Abstract
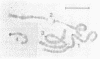
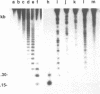
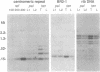
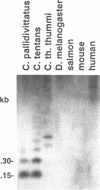
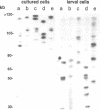
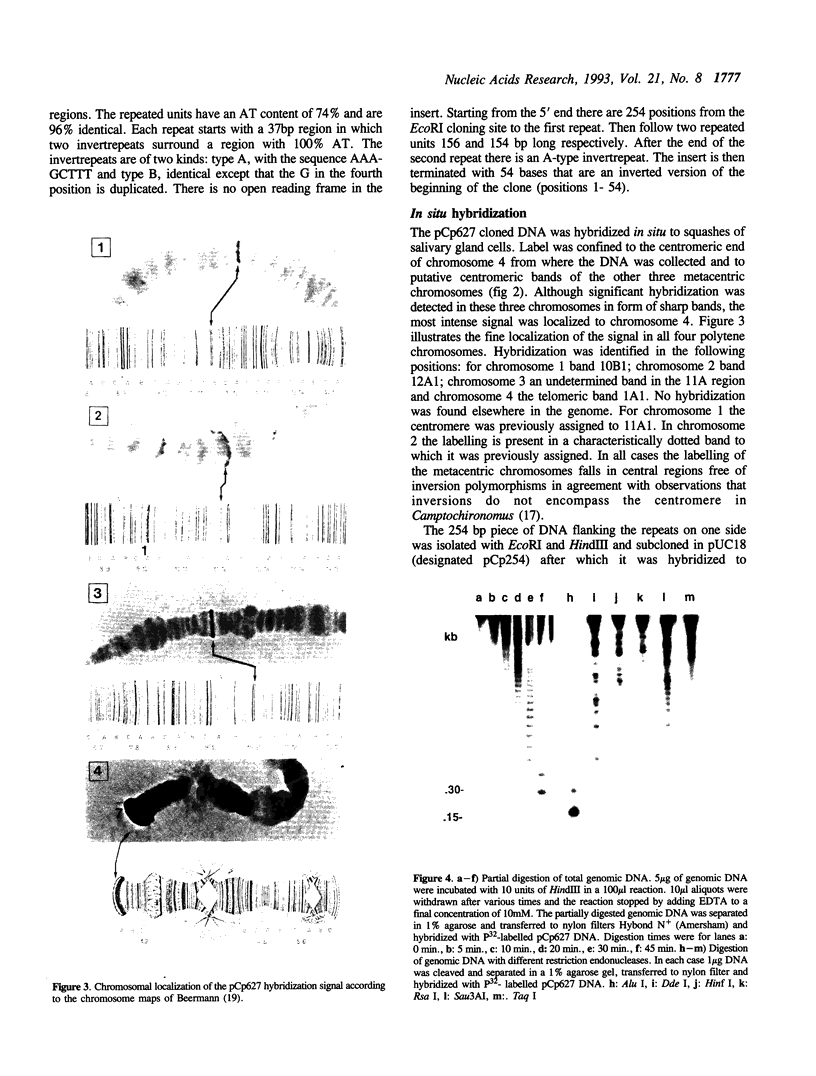
A clone containing centromere-associated DNA from Chironomus pallidivittatus was obtained by microdissection-microcloning. It hybridizes to the centromeric end of one chromosome and exclusively to regions in the three remaining, metacentric chromosomes to which centromeres have previously been localized on cytological grounds. In the metacentric positions the hybridization can be assigned to thin bands. The clone contains 155bp tandem repeats and short flanking regions represented in all of the centromeres. Titration experiments show that the four centromeres together contain 200kb of 155bp repeat per genome. In a line of tissue culture cells the amounts are increased by a factor 1.5-2, resulting in proportionately extended arrays of tandem repeats. Each repeat contains two invertrepeats surrounding a region containing only AT base pairs, a feature with some similarity to functionally essential elements in the Saccharomyces cerevisiae centromere.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERMANN W. Cytologische Analyse eines Camptochironomus-Artbastards. I. Kreuzungsergebnisse und die Evolution des Karyotypus. Chromosoma. 1955;7(2-3):198–259. doi: 10.1007/BF00329725. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Levis R., Rubin G. M. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981 Sep;25(3):693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Clarke L., Amstutz H., Fishel B., Carbon J. Analysis of centromeric DNA in the fission yeast Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8253–8257. doi: 10.1073/pnas.83.21.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Baum M. P. Functional analysis of a centromere from fission yeast: a role for centromere-specific repeated DNA sequences. Mol Cell Biol. 1990 May;10(5):1863–1872. doi: 10.1128/mcb.10.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature. 1980 Oct 9;287(5782):504–509. doi: 10.1038/287504a0. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Cohn M., Edström J. E. Chromosome ends in Chironomus pallidivittatus contain different subfamilies of telomere-associated repeats. Chromosoma. 1992 Oct;101(10):634–640. doi: 10.1007/BF00360541. [DOI] [PubMed] [Google Scholar]
- Daneholt B., Edström J. E. The content of deoxyribonucleic acid in individual polytene chromosomes of Chironomus tentans. Cytogenetics. 1967;6(5):350–356. doi: 10.1159/000154939. [DOI] [PubMed] [Google Scholar]
- Dreesen T. D., Bower J. R., Case S. T. A second gene in a Balbiani ring. Chironomus salivary glands contain a 6.5-kb poly(A)+ RNA that is transcribed from a hierarchy of tandem repeated sequences in Balbiani ring 1. J Biol Chem. 1985 Sep 25;260(21):11824–11830. [PubMed] [Google Scholar]
- Earnshaw W. C., Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91(3-4):313–321. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- Gall J. G., Atherton D. D. Satellite DNA sequences in Drosophila virilis. J Mol Biol. 1974 Jan 5;85(4):633–664. doi: 10.1016/0022-2836(74)90321-0. [DOI] [PubMed] [Google Scholar]
- Galler R., Saiga H., Widmer R. M., Lezzi M., Edström J. E. Two genes in Balbiani ring 2 with metabolically different 75S transcripts. EMBO J. 1985 Nov;4(11):2977–2982. doi: 10.1002/j.1460-2075.1985.tb04032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R. L., Karpen G. H., Spradling A. C. Replication forks are not found in a Drosophila minichromosome demonstrating a gradient of polytenization. Chromosoma. 1992 Dec;102(1):15–19. doi: 10.1007/BF00352285. [DOI] [PubMed] [Google Scholar]
- Grady D. L., Ratliff R. L., Robinson D. L., McCanlies E. C., Meyne J., Moyzis R. K. Highly conserved repetitive DNA sequences are present at human centromeres. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1695–1699. doi: 10.1073/pnas.89.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaf T., Warburton P. E., Willard H. F. Integration of human alpha-satellite DNA into simian chromosomes: centromere protein binding and disruption of normal chromosome segregation. Cell. 1992 Aug 21;70(4):681–696. doi: 10.1016/0092-8674(92)90436-g. [DOI] [PubMed] [Google Scholar]
- Joseph A., Mitchell A. R., Miller O. J. The organization of the mouse satellite DNA at centromeres. Exp Cell Res. 1989 Aug;183(2):494–500. doi: 10.1016/0014-4827(89)90408-4. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto H., Masukata H., Muro Y., Nozaki N., Okazaki T. A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J Cell Biol. 1989 Nov;109(5):1963–1973. doi: 10.1083/jcb.109.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaseko Y., Adachi Y., Funahashi S., Niwa O., Yanagida M. Chromosome walking shows a highly homologous repetitive sequence present in all the centromere regions of fission yeast. EMBO J. 1986 May;5(5):1011–1021. doi: 10.1002/j.1460-2075.1986.tb04316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Chromosomal localization of mouse satellite DNA. Science. 1970 Jun 12;168(3937):1356–1358. doi: 10.1126/science.168.3937.1356. [DOI] [PubMed] [Google Scholar]
- Scalenghe F., Turco E., Edström J. E., Pirrotta V., Melli M. Microdissection and cloning of DNA from a specific region of Drosophila melanogaster polytene chromosomes. Chromosoma. 1981;82(2):205–216. doi: 10.1007/BF00286105. [DOI] [PubMed] [Google Scholar]
- Schmidt E. R. Clustered and interspersed repetitive DNA sequence family of Chironomus. The nucleotide sequence of the Cla-elements and of various flanking sequences. J Mol Biol. 1984 Sep 5;178(1):1–15. doi: 10.1016/0022-2836(84)90227-4. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. The organization of repetitive sequences in a cluster of rabbit beta-like globin genes. Cell. 1980 Feb;19(2):379–391. doi: 10.1016/0092-8674(80)90512-7. [DOI] [PubMed] [Google Scholar]
- Sullivan K. M., Lilley D. M. A dominant influence of flanking sequences on a local structural transition in DNA. Cell. 1986 Dec 5;47(5):817–827. doi: 10.1016/0092-8674(86)90524-6. [DOI] [PubMed] [Google Scholar]
- Sümegi J., Wieslander L., Daneholt B. A hierarchic arrangement of the repetitive sequences in the Balbiani ring 2 gene of Chironomus tentans. Cell. 1982 Sep;30(2):579–587. doi: 10.1016/0092-8674(82)90254-9. [DOI] [PubMed] [Google Scholar]
- Wong A. K., Rattner J. B. Sequence organization and cytological localization of the minor satellite of mouse. Nucleic Acids Res. 1988 Dec 23;16(24):11645–11661. doi: 10.1093/nar/16.24.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss C. Chironomus tentans epithelial cell lines sensitive to ecdysteroids, juvenile hormone, insulin and heat shock. Exp Cell Res. 1982 Jun;139(2):309–319. doi: 10.1016/0014-4827(82)90255-5. [DOI] [PubMed] [Google Scholar]