Summary
In a previous study, we reported that a deficiency in MnSOD activity (approximately 80% reduction) targeted to type IIB skeletal muscle fibers was sufficient to elevate oxidative stress and to reduce muscle function in young adult mice (TnIFastCreSod2fl/fl mice). In the present study, we used TnIFastCreSod2fl/fl mice to examine the effect of elevated oxidative stress on mitochondrial function and to test the hypothesis that elevated oxidative stress and decreased mitochondrial function over the lifespan of the TnIFastCreSod2fl/fl mice would be sufficient to accelerate muscle atrophy associated with aging. We found that mitochondrial function is reduced in both young and old TnIFastCreSod2fl/fl mice, when compared with control mice. Complex II activity is reduced by 47% in young and by ~90% in old TnIFastCreSod2fl/fl mice, associated with reduced levels of the catalytic subunits for complex II, SDHA and SDHB. Complex II-linked mitochondrial respiration is reduced by approximately 70% in young TnIFastCreSod2fl/fl mice. Complex II-linked mitochondrial ATP production is reduced by 39% in young and was found to be almost completely absent in old TnIFastCreSod2fl/fl mice. Furthermore, in old TnIFastCreSod2fl/fl mice, aconitase activity is almost completely abolished; mitochondrial superoxide release remains greater than 2-fold elevated; and oxidative damage (measured as F2 isoprostanes) is increased by 30% relative to age-matched controls. These data show that despite elevated skeletal muscle-specific mitochondrial oxidative stress, oxidative damage and complex II-linked mitochondrial dysfunction, age-related muscle atrophy was not accelerated in old TnIFastCreSod2fl/fl mice, suggesting mitochondrial oxidative stress may not be causal for age-related muscle atrophy.
Introduction
Elevated oxidative stress (Lopez et al., 2000; Wanagat et al., 2001; Fulle et al., 2004, Mansouri et al., 2006; Muller et al., 2007) and decreased mitochondrial function (Short et al., 2005, Marcinek et al., 2005; Mansouri et al., 2006) have been shown to be associated with age-related skeletal muscle atrophy yet, a causal role for increased oxidative stress and mitochondrial dysfunction has not been established. Knockout mouse models with alterations in mitochondrial antioxidant defense provide a mechanism for directly testing the effect of elevated mitochondrial oxidative stress and mitochondrial dysfunction on skeletal muscle atrophy. Mice deficient in MnSOD, the first line of antioxidant defense against superoxide radicals in the mitochondrial matrix, are an excellent model to study the effects of elevated mitochondrial oxidative stress. However, complete deletion of Sod2−/− leads to lethality within days to just a few weeks depending on genetic background (Huang et al., 2006), and therefore Sod2−/− are not a useful model for aging studies.
To circumvent the lethality of complete deficiency of MnSOD, investigators have generated mouse models with targeted deletion of MnSOD in specific tissues using Cre-Lox approaches. Young mice with combined heart and skeletal muscle deletion of Sod2 (H/M-Sod2−/− mice, Nojiri et al., 2006), or, skeletal muscle-specific MnSOD knockout mice (muscle-Sod2−/−; Kuwahara et al., 2010) have been shown to have elevated mitochondrial oxidative stress in the affected tissues. Despite the elevated mitochondrial oxidative stress in the skeletal muscle of these two mouse models, there was no indication of muscle atrophy in either animal model (Nojiri et al., 2006; Kuwahara et al., 2010). Similarly, in a previous study, we reported that young adult TnIFastCreSod2fl/fl mice that have a 70% reduction in MnSOD content specifically in type IIB fibers, elevated mitochondrial oxidative stress and oxidative damage, show no loss of muscle mass (Lustgarten et al., 2009). Thus, collective evidence from the H/M-Sod2−/−, muscle-Sod2−/−, and TnIFastCreSod2fl/fl mice suggests that elevated mitochondrial oxidative stress and mitochondrial dysfunction are not sufficient to initiate muscle atrophy, at least in young mice. However, it is possible that over the lifespan chronic elevation of mitochondrial dysfunction and oxidative stress might contribute to muscle atrophy in older mice. To address this question, we asked whether elevated mitochondrial dysfunction and oxidative stress resulting from MnSOD deficiency targeted to type IIB enriched skeletal muscle in TnIFastCreSod2fl/fl mice leads to an accelerated loss of muscle mass during aging.
Results
Mitochondrial function is reduced in young TnIFastCreSod2fl/fl mice
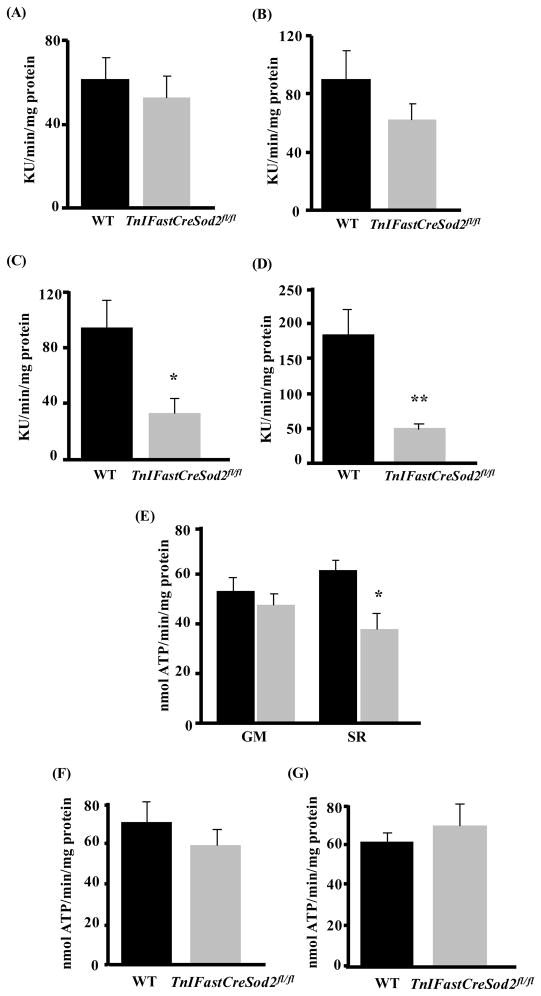
We have previously shown that mitochondria isolated from type IIB enriched skeletal muscle in young TnIFastCreSod2fl/fl mice have a greater than 80% decrease in MnSOD activity, elevated intra-mitochondrial superoxide, elevated mitochondrial superoxide release, and increased lipid oxidative damage, relative to wild type (Lustgarten et al., 2009). However, the role of elevated type IIB enriched skeletal muscle mitochondrial oxidative stress on mitochondrial function in TnIFastCreSod2fl/fl mice was not addressed. To determine the effect of the targeted reduction of MnSOD in type IIB muscle fibers on muscle mitochondrial function, we measured mitochondrial oxygen consumption and ATP production in skeletal muscle mitochondria isolated from type IIB enriched skeletal muscle in young adult (6–8 month) TnIFastCreSod2fl/fl and wild type mice. We found no significant difference in the glutamate plus malate (GM) supported basal rate of mitochondrial oxygen consumption (in the absence of ADP) in mitochondria isolated from type IIB enriched skeletal muscle from TnIFastCreSod2fl/fl when compared with wild type mice (Figure 1A). GM-stimulated maximal respiration (in the presence of exogenously added ADP) was reduced by 32% in mitochondria isolated from type IIB enriched muscle from TnIFastCreSod2fl/fl mice, but this difference was not statistically significant (Figure 1B). Succinate plus rotenone (SR)-linked basal and maximal respiration were reduced by 65% (Figure 1C) and 74% (Figure 1D), respectively, in type IIB enriched muscle from TnIFastCreSod2fl/fl mice when compared with wild type. The rate of mitochondrial ATP production by mitochondria isolated from type IIB enriched skeletal muscle respiring on several substrates including GM (Figure 1E), α-ketoglutarate (Figure 1F) and α-glycerol phosphate (Figure 1G) was not significantly different between TnIFastCreSod2fl/fl and wild type mice. In contrast, the rate of mitochondrial ATP production with SR used as substrate was reduced by 39% in type IIB enriched muscle from TnIFastCreSod2fl/fl mice (Figure 1E), indicating a complex II-specific defect in type IIB enriched skeletal muscle from TnIFastCreSod2fl/fl. Thus, mitochondrial function with complex II-linked substrate was altered in young mice with a targeted reduction of MnSOD.
Figure 1. Mitochondrial function in young TnIFastCreSod2fl/fl and wild type mice.
A) Basal and maximal mitochondrial oxygen consumption were measured in mitochondrial isolated from type IIB enriched muscle fibers isolated from young (6–8 month) TnIFastCreSod2fl/fl and wild type mice using the oxygen sensing probe, A65N-1, as described in Experimental Procedures. Basal respiration (A, C; n=5) was measured in the absence of exogenously added ADP with glutamate plus malate (GM, A) and succinate plus rotenone (SR, C) used as substrates. Maximal respiration was measured in the presence of exogenously added ADP with GM (B, n = 9) and SR (D, n = 10) used as substrates. Data are expressed as Kilo Units (KU, 103)/min/mg protein. *denotes a significant difference (p < 0.05) from wild type values. **denotes a significant difference (p < 0.01) from wild type values. Values represent means ± SEM. E–G) Rate of mitochondrial ATP production in mitochondrial isolated from type IIB enriched hindlimb skeletal muscle of 6–8 month TnIFastCreSod2fl/fl (grey bars) and wild type (black bars). ATP production was measured using a luciferase based assay as described in Experimental Procedures. GM and SR (E) were used as substrate. α-ketoglutarate (F) and α-glycerolphosphate (G) were used as substrates. Values represent means ± SEM for five samples.
Activities of Complex I and II are reduced in TnIFastCreSod2fl/fl mice
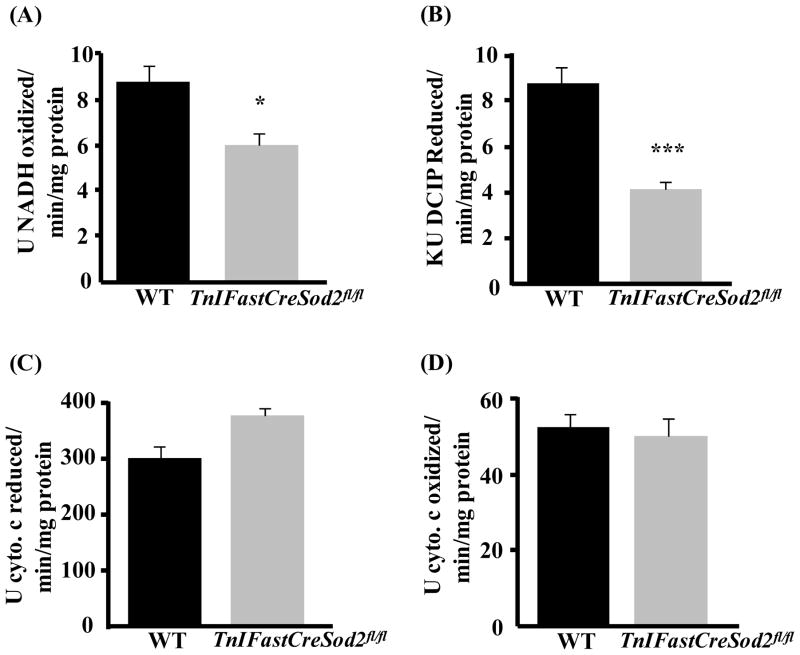
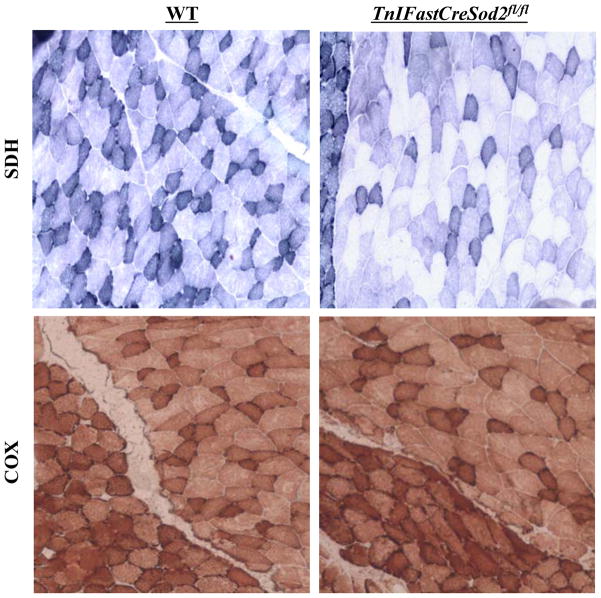
The electron transport chain (ETC), consisting of 5 protein complexes: complex I (NADH-ubiquinone oxidoreductase), complex II (succinate-ubiquinone oxidoreductase), complex III (ubiquinol-cytochrome c reductase), complex IV (cytochrome c oxidase) and complex V (ATP synthase) is located in the inner mitochondrial membrane. Therefore, the lack of MnSOD could potentially increase oxidative stress induced damage to the ETC complexes. As shown in Figure 2, the activities of complexes I and II were reduced by 32% and 47%, respectively, in mitochondria isolated from type IIB enriched skeletal muscle in TnIFastCreSod2fl/fl compared to muscle mitochondria from wild type. Activities of complex III and IV were not different in the two mouse models. In addition, there was no difference in the activities of complexes I, II, III, or IV in homogenates of type I fiber enriched skeletal muscle (soleus) from TnIFastCreSod2fl/fl or wild type mice (data not shown). We confirmed the changes in ETC activity using histological analysis of gastrocnemius muscle sections. As shown in Figure 3, complex IV activity is not changed, but clear zones indicative of reduced complex II activity are evident in muscle from TnIFastCreSod2fl/fl mice, when compared to muscle from wild type mice.
Figure 2. Biochemical measurement of ETC complex activity in young TnIFastCreSod2fl/fl and wild type mice.
Electron transport chain respiratory complex activity was measured spectrophotometrically as described in Experimental Procedures. A–D) ETC activity in mitochondria isolated from type IIB enriched skeletal muscle from young (6–8 month) TnIFastCreSod2fl/fl and wild type mice for A) complex I, B) complex II, C) complex III, D) complex IV. Values represent means ± SEM for 5 samples. *denotes a significant difference (p < 0.05) from wild type values. ***denotes a significant difference (p < 0.001) from wild type values.
Figure 3. Histological measurement of complex II and complex IV activity in young TnIFastCreSod2fl/fl and wild type mice.
Gastrocnemius (containing the attached soleus) was frozen in liquid nitrogen-cooled isopentane and sectioned using a cryostat. Muscle sections were incubated with substrates specific for complex II (Top) and complex IV (Bottom), as described in Experimental Procedures. Each image is a representative section of four independent samples for both wild type (left) and TnIFastCreSod2fl/fl (right).
SDHA and SDHB protein content are reduced but levels of SdhA and SdhB mRNA are not significantly different in young TnIFastCreSod2fl/fl mice
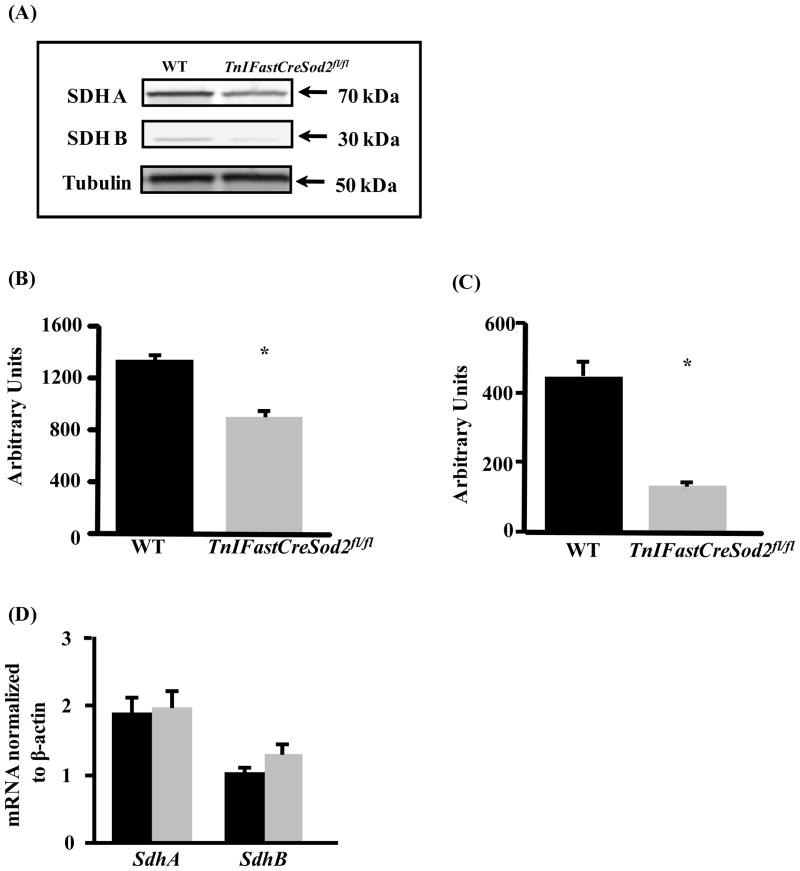
Complex II is composed of two hydrophilic (SDHA and SDHB) and two hydrophobic membrane localized subunits (SDHC and SDHD). To determine whether the reduced activity of complex II is associated with a reduction in the level of complex II protein, we measured the level of SDHA and SDHB in mitochondria isolated from type IIB enriched muscle in TnIFastCreSod2fl/fl and wild type control mice. SDHA and SDHB protein content were reduced by 32% and 70%, respectively (Figure 4A-C). SDHA and SDHB protein levels were not different in homogenates of type I fiber enriched soleus muscles isolated from TnIFastCreSod2fl/fl and wild type mice (data not shown). We also measured SdhA and SdhB mRNA content to determine whether the deficit in SDHA and SDHB protein content found in TnIFastCreSod2fl/fl was associated with a decrease in gene transcription. The mRNA content for SdhA and SdhB found in type IIB enriched skeletal muscle was not significantly different in muscle isolated from TnIFastCreSod2fl/fl and wild type mice (Figure 4D).
Figure 4. SdhA and SdhB mRNA and protein content in young TnIFastCreSod2fl/fl and wild type mice.
SDHA and SDHB protein content found in type IIB enriched skeletal muscle (A) in young (6–8 month) TnIFastCreSod2fl/fl and wild type mice. Tubulin was used as the loading control. Quantification of SDHA and SDHB is shown in Figure 4B and 4C, respectively. Each image is representative ofseven samples for both TnIFastCreSod2fl/fl and wild type mice. mRNA expression of SdhA and SdhB in type IIB enriched skeletal muscle (D) in TnIFastCreSod2fl/fl (grey bars) and wild type (black bars) mice was measured as described in Experimental Procedures. Values represent means ± SEM for five samples.
Activities of other TCA cycle enzymes are not decreased in young TnIFastCreSod2fl/fl mice
To determine whether the reduction in the enzymatic activities of aconitase and succinate dehydrogenase (SDH) was selective, we measured the activity of the mitochondrial matrix-localized citric acid cycle (TCA) enzymes citrate synthase, isocitrate dehydrogenase, malate dehydrogenase and fumarase. No significant difference was found for the enzymatic activity of citrate synthase, isocitrate dehydrogenase, malate dehydrogenase, or fumarase in either type IIB enriched skeletal muscle (Table I) or in the type I enriched soleus muscle (data not shown) when comparing the values obtained in TnIFastCreSod2fl/fl with wild type mice. These data indicate that the TCA cycle enzymes aconitase and SDH are preferentially sensitive to MnSOD deficiency.
Table I. Citric acid cycle (TCA) enzymatic activity in type IIB enriched skeletal muscle isolated from young (6–8 month) TnIFastCreSod2fl/fl and wild type mice.
TCA enzymatic activity was measured as described in Experimental Procedures. For citrate synthase, aconitase, isocitrate dehydrogenase, fumarase, and malate dehydrogenase (n = 4). For succinate dehydrogenase (n = 5). Data are expressed as Kilo Units (KU, 103)/min/mg protein for citrate synthase, succinate dehydrogenase and fumarase. Data are expressed as Mega Units (MU, 106)/min/mg protein for aconitase, isocitrate dehydrogenase and malate dehydrogenase.
| Citrate Synthase | Aconitase | Isocitrate Dehydrogenase | Succinate Dehydrogenase | Fumarase | Malate Dehydrogenase | |
|---|---|---|---|---|---|---|
| WT | 12.8 ± 0.1 | 0.41 ± 0.02 | 6.7 ± 0.4 | 8.7 ± 0.7 | 12.3 ± 0.3 | 3.8 ± 0.5 |
| TnIFastCreSod2fl/fl | 11.8 ± 0.9 | 0.18 ± 0.02* | 6.1 ± 0.5 | 4.1 ± 0.4* | 12.6 ± 0.2 | 3.7 ± 0.3 |
Values represent means ± SEM.
denotes a significant difference (p < 0.05) from wild type values.
SDH is sensitive to inactivation by superoxide
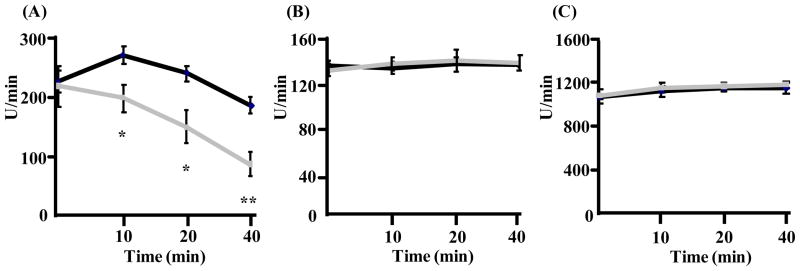
Aconitase inactivation was previously shown to be due to superoxide mediated Fe-S cluster damage (Gardner et al., 1995). The SDHB subunit of complex II contains three Fe-S clusters (Hagerhall 1997). Therefore, we asked whether the decrease in SDH activity found in TnIFastCreSod2fl/fl muscle was related to superoxide mediated inactivation. As shown in Figure 5A, SDH activity is reduced in the presence of potassium superoxide in vitro, demonstrating that SDH is also susceptible to inactivation by superoxide. In contrast, the activities of isocitrate dehydrogenase (Figure 5B) and fumarase (Figure 5C) are not altered by exposure to potassium superoxide.
Figure 5. Sensitivity of SDH, isocitrate dehydrogenase and fumarase to inactivation after incubation with superoxide for 0, 10, 20, and 40 minutes.
SDH (A), isocitrate dehydrogenase (B) and fumarase (C) were incubated for 0, 10, 20 and 40 minutes with an amount of potassium superoxide intermediate to that produced by actively respiring mitochondria isolated from type IIB enriched skeletal muscle in young (6–8 month) TnIFastCreSod2fl/fl and wild type mice. Superoxide was measured via use of DIPPMPO and EPR. Addition of CuZnSOD eliminated the EPR-derived signal, indicating that superoxide was responsible for the enzymatic inactivation of SDH (Lustgarten et al., 2009). Values represent means ± SEM for five samples for SDH, four samples each for isocitrate dehydrogenase and fumarase. *denotes a significant difference (p < 0.05) from wild type values. **denotes a significant difference (p < 0.01) from wild type values.
Oxidative stress and oxidative damage are elevated in old TnIFastCreSod2fl/fl mice
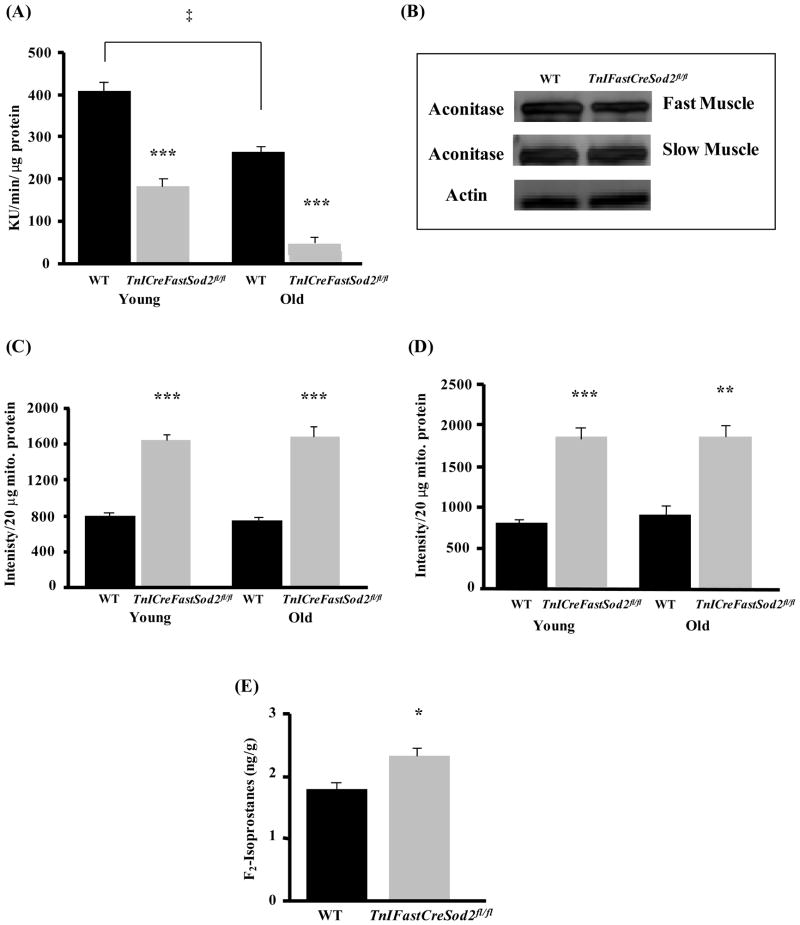
In a previous study, we demonstrated that type IIB enriched muscle from the TnIFastCreSod2fl/fl mice had increased levels of oxidative stress as measured by reduced aconitase activity, increased superoxide release and increased oxidative damage (measured as F2 isoprostanes; Lustgarten et al., 2009). We hypothesized that a lifelong reduction in MnSOD content would lead to an increase in mitochondrial matrix-localized superoxide and potentially exacerbate oxidative stress in older animals. To ask whether mitochondrial oxidative stress is elevated during aging in muscle from TnIFastCreSod2fl/fl mice, we measured aconitase activity in young and old wild type and TnIFastCreSod2fl/fl mice. As shown in Figure 6A, aconitase activity was reduced by approximately 90% in old (27–34 month) TnIFastCreSod2fl/fl mice when compared with the value obtained in either young TnIFastCreSod2fl/fl or in old wild type mice. Furthermore, aconitase activity is decreased by more than 35% during aging in wild type mice. Interestingly, the aconitase protein level is also reduced by more than 50% in type IIB enriched muscle from old TnIFastCreSod2fl/fl mice when compared with young TnIFastCreSod2fl/fl mice (Figure 6B). This effect is specific to type IIB enriched skeletal muscle in the TnIFastCreSod2fl/fl mice as we found no significant difference in aconitase protein in Type I fiber enriched soleus muscle from old mice of either genotype (Figure 6B). These data demonstrate an increase in type IIB enriched skeletal muscle mitochondrial matrix oxidative stress during aging that occurs in old wild type mice but to a greater extent in old TnIFastCreSod2fl/fl mice.
Figure 6. Oxidative stress in old TnIFastCreSod2fl/fl and wild type mice.
Aconitase activity (A) was measured in homogenates of type IIB enriched skeletal muscle from old (27–34 months) wild type and TnIFastCreSod2fl/fl mice. Values represent means ± SEM for five samples. ***denotes a significant difference (p < 0.001) from wild type values. ‡Significantly (p < .05) different as a function of age. Aconitase protein content (B) in type IIB enriched skeletal muscle and oxidative skeletal muscle (soleus) isolated from old (27–34 months) TnIFastCreSod2fl/fl and wild type mice. Each image is representative of three samples each for both old wild type and TnIFastCreSod2fl/fl mice. Actin was used as a loading control in type II enriched muscle. Mitochondrial superoxide release was measured in mitochondria that respired on GM (C) or SR (D), as isolated from young (6–8 months) and old (27–34 months) wild type (black bars) and TnIFastCreSod2fl/fl (grey bars). Values represent means ± SEM for five samples. ***denotes a significant difference (p < 0.001) from wild type values. E) Lipid peroxidation (F2-isoprostanes) in tibialis anterior muscles isolated from middle aged (21 months) TnIFastCreSod2fl/fl and wild type mice, measured as described in Experimental Procedures, and, expressed as nanograms per gram of tissue. Values represent means ± SEM for three samples for wild type and four samples for TnIFastCreSod2fl/fl mice. *denotes a significant difference (p < 0.05) from wild type values.
Because aconitase activity is an indirect indicator of mitochondrial matrix superoxide content, we directly measured mitochondrial superoxide release with use of electron paramagnetic resonance (EPR). Mitochondria isolated from type IIB enriched skeletal muscle from old TnIFastCreSod2fl/fl or old wild type mice do not release more superoxide than mitochondria isolated from their young, genotype-matched counterparts (Figure 6C, 6D). However, mitochondria isolated from both young and old TnIFastCreSod2fl/fl mice release greater than 2-fold more superoxide than mitochondria isolated from age-matched wild type mice in the presence of either GM or SR as respiratory substrates. Thus, the reduction in MnSOD results in elevated levels of superoxide that can be detected extra-mitochondrially.
To determine whether a reduction in MnSOD content leads to an increase in oxidative damage during aging in TnIFastCreSod2fl/fl mice, we measured lipid peroxidation (F2-Isoprostanes) in the tibialis anterior muscle (35% type IIB fibers) isolated from 21-month-old TnIFastCreSod2fl/fl mice. We had previously shown that young adult TnIFastCreSod2fl/fl mice have elevated levels of F2- isoprostanes (Lustgarten et al., 2009). Here we found a significant elevation (30%) of F2-Isoprostanes in tibialis anterior muscle isolated from middle-aged TnIFastCreSod2fl/fl mice, when compared with the value obtained in middle-aged wild type mice (Figure 6E).
Complex II-linked ATP production, complex II activity and protein content are each reduced in old TnIFastCreSod2fl/fl mice
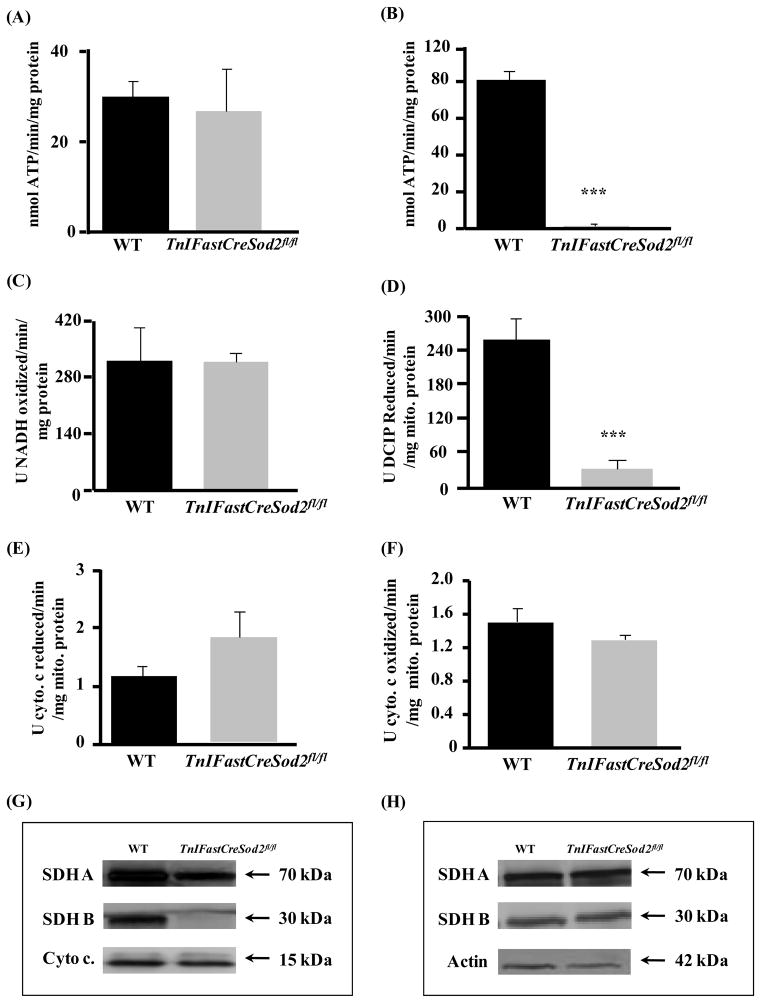
The rate of mitochondrial ATP production with GM used as substrate was not significantly different in mitochondria isolated from type IIB enriched skeletal muscle in old TnIFastCreSod2fl/fl mice, when compared with the corresponding value in age-matched wild type mice (Figure 7A). However, in response to SR as substrate, the rate of mitochondrial ATP production was reduced by 98% in old TnIFastCreSod2fl/fl mice, relative to age-matched wild type (Figure 7B). The activities of complexes I through IV were measured to investigate whether mitochondrial respiratory complex activity was impaired during aging in TnIFastCreSod2fl/fl. Complex II activity was selectively reduced by 88% (Figure 7D), but the activity of the other three protein complexes was not different when comparing old mutant mice with age-matched wild type. SDHA and SDHB protein content were found to be reduced by more than 50% and 90%, respectively (Figure 7G) in type IIB enriched muscle isolated from TnIFastCreSod2fl/fl mice, when compared with age-matched wild type. In contrast, levels of SDHA and SDHB were not significantly different in homogenates of type I fiber enriched soleus muscles isolated from old TnIFastCreSod2fl/fl, when compared withold wild type mice (Figure 7H).
Figure 7. Deficits in complex II-linked ATP production, complex II activity and protein content in old TnIFastCreSod2fl/fl mice.
The rate of mitochondrial ATP in old (27–34 months) wild type and TnIFastCreSod2fl/fl mice, with GM (A) and SR (B) used as respiratory substrates. Values represent means ± SEM for five samples. ***denotes a significant difference (p < 0.001) from wild type values. Biochemical measurement of ETC complex activity (complex I, (C); complex II, (D); complex III, (E); complex IV, (F) in mitochondria isolated from type IIB enriched skeletal muscle in old TnIFastCreSod2fl/fl and wild type mice, measured as described in Experimental Procedures. Values represent means ± SEM for four samples. ***denotes a significant difference (p < 0.001) from wild type values. SDHA and SDHB protein content in homogenates of type IIB enriched skeletal muscle (G) and oxidative (H) skeletal muscles isolated from old TnIFastCreSod2fl/fl and wild type mice. Each image is representative of three samples for both wild type and TnIFastCreSod2fl/fl mice. Cytochrome c and actin were used as loading controls for type II enriched skeletal muscle and in type I enriched muscle, respectively.
Muscle atrophy is not greater during aging in TnIFastCreSod2fl/fl mice
The preceding experiments demonstrated an increase in oxidative stress and mitochondrial dysfunction in mitochondria isolated from type IIB enriched muscle in TnIFastCreSod2fl/fl mice during aging. If mitochondrial dysfunction and oxidative stress play a casual role in muscle atrophy during aging, we would predict an increase in muscle atrophy in muscle with high type IIB content (and therefore low MnSOD activity) in the TnIFastCreSod2fl/fl mice. As shown in Table II, the mass of the type IIB rich gastrocnemius muscle (normalized to body mass, G/BW) and type I rich soleus muscle (S/BW) was examined in young (3–4 months) and old (27–34 months) TnIFastCreSod2fl/fl and wild type mice. G/BW declined significantly during aging for both wild type and TnIFastCreSod2fl/fl female but not male mice (data not shown). S/BW did not change during aging for either genotype or gender during aging. Significant increases in body mass and the percentage of body fat were identified for both old wild type and TnIFastCreSod2fl/fl female, but not male mice (data not shown) during aging. In addition, the percentage of lean mass significantly declined during aging for both wild type and TnIFastCreSod2fl/fl female mice. However, lifelong elevated oxidative stress, oxidative damage and mitochondrial dysfunction targeted to type IIB enriched-skeletal muscle was not sufficient to further alter G/BW, S/BW, body mass, or the respective percentages of body fat and lean mass during aging in female or male (data not shown) TnIFastCreSod2fl/fl, when compared to age-matched wild type mice.
Table II. Phenotypic data in young and old TnIFastCreSod2fl/fl and wild type mice.
Shown are values for body mass, percent lean mass or body fat, and muscle mass normalized to body mass for soleus and gastrocnemius muscle during aging in young female (6–8 months, n = 5) and old (27–34 months, n = 5) wild type and TnIFastCreSod2fl/fl mice. Quantitative magnetic resonance imaging (qMRI) was used for the determination of the percentages of body fat and lean mass based on Taicher et al., (2003), Tinsley et al., (2004) and as described by Lustgarten et al., (2009).
| Body Mass (g) | Lean Mass (%) | Body Fat (%) | Sol./BW (mg/g) | Gast./BW (mg/g) | ||
|---|---|---|---|---|---|---|
| Young | WT | 21.3 ± 0.3 | 83.2 ± 0.6 | 16.5 ± 0.6 | 0.32 ± 0.01 | 5.9 ± 0.1 |
| TnIFastCreSod2fl/fl | 20.9 ± 0.3 | 81.7 ± 0.6 | 17.8 ± 0.6 | 0.33 ± 0.02 | 5.7 ± 0.1 | |
| Old | WT | 23.4 ± 0.8a | 78.2 ± 2.1a | 21.2 ± 2.1a | 0.34 ± 0.02 | 4.1 ± 0.2a |
| TnIFastCreSod2fl/fl | 25.7 ± 1.6a | 76.1 ± 1.9a | 23.4 ± 1.9a | 0.30 ± 0.02 | 4.4 ± 0.2a |
Values represent means ± SEM.
denotes a significant difference (p < 0.05) as a function of age.
Discussion
The goal of the present study was to investigate the role of chronic elevations mitochondrial oxidative stress, oxidative damage and mitochondrial dysfunction on age-related muscle atrophy in a mouse model with targeted deletion of the mitochondrial antioxidant, MnSOD in type IIB muscle fibers. The data presented in this report show that increases in mitochondrial oxidative stress and oxidative damage, and alterations in mitochondrial function caused by MnSOD deficiency in type IIB fibers does not lead to skeletal muscle fiber atrophy in either young or old mice. In young mice with a greater than 80% reduction in MnSOD activity in type IIB enriched skeletal muscle, we found reduced aconitase activity (Lustgarten et al., 2009), decreased rates of mitochondrial oxygen consumption and ATP production, and a specific defect in complex II activity and protein content. In old TnIFastCreSod2fl/fl mice, aconitase activity was further reduced, mitochondrial superoxide release was elevated, oxidative damage was increased, and mitochondrial function was decreased, but, there was no acceleration of the rate of muscle atrophy, relative to the values found in age-matched wild type mice. These results are consistent with previous studies in mice with a 50% reduction in MnSOD (Sod2+/− mice, Van Remmen et al., 2001; Van Remmen et al., 2003; Mansouri et al., 2006), in mice deficient in MnSOD in both skeletal muscle and heart (H/M-Sod2−/−, Nojiri et al., 2006), and in mice lacking MnSOD in skeletal muscle (M-Sod2−/−, Kuwahara et al., 2010), as none of these models show increases in muscle atrophy despite elevated mitochondrial oxidative stress and mitochondrial dysfunction.
Skeletal muscle is a heterogeneous tissue composed of several different fiber types that are characterized on the basis of the content of myosin heavy chain type I, IIA, IIX, or IIB (Schiaffino et al., 1986; Schiaffino and Reggiani, 1994). The fiber types also differ in terms of metabolism. Mitochondrial content and oxidative metabolism are high in type I muscle fibers, while type IIB muscle fibers contain low levels of oxidative enzymes and mitochondria, but have a high abundance of glycolytic proteins (Pette and Staron, 1990). Type IIA and IIX fibers are intermediate in terms of combined oxidative and glycolytic capacity. Furthermore, mitochondria from type I and type II skeletal muscle have previously been shown to differ with respect to production of reactive oxygen species. Mitochondrial free radical leak (expressed as moles of H2O2 produced/mole O2 consumed), as found in the lateral portion of the gastrocnemius that is enriched in type IIB fiber content) was reported to be higher than mitochondrial free radical leak in muscles comprised mostly of type IIA fibers (medial gastrocnemius) or type I fibers (soleus) (Anderson and Neufer, 2006; Picard et al., 2008). Consistent with these findings, muscles comprised primarily of type II fibers have been shown to have increased levels of mitochondrial protein oxidative damage and decreased mitochondrial function relative to muscle (soleus) enriched in type I muscle fibers (Feng et al., 2008; Conley et al., 2007). Furthermore, type II muscle fibers have been shown to be more susceptible to age-related changes than type I skeletal muscle (Tomonaga 1977; Larsson et al., 1978; Jakobsson et al., 1990; Lexell and Taylor, 1991; Fulle et al., 2004). Collectively, these data suggest that mitochondrial oxidative stress and damage and mitochondrial dysfunction in type II fibers may be important factors in age-related muscle atrophy. The TnIFastCreSod2fl/fl mouse model targets deletion of MnSOD specifically in type IIB muscle fibers and is therefore particularly relevant for studying age-related changes in skeletal muscle.
Our data also strengthen the direct link between reduced MnSOD content and specific deficits in the activity of aconitase (Gardner et al., 1994; Li et al., 1995; Longo et al., 1999; Powell and Jackson, 2003, Hinerfeld et al., 2004; Lustgarten et al., 2009), complex I (Longo et al., 1999; Powell et al., 2003; Hinerfeld et al., 2004; Nojiri et al., 2006; Kuwahara et al., 2010), and complex II (Li et al., 1995; Longo et al., 1999; Powell et al., 2003; Hinerfeld et al., 2004; Nojiri et al., 2006; Kuwahara et al., 2010). Complex I supported respiration (GM) and activity were both decreased by 30%, but this amount was insufficient to adversely affect the rate of mitochondrial ATP production found in young TnIFastCreSod2fl/fl mice when compared with the corresponding value in wild type. One possible explanation involves the mitochondrial threshold effect (Mazat et al., 2001), in which 35–40% of complex I must be inactivated before mitochondrial function is affected (Barrientos and Moraes, 1999). In contrast, the reduction in complex II activity found in TnIFastCreSod2fl/fl mice was sufficient to negatively affect its respective rates of mitochondrial respiration, ATP and H2O2 production (Lustgarten et al., 2009). Succinate dehydrogenase is comprised of four nuclear encoded subunits: SdhA, SdhB, SdhC, and SdhD. SDHA is a 70 kDA protein and contains the FAD binding site. SDHB is a 30 kDA protein and contains three iron-sulphur clusters. Both SDHA and SDHB are soluble in the mitochondrial matrix, and are anchored to the inner mitochondrial membrane by two 15 kDA transmembrane proteins, SDHC and SDHD (Hagerhall, 1997). The activity of complex II has been previously reported to be directly proportional to the content of its catalytic subunits, SDHA and SDHB (Hinerfeld et al., 2004). SDHA and SDHB protein content found in type IIB enriched skeletal muscle were each significantly reduced but, no significant difference was observed for the mRNA levels of either SdhA or SdhB in TnIFastCreSod2fl/fl mice. These results are consistent with a previous study in mice lacking MnSOD in both heart and skeletal muscle (H/M-Sod2−/− mice, Nojiri et al., 2006). SDHB contains three iron-sulphur clusters, leaving open the possibility that SDHB is translated, oxidized, and subsequently degraded, as it may pass through the same metabolic fate as aconitase-reversible posttranslational inactivation, release of a labile iron from the [4Fe-4S]2+ cluster, disassembly of the [4Fe-4S]2+ cluster, carbonylation, and, protein degradation (Bulteau et al., 2003). SDHB contains one [4Fe-4S]2+ cluster, and the [3Fe–4S]+ cluster found in SDHB has been shown to be sensitive to oxidation (Beinert et al., 1977). Here we show that purified SDH is sensitive to inactivation by superoxide, with significant time-dependent reductions in SDH activity following incubation with superoxide. Prior to our study, superoxide had been shown to be only a mild inactivator of SDH (Zhang et al., 1990). Inactivation by superoxide is not a general effect as the enzymatic activity of fumarase and isocitrate dehydrogenase were not sensitive to inactivation when assayed under the same experimental conditions used to test the superoxide sensitivity of SDH. Mammalian fumarase activity was previously shown to be insensitive to superoxide-mediated inactivation (Patel et al.,1996), and our data validates that result.
One possible explanation for the reduction in SDHA and SDHB protein content may involve mRNA oxidation. Mitochondria isolated from type IIB enriched muscle in TnIFastCreSod2fl/fl mice respiring on complex I or complex II-linked substrate release greater than 2-fold more superoxide than mitochondria isolated from wild type mice (Lustgarten et al., 2009). As a result of potentially elevated cytosolic superoxide (or H2O2, after conversion by CuZnSOD), an increase in SdhA or SdhB mRNA oxidation may occur, thereby resulting in a reduction in protein expression (Shan et al., 2007) that may be responsible for the decrease in SDHA and/or SDHB protein content. Future studies aimed at investigating the association between a decrease in MnSOD content and the reduction in SDHA and SDHB subunit levels should investigate whether SdhA and SdhA mRNA are oxidized and are unable to be translated.
In summary, we report that mitochondrial oxidative stress and damage and mitochondrial dysfunction specifically targeted to type IIB skeletal muscle fibers over the lifespan of mice are not sufficient to accelerate age-related muscle atrophy. The fact that reductions in MnSOD activity of 50% in both young and old Sod2+/− mice (Mansouri et al., 2006), greater than 80% (present report, young and old) and 100% in both M-Sod2−/− (Kuwahara et al., 2010) and H/M-Sod2−/− mice (Nojiri et al., 2006) all result in an increase in mitochondrial oxidative stress and compromised mitochondrial function without affecting skeletal muscle mass strongly argue that mitochondrial oxidative stress and mitochondrial dysfunction are not involved in the maintenance of muscle mass in either young or old mice.
Experimental Procedures
Animals
Young (6–8 months) and old female (27–34 months) wild type and TnIFastCreSod2fl/fl mice (described previously in Lustgarten et al. 2009) were used for all experiments, with the exception of the assay of F2-isoprostanes, in which 21-month-old wild type and TnIFastCreSod2fl/fl male mice were used. All procedures involving the mice were approved by the Subcommittee for Animal Studies at the Audie L. Murphy Veterans Administration Hospital and the University of Texas Health Science Center at San Antonio.
Measurement of mitochondrial function
Because the TnI promoter is expressed predominantly in type IIB muscle fibers (Hallauer and Hastings, 2002), we used portions of mouse hindlimb muscle that are predominantly type II in composition (the white portion of the quadriceps and the gastrocnemius) for isolation of mitochondria for our experiments and refer to this as type IIB enriched muscle throughout the manuscript. Burkholder et al., (1994) reported the type IIB fiber composition of the gastrocnemius and quadricep group to range from 55–70%. Red portions of both muscles were removed to maximize type IIB fiber content in our muscle preparations. We have previously shown that MnSOD activity is reduced by greater than 80% in this combined muscle preparation (Lustgarten et al. 2009).
Mitochondria were isolated based on the method of King et al., (2007) and as described by Lustgarten et al., (2009). Mitochondrial respiration was measured using the method of Hynes et al., (2006). This assay is based on measuring the fluorescence of the oxygen-sensing probe (A65N-1, Axxora, San Diego, CA) which is quenched in the presence of oxygen. The quenching of the signal declines as oxygen is consumed, and the corresponding increase in fluorescence is directly proportional to the rate of mitochondrial oxygen consumption. 40–80 μg of mitochondria isolated from type IIB enriched muscle from wild type and TnIFastCreSod2fl/fl mice was resuspended in 150 μL of respiration buffer containing 125 mM KCl, 10 mM MOPS, 5 mM MgCl2, 2 mM K2HPO4, pH 7.44, with substrates: GM (12.5 mM glutamate, 12.5 mM malate) or SR (25 mM succinate, 0.5 μM rotenone) and A65N-1 probe, 67 nM. 100 μL of mineral oil was used to seal each well from atmospheric oxygen. Measurements were performed in a Spectramax 384 spectraphotometer (Molecular Devices, U.S.A.).
The rate of mitochondrial ATP production was measured in isolated muscle mitochondria as we have previously described (Lustgarten et al., 2009). The respiratory substrates GM (2.5 mM), SR (5 mM succinate, 0.5 μM rotenone), α-ketoglutarate (2.5 mM) and α-glycerol phosphate (2.5 mM) were added as indicated. Mitochondrial electron transport respiratory complex activity (ETC, complexes I-IV) was measured using spectrophotometric assays. Mitochondria isolated from type IIB enriched skeletal muscle isolated from wild type and TnIFastCreSod2fl/fl mice were solubilized in buffer containing 750 mM 6-aminocaproic acid (hexanoic acid), 50 mM Bis Tris and 1% L-dodecyl maltoside, pH 7.0. The samples were then placed on a rotator for 1 hour at 4°C, followed by centrifugation at 100,000xg for 15 minutes. The supernatant was then assayed for protein content and resuspended in respiration buffer (250 mM sucrose, 10 mM KH2PO4, 10 mM Tris, 1 mM EGTA, pH 7.4). Activity measurements for complexes I, II and IV were performed on a Spectramax 384 Spectraphotometer (Molecular Devices, U.S.A). Complex III activity was measured via a Beckman spectrophotometer. For complex I, 3.3 μg of mitochondrial protein was added to 150 μL of respiration buffer containing 0.1 mM NADH, 0.05 mM decylubiquinone and 0.05 mM dichloroindophenol (DCIP). Complex I activity was determined by monitoring the oxidation of NADH at 340 nm, at 30°C (Birch-Machin and Turnbull, 2001). A separate sample was measured in parallel with the addition of 640 nM rotenone. Subtraction of this data is indicative of rotenone-sensitive complex I activity. Complex I data was expressed as Units of NADH oxidized/min/mg protein. For complex II, 3.3 μg of mitochondrial protein was added to 150 μL of respiration buffer containing 20 mM succinate, 50 uM ubiquinone, 2 mM KCN and 50 μM DCIP. Complex II activity was measured via the succinate-dependent reduction of DCIP, as measured at 600 nm, at 30°C (Boveris and Cadenas, 1975). A separate sample containing 3 mM malonate was measured in parallel. Subtraction of this data is indicative of malonate-sensitive complex II activity. Complex II data are expressed as Units DCIP reduced/min/mg protein. For complex III, 3.3 μg of mitochondrial protein was added to 1 mL of buffer containing 100 μM (Fe3+) cytochrome c, 100 μM decylubiquinol and 2 mM KCN. Complex III activity was measured by following the reduction of cytochrome c at 550 nm, at 25°C. Complex III data was expressed as Units of cytochrome c reduced/min/mg protein. Complex IV activity was measured by addition of 1.65 μg of mitochondrial protein to 150 μL of respiration buffer containing 90 μM (Fe2+) cytochrome c. Complex IV activity was determined by following the oxidation of cytochrome c at 550 nm, at 30°C. Complex IV data are expressed as Units of cytochrome c oxidized/min/mg protein.
Histology
Cryostat sections of gastrocnemius muscles isolated from young female wild type and TnIFastCreSod2 fl/fl mice were used for the histologic analysis of complex II and complex IV activity and were prepared as previously described by Lustgarten et al., (2009). Complex II activity was assayed by incubating sections for 1 hour at 37°C in a buffer containing 20 uM NaPO4, 40 μM succinate, 0.1% NBT, pH 7.4 (Nachlas et al., 1957). Sections were then rinsed in saline, followed by fixation in formalin-saline (10% neutral buffered formalin containing 10 mM NaCl) for 10 minutes. Sections were placed in 15% ethanol for 15 minutes, followed by addition of mounting media. A dark blue formazan color is indicative of muscle fibers rich in complex II activity. Complex IV activity was assayed by incubating sections for 1 hour at room temperature in buffer consisting of 200 mM sucrose, 20 mM NaPO4, 1.3 mM 3, 3′ diaminobenzidine tetrahydrochloride (DAB), 80 μM cytochrome c, 8 nM catalase, pH 7.6. Sections were then washed three times with double distilled water, followed by muscle section dehydration in ascending alcohols (50%, 70%, 80%, 95% × 2 washes, 100% × 2 washes). Sections were cleared with two additions of xylene. Following the addition of mounting media, sections were visualized for dark brown staining, which is indicative of muscle fibers with high complex IV activity (Seligman, 1972).
Measurement of SDH mRNA
SdhA and SdhB mRNA were isolated from type IIB enriched skeletal muscle and the content was measured using quantitative real time PCR as described by Dahia et al., (2005). For determination of SdhA mRNA content, GTGCGGATTGATGAGTACGATT and CACATGCATGAGCTATTATACATAA were used as the forward and reverse primers, respectively. SdhB mRNA content was measured with use of CGACTCCAGAGACGACTTCAC and GCTCGCTTCTCCTTGTAGGTC as the forward and reverse primers, respectively.
Western Blotting
Homogenates of type IIB enriched or type I enriched (soleus) skeletal muscle isolated from TnIFastCreSod2fl/fl and wild type mice were prepared in assay buffer containing 50 mM Tris-HCl buffer with 150 mM NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate and 1x protease inhibitor. Equivalent amounts of protein (40–80 μg) for each sample were resolved in 4–20% Tris-HCl SDS-PAGE gels in triplicate. After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane. The membrane was then incubated in Tris-buffered saline, pH 7.4, with 0.05% Tween 20 (TBS-T) containing 10% nonfat milk for 1 hour at room temperature. The blots were then reacted with antibodies specific for aconitase (1:2000, a kind gift from Luke Szweda, Oklahoma Medical Research Foundation, Oklahoma, U.S.A.), SDHA (1:1000, Invitrogen, U.S.A.) and SDHB (1:500, Invitrogen, U.S.A.) at 4°C overnight. After washing with TBS-T, the blots were incubated with either goat anti-rabbit IgG horseradish peroxidase or goat anti-mouse IgG-horseradish peroxidase (1:1000, Sigma) for 2 hours at room temperature. The blots were washed five times with TBS-T and the bands corresponding to each respective protein was visualized using chemiluminescent detection reagents obtained from Amersham Biosciences.
Citric acid cycle enzymatic activity
Citric acid cycle enzymatic activity was measured in mitochondria isolated from type IIB enriched-skeletal muscle from wild type and TnIFastCreSod2fl/fl mice. To obtain soluble mitochondrial proteins, mitochondria were resuspended in 30 mM potassium phosphate buffer containing 0.2% Triton-X-100, pH 7.4, followed by rotation for 45 minutes at 4°C and centrifugation at 16100xg. The activity of citrate synthase (1 μg), isocitrate dehydrogenase (13 μg), fumarase (30 μg) and malate dehydrogenase (0.1) was measured by addition of mitochondrial extract (with the amount of protein added in parenthesis) to 200 μL of enzyme activity buffer as described by Robinson et al., (1987). All enzyme activity assays were conducted at 30°C.
Identification of proteins sensitive to superoxide
Purified E. Coli succinate dehydrogenase (SDH, a kind gift from Dr. Gary Ceccini, UCSF), fumarase (Sigma-Aldrich, U.S.A.) and isocitrate dehydrogenase (Sigma-Aldrich, U.S.A.) were used to determine their potential sensitivity to inactivation in the presence of potassium superoxide. E. Coli SDH is structurally and functionally homologous to mammalian SDH (Cecchini et al., 2002). Potassium superoxide solution was prepared by adding 0.5 g of KO2 to 1.5 mL anhydrous dimethyl sulfoxide (DMSO). This solution was vortexed for 5 minutes, followed by centrifugation at 500xg for 5 minutes. The supernatant containing soluble KO2 was removed. Catalase was added (1/100) to degrade any hydrogen peroxide that had formed from the spontaneous dismutation of superoxide. Electron paramagnetic resonance (EPR) was used to quantify the amount of superoxide present in the KO2 solution. The KO2 stock was then further diluted in DMSO (1/5) to obtain an EPR spectrum intermediate in magnitude to that produced by mitochondria isolated from both wild type and TnIFastCreSod2fl/fl mice (Lustgarten et al., 2009). Non-diluted KO2 produces an EPR signal that is approximately 10–12 and 5–6 fold higher than mitochondria respiring on SR and isolated from wild type and TnIFastCreSod2fl/fl, respectively (data not shown). Addition of CuZnSOD (1 U/μL) eliminated the observed EPR spectra (Lustgarten et al., 2009), thereby indicating the specificity of the EPR-derived signal for detecting superoxide. Individual enzymes were assayed for their sensitivity to inactivation in the presence of superoxide via room temperature incubation. For example, 10 μg of purified SDH was added to either 1 μL of diluted KO2 or 1 μL of DMSO in a final volume of 10 μL. For measurement of SDH activity, 200 mM succinate, 4 mM ubiquinone and 3 mM DCIP were added to a reaction buffer consisting of 250 mM sucrose, 10 mM KH2PO4, 10 mM Tris, 1 mM EGTA, 0.4 U/μL CuZnSOD, 7.5U/μL catalase, pH 7.4. CuZnSOD and catalase were added to the reaction buffer to degrade any superoxide (and potentially, H2O2) that remained after incubation with SDH. At the indicated time points (0, 10, 20, 40 minutes), 0.6 μL of succinate dehydrogenase incubated with either KO2 or DMSO was removed and added to 150 μL of reaction buffer. SDH activity was then measured based the method of Boveris and Cadenas (1975).
To test the sensitivity of other mitochondrial matrix-localized proteins to inactivation by superoxide, we measured the enzymatic activity of fumarase and isocitrate dehrdrogenase. Three μg of fumarase was incubated with 4 μL of diluted KO2 stock or 4 μL of DMSO in 50 μL of reaction buffer. At the indicated time points (0, 10, 20, 40 minutes) 10 μL of superoxide or DMSO incubated-fumarase was added to150 μL of reaction buffer containing 100 mM malate, 0.4 U/μL CuZnSOD and 7.5 U/μL catalase, pH 7.4. Fumarase activity was measured based on the method of Robinson et al., (1987). Similarly, 55 μg of isocitrate dehydrogenase was incubated with 3.75 μL of diluted KO2 or 3.75 μL of DMSO in 50 μL of reaction buffer. At the indicated time points (0, 10, 20, 40 minutes), 10 μL of superoxide or DMSO-incubated isocitrate dehydrogenase was added to 150 uL of reaction buffer containing 50 mM Tris pH 7.4, 1 mM MnCl2, 0.5 mM EDTA, 0.25 mM NADP+, 1.6 mM isocitrate, 0.4 U/μL CuZnSOD and 7.5U/μL catalase, pH 7.4. Isocitrate dehydrogenase activity was then measured based on the method of Robinson et al., (1987).
Measurement of oxidative stress status during aging in TnIFastCreSod2fl/fl mice
Measurement of aconitase activity is a sensitive indicator of mitochondrial matrix superoxide content (Gardner and Fridovich, 1991; Gardner et al., 1994, 1995, Gardner 2002). Aconitase activity was measured in homogenates of type IIB enriched skeletal muscle isolated from old female TnIFastCreSod2fl/fl and wild type mice, based on the method of Gardner et al., (1994) and as described by Lustgarten et al., (2009). Mitochondrial superoxide release was measured via electron paramagnetic resonance (EPR) with use of the spin trap, 5-diisopropoxyphosphoryl-5-methyl-1-pyrroline-N-oxide, (DIPPMPO; Chalier and Tordo, 2002), as described by Lustgarten et al., (2009). Levels of F2-isoprostanes in tibialis anterior (TA) muscles isolated from middle aged (21 months) mice were determined as described by Lustgarten et al., (2009). TA muscle has been shown to contain 35% type IIB and 65% IIA fibers (Burkholder et al., 1994)
Statistics
Unpaired student’s t test was used for all analyses, unless otherwise indicated.
Chemicals
All chemicals were purchased from Sigma-Aldrich Chemical Co., unless otherwise indicated.
Acknowledgments
We thank Corinne Price for editing the manuscript.
Grants
This work was funded by NIH grant P01AG020591, a VA Merit grant (HVR) and NIA Training Grant 5T3-AG021890-02.
References
- Anderson EJ, Neufer PD. type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol. 2006;290:C844–851. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- Barrientos A, Moraes CT. Titrating the effects of mitochondrial complex I impairment in the cell physiology. J Biol Chem. 1999;274:16188–16197. doi: 10.1074/jbc.274.23.16188. [DOI] [PubMed] [Google Scholar]
- Beinert H, Ackrell BA, Vinogradov AD, Kearney EB, Singer TP. Interrelations of reconstitution activity, reactions with electron acceptors, and iron-sulfur centers in succinate dehydrogenase. Arch Biochem Biophys. 1977;182:95–106. doi: 10.1016/0003-9861(77)90287-9. [DOI] [PubMed] [Google Scholar]
- Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. Methods Cell Biol. 2001;65:97–117. doi: 10.1016/s0091-679x(01)65006-4. [DOI] [PubMed] [Google Scholar]
- Boveris A, Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 1975;54:311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Ikeda-Saito M, Szweda LI. Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry. 2001;42:14846–14855. doi: 10.1021/bi0353979. [DOI] [PubMed] [Google Scholar]
- Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221(2):177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- Cecchini G, Schroder I, Gunsalus RP, Maklashina E. Succinate dehydrogenase and fumarate reductase from Escherichia coli. Biochim Biophys Acta. 2002;1553:140–157. doi: 10.1016/s0005-2728(01)00238-9. [DOI] [PubMed] [Google Scholar]
- Chalier F, Tordo P. 5-(Diisopropoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide, DIPPMPO, a crystalline analog of the nitrone DEPMPO: synthesis and spin trapping properties. J Chem Soc, Perkin Trans. 2002;2:2110–2117. [Google Scholar]
- Conley KE, Jubrias SA, Amara CE, Marcinek DJ. Mitochondrial dysfunction: impact on exercise performance and cellular aging. Exerc Sport Sci Rev. 2007;35:43–49. doi: 10.1249/JES.0b013e31803e88e9. [DOI] [PubMed] [Google Scholar]
- Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS, Hodin R, Heitritter S, Moore F, Dluhy R, Sosa JA, Ocal IT, Benn DE, Marsh DJ, Robinson BG, Schneider K, Garber J, Arum SM, Korbonits M, Grossman A, Pigny P, Toledo SP, Nose V, Li C, Stiles CD. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Navratil M, Thompson LV, Arriaga E. A principal component analysis reveals age-related and muscle-type-related differences in protein carbonyl profiles of muscle mitochondria. J Gerontol A Biol Sci Med Sci. 2008;63:1277–1288. doi: 10.1093/gerona/63.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fano G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- Gardner PR, Nguyen DH, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci. 1994;91:12248–12252. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner PR, Raineri I, Epstein LB, White CW. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem. 1995;270:13399–405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- Gardner PR. Aconitase: sensitive target and measure of superoxide. Methods Enzymol. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- Hagerhall C. Succinate: quinone oxidoreductases. Variations on a conserved theme. Biochim Biophys Acta. 1997;1320:107–141. doi: 10.1016/s0005-2728(97)00019-4. [DOI] [PubMed] [Google Scholar]
- Hallauer PL, Hastings KE. TnIfast IRE enhancer: multistep developmental regulation during skeletal muscle fiber type differentiation. Dev Dyn. 2002;224(4):422–31. doi: 10.1002/dvdy.10122. [DOI] [PubMed] [Google Scholar]
- Hinerfeld D, Traini MD, Weinberger RP, Cochran B, Doctrow SR, Harry J, Melov S. Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. J Neurochem. 2004;88:657–667. doi: 10.1046/j.1471-4159.2003.02195.x. [DOI] [PubMed] [Google Scholar]
- Huang TT, Naeemuddin M, Elchuri S, Yamaguchi M, Kozy HM, Carlson EJ, Epstein CJ. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum Mol Genet. 2006;15:1187–1194. doi: 10.1093/hmg/ddl034. [DOI] [PubMed] [Google Scholar]
- Hynes J, Marroquin LD, Ogurtsov VI, Christiansen KN, Stevens GJ, Papkovsky DB, Will Y. Investigation of drug-induced mitochondrial toxicity using fluorescence-based oxygen-sensitive probes. Toxicol Sci. 2006;92:186–200. doi: 10.1093/toxsci/kfj208. [DOI] [PubMed] [Google Scholar]
- Jakobsson F, Borg K, Edstrom L. Fibre-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Acta Neuropathol. 1990;80:459–468. doi: 10.1007/BF00294604. [DOI] [PubMed] [Google Scholar]
- King KL, Stanley WC, Rosca M, Kerner J, Hoppel CL, Febbraio M. Fatty acid oxidation in cardiac and skeletal muscle mitochondria is unaffected by deletion of CD36. Arch Biochem Biophys. 2007;467:234–238. doi: 10.1016/j.abb.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara H, Horie T, Ishikawa S, Tsuda C, Kawakami S, Noda Y, Kaneko T, Tahara S, Tachibana T, Okabe M, Melki J, Takano R, Toda T, Morikawa D, Nojiri H, Kurosawa H, Shirasawa T, Shimizu T. Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic Biol Med. 2010 Feb 13; doi: 10.1016/j.freeradbiomed.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Larsson L, Sjodin B, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. Acta Physiol Scand. 1978;103:31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor T. Variability in muscle fiber areas in whole human quadriceps muscle: effects of increasing age. J Anat. 1991;174:239–249. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–81. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Longo VD, Liou LL, Valentine JS, Gralla EB. Mitochondrial superoxide decreases yeast survival in stationary phase. Arch Biochem Biophys. 1999;365:131–142. doi: 10.1006/abbi.1999.1158. [DOI] [PubMed] [Google Scholar]
- Lopez ME, Van Zeeland NL, Dahl DB, Weindruch R, Aiken JM. Cellular phenotypes of age-associated skeletal muscle mitochondrial abnormalities in rhesus monkeys. Mutat Res. 2000;452(1):123–138. doi: 10.1016/s0027-5107(00)00059-2. [DOI] [PubMed] [Google Scholar]
- Lustgarten MS, Jang YC, Liu Y, Muller FL, Qi W, Steinhelper M, Shimizu T, Shirasawa T, Bhattacharya A, Richardson A, Van Remmen H. Conditional knockout of Mn-SOD targeted to type IIB skeletal muscle fibers increases oxidative stress and is sufficient to alter aerobic exercise capacity. Am J Physiol Cell Physiol. 2009;297(6):C1520–C1532. doi: 10.1152/ajpcell.00372.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev. 2006;127:298–306. doi: 10.1016/j.mad.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, Conley KE. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol. 2005;569(Pt 2):467–473. doi: 10.1113/jphysiol.2005.097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazat JP, Rossignol R, Malgat M, Rocher C, Faustin B, Letellier T. What do mitochondrial diseases teach us about normal mitochondrial functions that we already knew: threshold expression of mitochondrial defects. Biochim Biophys Acta. 2001;1504:20–30. doi: 10.1016/s0005-2728(00)00236-x. [DOI] [PubMed] [Google Scholar]
- Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Van Remmen H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1159–1168. doi: 10.1152/ajpregu.00767.2006. [DOI] [PubMed] [Google Scholar]
- Nachlas MM, Tsou KC, De Souza E, Cheng CS, Seligman AM. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957;5:420–36. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Nojiri H, Shimizu T, Funakoshi M, Yamaguchi O, Zhou H, Kawakami S, Ohta Y, Sami M, Tachibana T, Ishikawa H, Kurosawa H, Kahn RC, Otsu K, Shirasawa T. Oxidative stress causes heart failure with impaired mitochondrial respiration. J Biol Chem. 2006;281:33789–33801. doi: 10.1074/jbc.M602118200. [DOI] [PubMed] [Google Scholar]
- Patel M, Day BJ, Crapo JD, Fridovich I, McNamara JO. Requirement for superoxide in excitotoxic cell death. Neuron. 1996;16:45–55. doi: 10.1016/s0896-6273(00)80052-5. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:l–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Picard M, Csukly K, Robillard ME, Godin R, Ascah A, Bourcier-Lucas C, Burelle Y. Resistance to Ca21-induced opening of the permeability transition pore differs in mitochondria from glycolytic and oxidative muscles. Am J Physiol Regul Integr Comp Physiol. 2008;295:R659–R668. doi: 10.1152/ajpregu.90357.2008. [DOI] [PubMed] [Google Scholar]
- Powell CS, Jackson RM. Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: modulation of enzyme activities by MnSOD. Am J Physiol Lung Cell Mol Physiol. 2003;285:L189–98. doi: 10.1152/ajplung.00253.2002. [DOI] [PubMed] [Google Scholar]
- Robinson JB, Jr, Brent JB, Sumegi B, Srere PA. An enzymatic approach to the study of the Krebs tricarboxylic acid cycle. In: Darley-Usmar VM, Rickwood D, Wilson MT, editors. Mitochondria: A Practical Approach. 1987. pp. 153–169. [Google Scholar]
- Seligman AM. Histochemistry, theoretical and applied. In: Everson Pearse AG, editor. Histochemistry, theoretical and applied. 3. Vol. 2. Baltimore, MD: Williams & Wilkins; 1972. [Google Scholar]
- Schiaffino S, Saggin L, Viel A, Ausoni S, Sartore S, Gorza L. Muscle fiber types identified by monoclonal antibodies to myosin heavy chains. In: Benzi G, Packer L, Siliprandi N, editors. Biochemical Aspects of Physical Exercise. Amsterdam: Elsevier; 1986. pp. 27–34. [Google Scholar]
- Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol. 1994;77(2):493–501. doi: 10.1152/jappl.1994.77.2.493. [DOI] [PubMed] [Google Scholar]
- Shan X, Chang Y, Lin CL. Messenger RNA oxidation is an early event preceding cell death and causes reduced protein expression. FASEB J. 2007;21(11):2753–2764. doi: 10.1096/fj.07-8200com. [DOI] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377(6):990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- Tinsley FC, Taicher GZ, Heiman ML. Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res. 2004;12(1):150–60. doi: 10.1038/oby.2004.20. [DOI] [PubMed] [Google Scholar]
- Tomonaga M. Histochemical and ultrastructural changes in senile human skeletal Muscle. J Am Geriatr Soc. 1977;25:125–131. doi: 10.1111/j.1532-5415.1977.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Williams MD, Guo Z, Estlack L, Yang H, Carlson EJ, Epstein CJ, Huang TT, Richardson A. Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am J Physiol Heart Circ Physiol. 2001;281:H1422–1432. doi: 10.1152/ajpheart.2001.281.3.H1422. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- Wanagat J, Cao Z, Pathare P, Aiken JM. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001;15:322–332. doi: 10.1096/fj.00-0320com. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Marcillat O, Giulivi C, Ernster L, Davies KJ. The oxidative inactivation of mitochondrial electron transport chain components and ATPase. J Biol Chem. 1990;265:16330–16336. [PubMed] [Google Scholar]