases such as ERK1/2 and PPAR-γ. For example, it has been shown that ERK1/2-mediated serine phosphorylation is associated with a reduction in PPAR-γ translational activity in placental and kidney cell lines (3, 10). Furthermore, PPAR-γ activation is associated with inhibition of ERK1/2 in vascular smooth muscle cells (8); on the contrary, ERK1/2 inhibition has been shown to reactivate PPAR-γ in adipocytes exposed to cyclic stretch (32). Thus, following HS/R, C-peptide may impact cross-talk between ERK1/2 and PPAR-γ, resulting in enhanced anti-inflammatory effects in the lung.
Our work has limitations. The nature of the cell type being affected by C-peptide in our model is unclear. It appears that C-peptide may exert its effects by interacting with a receptor on the cell surface (28). However, despite some work suggesting that this may be a G-protein coupled receptor, the nature of its receptor remains unclear (35). Additionally, it is unclear whether the effects on cell signaling represent direct or indirect actions of this peptide. Further work will need to be performed to elucidate the mechanism by which C-peptide modulates cell signaling. Additionally, the effects of C-peptide on specific cell types in the lung will need to be defined.
In conclusion, our study represents the first to explore the effects of C-peptide in an in vivo model of hemorrhagic shock. C-peptide administered following hemorrhagic shock was associated with blunting of the systemic inflammatory response and a reduction in lung inflammation. In the lung these effects were associated with PPAR-γ activation and ERK1/2, NF-κB, and AP-1 inhibition. These findings are novel and clinically relevant because C-peptide was administered during resuscitation. Hence, C-peptide may represent a novel endogenous peptide with anti-inflammatory properties.
GRANTS
This study was supported by the Shock Society/Novo Nordisk grant to young investigators (R. S. Chima) and National Institutes of Health K12 HD028827 (to R. S. Chima) and R01 AG027990 (to B. Zingarelli).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
REFERENCES
- 1. Abraham E. Neutrophils and acute lung injury. Crit Care Med 31: S195–S199, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Abraham E. NF-kappaB activation. Crit Care Med 28: N100–N104, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor gamma is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. J Biol Chem 272: 5128–5132, 1997 [DOI] [PubMed] [Google Scholar]
- 4. Al-Rasheed NM, Chana RS, Baines RJ, Willars GB, Brunskill NJ. Ligand-independent activation of peroxisome proliferator-activated receptor-gamma by insulin and C-peptide in kidney proximal tubular cells: dependent on phosphatidylinositol 3-kinase activity. J Biol Chem 279: 49747–49754, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Al-Rasheed NM, Meakin F, Royal EL, Lewington AJ, Brown J, Willars GB, Brunskill NJ. Potent activation of multiple signalling pathways by C-peptide in opossum kidney proximal tubular cells. Diabetologia 47: 987–997, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Auten RL, Mason SN, Whorton MH, Lampe WR, Foster WM, Goldberg RN, Li B, Stamler JS, Auten KM. Inhaled ethyl nitrite prevents hyperoxia-impaired postnatal alveolar development in newborn rats. Am J Respir Crit Care Med 176: 291–299, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barlos D, Deitch EA, Watkins AC, Caputo FJ, Lu Q, Abungu B, Colorado I, Xu DZ, Feinman R. Trauma-hemorrhagic shock-induced pulmonary epithelial and endothelial cell injury utilizes different programmed cell death signaling pathways. Am J Physiol Lung Cell Mol Physiol 296: L404–L417, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benkirane K, Amiri F, Diep QN, El Mabrouk M, Schiffrin EL. PPAR-γ inhibits ANG II-induced cell growth via SHIP2 and 4E-BP1. Am J Physiol Heart Circ Physiol 290: H390–H397, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R, Cochin B, Lanken PN, Leeper KV, Marini J, Murray JF, Oppenheimer L, Pesenti A, Reid L, Rinaldo J, Villar J, Vanasbeck BS, Dhainaut JF, Mancebo J, Matthay M, Meyrick B, Payen D, Perret C, Fowler AA, Schaller MD, Hudson LD, Hyers T, Knaus W, Matthay R, Pinsky M, Bone RC, Bosken C, Johanson WG, Lewandowski K, Repine J, Rodriguez-Roisin R, Roussos C, Antonelli MA, Beloucif S, Bihari D, Burchardi H, Lemaire F, Montravers P, Petty TL, Robotham J, Zapol W. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J Biol Chem 272: 10811–10816, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 410: 37–40, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Chima RS, Hake PW, Piraino G, Mangeshkar P, Denenberg A, Zingarelli B. Ciglitazone ameliorates lung inflammation by modulating the increased inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. Crit Care Med 36: 2849–2857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chima RS, Maltese G, Lamontagne T, Piraino G, Denenberg A, O'Connor M, Zingarelli B. C-peptide ameliorates kidney injury following hemorrhagic shock. Shock 2011. EPub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. The role of the lung in postinjury multiple organ failure. Surgery 138: 749–757; discussion 757–748, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Durham RM, Moran JJ, Mazuski JE, Shapiro MJ, Baue AE, Flint LM. Multiple organ failure in trauma patients. J Trauma 55: 608–616, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol 281: L1037–L1050, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Fremont RD, Koyama T, Calfee CS, Wu W, Dossett LA, Bossert FR, Mitchell D, Wickersham N, Bernard GR, Matthay MA, May AK, Ware LB. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma 68: 1121–1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hills CE, Brunskill NJ. Intracellular signalling by C-peptide. Exp Diabetes Res 2008: 635158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu JT, Kan WH, Hsieh CH, Choudhry MA, Bland KI, Chaudry IH. Role of extracellular signal-regulated protein kinase (ERK) in 17beta-estradiol-mediated attenuation of lung injury after trauma-hemorrhage. Surgery 145: 226–234, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Ido Y, Vindigni A, Chang K, Stramm L, Chance R, Heath WF, DiMarchi RD, Di Cera E, Williamson JR. Prevention of vascular and neural dysfunction in diabetic rats by C-peptide. Science 277: 563–566, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Jarrar D, Song GY, Kuebler JF, Rue LW, Bland KI, Chaudry IH. The effect of inhibition of a major cell signaling pathway following trauma hemorrhage on hepatic injury and interleukin 6 levels. Arch Surg 139: 896–901, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391: 82–86, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Johansson BL, Sjoberg S, Wahren J. The influence of human C-peptide on renal function and glucose utilization in type 1 (insulin-dependent) diabetic patients. Diabetologia 35: 121–128, 1992 [DOI] [PubMed] [Google Scholar]
- 24. Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol 9: 240–246, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Luppi P, Cifarelli V, Tse H, Piganelli J, Trucco M. Human C-peptide antagonises high glucose-induced endothelial dysfunction through the nuclear factor-kappaB pathway. Diabetologia 51: 1534–1543, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active NFkappaB. Nucleic Acids Res 29: E21, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391: 79–82, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Rigler R, Pramanik A, Jonasson P, Kratz G, Jansson OT, Nygren P, Stahl S, Ekberg K, Johansson B, Uhlen S, Uhlen M, Jornvall H, Wahren J. Specific binding of proinsulin C-peptide to human cell membranes. Proc Natl Acad Sci USA 96: 13318–13323, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scalia R, Coyle KM, Levine BJ, Booth G, Lefer AM. C-peptide inhibits leukocyte-endothelium interaction in the microcirculation during acute endothelial dysfunction. FASEB J 14: 2357–2364, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Shenkar R, Abraham E. Hemorrhage induces rapid in vivo activation of CREB and NF-kappaB in murine intraparenchymal lung mononuclear cells. Am J Respir Cell Mol Biol 16: 145–152, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Steiner DF, Cunningham D, Spigelman L, Aten B. Insulin biosynthesis: evidence for a precursor. Science 157: 697–700, 1967 [DOI] [PubMed] [Google Scholar]
- 32. Tanabe Y, Koga M, Saito M, Matsunaga Y, Nakayama K. Inhibition of adipocyte differentiation by mechanical stretching through ERK-mediated downregulation of PPARgamma2. J Cell Sci 117: 3605–3614, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 173: 1008–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vish MG, Mangeshkar P, Piraino G, Denenberg A, Hake PW, O'Connor M, Zingarelli B. Proinsulin c-peptide exerts beneficial effects in endotoxic shock in mice. Crit Care Med 35: 1348–1355, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Wahren J, Ekberg K, Johansson J, Henriksson M, Pramanik A, Johansson BL, Rigler R, Jornvall H. Role of C-peptide in human physiology. Am J Physiol Endocrinol Metab 278: E759–E768, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Young LH, Ikeda Y, Scalia R, Lefer AM. C-peptide exerts cardioprotective effects in myocardial ischemia-reperfusion. Am J Physiol Heart Circ Physiol 279: H1453–H1459, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Zingarelli B. Nuclear factor-kappaB. Crit Care Med 33: S414–S416, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Zingarelli B, Cook JA. Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation. Shock 23: 393–399, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Zingarelli B, Hake PW, O'Connor M, Burroughs TJ, Wong HR, Solomkin JS, Lentsch AB. Lung injury after hemorrhage is age dependent: role of peroxisome proliferator-activated receptor gamma. Crit Care Med 37: 1978–1987, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zingarelli B, Hake PW, Yang Z, O'Connor M, Denenberg A, Wong HR. Absence of inducible nitric oxide synthase modulates early reperfusion-induced NF-kappaB and AP-1 activation and enhances myocardial damage. FASEB J 16: 327–342, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Zingarelli B, Ischiropoulos H, Salzman AL, Szabo C. Amelioration by mercaptoethylguanidine of the vascular and energetic failure in haemorrhagic shock in the anesthetised rat. Eur J Pharmacol 338: 55–65, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Zingarelli B, Sheehan M, Hake PW, O'Connor M, Denenberg A, Cook JA. Peroxisome proliferator activator receptor-gamma ligands, 15-deoxy-Delta(12,14)-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol 171: 6827–6837, 2003 [DOI] [PubMed] [Google Scholar]