Abstract
The superior olivary nucleus (SON) is the primary source of inhibition in the avian auditory brainstem. While much is known about the role of inhibition at the SON's target nuclei, little is known about how the SON itself processes auditory information or how inhibition modulates these properties. Additionally, the synaptic physiology of inhibitory inputs within the SON has not been described. We investigated these questions using in vivo and in vitro electrophysiological techniques in combination with immunohistochemistry in the chicken, an organism for which the auditory brainstem has otherwise been well characterized. We provide a thorough characterization of monaural response properties in the SON and the influence of inhibitory input in shaping these features. We found that the SON contains a heterogeneous mixture of response patterns to acoustic stimulation and that in most neurons these responses are modulated by both GABAergic and glycinergic inhibitory inputs. Interestingly, many SON neurons tuned to low frequencies have robust phase-locking capability and the precision of this phase locking is enhanced by inhibitory inputs. On the synaptic level, we found that evoked and spontaneous inhibitory postsynaptic currents (IPSCs) within the SON are also mediated by both GABAergic and glycinergic inhibition in all neurons tested. Analysis of spontaneous IPSCs suggests that most SON cells receive a mixture of both purely GABAergic terminals, as well as terminals from which GABA and glycine are coreleased. Evidence for glycinergic signaling within the SON is a novel result that has important implications for understanding inhibitory function in the auditory brainstem.
Keywords: GABAA receptor, sound localization, superior olivary nucleus, glycine receptor, inhibition, interaural time disparity
interaural time disparities (ITDs) are the main cue that animals use to localize low frequency sounds. Many features of neural circuitry that process this cue are similar between birds and mammals. For example, both systems involve specialized coincidence-detecting neurons that detect the timing differences of spikes arriving from both ears. These neurons comprise the medial superior olive in mammals (Goldberg and Brown 1969; Yin and Chan 1990) and nucleus laminaris (NL) in birds (Parks and Rubel 1975; Sullivan and Konishi 1986; Carr and Konishi 1990; Peña et al. 1996; Burger and Rubel 2008; Grothe et al. 2010). Additionally, both systems include inhibitory feedback pathways to monaural and binaural processing centers originating from one or more nuclei located in the superior olive (Caspary et al. 1994; Lachica et al. 1994; Ebert and Ostwald 1995a,b; Westerberg and Schwarz 1995; Smith et al. 1998; Backoff et al. 1999; Yang et al. 1999; Kopp-Scheinpflug et al. 2002; Burger et al. 2005).
In birds, the superior olivary nucleus (SON) is the primary source of inhibition to both cochlear nuclei, nucleus angularis (NA) and nucleus magnocellularis (NM), as well as the coincidence detecting neurons in NL (Carr et al. 1989; Lachica et al. 1994; Westerberg and Schwarz 1995; Yang et al. 1999; Burger et al. 2005). The SON receives excitatory inputs from the ipsilateral NA and NL and a putatively inhibitory input from the contralateral SON (Yang et al. 1999; Monsivais et al. 2000; Burger et al. 2005; Fukui et al. 2010). In vitro studies on chicken brainstem slices have shown that GABAergic input from the SON evokes a depolarizing GABAergic conductance in both NL and NM that improves ITD selectivity and phase locking to auditory stimuli (Yang et al. 1999; Monsivais et al. 2000). These studies suggested that GABAergic inhibition at NL and NM arising from the SON has relatively slow kinetic properties and is unlikely to contribute to temporal properties on a cycle-by-cycle basis. More recent work has shown that inhibitory signaling within the avian auditory brainstem is not solely GABAergic but also includes a glycinergic component endowed with faster kinetics (Kuo et al. 2009). The source of this glycinergic input is unknown but appears to derive from corelease of GABA and glycine from some inhibitory terminals (Kuo et al. 2009).
Recent studies have contributed much to our understanding of SON function in shaping ITD selectivity in birds. Peña et al. (1996) proposed that inhibition contributes to maintenance of ITD selectivity over a wide range of intensities (Peña et al. 1996; Viete et al. 1997), and more recent models based on new anatomical evidence expanded on this idea (Monsivais et al. 2000; Burger et al. 2005; Dasika et al. 2005). We proposed that in addition to the inhibitory feedback mechanism at the ipsilateral NA, NM, and NL, the putative inhibitory connection between the two SONs would serve to balance input strength for coincidence detecting neurons when stimulus amplitude was bilaterally unequal (Burger et al. 2005; Dasika et al. 2005). Recent work has supported this hypothesis by demonstrating that the SON modulates both ITD encoding in NL and rate/intensity coding in NM bilaterally (Nishino et al. 2008; Fukui et al. 2010).
Despite its central role in these pathways, the nature of acoustic response properties or inhibitory signaling within the SON has not been thoroughly investigated. While there has been much gained from investigation of the influence of inhibition and SON function at its targets, very little is known about how the SON itself processes auditory information. Only a few studies to date report in vivo recordings from SON cells (Moiseff and Konishi 1983; Lachica et al. 1994; Tabor et al. 2011), but these recordings were incidental to the primary goals of these studies. Thus a thorough characterization of chicken SON auditory response properties is lacking. Since the SON plays a central role in maintaining ITD selectivity as the primary source of inhibition for many targets in the avian auditory system, we sought: 1) to characterize the monaural response properties of SON cells to acoustic stimulation, 2) to investigate how these properties are modulated by inhibition, and 3) to investigate the nature of inhibitory synaptic transmission at SON neurons. We pursued these questions using in vivo and in vitro electrophysiological recording techniques and immunohistochemical analysis. We found that the SON contains a heterogeneous mixture of cell types with diverse monaural response patterns to acoustic stimulation and that these responses are modulated by both GABAergic and glycinergic inhibitory inputs in a majority of neurons. Surprisingly, many SON cells have strong phase-locking capability, and the precision of this phase-locking is enhanced by inhibitory inputs. On the synaptic level, we found in all SON neurons that we sampled evoked (eIPSCs) and spontaneous inhibitory postsynaptic currents (sIPSCs) were mediated by both GABAergic and glycinergic inhibition. Immunohistochemical evidence and analysis of sIPSCs revealed that most SON cells receive a mixture of both purely GABAergic terminals as well as terminals from which GABA and glycine are coreleased. Evidence for glycinergic signaling within the SON is a novel result that may have important implications for the role of inhibition in the auditory brainstem.
MATERIALS AND METHODS
Animals/surgery.
All procedures were approved by the Lehigh University Institutional Animal Care and Use Committee. Eighty White Leghorn chickens aged postnatal days 5–30 were used for the in vivo experiments. Fertilized eggs were obtained from a commercial poultry supplier (Moyer's Chicks, Quakertown, PA) and raised at Lehigh University's central animal facility. Chicks were initially anesthetized for surgery with a single dose of ketamine (Ketaset; Fort Dodge Animal Health, Fort Dodge, IA) at 80 mg/kg im and pentobarbital (Sigma-Aldrich, Saint Louis, MO) at 80 mg/kg ip. For recording, anesthesia was switched to urethane (full dose: 2.5 g/kg, given in quarter doses approximately every 30–45 min im). Small supplemental doses were administered if a chick showed any signs of discomfort. After chicks were deeply anesthetized, feathers were removed from the head and the guard feathers were removed from the opening of the ear canal with scissors and depilatory cream. A layer of skin was reflected on the neck to expose the trachea. A small incision was made and the trachea was intubated using a blunt-ended dispensing needle secured with thread. Following tracheotomy, chicks breathed normally during the experiment. Throughout experiments, body temperature was maintained at 39.5°C via a TC-1000 temperature controller and cloacal thermistor probe (CWE, Ardmore, PA). The head was secured in a stereotaxic apparatus (Stoelting, Wood Dale, IL) with ear bars and adjusted to approximately a 45° angle with respect to the horizontal plane. An incision was made in the scalp, and the tissue was reflected from the top of the skull. Any remaining fascia on the skull was cauterized, and the skull was covered with a thin layer of cyanoacrylate adhesive and small glass beads. A brass rod was then secured to the rostral skull at the midline with dental acrylic. After the dental acrylic hardened, the chick was transferred to a custom stereotaxic apparatus (Schuller et al. 1986) inside a sound-attenuation booth (Industrial Acoustics, Bronx, NY). The head was then adjusted to a 45° pitch angle, 0° roll, and 0° yaw with respect to the horizontal axis. A hole was drilled in the skull overlying the cerebellum ∼2.1 mm lateral to the midline. The dura was excised and reflected from the cerebellum. Speakers were coupled to the ears with tubes insulated with acoustic foam (Etymotic Research, Elk Grove Village, IL), and a ground wire was inserted into the exposed neck muscle at the base of the head.
In vivo recording configuration.
The SON was located using a single blunt-tipped glass electrode (filled with 3 M KCl, 0.5–1 MΩ resistance) advanced through the brainstem using a remotely driven actuator (MC1000e-1 Controller; Siskiyou Design Instruments) while presenting 50-ms noise bursts in the ispilateral ear. The SON was identifiable by characteristic responses to noise bursts that evoked strong sustained responses lasting over a penetration depth of ∼500 μm. The SON was typically located 2.1 to 2.2 mm from the midline, rostral to NM and ∼8–9 mm from the surface of the cerebellum. After the SON was located, the blunt electrode was replaced by either a single sharp-tipped borosilicate recording electrode or by a “piggy-back” multibarrel electrode (Havey and Caspary 1980) used for simultaneous recording and local iontophoresis of drugs. Recording electrodes were filled with 3 M KCl and typically had a resistance range of 5–15 MΩ. Multibarrel electrodes were fabricated from 3-barrel borosilicate glass blanks pulled to a sharp tip and then broken manually under a microscope to a tip with 10–20 μm total diameter (A-M Systems, Carlsborg, WA). A sharp single recording electrode was bent to approximately a 20° angle and glued to the multibarrel tip so that it protruded ∼20 μm. The electrodes were then secured with dental acrylic and allowed to dry. Multibarrels were loaded with drug solutions in two barrels, and the third barrel was filled with 3 M KCl for current balance. Drug solutions contained either: bicuculline methiodide (5 mM in 0.9% NaCl, pH 3), SR95531 hydrobromide (3 mM in 0.9% NaCl, pH 3), or strychnine HCl (10 mM in 0.9% NaCl, pH 3.5). Bicuculline methiodide and SR95531 hydrobromide were obtained from Tocris bioscience (Bristol UK), and strychnine HCl was obtained from Sigma-Aldrich. Drug/balance barrels were connected by silver-silver chloride wires to a headstage unit controlled by an MVCS-02 iontophoresis module (NPI Electronic, Tamm, Germany). Drugs were held in the pipette with −15 to −18 nA retaining current. To expel drugs, the current was switched to the positive range, typically 50–70 nA. Drugs were applied until their effects reached a stable saturated level, suggesting that as many receptors were blocked as were available. Recovery times ranged from 5 to 43 min for bicuculline and SR95531 and 10 to 87 min for strychnine treatment.
Acoustic stimuli and data acquisition.
Acoustic stimuli were created using freely available Spike software (Brandon Warren, University of Washington) and output to Eartone 3A Insert Earphones (Etymotic Research, Elk Grove Village, IL) through a HB7 Headphone Driver (Tucker-Davis Technologies, Alachua, FL). Speaker output was controlled by a PA5 Programmable Attenuator (Tucker-Davis Technologies, Alachua, FL). Speaker intensities were calibrated using a ¼-in. Free Field microphone (model 2520, Larson-Davis, Provo, UT) and microphone preamp (model 2221; Larson-Davis). Stimuli consisted of 50-ms tones with 5-ms rise/fall times with either frequency or intensity presented in pseudorandom order.
Extracellular signals were band-pass filtered between 0.3 and 3 kHz (model 3362 filter; Krohn-Hite, Brockton, MA) and amplified using a Neuroprobe Amplifier Model 1600 (A-M Systems). Action potential event times were recorded using a voltage window discriminator (model 121; World Precision Instruments, Sarasota, FL) and processed by an RX6 Multifunction Processor (Tucker-Davis Technologies).
Labeling of recording sites.
To anatomically confirm recording sites, neurobiotin (Vector Labs, Burlingame, CA) was iontophoresed from the recording electrode (5% Neurobiotin in 2 M K acetate, as described in Köppl and Carr 2008) with 0.5 μA of positive current for 60 s using an iontophoresis pump (BAB-501; Kation Scientific, Minneapolis, MN) in several experiments. This protocol ejected a minimal amount of neurobiotin, which was sufficient to label just a few (<10) local neurons (Fig. 1D). After a minimum rest time of 30 min to allow cellular uptake, the chick was transcardially perfused first with PBS and then with 4% paraformaldehyde (PFA) in PBS. Following perfusion, the brain was removed from the skull and postfixed in 4% PFA in PBS overnight at 4°C. Tissue was sliced on a vibratome and then processed using the Vectastain ABC kit (Vector Labs).
Fig. 1.
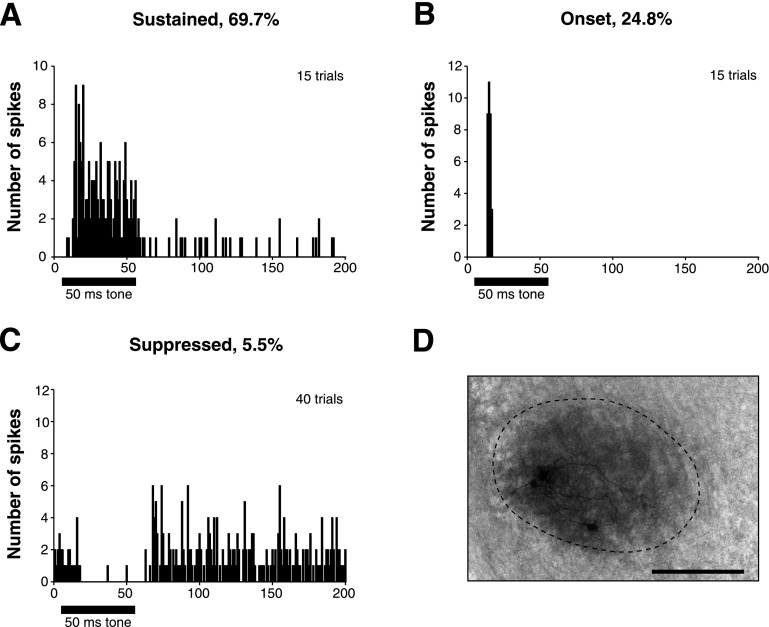
Superior olivary nucleus (SON) cells have diverse responses to tone stimulation. A: peristimulus (PST) histogram of a cell with a sustained response, showing the typical peak initial response followed by tonic firing throughout the duration of the stimulus. B: PST histogram of a cell with an onset response, which typically fires 1 or 2 spikes only at the beginning of a stimulus tone. C: PST histogram of a suppressed cell, which have high spontaneous rates that are reduced or eliminated during a tone stimulus. D: recording sites were confirmed by iontophoresis of neurobiotin from the recording electrode following several experiments. In this case, neurobiotin labeled just a few cells within the SON. Scale bar = 200 μm.
In vivo data analysis and statistics.
Data were analyzed using Spike software. Spike counts were obtained from a time window equal in duration to the stimulus and adjusted to the minimum first spike latency of the response. Response patterns of neurons were classified from peristimulus time (PST) histograms at a level 20 dB SPL above threshold. Neuron threshold was defined as either 1) the intensity at which the spike rate during the stimulus time exceeded 2 SDs of the average spontaneous rate, or 2) the intensity at which the spike rate during the stimulus time exceeded a 50% increase in the average spontaneous rate. The latter method was necessary to accommodate the small proportion of cells with high spontaneous rates, as the SD of the spontaneous rates in these cells was quite low compared with the actual spontaneous rate. Spontaneous rates were calculated from a time window preceding stimulation and averaged across all trials. Dynamic range was defined as the intensity range from threshold to the intensity at which firing rate is maximal. Rate-intensity slope values were calculated from linear fits of 10–90% of dynamic range. Input-output functions were classified as nonmonotonic if the spike rate decreased >10% of the peak rate. Characteristic frequency (CF) was defined as the frequency at which threshold was crossed at the lowest intensity. If multiple frequencies evoked threshold crossing at a single intensity, the frequency with the highest spike rate at that intensity was chosen. Frequency tuning curves were assessed by analyzing Q10 dB values, calculated as the CF divided by the response bandwidth 10 dB SPL above threshold. Vector strength (Goldberg and Brown 1969) was calculated as , where αi is the phase position of a single spike, and N is the number of spikes, as described by Fukui et al. (2010). Vector strength values were obtained from a time window excluding any onset peak response to the end of the stimulus time. This time window varied between cells but was always >20 ms (range 22–50 ms). Vector strength values were tested for statistical significance using the Rayleigh test. P values were approximated using equation 27.4 from Zar (1999) where R is vector strength, N is the number of spikes, and Rn is R·N, as described in Berens (2009). Unless otherwise noted, data values reported in text are presented as means ± SD, and all data shown in figures are presented as means ± SE. Data were tested for statistical significance using Student's paired t-test with two-tailed distribution.
In vitro brain-slice preparation.
For synaptic physiology, 12 white leghorn chickens aged E17-P5 were rapidly decapitated and the brainstem containing auditory nuclei was removed, blocked, and submerged in oxygenated artificial cerebrospinal fluid (aCSF; containing in mM: 130 NaCl, 3 KCl, 10 glucose, 1.25 NaH2PO4, 26 NaHCO3, 3 CaCl2, and 1 MgCl2) at 22°C. The brainstem was placed rostral surface down on the stage of a vibrating microtome (Microm 650V, Walldorf, Germany). Coronal sections (150–200 μm) containing the SON were collected, submerged in an incubation chamber of continuously oxygenated aCSF and incubated at 37°C for ∼1 h. Slices were then maintained at room temperature until used for recording.
Brainstem slices were placed in a custom recording chamber on a retractable chamber shuttle system (Siskiyou Design Instruments), and neurons were visualized with a Nikon FN-1 Physiostation microscope using infrared differential interference contrast optics. Video images were captured using a CCD camera (Hammamatsu C7500–50, Hamamatsu City, Japan) coupled to a video monitor. The recording chamber was continuously perfused with aCSF at a rate of 2–4 ml/min. An inline feedback temperature controller and heated stage were used to maintain chamber temperature at 35 ± 1°C (TC344B; Warner Instruments, Hamden, CT).
IPSC recordings.
For evoked IPSC recordings, a concentric bipolar electrode with tungsten core (WPI TM53CCINS, Sarasota, FL) was lowered to the tissue surface with a micromanipulator and placed in a position dorsomedial to the SON. Principal SON neurons were identified based on their characteristic round morphology. Patch pipettes were pulled from thick-walled borosilicate glass capillary tubes (WPI 1B120F-4) to a resistance of 4–8 MΩ using a two-stage puller (Narishige PC-10, Tokyo, Japan) and back-filled with internal solution (containing in mM: 130 CsCl, 1 CaCl2, 1 MgCl2, 10 EGTA, 10 HEPES, 2 ATP, 0.3 GTP, and 10 phosphocreatine, pH adjusted to 7.3 with CsOH). QX314 (5 mM) was added to the internal solution to prevent antidromic action potentials. In many cases, 0.4% biocytin was added to the internal solution to label the neurons following the protocol of Scott et al. (2005). SON principal neurons had an average whole-cell capacitance of 36.7 ± 14.3 pF and an average series resistance of 10.2 ± 4.1 MΩ. In voltage clamp, series resistance was compensated at 60–80%. eIPSCs and sIPSCs were recorded during bath application of 6,7-dinitroquinoxaline-2,3-dione (DNQX; 40 μM) and d-2-amino-5-phosphonopentanoic acid (AP5; 50 μM) to block glutamatergic input. Membrane voltage was clamped at −60 mV using a Multiclamp 700B amplifier. The signal was digitized with a Digidata 1440 data acquisition board and recorded using Clampex software (Molecular Devices, Sunnyvale, CA). IPSCs were evoked with 50-μs stimulus pulses with a stimulus isolation unit (Isoflex: A.M.P.I.) through a bipolar electrode. Stimulus magnitude (range 10–90 V) was gradually increased until IPSC amplitudes stabilized at their maximum. sIPSC data were collected by recording 30- to 60-s epochs while the membrane was clamped at −60 mV. Miniature IPSCs were also collected in the presence of 1 μM TTX to block the contribution of presynaptic action potentials to spontaneous events. In our recordings, no significant differences were observed between spontaneous and miniature events in any condition and therefore the data were pooled (Oleskevich and Walmsley 2002) and will be referred to as sIPSCs hereafter. After collection of control data, SR95531 (20 μM) and strychnine (500 nM) were sequentially applied to block GABAA and glycine receptors (GlyRs), respectively. In several cells, SR95531 and strychnine were applied simultaneously during data collection. IPSC amplitudes and kinetics were analyzed using Clampfit software. Rise and decay time constants, expressed hereafter as tau (τrise and τdecay) values, were calculated from standard exponential fits from 10–90% of the peak of IPSCs. The τdecay values were obtained using either single or double exponential fits. Goodness of fit was determined by comparing the sum of the squared errors. Double exponential fits were chosen if the sum of the squared errors was less than half that of the single exponential fit. A weighted τdecay value was calculated for double exponential fits using the equation: weighted τ = τ1 [A1(A1 + A2)] + τ2 [A2(A1 + A2)], as in Kuo et al. (2009). sIPSC frequency (Hz) was also calculated. Drug and recovery condition amplitudes and kinetic measures were normalized to control, and each treatment group was assessed for statistical significance using paired Student's t-tests. Data in the results are expressed as percentage of control ± SD. Error bars in figures represent SE. Raw data values for eIPSCs, sIPSCs, and miniature IPSCs are found in Table 1.
Table 1.
Raw data values for evoked, spontaneous, and miniature IPSCs during in vitro whole cell voltage clamp experiments
| Condition | Peak Amplitude, pA | Area, pA | Halfwidth, ms | τrise, ms | τdecay, ms | Frequency, Hz |
|---|---|---|---|---|---|---|
| Evoked IPSCs | ||||||
| Control (n = 20) | −947 ± 957 | −4339 ± 3557 | 3.92 ± 2.29 | 1.56 ± 0.83 | 4.41 ± 2.93 | – |
| Strychnine (n = 12) | −510 ± 611 | −2807 ± 2513 | 5.45 ± 3.26 | 1.70 ± 0.85 | 7.03 ± 3.55 | – |
| SR95531 (n = 14) | −310 ± 216 | −741 ± 353 | 2.35 ± 1.03 | 1.23 ± 0.77 | 2.34 ± 0.88 | – |
| SN + SR (n = 13) | −35.6 ± 48.0 | −135 ± 108 | – | – | – | – |
| Recovery (n = 15) | −676 ± 672 | −3227 ± 2584 | 3.83 ± 2.25 | 1.53 ± 0.58 | 5.35 ± 3.82 | – |
| Spontaneous IPSCs | ||||||
| Control (n = 18) | −114 ± 48.2 | −428 ± 192 | 1.93 ± 0.61 | 0.91 ± 0.22 | 4.35 ± 1.61 | 7.45 ± 6.51 |
| Strychnine (n = 12) | −73.3 ± 35.6 | −329 ± 147 | 2.32 ± 1.10 | 0.99 ± 0.31 | 6.48 ± 4.41 | 8.49 ± 4.58 |
| SR95531 (n = 16) | −95.3 ± 53.6 | −278 ± 169 | 1.39 ± 0.43 | 0.84 ± 0.37 | 2.91 ± 1.69 | 1.87 ± 1.38 |
| Recovery (n = 14) | −103 ± 61.5 | −398 ± 209 | 2.09 ± 1.00 | 1.06 ± 0.55 | 5.59 ± 4.10 | 7.76 ± 6.75 |
| Miniature IPSCs | ||||||
| Control (n = 6) | −141 ± 48.7 | −496 ± 112 | 2.26 ± 0.48 | 0.90 ± 0.33 | 4.71 ± 1.26 | 11.3 ± 7.02 |
| Strychnine (n = 5) | −63.1 ± 17.2 | −244 ± 60.4 | 2.11 ± 0.44 | 1.06 ± 0.32 | 7.46 ± 1.12 | 9.11 ± 6.61 |
| SR95531 (n = 5) | −92.2 ± 41.2 | −308 ± 113 | 1.81 ± 0.26 | 0.87 ± 0.24 | 4.34 ± 1.01 | 1.81 ± 0.98 |
| Recovery (n = 5) | −122 ± 84.6 | −436 ± 255 | 2.20 ± 0.39 | 0.97 ± 0.20 | 5.09 ± 1.08 | 10.8 ± 6.51 |
Values indicate mean ± SD. IPSCs, inhibitory postsynaptic currents; SN, strychnine, SR, SR95531.
Immunohistochemistry, receptor staining.
Four chickens aged embryonic day 19 to postnatal day 21 were deeply anesthetized with ketamine (Ketaset; Fort Dodge Animal Health) at 160 mg/kg im and pentobarbital (Sigma-Aldrich) at 80 mg/kg ip. Chickens were transcardially perfused first with PBS and then 4% PFA in PBS, pH 7.4. The brain was removed from the skull and postfixed in 4% PFA overnight at 4°C. Following PBS rinse, a section of brainstem containing the auditory nuclei was sliced into 50-μm coronal sections using a vibratome. An additional fixation step of methanol:acetic acid (95:5) for 10 min at −20°C (Dumoulin et al. 2001) was applied to the sections. Sections were blocked in 10% normal goat serum, then incubated overnight at 4°C in primary antibody solution [0.3% PBST, 5% normal goat, and mAb4a (1:1,000; Synaptic Systems; Göttingen, Germany; lot no. 146011/13)]. This antibody was raised against the glycine receptor from rat spinal cord (Pfeiffer et al. 1984) and recognizes amino acids 96–105 of the α1 subunit (Schröder et al. 1991). A BLAST search revealed that this amino acid sequence is identical in the chicken GlyR α1-subunit (Altschul et al. 1997). Additionally, this antibody has been previously used in the chicken brain (Tsen et al. 2000). After PBS rinses, the sections were incubated for 2 h with secondary antibody conjugated to a fluorophore (1:200 AlexaFluor 488 goat anti-mouse lot no. 558866; Invitrogen, Eugene, OR). A fluorescent Nissl stain (NeuroTrace 640/660) was applied to visualize nuclei (1:100, for 25 min). Sections were mounted between coverslips in Glycergel (Dako, Carpenteria, CA), and images were obtained with a confocal microscope (Zeiss LSM 510 Meta, Thornwood, NY). Images were processed using Photoshop (Adobe Systems, San Jose, CA) to match pixel intensity distributions between color channels. No staining was observed when primary antibodies were absent. Additionally, antibody preabsorption experiments were performed using a custom peptide (GenScript, Piscataway, NJ) matching the GlyR sequence 96–105 (WNDPRLAYNE) with amidation at the C terminus, as described in Schröder et al. (1991). Peptide concentrations at a 400-fold ratio to primary concentration eliminated cell specific staining, confirming the specificity of the mAb4a antibody (data not shown).
Immunohistochemistry, neurotransmitter staining.
To anatomically confirm GABA and glycine neurotransmitter at terminals, immunohistochemistry following the protocol of Kuo et al. (2009) was performed. Briefly, three mature chickens ranging in age from P17-P23 were deeply anesthetized as previously described and transcardially perfused with PBS then 2% PFA and 2% glutaraldehyde (Grade 1; Sigma) in PBS, pH 7.4. The brains were removed from the skulls and rinsed in PBS, and the section of brainstem containing the auditory nuclei was sliced into 30-μm coronal sections using a vibratome. Sections were rinsed thoroughly in PBS and then incubated in freshly made 1% sodium borohydride (Sigma) in PBS at room temperature for 30 min to reduce glutaraldehyde autofluorescence. After thorough PBS rinses, the sections were blocked in 2% normal goat serum, 1% BSA, and 0.1% saponin in PBS for 1 h at room temperature. Primary antibodies were raised with neurotransmitter conjugated to BSA and glutaraldehyde. Anti-glycine (polyclonal rabbit anti-glycine; 1:500; AB 139, lot no. NG1740903; Millipore) and anti-GABA (monoclonal mouse anti-GABA; 1:5000; mAB 3A12, lot ps2; Swant) primary antibodies were incubated concurrently overnight at 4°C in block solution. Both antibodies have been used in chick tissue previously (Matute and Streit 1986; Kalloniatis and Fletcher 1993; Kuo et al. 2009). After primary incubation, sections were rinsed in PBS and incubated in secondary antibodies (Alexa Fluor 488 goat anti-mouse; lot no. 774904; Alexa Fluor 568 goat anti-rabbit; lot no. 514959; 1:500; Invitrogen) for 2 h at room temperature. Sections were mounted on gelatin coated slides, allowed to dry overnight, dehydrated in ascending alcohols, and cleared in xylene. Sections were then rehydrated in descending alcohols and coverslipped with Vectashield mounting medium (Vector Laboratories). Confocal images were obtained and edited as previously described.
RESULTS
Monaural response properties of SON cells to acoustic stimulation were investigated using in vivo recording techniques. The modulation of these properties by inhibitory signaling was also evaluated. Additionally, the nature of inhibitory synaptic transmission at these neurons was investigated using whole cell voltage-clamp recording techniques. In vivo data were collected from 109 SON cells, from 80 white leghorn chicks age range P5-P30. The CFs of sampled cells ranged from 0.19 to 4.8 kHz. In vitro data were obtained from late stage embryos and early posthatch chicks aged E17-P5. A total of 27 SON neurons from 12 chicks was used for analysis of synaptic physiology.
SON neurons have diverse response patterns to acoustic stimulation.
SON neurons were stimulated at BF with 50-ms tone bursts. The responses of SON cells to acoustic stimulation segregated into two broad categories. The great majority (94.5%) of SON cells were driven by acoustic stimulation, while the remaining population was suppressed by acoustic stimulation (Fig. 1). Of neurons with acoustically driven responses, 69.7% had a sustained response. These cells typically had a strong initial peak response followed by tonic firing throughout a stimulus tone (Fig. 1A); 24.8% had an onset response during acoustic stimulation in which only one or two spikes fired at the beginning of a stimulus tone (Fig. 1B). The spike rates of most cells increased monotonically with intensity (64% of all cells), while the remainder of cells were nonmonotonic, exhibiting >10% depression from the peak rate at high intensities. The proportion of cells exhibiting nonmonotonic rate functions was greater for sustained cells (41% of sustained cells, 22% of onset cells). Acoustically driven neurons had an average threshold of 26 dB SPL ± 21, average dynamic range of 40 dB SPL ± 17, and an average first spike latency of 9.3 ms ± 4.2.
We recorded from six neurons that were suppressed by acoustic stimuli. This population tended to have relatively high spontaneous firing rates (29 spikes/s ± 13, n = 6) that were reduced or eliminated during acoustic stimulation (Fig. 1C). It should be noted that because this cell type was encountered infrequently, a thorough characterization of this population was not possible. However, in several neurons we observed that this spike suppression was dependent on both stimulus intensity and frequency. A representative example of a suppressed neuron is shown in Fig. 2. The acoustically driven suppression of spiking in this SON cell occurred over a 1.4-octave frequency range (Fig. 2A). The suppressive effect occurred over a relatively broad frequency range for all neurons where tuning information was obtained (1.8 octaves ± 0.9, n = 3). In every neuron tested (n = 6), the suppressive effect increased with sound intensity (Fig. 2B). Our observations are consistent with those reported for two neurons in Tabor et al. (2011).
Fig. 2.
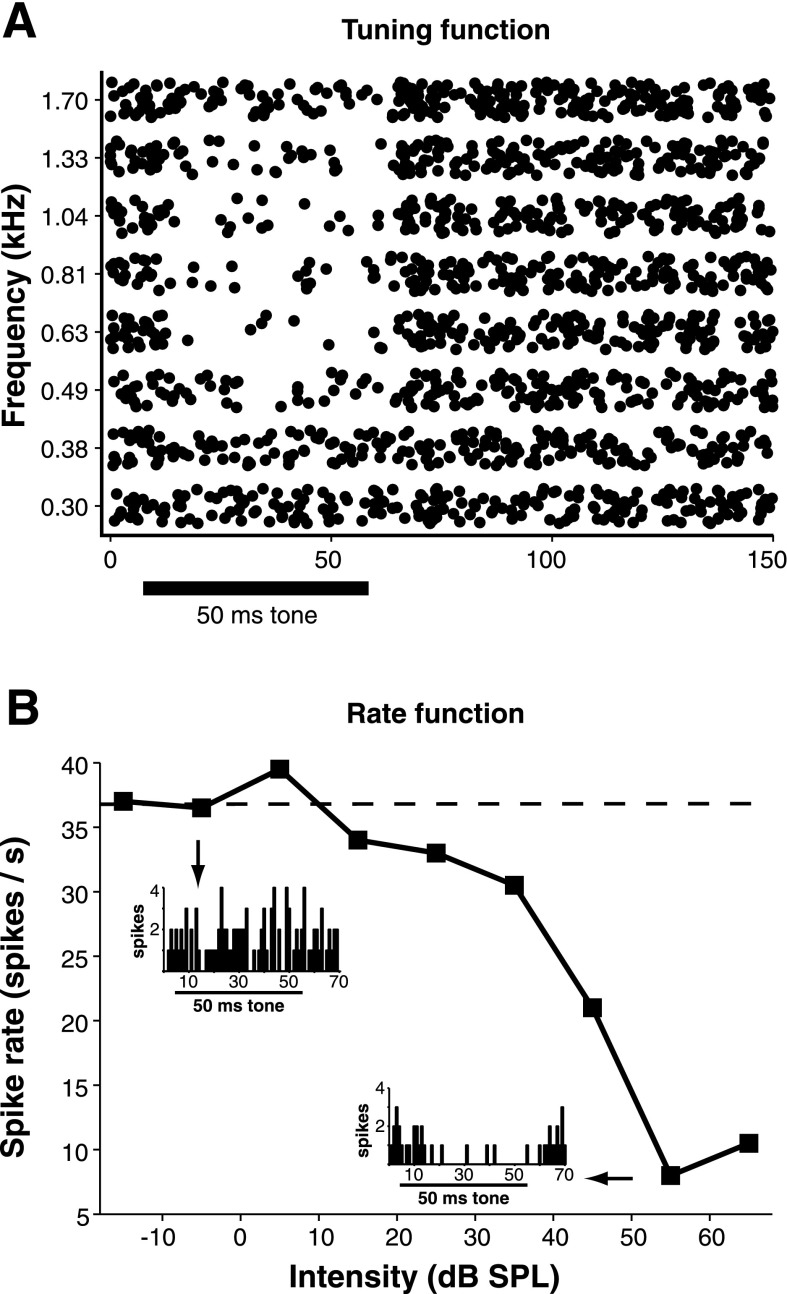
Spike rate reduction of suppressed SON cells is frequency and intensity dependent. A: raster plot demonstrating the stimulus frequency dependence of acoustically driven reduction in spike rate in a suppressed SON cell. The cell was stimulated using 50-ms tones (black bar) at 20 dB SPL above suppression threshold at 0.7 kHz, our estimate of characteristic frequency (CF). B: rate-level function for the same cell in A at 0.7 kHz. Degree of spike suppression increases with intensity. Inset: PST histograms showing the number of spikes before the threshold for suppression has been crossed (top left, 101 spikes) and when suppression of spike rate is maximal (bottom right, 39 spikes). Spike counts were obtained from the entire histogram shown (40 trials).
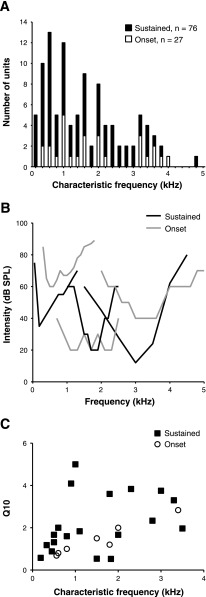
We also investigated the frequency-tuning properties of 27 SON cells with sustained or onset responses. The population's CFs were distributed across the entire chicken audiogram, and the frequency range did not differ for sustained and onset cells (Fig. 3A). Tuning curves for both types were generally broad, and representative examples are shown in Fig. 3B (mean Q10: 2.0 ± 1.2). The distribution of Q10 values for all neurons for which tuning data were obtained demonstrates that most SON cells were broadly tuned, regardless of response type (Fig. 3C).
Fig. 3.
SON neurons are broadly tuned. A: CFs of sampled SON cells are distributed across the entire chicken audiogram and include both sustained (filled bars) and onset neurons (open bars). B: example tuning curves from cells with sustained and onset responses. Tuning curves are similar and generally broad for both cell types. C: distribution of Q10 values re: CF from sustained and onset cells.
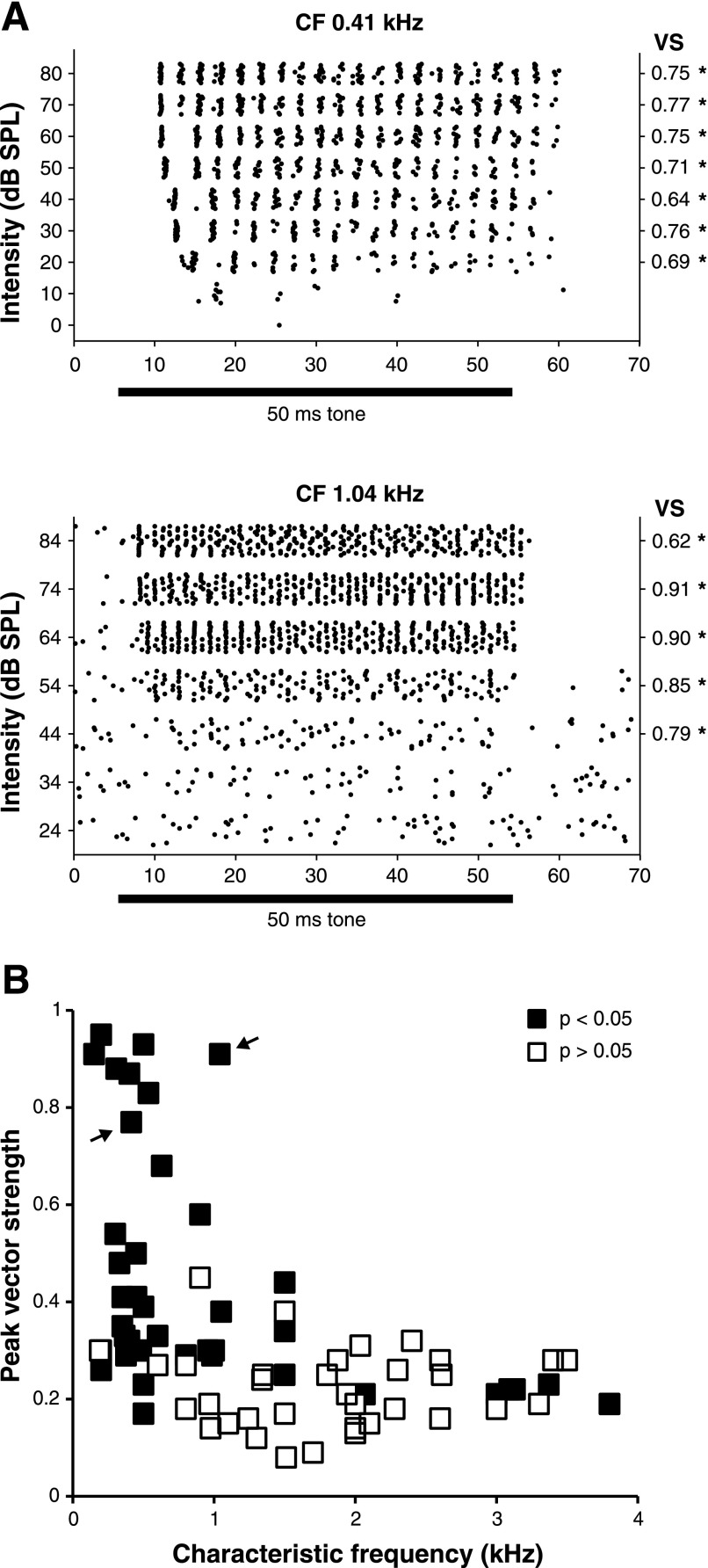
We also investigated whether SON neurons were capable of encoding temporal aspects of auditory signals by analyzing phase-locking to tone stimuli. Previous studies have suggested that temporal information is not likely to be encoded by SON cells (Lachica et al. 1994; Yang et al. 1999; Monsivais et al. 2000). Surprisingly, we observed that many SON cells have robust phase-locking capabilities. We calculated vector strength values from a time window that excluded the initial peak response (as judged from PST histograms) through the end of the stimulus for 74 cells with sustained responses. Raster plots of phase-locked discharges and their corresponding vector strengths are shown for two neurons with CFs of 0.41 and 1.04 kHz in Fig. 4A. Figure 4B shows a scatter plot of peak vector strength values as a function of CF for the entire population. Vector strength values were tested for significance using the Rayleigh test (see materials and methods): 40/74 cells had peak vector strengths with P values < 0.05 according to this test (filled squares in Fig. 4B). Of these 40 cells, 6 had CFs >1.5 kHz.
Fig. 4.
Some SON neurons respond to acoustic stimulation with phase-locked discharges. A: raster plots of responses to CF tones for 2 SON cells that demonstrated high vector strength values. Values were computed for responses at all suprathreshold intensities for neurons with robust sustained responses. *P < 0.05 by Rayleigh test (see materials and methods). B: distribution of peak vector strength re: CF. Many of the sustained cells with CF <1.5 kHz have peak vector strength values 0.3 or higher. ■: P < 0.05 by Rayleigh test (see materials and methods). Arrows denote the peak vector strengths of the neurons shown in A.
Response properties of SON neurons are modulated by both GABAergic and glycinergic inhibitory inputs.
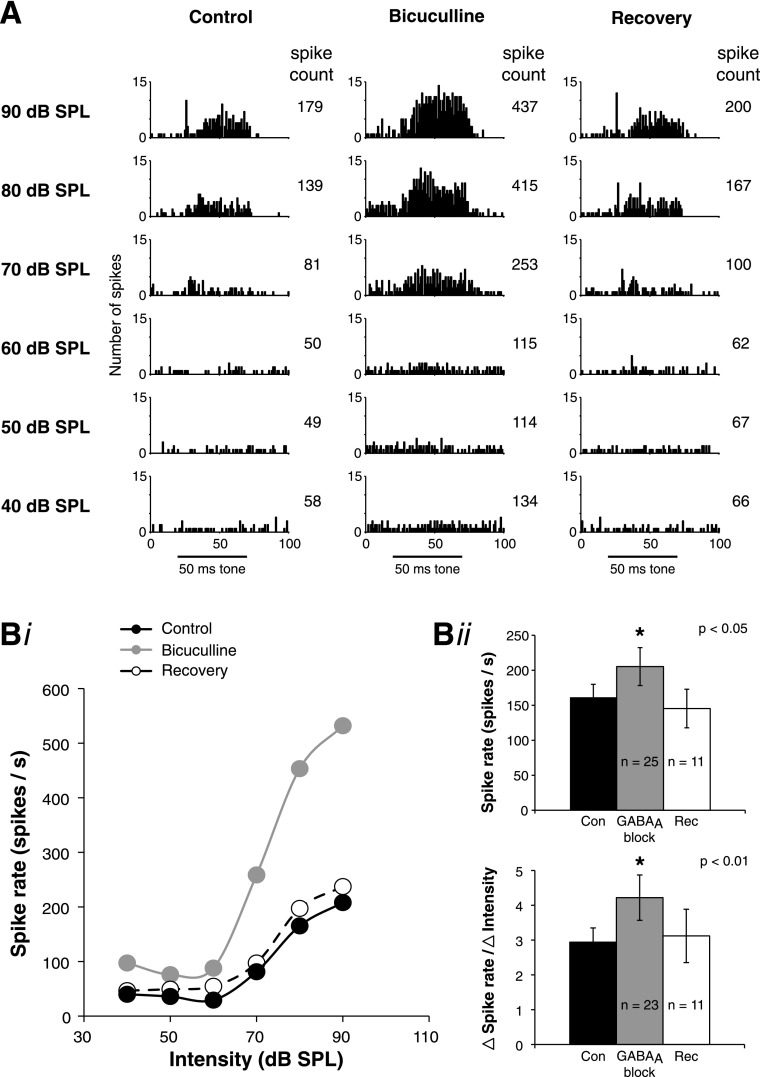
It has not previously been investigated whether inhibition influences SON responses. The SON is known to receive a putatively inhibitory input from its contralateral counterpart, and immunostaining against GABAA receptors or glutamic acid decarboxylase showed strong reactivity in the SON (Carr et al. 1989; Code and Churchill 1991). We next investigated how the monaural response properties of SON cells are modulated by inhibitory inputs. To test whether SON neurons are modulated by GABAergic inputs, we employed pharmacological block of GABAA receptors by iontophoresis of either SR95531 hydrobromide or bicuculline methiodide using multibarrel electrodes.
Both SR95531 and bicuculline had similar effects on response properties, so data for GABAA receptor block were pooled for population analysis. GABAA antagonist application increased acoustically driven spike rates in every cell tested (25/25 cells). A representative example of the effects of GABAA block on the response properties of an SON neuron is shown in Fig. 5. Figure 5A demonstrates that application of bicuculline increased the number of spikes in this neuron at every intensity. In this case, the spike count increased by 130% at 60 dB SPL and by 200% at 80 dB SPL. Following cessation of bicuculline application, the spike count recovered to control levels within 5 min for this neuron. Rate-level functions during the control, bicuculline, and recovery conditions for the neuron shown in Fig. 5A are shown in Fig. 5Bi. Two important features of GABAA function are demonstrated in this example. First, bicuculline application increased the spike rate, particularly at suprathreshold intensities. Across the population of tested cells, GABAA block significantly increased the spike rate at the stimulus intensity that evoked the peak firing rate in the control condition (P < 0.05; Fig. 5Bii). However, GABAA block did not significantly affect the threshold (22 dB SPL ± 20 for control; 21 dB SPL ± 22 for GABAA block; P > 0.05; n = 23). The effect of GABAA block on spontaneous firing rate was variable, increasing in some cells but not changing in others. Across the population, there was a mild trend for the spontaneous rate to increase during GABAA block, but this effect was not statistically significant (24 spikes/s ± 27 for control; 29 spikes/s ± 30 for GABAA block; P > 0.05; n = 23). GABAA block did not affect the first spike latency (8.7 ms ± 2.9 for control; 8.0 ms ± 2.0 for GABAA block; P > 0.05; n = 22) of SON neurons.
Fig. 5.
Response properties of SON neurons to acoustic stimulation are modulated by GABAergic inhibitory inputs. A: example of SON responses during local iontophoresis of bicuculline during acoustic stimulation. PST histograms are shown for the control, bicuculline, and recovery conditions. Number of spikes increases in the presence of bicuculline, particularly during suprathreshold stimulation and then recovers when iontophoresis of the drug is turned off. Black bar represents the 50-ms stimulus tone. Spike counts are the total for entire PST histogram shown. Bi: rate-level functions for control (Con), bicuculline, and recovery (Rec) conditions from the same cell as in A. Bii: summary population data for GABAA receptor block. GABAA block significantly increases the peak spike rate (re: the peak control rate; *P < 0.05). GABAA block also significantly increases the slope of the dynamic range (calculated over the intensity range derived from 10–90% of threshold to maximum rate in control; *P < 0.01).
The second major feature of GABA function is that it decreases the slope of the input-output function. Across the population, GABAA block significantly steepened the input-output function. We quantified this change by calculating response slope over 10–90% of the dynamic range of the response for each condition (see materials and methods). The intensity boundaries of the dynamic range were unchanged by GABAA antagonist application (45 dB SPL ± 16 for control; 47 dB SPL ± 18 for GABAA block; P > 0.05; n = 23). However, GABAA block significantly increased the slope of the response through the dynamic range (P < 0.01; Fig. 5Bii).
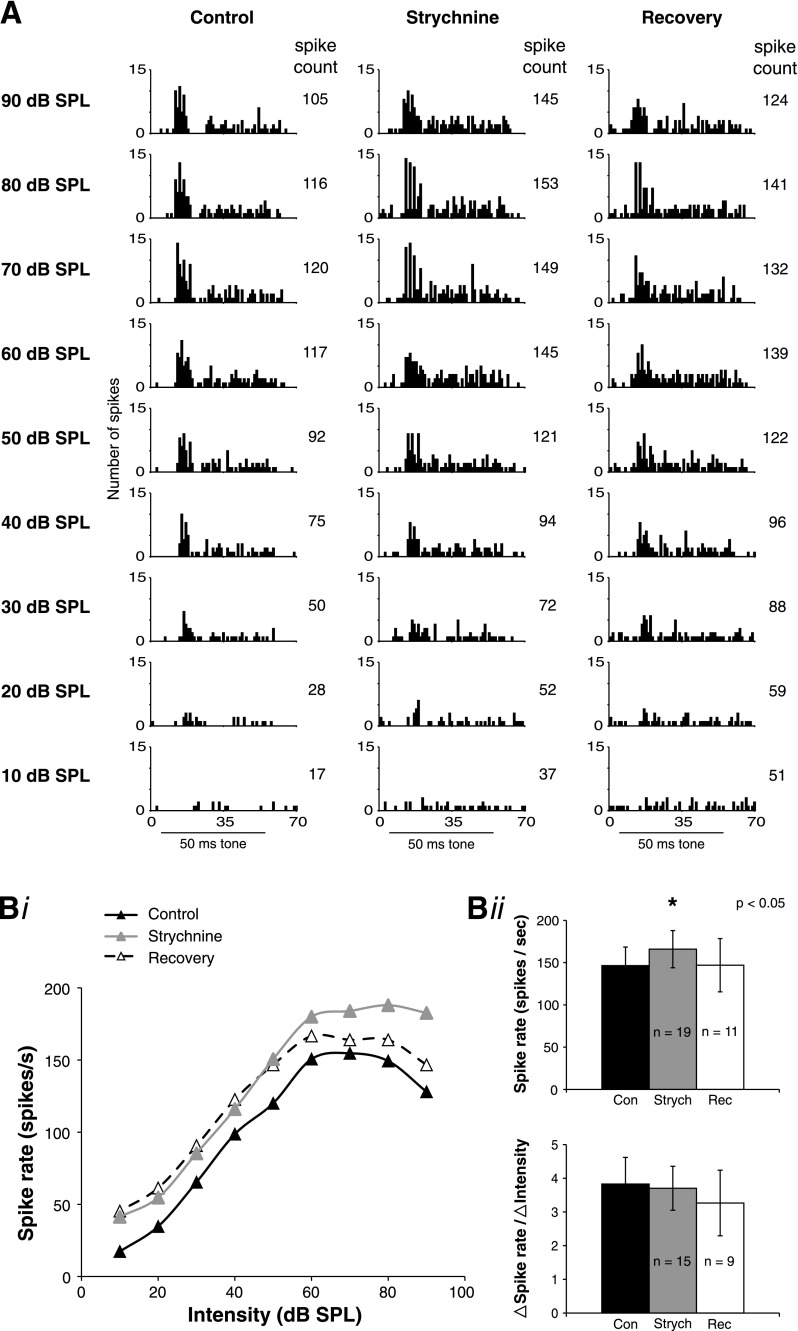
Glycinergic signaling is prominent in SON.
A recent study showed that in addition to GABAergic signaling, prominent glycinergic signaling also exists in NA (Kuo et al. 2009), although the source of the glycinergic inhibition is not known. To test whether SON neurons also receive glycinergic inhibitory inputs, we locally iontophoresed strychnine (a GlyR antagonist) while recording acoustic responses. Surprisingly, we found that similar to GABAA block, GlyR block also increased acoustically driven spikes in 16/19 cells. A representative SON neuron in which glycinergic transmission was manipulated is shown in Fig. 6. Figure 6A shows that strychnine treatment increased the spike count similarly throughout most of the dynamic range of this cell (∼26%) but had a slightly larger effect at the loudest intensity tested (90 dB SPL, 38% increase). After iontophoresis of strychnine was turned off, the spike count recovered nearly to control levels at higher intensities within 40 min; this cell was lost before complete recovery could be obtained. Figure 6Bi shows rate-level functions during the control, strychnine, and recovery conditions for the same cell in Fig. 6A, illustrating two important features of glycinergic modulation. First, despite a general rise in spike rate, the slope of the dynamic range did not change for this neuron. Across the population, and in contrast to GABAA block, strychnine did not significantly affect the slope of the input-output function. The second feature of interest is that nonmonotonic responses are frequently mediated by inhibitory input to SON neurons. For this cell, the control input-output function was nonmonotonic but became monotonic during strychnine treatment. This change from nonmonotonic to monotonic input-output functions was observed for two out of two neurons treated with strychnine as well as five out of eight GABAA block neurons.
Fig. 6.
Response properties of SON neurons to acoustic stimulation are modulated by glycinergic inhibitory inputs. A: example of SON acoustic responses during local iontophoresis of strychnine. PST histograms are shown for the control, strychnine, and recovery conditions. Spike count increases at all intensities in the presence of strychnine and then recovers when iontophoresis of the drug is turned off. For this cell, the input-output function that was nonmonotonic in the control condition became monotonic during strychnine treatment. Black bar indicates 50-ms stimulus tone. Spike counts are the total for entire PST histogram shown. Bi: rate-level functions for control, strychnine, and recovery conditions from the same cell as in A. Bii: population data for glycine receptor (GlyR) block. GlyR block significantly increases the peak spike rate (re: the peak control rate; *P < 0.05). GlyR block did not significantly affect the slope of the dynamic range.
Across the population of tested cells, GlyR block significantly increased the spike rate at the intensity evoking the peak rate in the control condition (P < 0.05; Fig. 6Bii). Similar to GABAA block, GlyR block did not affect the threshold intensity (21 dB SPL ± 21 for control; 21 dB SPL ± 20 for strychnine; P > 0.05; n = 15), the intensity limits of the dynamic range (40 dB SPL ± 18 for control; 43 dB SPL ± 19 for strychnine; P > 0.05; n = 15), or the first spike latency (8.4 ms ± 1.8 for control; 8.5 ms ± 2.1 for strychnine; P > 0.05; n = 15) of SON neurons. Unlike GABAA block, GlyR block had no affect on the slope of the dynamic range (Fig. 6Bii). The effect of GlyR block on spontaneous firing rate was variable between neurons, similar to GABAA block. Across the population, there was a mild trend for the spontaneous rate to increase during strychnine treatment, but this effect was not statistically significant (11 spikes/s ± 14 for control; 17 spikes/s ± 21 for strychnine; P > 0.05; n = 15).
GABAergic and glycinergic inhibitory inputs enhance phase-locking in SON neurons.
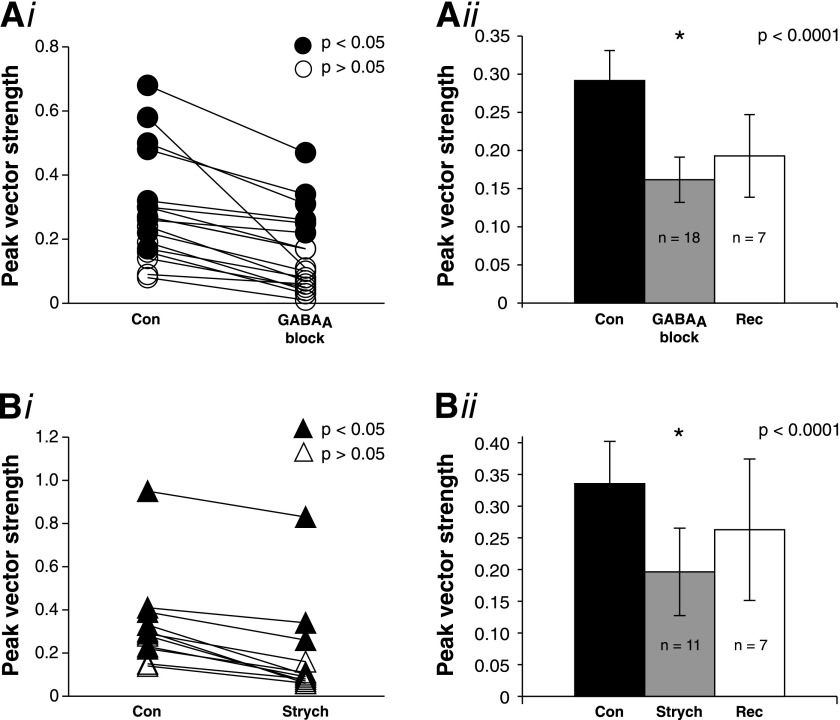
In this study, we show that some SON cells show strong phase-locking capabilities (Fig. 4). To investigate how inhibitory inputs may influence the phase-locking of SON neurons, we measured changes in peak vector strength during pharmacological block of GABAergic and glycinergic inhibitory receptors (using similar methods as in the previous experiments). We found that both types of inhibitory inputs enhance the precision of phase-locking in SON cells. GABAA block reduces the peak vector strength in every cell tested, and this effect is significant across the population (P < 0.0001; Fig. 7Ai, ii). Similar to GABAA block, GlyR block with strychnine also significantly reduces the peak vector strength of SON cells (P < 0.0001; Fig. 7Bi, ii).
Fig. 7.
Phase-locking of sustained SON neurons is enhanced by both GABAergic and glycinergic inhibitory inputs. Ai: GABAA block decreases the peak vector strength of sustained SON cells (pooled bicuculline and SR95531 data). ●: P < 0.05 by Rayleigh test (see materials and methods). Aii: summary population data of the effect of GABAA block on peak vector strength. GABAA block significantly decreases the peak vector strength (*P < 0.0001). Bi: GlyR block also decreases the peak vector strength of sustained SON cells. ▲: P < 0.05 by Rayleigh test (see materials and methods). Bii: summary population data of the effect of GlyR block on peak vector strength. GlyR block significantly decreases the peak vector strength (*P < 0.0001).
Evoked inhibitory synaptic transmission in the SON is mediated by both GABAergic and glycinergic components.
The in vivo experiments demonstrated modulation of SON response properties with application of both glycine and GABAA receptor antagonists. Both antagonists increased acoustically driven spike rates and diminished the precision of phase-locking. Additionally, GABAA block increased the slope of the input-output function through the dynamic range. To further characterize inhibitory transmission in the SON, we used in vitro whole cell voltage clamp techniques to record eIPSCs and sIPSCs while pharmacologically manipulating GABAergic and/or glycinergic transmission.
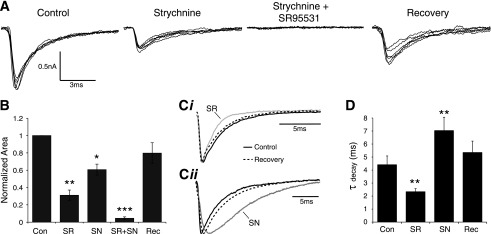
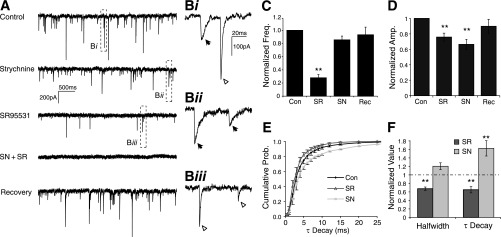
IPSCs were evoked by placing a stimulating electrode on input fibers dorsomedial to the border of SON while excitatory inputs were blocked with 40 μM DNQX and 50 μM AP5. eIPSCs were observed in 20/21 neurons tested. eIPSC amplitude was variable with an average peak amplitude of −975 ± 957 pA (Table 1). To isolate the GABAergic component of eIPSCs, 500 nM strychnine was bath applied. Peak eIPSC amplitude was reduced in 12/12 cells on average to 50.6 ± 16.8% of the control value (Fig. 8A). Similarly, blocking GABAA receptors with SR95531 (20 μM) reduced eIPSC amplitude to 45.2 ± 19.4% of control (n = 14). Simultaneous application of both SR95531 and strychnine completely abolished eIPSCs in 9 of 13 cells. The residual current for the population was 5.3 ± 6.9% of control (Table 1). In addition to amplitude modulation, changes in the eIPSC waveform kinetics were also evident (Fig. 8A). We compared the halfwidth and τdecay values of isolated GABAergic and glycinergic eIPSC components. Blocking GlyRs broadened the eIPSC and increased both the halfwidth (145.3 ± 59.9% of control; P < 0.05; n = 12) and τdecay (158.1 ± 72.1% of control; P < 0.05; n = 12; Fig. 8, Cii and D). Blocking GABAA receptors with SR95531 had a complementary effect on kinetics, narrowing the eIPSC waveform by reducing halfwidth (65.3 ± 25.6% of control; P < 0.01; n = 14) and τdecay (66.5 ± 19.7% of control; P < 0.01; n = 14; Fig. 8, Ci and D). Area under the eIPSC waveform was also reduced in both drug conditions (strychnine: 60.7 ± 22.1% of control; P < 0.01; n = 12; SR95531: 31.1 ± 22.4%; P < 0.01; n = 14; Fig. 8B). These data suggest that both GABAergic and glycinergic transmission occur in the SON, that each contribute equally to the peak eIPSC amplitude, but that the GABAergic transmission provides the majority of the total eIPSC current.
Fig. 8.
Evoked inhibitory postsynaptic currents (eIPSCs) in the SON contain GABAergic and glycinergic components. A: eIPSC traces from a representative SON neuron during pharmacological treatments. B: population data showing the effect of treatments on eIPSC area. C: Normalized eIPSC traces showing kinetic modulations during drug treatments (average of 10 traces in each condition). Ci: bath application of SR95531 (SR), a GABAA receptor antagonist results in faster eIPSC kinetics. Cii: blockade of GlyRs with strychnine (SN) yielded slower eIPSC kinetics. D: Population data for τdecay measured in each condition. *P < 0.05, **P < 0.01, significantly different from control. ***P < 0.01, significantly different from all other conditions.
Glycine and GABA are coreleased at some inhibitory terminals in SON.
Kuo et al. (2009) showed evidence suggesting that the most likely source of glycine in NA was from terminals that corelease GABA and glycine. Given our findings that both GABAergic and glycinergic synaptic transmission act on SON neurons, several possible input arrangements exist for these two modes of inhibition: 1) SON neurons could receive independent, purely GABAergic and purely glycinergic inputs; 2) these inputs could be provided by GABA/glycine corelease terminals as reported in NA (Kuo et al. 2009); or 3) there could be a mixture of single transmitter and corelease terminals. To differentiate between these possible input arrangements, we evaluated the properties of sIPSCs and miniature IPSCs. Statistical analysis of spontaneous and miniature events revealed no significant differences so the data sets were pooled and will be referred to as sIPSCs (see materials and methods).
We held SON cells at −60 mV for 30- or 60-s epochs while blocking excitatory input with DNQX and AP5 in 24 neurons. sIPSCs were common with an average frequency of 8.42 ± 6.25 Hz (Table 1). Blocking GABAA receptors significantly reduced the sIPSC frequency (27.8 ± 23.5% of control; P < 0.01; n = 21), suggesting that many sIPSCs were purely GABAergic. SR95531 application also reduced the average peak amplitude of the remaining sIPSCs (76.4 ± 19.1% of control; P < 0.001; n = 21; Fig. 9, A, C, and D). In contrast, strychnine application had no significant effect on sIPSC frequency, but there was a slight trend toward fewer events (85.6 ± 25.6% of control; P > 0.05; n = 17; Fig. 9C). However, peak sIPSC amplitude was significantly reduced (66.2 ± 32.3% of control; P < 0.01; n = 17; Fig. 9, A and D). No sIPSCs were observed during simultaneous application of strychnine and SR95531 (n = 13). One additional observation was that during the control condition there was typically a few very large (>400 pA) events as seen in Fig. 9A. These events were absent in the drug conditions but returned with recovery. These data taken together are consistent with an input arrangement where each SON neuron receives a substantial portion of purely GABAergic synaptic terminals but few, if any, purely glycinergic terminals. Rather, the glycinergic component appears to be coreleased with GABA at some terminals. We further analyzed the distributions of sIPSC kinetics in each condition to confirm these findings.
Fig. 9.
Spontaneous postsynaptic currents are modulated by blockade of both GABA and glycine receptors. A: representative 5-s epochs of spontaneous (s)IPSC records in each experimental condition. B: expanded 100-ms segments from the traces in A showing condition dependent decay kinetics. Bi: control condition showing heterogeneous kinetic profiles including slow arrow and fast arrowhead events. Bii: isolated GABAergic sIPSCs tended to have slower kinetics (quantified in F). Biii: isolated glycinergic sIPSCs had faster kinetics. C: population data for normalized frequency of sIPSCs. Frequency was significantly reduced during application of SR95531 but not strychnine. D: population data for normalized amplitude of sIPSCs. Both SR95531 and strychnine significantly reduced sIPSC amplitude when bath applied. E: cumulative probability analysis of τdecay values revealed a monophasic rise in control conditions that was significantly shifted to shorter or longer values in SR95531 and strychnine, respectively (P < 0.01, Kolmogorov-Smirnov test). F: quantification of kinetic measures show a decrease in halfwidth and τdecay values in SR95531 and an increase τdecay values in strychnine. Strychnine did not significantly change halfwidth. *P < 0.05, **P < 0.01, significantly different from control; pooled data include both sIPSCs and miniature IPSCs.
The eIPSC data show that isolated GABAergic and glycinergic conductances have very different decay kinetics. If sIPSCs arose from purely GABAergic or glycinergic terminals, then we would expect a bimodal distribution of sIPSC kinetics. However, τdecay values for sIPSCs recorded in the control condition typically had a broad continuous distribution from slow to fast events. Figure 9Bi shows example events from the control condition in Fig. 9A illustrating this heterogeneity. The first event (arrow) has a slow decay, whereas the second event (arrowhead) is notably faster. Application of strychnine shifted the sIPSC population towards slower events (Fig. 9, Bii, E, and F). Conversely, SR95531 application resulted in mostly faster sIPSCs (Fig. 9, Biii, E, and F). Analysis of the cumulative probability plot for all τdecay values from 14 cells in which both drugs were applied shows a monophasic rise in the control condition consistent with the broad and continuous distribution of values (Fig. 9E). The τdecay kinetics of sIPSCs in both strychnine and SR95531 were significantly different from the control condition and different from each other (P < 0.01, Kolmogorov-Smirnov test). Taken together the observations of antagonist effects on τdecay distributions in conjunction with their influences on sIPSC frequency and amplitude modulation shown above suggests that the most parsimonious model of SON input arrangements includes both purely GABAergic terminals and some GABA/glycine corelease terminals. While we cannot rule out the possibility that SON neurons receive some purely glycinergic terminals, our data do not support this hypothesis.
Immunohistochemical analysis of glycinergic signaling in SON.
Glycinergic signaling in the avian brainstem auditory system has only been reported in two studies that we know of (Code and Rubel 1989; Kuo et al. 2009), but our physiological results suggest glycinergic transmission is an important transmitter system in the SON. Further, analysis of sIPSCs suggest that glycine is coreleased with GABA. We performed immunohistochemical analysis to confirm these two sets of physiological findings.
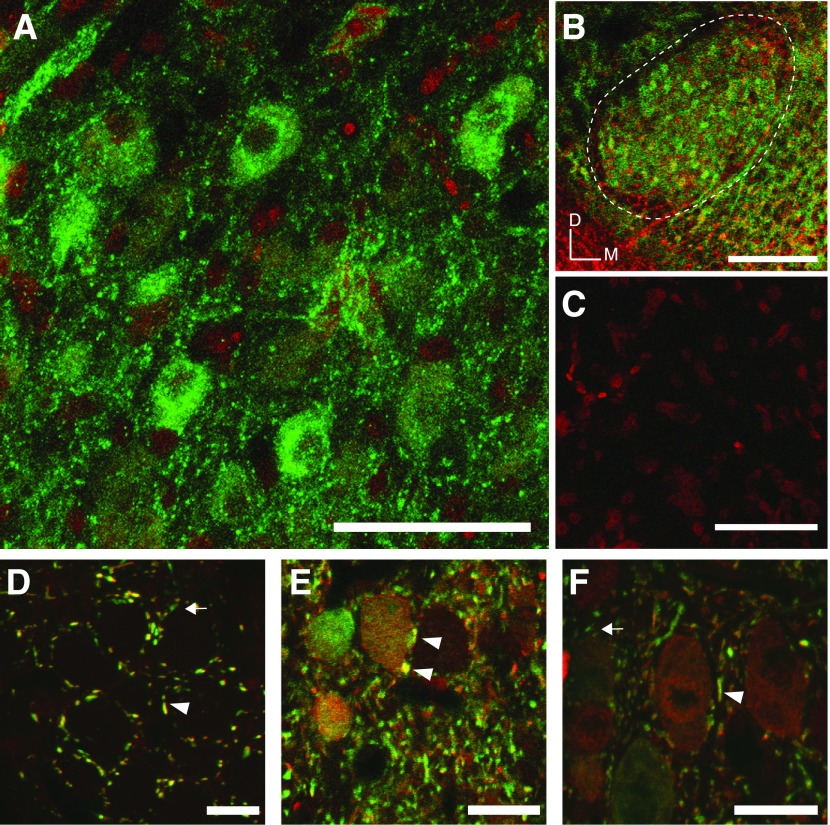
First, we performed immunohistochemistry using an antibody originally raised against glycine receptor (GlyR) from rat spinal cord (Pfeiffer et al. 1984; see materials and methods) in four chickens aged E19-P21. GlyR immunoreactivity was abundant throughout the SON and intensely stained individual neurons. Figure 10, A–C, shows representative staining from a P7 brain. Figure 10A shows at high magnification individual neurons in the SON positively stained for GlyR (green label). Figure 10B shows a low power image of a 50-μm section through the SON (dashed white line) with widespread GlyR immunofluorescence. Figure 10C shows only nissl staining (red nuclei) and the absence of nonspecific staining when primary antibody was excluded.
Fig. 10.
Immunohistochemistry of glycinergic signaling in the SON. A-C: images of coronal sections of a P7 chicken SON: images are maximum intensity projections from confocal z-stacks through 50 μm slices. A: GlyR immunoreactivity (green) is abundant among SON somas and processes. Nissl stain is shown in red. B: lower magnification image from the same SON as in A, GlyR is broadly distributed throughout the SON. Dashed line denotes the borders of the SON. Orientation bars indicate dorsal (D) and medial (M). C: immunofluorescence for GlyR is absent in tissue processed without primary antibody (Nissl staining shown in red). D–F: staining for neurotransmitters glycine (red) and GABA (green), images are single confocal sections from a P23 chicken brainstem sectioned at 30 μm. D: GABA/glycine immunostaining in nucleus magnocellularis (NM) show singly labeled GABA terminals arrow and robust double-labeled terminals arrowheads. Somatic staining was completely absent in NM. E and F: SON neurons also have both doubly labeled GABA/Gly immunopositive terminals as well as singly labeled GABAergic terminals. In contrast to NM, SON somas are differentially immunopositive for GABA or glycine or both. Scale bars: A and C = 50 μm, B = 200 μm, and D–F = 20 μm.
Second, we used antibodies targeting GABA and glycine following the method of Kuo et al. (2009) to examine the distribution of these neurotransmitters in terminals and somas of SON cells in three animals aged P17–23. Figure 10D shows NM with both doubly and singly labeled terminals at high magnification confirming the pattern observed in Kuo et al., (2009), while two images from the SON are shown in Fig. 10, E and F. Singly labeled GABAergic terminals appear green and are labeled with arrows while terminals positive for both GABA and glycine (red label) appear yellow and are indicated with arrowheads in Fig. 10, D-F. SON somas were previously reported to be immunopositive for GABA (von Bartheld et al. 1989), and green labeled somas shown in Fig. 10, E and F, confirm this earlier finding. This pattern is in marked contrast to NM, where somatic staining is absent in Fig. 10D. We also observed positive soma staining for both transmitters in SON as shown in Fig. 10, E and F. The relative intensity of staining for GABA and/or glycine was highly variable between cells. These results suggest that a candidate source of GABA and glycine corelease to NM, NA, NL, and SON is the SON itself.
DISCUSSION
In this study, we characterized the monaural response properties of SON cells during acoustic stimulation and the modulation of these responses by GABAergic and glycinergic inhibition. We showed that GlyR immunoreactivity is prominent in the SON. We also investigated the synaptic physiology of inhibition in the SON. We found that SON neurons demonstrate a heterogeneous mixture of acoustic response patterns. These responses are modulated by both GABAergic and glycinergic inhibitory inputs in the majority of SON neurons. Many SON neurons have robust phase-locking capability, and the precision of this phase-locking is enhanced by inhibitory inputs. On the synaptic level, we found that evoked and sIPSCs within the SON are also mediated by GABAergic and glycinergic inhibition. Analysis of sIPSC data suggests that the most likely input arrangement for most SON cells is a mixture of purely GABAergic terminals and terminals from which GABA and glycine are coreleased. These results were supported with immunohistochemistry demonstrating that many synaptic terminals in the SON are positive for both GABA and glycine.
In vivo SON physiology.
To date, only a few studies have investigated SON response characteristics in vivo. Moiseff and Konishi (1983) reported a general characterization of SON cells in the barn owl in which most cells were only excited monaurally. Lachica et al. (1994) reported PST histograms of SON cells from chicken that demonstrated a primary-like response pattern and cells with a strong phasic onset response followed by a steadily decreasing tonic response. Additionally, this study reported a few sample tuning curves that were relatively broad. The present study has expanded upon the previous data by showing that in addition to neurons with a sustained response that compose the majority of the tested population, other response patterns to acoustic stimulation also exist within the SON. Specifically, we found that many cells only respond with a phasic onset pattern to tones (onset cells). Interestingly, we also observed a third class comprising a small proportion of SON neurons that were suppressed in response to acoustic stimulation.
We investigated the frequency tuning properties of 27 sustained and onset cells. The majority of these cells were very broadly tuned, with average Q10 values of ∼2. This result agrees well with recent anatomical and physiological studies that investigated inhibitory feedback to SON's ipsilateral targets. Fukui et al. (2010) recorded from individual NM neurons and demonstrated tuning of inhibitory responses was broader than that of excitatory responses. Tabor et al. (2011) recently showed that individual SON neurons innervate NL broadly across the tonotopic domain. These data taken together with the data presented here suggest that SON derived inhibitory feedback in the chicken auditory system functions broadly in the frequency domain.
Possible functions of the different SON cell types.
It has previously been shown that the SON forms a negative feedback circuit in the avian auditory brainstem. Excitatory inputs from NA and NL drive the ipsilateral SON (Conlee and Parks 1986; Takahashi and Konishi 1988; Westerberg and Schwarz 1995), which in turn sends inhibitory projections back to the ipsilateral NA, NM, and NL (Yang et al. 1999; Monsivais et al. 2000; Burger et al. 2005). Additionally, a second output pathway from the SON forms reciprocal connections between the two SONs (Yang et al. 1999; Monsivais et al. 2000). Our previous study demonstrated that these two output pathways from the SON arise from distinct populations of SON neurons (Burger et al. 2005). This and other studies proposed that in addition to the inhibitory feedback mechanism at the ipsilateral NA, NM, and NL, the putative inhibitory connection between the two SONs would serve to balance input strength for coincidence detecting neurons in NL by coupling inhibitory feedback to their inputs from NM (Burger et al. 2005; Dasika et al. 2005). More recent studies have supported this hypothesis by demonstrating that the SON modulates ITD encoding in NL (Nishino et al. 2008). Additionally, a given SON will suppress responses in the ipsilateral NM, but disinhibit the contralateral NM (Fukui et al. 2010). While the targets of the various cell types could not be identified in the current study, the response properties of the majority of neurons reported in this study are consistent with the current understanding of SON function. The acoustically driven SON cells reported in this study had an average dynamic range of 40 dB SPL ± 16, with ∼60% of cells responding monotonically with increasing stimulation intensity. Fukui et al. (2010) reported that the effects of GABAergic inhibitory inputs were strongest at higher intensities (>60 dB) in NM. Most SON cells responded robustly at high intensities and thus are well suited to provide an intensity dependent inhibitory signal to their targets.
We found a small population of SON cells that were suppressed by acoustic stimulation, consistent with observations of two neurons reported in Tabor et al. (2011). These neurons had high spontaneous rates and discharges were reduced or eliminated by tone stimulation. The suppression that we observed was dependent on stimulus frequency and intensity, and typically the suppression appeared to last for the duration of the stimulus. These neurons were encountered relatively rarely, and we did not have the opportunity to fully characterize this population. For example, it was not determined in this study if the suppression in these cells is mediated by inhibitory inputs to the suppressed neurons themselves. It is possible that the suppressed cells inherit their response properties from the NA (a primary source of excitatory input to the SON), as a similar response type (type IV) has been reported in the NA of barn owl (Köppl and Carr 2003). The aforementioned study suggested that these cells may be involved in encoding spectral information. The functional role of the SON cells with the suppressed response type is not clear and it is unknown if these cells are also inhibitory neurons as are the majority of SON neurons.
Temporal properties of SON cells.
Previous physiological investigations suggest that the inhibitory input from the SON to the NM and NL are GABAergic in nature, depolarizing, and have remarkably slow kinetics. These inputs readily summate and are likely to have a relatively long-lasting effect on postsynaptic neurons (Yang et al. 1999; Lu and Trussell 2000; Monsivais et al. 2000; Howard et al. 2007; Howard and Rubel 2010). A limited sample of whole cell physiological recordings suggested that SON neurons do not respond to electrical input with temporal precision (Yang et al. 1999). These results suggested that SON cells were unlikely to convey a temporally structured inhibition on a cycle-by-cycle basis with respect to sound stimuli. Surprisingly, we found that many low CF SON neurons respond to acoustic stimulation with phase-locked discharges, and this phase-locking was enhanced by inhibitory inputs. These results suggest that some SON neurons may be providing temporally patterned inhibition to their targets. Interestingly, six cells with CF higher >1.5 kHz were found to be significant by the Rayleigh test. While the peak vector strengths for these neurons were relatively low (0.19–0.23), significance by the Rayleigh test suggests that the occurrence of sustained spikes in these cells is not random with respect to the stimulus waveform. Recently, Kuo et al. (2009) demonstrated that rapid glycinergic signaling within the NA is quite prominent. This study did not identify the source of the glycine but showed that many terminals immunoreactive to antisera against GABA also appeared to contain glycine. One intriguing possibility is that the glycinergic input arises from the SON.
Our immunostaining for GABA and glycine revealed SON somas positive for both transmitters, in contrast to the glutamatergic NM, where somatic staining is absent. The GABA-positive somatic staining was reported in von Bartheld et al. (1989) but not shown. Our GABA staining confirms this finding and is complemented with glycine immunoreactivity in some SON neurons. The somatic staining pattern in SON, as well as coimmunopositive terminal staining among the target nuclei of SON projections shown in both Kuo et al. (2009) and the current study, support the hypothesis that the SON is a source of GABA/glycine corelease terminals in the chicken auditory brainstem.
If indeed the SON is a major source of glycinergic inhibition to its target nuclei, the SON may provide phase-locked release of glycine, a neurotransmitter that has rapid kinetics and therefore provide a source of temporally patterned inhibition to targets in NA or elsewhere. Rapid glycinergic transmission is a hallmark of many lower auditory nuclei including those involved in temporal processing in mammals (Grothe and Sanes 1993; Brand et al. 2002; Smith et al. 2000; Awatramani et al. 2004; Magnusson et al. 2005). Similar function for glycinergic transmission has not been demonstrated in vivo in birds. Future studies will be necessary to investigate whether the glycine in the avian auditory brainstem arises from the SON and what the functional consequences of this output are for its targets.
Inhibitory inputs to the SON.
The in vivo data reported in this study demonstrate that most SON cells receive both GABAergic and glycinergic inhibitory inputs. Our in vitro data and immunohistochemistry supported these findings. During tone stimulation, both modes of inhibition influenced acoustically driven spike rate but not the spontaneous rate. Further, both modes of inhibition contribute to the precision of phase-locking. Finally, GABAergic signaling influenced the slope of the dynamic range; however, glycinergic signaling had no effect on this property. While the source of the inhibitory inputs to SON neurons observed in this study remains unknown, one possibility is that the inhibition arises from the contralateral SON. Although we presented tones monaurally, the contralateral SON could have been driven in one of two ways. First, it has long been known that the middle ears of the chicken are acoustically coupled by the interaural canal, through which low frequency sound waves can readily pass and thereby influence the opposite ear drum (for review, see Hyson 2005). Second, ipsilateral NM cells project bilaterally to each NL (Parks and Rubel 1975; Rubel and Parks 1975), and the contralateral NL that projects in part to the contralateral SON (Conlee and Parks 1986) can be monaurally driven (Peña et al. 1996). Alternatively, intrinsic SON inhibitory connections cannot presently be ruled out. Further experiments will be necessary to investigate these different possibilities.
Our in vitro data provide additional insights into the nature of GABA and glycinergic input to SON neurons. In all of the cells tested, we observed a GABAergic and glycinergic component during eIPSCs. Isolated GABAergic currents had a longer duration while the glycinergic component was shorter compared with control. In the SON, the GABAergic component contributed ∼65% of the total inhibitory current. eIPSCs in the control condition were similar in kinetics but larger in magnitude than eIPSCs seen in the NA (Kuo et al. 2009) where GABA and glycine appear to be coreleased. Our analysis of sIPSCs suggested a similar input arrangement of inhibitory transmission in the SON. We found that blocking GABAergic transmission resulted in a significant decrease in the frequency of spontaneous events, indicating that the SON receives many inhibitory inputs that are purely GABAergic. In contrast blocking GlyRs did not reduce sIPSC frequency but did significantly reduce sIPSC amplitudes, suggesting that the glycinergic component of sIPSCs was not independent of GABA release. Kinetic analysis of sIPSCs was consistent with a corelease model of GABA and glycine. First, if GABA and glycine were released in separate vesicles, one would predict a bimodal distribution of sIPSC decay kinetics reflecting each of the two putative sources of input. Instead we observed that sIPSCs in the control condition had a continuous distribution of τdecay values. Second, sIPSC amplitude decreased significantly in both drug conditions consistent with GABA and glycine corelease. Finally, the control condition was the only condition where we saw very large (>400 pA) events (Fig. 9A). We hypothesize that these larger events, which tended to have fast kinetics (data not shown), are the result of summation of coreleased transmitters.
Role of corelease in the avian brainstem.
Corelease of GABA and glycine has been demonstrated at many synapses in the brain (Jonas et al. 1998; Tanaka and Ezure 2004; Dugué et al. 2005; Wojcik et al. 2006; Lu et al. 2008). Kuo et al., (2009) reported coimmunolocalization of GABA and glycine in NA, NM, and NL; however, physiological recording of glycinergic transmission was only observed in the NA. Burger et al. (2005) showed that many inhibitory terminals across NA, NM, and NL arise from collateral branches of single SON neurons. In the present study, we observed GlyR staining in the SON as well as mixed GABA/glycinergic synaptic transmission in vitro. In the mammalian auditory brainstem circuit one source of inhibitory input, the medial nucleus of the trapezoid body (MNTB), is known to corelease GABA, glycine and glutamate from terminals innervating the lateral superior olive early in development (Gillespie et al. 2005). Release from MNTB terminals shifts to primarily glycinergic output following hearing onset (Kandler and Friauf 1995; Kotak et al. 1998; Kim and Kandler 2003; Nabekura et al. 2004; Gillespie et al. 2005). Our data suggest that corelease in the SON is not likely due to developmental processes since we observed the physiological and anatomical hallmarks of corelease in animals up to P23. Hearing onset is around E11 in chickens, and the auditory system is considered mature before hatching (by E18; for review, see Rubel and Fritzsch 2002). However, a comprehensive developmental study will be required to completely rule out further developmental changes that may occur throughout maturation.
While the source of the GABA/Gly corelease terminals remains unknown, an appealing hypothesis is that SON neurons are providing both GABA and glycine to their targets. Coimmunostaining for GABA and glycine was observed in NM, NA, and NL (Kuo et al. 2009), and the SON is known to provide the dominant inhibition to these targets. However, at present, it appears that the mode of inhibition in those targets is determined by the complement of receptors expressed by the postsynaptic cells. A similar multitarget corelease arrangement has been described in a mammalian hindbrain circuit (Dugué et al. 2005). This arrangement seems plausible in the avian auditory brainstem where the primary source of inhibition (SON) provides input to both nuclei that process timing information and areas involved in intensity processing (Takahashi and Konishi 1988). The kinetically slow GABAergic input to NM and NL has been shown to improve temporal selectivity and coincidence detection (Funabiki et al. 1998; Yang et al. 1999; Monsivais et al. 2000; Fukui et al. 2010). The functional significance of GABA/glycine corelease within the SON is at present unknown. The in vivo experiments performed in this study demonstrated that GABAergic and glycinergic inhibitory inputs modulated the response properties of SON neurons similarly. The sIPSC analysis strongly suggests that SON neurons receive mixed inhibitory inputs composed of both corelease and purely GABAergic terminals. The possibility for diverse functional roles for this complement of inhibitory circuitry in the avian auditory brainstem should be investigated in future studies.
Summary.
We have reported several major findings in this study. First, we characterized the monaural response properties of chicken SON cells in vivo, which until now has been a notable gap in our understanding of auditory processing in the avian brainstem. SON cells have diverse response properties to acoustic stimulation, and some have strong phase-locking capabilities. Second, we have shown that these response properties are modulated by both GABAergic and glycinergic inhibitory inputs. On the synaptic level, we have shown that eIPSCs and sIPSCs are modulated by GABAergic and glycinergic inputs. Third, analysis of sIPSCs was consistent with a model of inhibitory input to SON neurons composed of both purely GABAergic terminals and terminals from which GABA and glycine are coreleased. Glycinergic signaling within the SON is a novel result with important implications for our understanding of acoustic processing in birds. The source(s) of inhibitory input to the SON and further investigation of the role of glycinergic signaling in avian brainstem auditory circuitry will be a focus of future study.
GRANTS
This work was supported by grants from the Deafness Research Foundation and the National Institute on Deafness and Other Communication Disorders Grant DC-008989.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Lynne Cassimeris for help with confocal microscopy, Dr. Martin Richter for comments on statistical analysis, and Gina Notaro for help in processing tissue following the in vivo experiments. We would like to thank Stefan Oline for editorial comments. We also thank the two anonymous reviewers whose expert comments and suggestions greatly improved our manuscript.
REFERENCES
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awatramani GB, Turecek R, Trussell LO. Inhibitory control at a synaptic relay. J Neurosci 24: 2643–2647, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backoff PM, Shadduck Palombi P, Caspary DM. Gamma-aminobutyric acidergic and glycinergic inputs shape coding of amplitude modulation in the chinchilla cochlear nucleus. Hear Res 134: 77–88, 1999. [DOI] [PubMed] [Google Scholar]
- Berens P. CircStat: A MATLAB toolbox for circular statistics. J Stat Softw 31: 1–21, 2009. [Google Scholar]
- Brand A, Behrend O, Marquardt T, McAlpine D, Grothe B. Precise inhibition is essential for microsecond interaural time difference coding. Nature 417: 543–547, 2002. [DOI] [PubMed] [Google Scholar]
- Burger RM, Rubel EW. Encoding of interaural timing for sound localization. In: The Senses: A Comprehensive Reference, edited by Dallos P, Oertel D. Oxford, UK: Academic, 2008, chapt. 3.35, p. 613. [Google Scholar]
- Burger RM, Cramer KS, Pfeiffer JD, Rubel EW. Avian superior olivary nucleus provides divergent inhibitory input to parallel auditory pathways. J Comp Neurol 481: 6–18, 2005. [DOI] [PubMed] [Google Scholar]
- Carr CE, Fujita I, Konishi M. Distribution of GABAergic neurons and terminals in the auditory system of the barn owl. J Comp Neurol 286: 190–207, 1989. [DOI] [PubMed] [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci 10: 3227–3246, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Backoff PM, Finlayson PG, Palombi PS. Inhibitory inputs modulate discharge rate within frequency receptive fields of anteroventral cochlear nucleus neurons. J Neurophysiol 72: 2124–2133, 1994. [DOI] [PubMed] [Google Scholar]
- Code RA, Churchill L. GABAA receptors in auditory brainstem nuclei of the chick during development and after cochlea removal. Hear Res 54: 281–295, 1991. [DOI] [PubMed] [Google Scholar]
- Code RA, Rubel EW. Glycine-immunoreactivity in the auditory brain stem of the chick. Hear Res 40: 167–172, 1989. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Parks TN. Origin of ascending auditory projections to the nucleus mesencephalicus lateralis pars dorsalis in the chicken. Brain Res 367: 96–113, 1986. [DOI] [PubMed] [Google Scholar]
- Dasika VK, White JA, Carney LH, Colburn HS. Effects of inhibitory feedback in a network model of avian brain stem. J Neurophysiol 94: 400–14, 2005. [DOI] [PubMed] [Google Scholar]
- Dugué GP, Dumoulin A, Triller A, Dieudonne S. Target-dependent use of co-released inhibitory transmitters at central synapses. J Neurosci 25: 6490–6498, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Dieudonne S. IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J Neurosci 21: 6045–6057, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert U, Ostwald J. GABA alters the discharge pattern of chopper neurons in the rat ventral cochlear nucleus. Hear Res 91: 160–166, 1995a. [DOI] [PubMed] [Google Scholar]
- Ebert U, Ostwald J. GABA can improve acoustic contrast in the rat ventral cochlear nucleus. Exp Brain Res 104: 310–322, 1995b. [DOI] [PubMed] [Google Scholar]
- Fukui I, Burger RM, Ohmori H, Rubel EW. GABAergic inhibition sharpens the frequency tuning and enhances phase locking in chicken nucleus magnocellularis neurons. J Neurosci 30: 12075–12083, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki K, Koyano K, Ohmori H. The role of GABAergic inputs for coincidence detection in the neurones of nucleus laminaris of the chick. J Physiol 508: 851–869, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci 8: 332–338, 2005. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J Neurophysiol 32: 613–636, 1969. [DOI] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev 90: 983–1012, 2010. [DOI] [PubMed] [Google Scholar]
- Grothe B, Sanes DH. Bilateral inhibition by glycinergic afferents in the medial superior olive. J Neurophysiol 69: 1192–1196, 1993. [DOI] [PubMed] [Google Scholar]
- Havey DC, Caspary DM. A simple technique for constructing “piggy back” multibarrel microelectrodes. Electroencephalogr Clin Neurophysiol 48: 249–251, 1980. [DOI] [PubMed] [Google Scholar]
- Howard MA, Burger RM, Rubel EW. A developmental switch to GABAergic inhibition dependent on increases in Kv1-type K+ currents. J Neurosci 27: 2112–2123, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MA, Rubel EW. Dynamic spike thresholds during synaptic integration preserve and enhance temporal response properties in the avian cochlear nucleus. J Neurosci 30: 12063–12074, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyson RL. The analysis of interaural time differences in the chick brain stem. Physiol Behav 86: 297–305, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science 281: 419–424, 1998. [DOI] [PubMed] [Google Scholar]
- Kalloniatis M, Fletcher EL. Immunocytochemical localization of the amino acid neurotransmitters in the chicken retina. J Comp Neurol 336: 174–193, 1993. [DOI] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J Neurosci 15: 6890–6904, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci 6: 3282–3290, 2003. [DOI] [PubMed] [Google Scholar]
- Köppl C, Carr CE. Maps of interaural time difference in the chicken's brainstem nucleus laminaris. Biol Cybern 98: 541–559, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppl C, Carr CE. Computational diversity in the cochlear nucleus angularis of the barn owl. J Neurophysiol 89: 2313–2329, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp-Scheinpflug C, Dehmel S, Dorrscheidt GJ, Rubsamen R. Interaction of excitation and inhibition in anteroventral cochlear nucleus neurons that receive large endbulb synaptic endings. J Neurosci 22: 11004–11018, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci 18: 4646–4655, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SP, Bradley LA, Trussell LO. Heterogeneous kinetics and pharmacology of synaptic inhibition in the chick auditory brainstem. J Neurosci 29: 9625–9634, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica EA, Rubsamen R, Rubel EW. GABAergic terminals in nucleus magnocellularis and laminaris originate from the superior olivary nucleus. J Comp Neurol 348: 403–418, 1994. [DOI] [PubMed] [Google Scholar]
- Lu T, Rubio ME, Trussell LO. Glycinergic transmission shaped by the corelease of GABA in a mammalian auditory synapse. Neuron 57: 524–535, 2008. [DOI] [PubMed] [Google Scholar]
- Lu T, Trussell LO. Inhibitory transmission mediated by asynchronous transmitter release. Neuron 26: 683–694, 2000. [DOI] [PubMed] [Google Scholar]
- Magnusson AK, Kapfer C, Grothe B, Koch U. Maturation of glycinergic inhibition in the gerbil medial superior olive after hearing onset. J Physiol 568: 497–512, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Streit P. Monoclonal antibodies demonstrating GABA-like immunoreactivity. Histochemistry 86: 147–157, 1986. [DOI] [PubMed] [Google Scholar]
- Moiseff A, Konishi M. Binaural characteristics of units in the owl's brainstem auditory pathway: precursors of restricted spatial receptive fields. J Neurosci 3: 2553–2562, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsivais P, Yang L, Rubel EW. GABAergic inhibition in nucleus magnocellularis: implications for phase locking in the avian auditory brainstem. J Neurosci 20: 2954–2963, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabekura J, Katsurabayashi S, Kakazu Y, Shibata S, Matsubara A, Jinno S, Mizoguchi Y, Sasaki A, Ishibashi H. Developmental switch from GABA to glycine release in single central synaptic terminals. Nat Neurosci 7: 17–23, 2004. [DOI] [PubMed] [Google Scholar]
- Nishino E, Yamada R, Kuba H, Hioki H, Furuta T, Kaneko T, Ohmori H. Sound-intensity-dependent compensation for the small interaural time difference cue for sound source localization. J Neurosci 28: 7153–7164, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleskevich S, Walmsley B. Synaptic transmission in the auditory brainstem of normal and congenitally deaf mice. J Physiol 540: 447–455, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks TN, Rubel EW. Organization and development of brain stem auditory nuclei of the chicken: organization of projections from n magnocellularis to n laminaris. J Comp Neurol 164: 435–448, 1975. [DOI] [PubMed] [Google Scholar]
- Peña JL, Viete S, Albeck Y, Konishi M. Tolerance to sound intensity of binaural coincidence detection in the nucleus laminaris of the owl. J Neurosci 16: 7046–7054, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer F, Simler R, Grenningloh G, Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci USA 81: 7224–7227, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci 25: 51–101, 2002. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Parks TN. Organization and development of brain stem auditory nuclei of the chicken: tonotopic organization of n magnocellularis and n laminaris. J Comp Neurol 4: 411–433, 1975. [DOI] [PubMed] [Google Scholar]
- Schröder S, Hoch W, Becker CM, Grenningloh G, Betz H. Mapping of antigenic epitopes on the alpha 1 subunit of the inhibitory glycine receptor. Biochemistry 30: 42–47, 1991. [DOI] [PubMed] [Google Scholar]
- Schuller G, Radtke-Schuller S, Betz M. A stereotaxic method for small animals using experimentally determined reference profiles. J Neurosci Methods 18: 339–350, 1986. [DOI] [PubMed] [Google Scholar]
- Scott LL, Mathews PJ, Golding NL. Posthearing developmental refinement of temporal processing in principal neurons of the medial superior olive. J Neurosci 25: 7887–7895, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Owens S, Forsythe ID. Characterisation of inhibitory and excitatory postsynaptic currents of the rat medial superior olive. J Physiol 529: 681–698, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Anatomy and physiology of principal cells of the medial nucleus of the trapezoid body (MNTB) of the cat. J Neurophysiol 79: 3127–3142, 1998. [DOI] [PubMed] [Google Scholar]
- Sullivan WE, Konishi M. Neural map of interaural phase difference in the owl's brainstem. Proc Natl Acad Sci USA 83: 8400–8404, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor KM, Wong RO, Rubel EW. Topography and morphology of the inhibitory projection from superior olivary nucleus to nucleus laminaris in chickens (Gallus gallus). J Comp Neurol 519: 358–375, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi TT, Konishi M. Projections of nucleus angularis and nucleus laminaris to the lateral lemniscal nuclear complex of the barn owl. J Comp Neurol 274: 212–238, 1988. [DOI] [PubMed] [Google Scholar]
- Tanaka I, Ezure K. Overall distribution of GLYT2 mRNA-containing versus GAD67 mRNA-containing neurons and colocalization of both mRNAs in midbrain, pons, and cerebellum in rats. Neurosci Res 49: 165–178, 2004. [DOI] [PubMed] [Google Scholar]
- Tsen G, Williams B, Allaire P, Zhou YD, Ikonomov O, Kondova I, Jacob MH. Receptors with opposing functions are in postsynaptic microdomains under one presynaptic terminal. Nat Neurosci 3: 126–132, 2000. [DOI] [PubMed] [Google Scholar]
- Viete S, Peña JL, Konishi M. Effects of interaural intensity difference on the processing of interaural time difference in the owl's nucleus laminaris. J Neurosci 17: 1815–1824, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Code RA, Rubel EW. GABAergic neurons in brainstem auditory nuclei of the chick: distribution, morphology, and connectivity. J Comp Neurol 4: 470–483, 1989. [DOI] [PubMed] [Google Scholar]
- Westerberg BD, Schwarz DW. Connections of the superior olive in the chicken. J Otolaryngol 24: 20–30, 1995. [PubMed] [Google Scholar]
- Wojcik SM, Katsurabayashi S, Guillemin I, Friauf E, Rosenmund C, Brose N, Rhee JS. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron 50: 575–587, 2006. [DOI] [PubMed] [Google Scholar]
- Yang L, Monsivais P, Rubel EW. The superior olivary nucleus and its influence on nucleus laminaris: a source of inhibitory feedback for coincidence detection in the avian auditory brainstem. J Neurosci 19: 2313–2325, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin TC, Chan JC. Interaural time sensitivity in medial superior olive of cat. J Neurophysiol 64: 465–488, 1990. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis (4th ed.). Prentice Hill, 1999. [Google Scholar]