SUMMARY
The ZntB Zn2+ efflux system is important for maintenance of Zn2+ homeostasis in Enterobacteria. We report crystal structures of ZntB cytoplasmic domains from Salmonella enterica serovar Typhimurium (StZntB) in dimeric and physiologically relevant homopentameric forms at 2.3 Å and 3.1 Å resolutions, respectively. The funnel-like structure is similar to that of the homologous Thermotoga maritima CorA Mg2+ channel and a Vibrio parahaemolyticus ZntB (VpZntB) soluble domain structure. However, the central α7 helix forming the inner wall of the StZntB funnel is oriented perpendicular to the membrane instead of the marked angle seen in CorA or VpZntB. Consequently, the StZntB funnel pore is cylindrical, not tapered, which may represent an “open” form of the ZntB soluble domain. Our crystal structures and isothermal titration calorimetry data indicate that there are three Zn2+ binding sites in the full-length ZntB, two of which could be involved in Zn2+ transport.
INTRODUCTION
Zn2+ homeostasis is critical for cellular function (Blencowe et al., 2003; Devirgiliis et al., 2007; Hantke, 2005; Lichten et al., 2009). In bacteria, it is maintained by multiple influx and efflux transport systems, including the ZnuABC and ZupT influx and ZntA and ZitB efflux systems (Ammendola et al., 2007; Chao et al., 2004a; Chao et al., 2004b; Grass et al., 2005; Pasquali et al., 2008; Patzer et al., 1998; Yatsunyk et al., 2008). An additional widespread Zn2+ transporter is ZntB (Caldwell et al., 2003; Worlock et al., 2002), a member of the CorA superfamily of ion transport systems, which consists, in the bacteria and archaea, of CorA, ZntB and a third group of unknown function (Maguire, 2008; Moomaw et al., 2008; Papp-Wallace et al., 2007; Knoop et al., 2005).
The CorA family forms selective Mg2+ channels with extremely high conductances of >100 pS (Schindl et al., 2007). CorA, the first divalent cation channel to have its structure determined (Lunin et al., 2006; Payandeh et al., 2006; Eshaghi et al., 2006), is present in about half of all bacteria and archaea with fully sequenced genomes (Maguire, 2006a; Maguire, 2008). The crystal structure of CorA shows a funnel-shaped homopentamer with the stem of the funnel in the membrane. The soluble cytosolic domain of each CorA monomer consists of the first ~250 amino acids of the protein and is followed by 2 transmembrane segments (TM) connected by a 9 amino acid periplasmic loop with a short 6 amino acid cytosolic C-terminus.
The ZntB branch of the CorA superfamily is widespread within the Enterobacteria but does not appear to be present in most other bacterial species or the archaea. Within the many Enterobacteria that carry both ZntB and CorA, there is typically 15–20% sequence identity between ZntB and CorA and a similar topology. Previous work on StZntB directly demonstrated that it mediates Zn2+ efflux (Worlock et al., 2002). Secondary structure prediction (Caldwell et al., 2003) and threading of ZntB sequence onto the CorA crystal structure (Maguire, 2006b) strongly suggested that ZntB and CorA have the same overall structure. This prediction was confirmed by solution of the Vibrio parahaemolyticus ZntB (VpZntB) soluble domain structure (Tan et al., 2009), showing a homopentamer whose monomers have the same overall structure as the CorA monomer. Thus, the CorA superfamily consists of both ion channels and transporters, similar to the ClC family of chloride transport systems (Mindell, 2008; Mindell et al., 2001).
To further investigate the molecular basis of zinc transport, the soluble domain of ZntB from S. Typhimurium (StZntB) was purified and crystallized, and the capacity for zinc binding by the soluble domain and full-length transporter was measured using isothermal titration calorimetry (ITC). The ZntB monomer structure is similar to CorA consisting of an αβα sandwich followed by a long “stalk” α-helix contiguous with TM1. Like CorA, ZntB forms a homopentamer. Unlike the CorA homopentamer, which is partially stabilized by Mg2+ ions within the cytosolic domain, the StZntB homopentamer is stabilized by extensive salt-bridges between the monomers. The interior of the funnel in ZntB is essentially cylindrical with a large diameter near the membrane, unlike that of CorA and VpZntB, which are V-shaped with small diameters near the membrane. This suggests the possibility that this StZntB structure represents an “open” form of the soluble domain in contrast to VpZntB and CorA, which are apparently closed forms. The ITC data reveal the presence of three zinc binding sites on the full-length receptor. Two of the sites appear equivalent to those observed in the X-ray structure of the soluble domain, while the third appears to reside in the membrane domain. Thus, through evolution, the same basic funnel-shaped homopentameric structure has been used to form a very high conductance Mg2+ channel and a transporter that mediates flux of a different divalent cation in the opposite direction.
RESULTS
Structure of the ZntB cytoplasmic domain
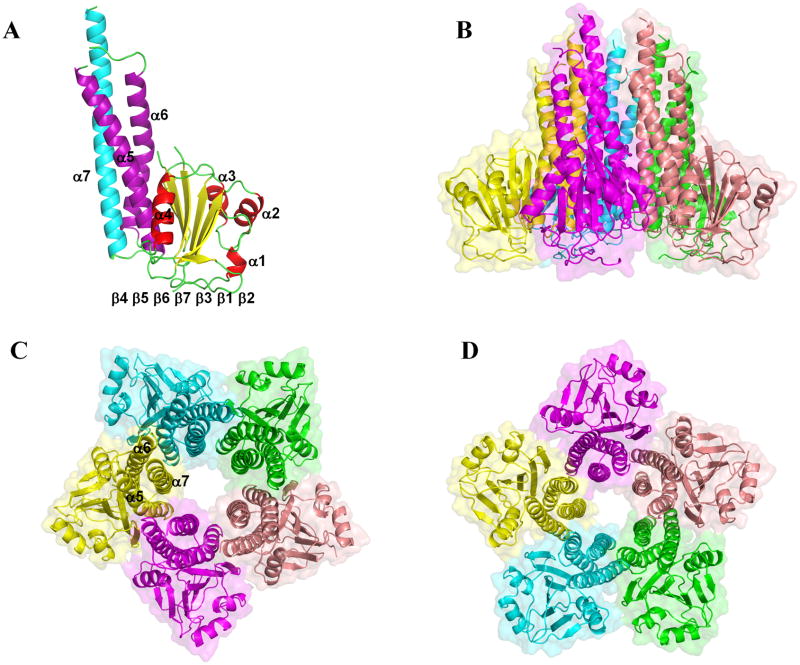
We report the X-ray structures of the soluble domain of StZntB in two crystal forms to 2.3 Å and 3.1 Å resolution (Figure 1, Table 1 and Supplementary Movie SM1). The StZntB structure at 2.3 Å resolution has two anti-parallel monomers per asymmetric unit. The StZntB structure at 3.1 Å resolution has five monomers per asymmetric unit, forming the physiologically relevant pentamer, which is not formed by crystallographic packing. In StZntB, two loop regions (residues 96–105 between β5 and β6, and residues 178–181 between α5 and α6) are not visible in the 2Fo-Fc electron density maps.
Figure 1. Structure of the cytoplasmic domain of StZntB.
(A) The ribbon diagram of the monomer structure at 2.3 Å. The α1-α4 helices in the αβα subdomain are colored in red, β1-β7 strands are colored in yellow, α5 and α6 in the coiled-coil domain are colored in purple, and α7 is colored in cyan. (B) Side view of the StZntB pentamer structure. (C) View of the funnel of the StZntB pentamer from the cytosol. (D) Rotation of 180 ° with respect to C, showing the funnel of the StZntB pentamer from the membrane. See also Supplementary Figure 1 and Supplementary Movie 1.
Table 1.
Data collection and refinement statistics
| Protein source | Salmonella enterica serovar Typhimurium | |||
|---|---|---|---|---|
| Data collection | Se-Met | Native (dimer) | Native (pentamer) | Zn peak absorption |
| space group | P1 | P21 | C2 | P21 |
| unit cell (Å) | a=44.36 b=56.69 | a=45.0 b=93.8 | a=173.6.1 b=101.9 | a=44.7 b=91.4 |
| c=58.45 | c=68.2 | c=90.3 | c=67.8 | |
| α=61.06 β=82.01 γ=82.74° | β=91.6° | β=110.4° | β=92.5° | |
| mol per asu | 2 | 2 | 5 | 2 |
| wavelength (Å) | 0.9793 (Se peak) | 1 | 1.282 (Zn peak) | 1.284 (Zn peak) |
| resolution (Å) | 2.7 | 2.3 | 3.15 | 2.95 |
| unique reflections | 13366 | 24331 | 25929 | 11337 |
| Redundancy | 3.9 (3.8)a | 3.5 (2.1)a | 3.8 (3.8)a | 3.4(1.8)a |
| completeness (%) | 98.9 (98.2)a | 96.0 (74.4)a | 99.8 (100)a | 97.8 (81.2)a |
| Rsym (%) b | 0.121 (0.574)a | 0.085 (0.490)a | 0.092 (0.590)a | 0.093 (0.312)a |
| I/σ(I) | 10.7 (2.6)a | 25.2 (1.6)a | 11.9(2.2)a | 17.5 (3.1)a |
| Refinement | ||||
| Rwork/Rfree (%) c | 20.1/25.4 | 22.2/27.1 | ||
| R.M.S.D. from ideal geometry | 0.006/0.944 | 0.007/1.034 | ||
| No. of atoms | ||||
| protein | 3802 | 9549 | ||
| Zn2+ | 8 | 15 | ||
| SO42− | 4 | |||
| water | 94 | |||
| Cl− | 2 | |||
| Mean B-value (Å2) | ||||
| main chain/side chain | 45.7/50.1 | |||
| Zn2+/Cl-/H2O | 66.8/40/43.6 | |||
| Ramachandron Plot statistics (%) | ||||
| most favorable region | 96.29 | 90.98 | ||
| allowed region | 3.71 | 7.20 | ||
| disallowed region | 0 | 1.82 | ||
Numbers in parenthesies represent values in the highest resolution shell.
Rsym=Σ(|Ii − < I>|)/Σ(Ii), where Ii is the measured intensities and <I> is the mean intensity of all measured observations equivalent to reflection Ii.
Rwork=Σ||Fobs| − |Fcalc||/Σ|Fobs|, where |Fobs| are the observed diffraction amplitude, |Fcalc| is the corresponding calculated structure factor amplitude. Rfree is defined by Rwork, but involved 10% of the measured reflections not used in refinement and set aside for cross-validation purpose.
See also Supplementary Movie 1.
Comparison of ZntB and CorA
The monomer structures of the soluble domain of StZntB cytoplasmic domains are similar both to the orthologous VpZntB (Tan et al., 2009) and the homologous T. maritima CorA (Lunin et al., 2006; Payandeh et al., 2006; Eshaghi et al., 2006). In StZntB the N-terminal seven-stranded mixed β-sheets (β2 ▲β1 ▼β3 ▲β7 ▲β6 ▼β5 ▲β4 ▼) are sandwiched by two sets of α-helices (α1-α3) and (α4-α6) (Figure 1A and Supplementary Figure S1A). However, there are topological differences in the secondary structure connectivity at the N-terminus among CorA, StZntB, and VpZntB. In CorA, the N-terminus proceeds β1, β2, α1 and β3. In contrast, VpZntB lacks the α1 helix and hence the N-terminus proceeds β1, β2, β3 and α2 (Supplementary Figure S1B), while in StZntB, the N-terminus proceeds α1, β1, β2 and β3 (Supplementary Figure S1C). In addition, VpZntB has a short α-helix (α6b) between and at right angles to α6 and α7 at the mouth of the funnel that is not present in StZntB or CorA.
The C-terminal stalk helix (α7) starts in the cytoplasm and moves towards the cell membrane. In full-length StZntB, the α7 helix is predicted by HMMTOP (Tusnady et al., 2001) to be a transmembrane helix as it is in CorA (Lunin et al., 2006; Payandeh et al., 2006; Eshaghi et al., 2006). The three anti-parallel αhelices, α5, α6 and α7 in StZntB, as in VpZntB and CorA, form a coiled-coil domain with heptad sequence repeats that promote hydrophobic interactions.
Results of a pair-wise superposition of the soluble domain structures of the two ZntB structures and T. maritima CorA are shown in Table 2. The two ZntB structures and T. maritima CorA are similar to each other in structure, with RMSDs within 2.0 Å over 194 residues. However, about 70 residues that are non-equivalent were omitted from this RMSD calculation. Taking these residues into account, the overall RMSD between VpZntB and StZntB monomers is 18.5 Å. The overall RMSD between the CorA and StZntB pentamers is even greater at ~20 Å. Despite sharing similar structural topologies, there are significant differences between StZntB and VpZntB, particularly in the N-terminal region (Supplementary Figure S2). For example, the α2 helix in VpZntB (residues 39–47) is out of register and tilted compared to the α2 helix of StZntB (residues 52–60). The same tilt and lack of registry is also apparent for the α3, α6 and α7 helices of each protein. Residues 199–205 in VpZntB form an α-helix, whereas in StZntB the corresponding region (218–223) forms a loop. These structural and topological differences help explain why molecular replacement alone could not be used to solve the structure of StZntB using VpZntB as the search model.
Table 2. Structural alignment of the soluble domains of monomeric StZntB, VpZntB and TmCorA.
Pair-wise root mean square deviations (RMSDs in Å) of Cα atoms for the indicated structures calculated using the program O are shown in the lower quadrant. The numbers of Cα atoms used for alignment are in parentheses. The percent sequence identity with respect to StZntB calculated by BLAST (Altschul et al., 1997) is shown in the upper quadrant. Percent sequence identity to StZntB is also shown for KpZntB and S. Typhimurium CorA (StCorA, accession number gi:60391925).
| StZntB | VpZntB | TmCorA | KpZntB | StCorA | |
|---|---|---|---|---|---|
| StZntB | 21 | 18 | 85 | 11 | |
| VpZntB | 1.81 (210) | ||||
| TmCorA | 1.85 (194) | 1.91 (206) | |||
| KpZntB | 0.97 (234) | 1.71 (198) | 1.78 (182) |
Comparison of ZntB and CorA pentamers
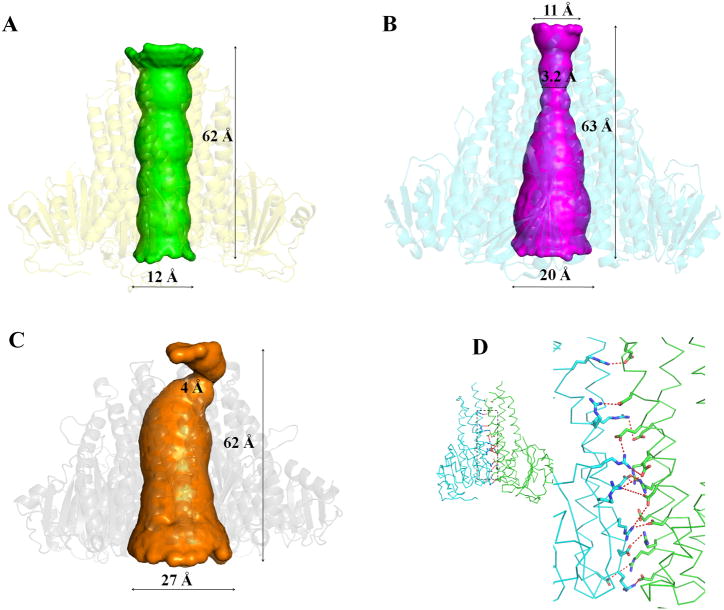
The StZntB soluble domain assembles into a homopentamer that resembles a funnel as previously observed with T. maritima CorA (Lunin et al., 2006; Payandeh et al., 2006; Eshaghi et al., 2006) and VpZntB (Tan et al., 2009). The inner wall of the funnel in CorA is formed by the α7 helix with a contribution from α6 at the cytosolic end while the outer wall is formed by α5 and α6 (Figure 1C). However, the funnel in the StZntB homopentamer has striking differences compared to those of CorA and VpZntB. In StZntB, the interior wall of the funnel is formed entirely by α7, and the pore within the funnel has a cylindrical shape. In both CorA and VpZntB the interior of the funnel is tapered with a wide mouth at the cytosolic end and a narrow neck proximal to the membrane (Figure 2). The cylinder inside the StZntB funnel has dimensions of ~12 Å at each end (Figure 2A). In contrast, CorA and VpZntB have respective dimensions of 20 Å and 27 Å at the funnel mouth and 3.2 Å and 4 Å at the narrowest position near the membrane (Figure 2B and 2C). Consistent with these differences, packing at the pentameric interface within StZntB is quite different from CorA and VpZntB. For example, the CorA pentameric interface consists of helices α3 to α6 and the β4 strand, while the VpZntB interface consists of helices α3, α5 to α7, the β4-β5 strand and Loop 5 (between α3 and β4). In contrast, the StZntB interface consists of α5, α6, α7 and Loop 5. The α3 helix and β strands are not involved in StZntB. In particular, the five α7 helices in StZntB that form the inner wall of the cylinder stand parallel to each other (Figure 1C). In VpZntB and CorA, the corresponding α7 helices tilt toward each other at angles of about 22° and 16°, respectively. The total volumes of the cytoplasmic domains of StZntB, VpZntB and T. maritima CorA are ~1.83 × 105 Å3, ~1.39 × 105 Å3 and 1.86 × 105 Å3, respectively. VpZntB is the most compact of the three structures. StZntB and CorA are close in total volume although StZntB has a broader base at the membrane than CorA, while CorA is much larger in diameter at the top of the funnel in the cytosol.
Figure 2. Funnel shape and salt-bridges of StZntB.
Shape of the interior of the funnel of (A) StZntB compared to (B) T. maritima CorA and (C) VpZntB. Shapes were calculated with HOLE (Smart et al., 1996). (D) Side view of the salt- bridges between two neighboring monomers in the StZntB pentamer structure. The inset (left) shows the entire monomers with dashed box highlighting the area enlarged on the right. See also Supplementary Table 1 and Supplementary Figures 3, 4 and 5.
The pentamer interface in StZntB consists of hydrogen bonds, salt-bridges, and hydrophobic and van der Waals interactions. In StZntB, only two residues contribute to hydrophobic interactions. Instead, multiple putative inter-monomer salt-bridges in StZntB (Figure 2D and Supplementary Table S1) contribute much more to stabilization of the pentamer than in VpZntB or CorA. Twelve oppositely charged ion-pairs in the monomer-monomer interface are within 5 Å (Supplementary Figure S3A). Size exclusion chromatography shows that the oligomeric state of StZntB soluble domain is disrupted at higher salt concentrations (Supplementary Figure S4). Further, the StZntB soluble domain crystallized as the dimeric form in the presence of 500 mM NaCl but as the pentameric form in the presence of 150 mM NaCl. Thus, both size exclusion chromatography and crystallography results suggest that the salt-bridges are important for stability of the oligomeric state. In contrast, VpZntB and CorA have, respectively, only 5 and 7 pairs of residues forming ion-pairs between monomers (Supplementary Figure S3B and S3C).
The interactions between adjacent α7 helices differ in StZntB compared to both VpZntB and CorA. Only one residue pair, proximal to the cytosolic end of α7, exhibits ionic attraction between adjacent α7 helices in StZntB (Supplementary Figure S5A). Thus in StZntB, there are no significant interactions to bring two adjacent α7 helices close to each other, which presumably explains why the funnel pore size maintains the same ~12 Å diameter from the cytosolic to the membrane end. In VpZntB and CorA, additional interactions, including H-bonds and hydrophobic interactions, are observed between adjacent α7 helices. All of these are proximal to the membrane (Supplementary Figure S5B and S5C). These interactions function to bring the membrane end of the α7 helices close to each other in VpZntB and CorA, while the cytosolic ends of α7 are further apart, lacking such interactions. Such different interactions help explain why the pore size close to the membrane end is much narrower in CorA and VpZntB compared to StZntB.
These interactions between monomers affect the apparent free energy of monomer-monomer association (Table 3). StZntB and CorA have comparable free energy contributions from electrostatic interactions and desolvation energy, with an overall ΔG of about −30 Kcal/mol for monomer-monomer interactions. In contrast, VpZntB is significantly less stable, with an overall ΔG of about −11 Kcal/mol.
Table 3.
Free Energies of Monomer-Monomer Interactions in the CorA Superfamily
| Transport System | ΔGelectrostatics (Kcal/mol) | ΔGdesolvation (Kcal/mol) | ΔGbinding (Kcal/mol) |
|---|---|---|---|
| StZntB | −50.4 | 22.4 | −28.0 |
| VpZntB | −20.1 | 9.4 | −10.7 |
| CorA | −56.8 | 25.7 | −31.1 |
See also Supplementary Figure 5.
Zn2+ binding sites
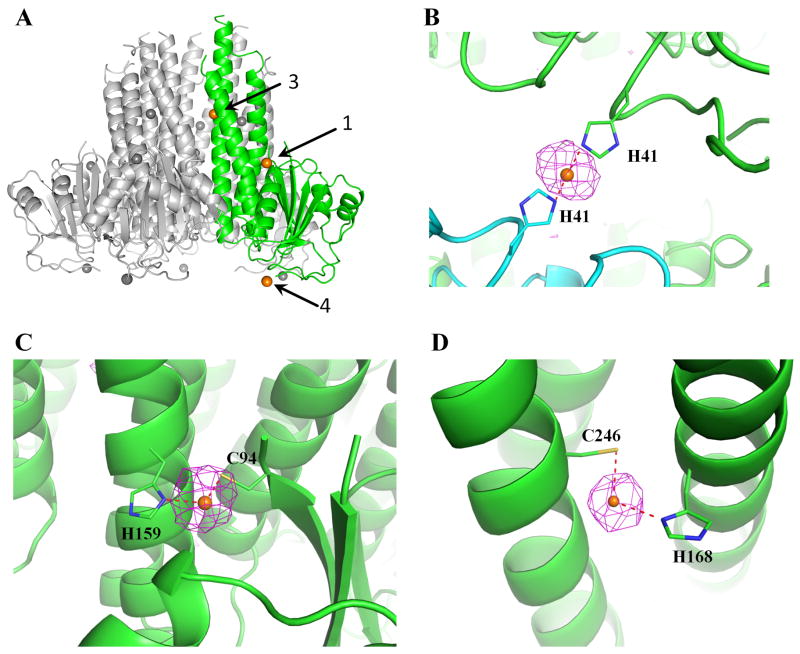
StZntB was co-crystallized in the presence of 1 mM ZnSO4. From the anomalous difference map calculated at the Zn absorption edge peak data, three Zn2+ ions bound to each monomer were identified in both the pentameric and monomeric states of the StZntB soluble domain (labeled sites 1, 3 and 4 in Figure 3A). ITC data on the full length StZntB indicated the presence of an additional Zn2+, termed site 2 (see below). In the soluble domain pentamer structure, one Zn2+ is coordinated by two H41 residues from adjacent crystallographic symmetry-related monomers located within the β2-β3 connecting loop (site 4) and is clearly not relevant physiologically (Figure 3A–B). A second Zn2+ is outside the funnel (site 1), coordinated by C94 in β5 and H159 in α5 from the same monomer (Figure 3C). The third Zn2+ in the soluble domain structure is located within the wall of the funnel (site 3) and is coordinated by H168 in α5 and C246 in α7 (Figure 3D and Supplementary Figure S6). In contrast, the soluble domain of VpZntB has no bound Zn2+. Instead, 5 Cl− ions were seen bound to each monomer (Tan et al., 2009). To investigate this difference further, crystals of the homopentameric StZntB were soaked in a stabilizing solution containing 100 mM NaBr for 1 hr. However, no Br− could be identified in the anomalous difference map calculated from the Br adsorption edge peak data (data not shown).
Figure 3. Zn2+ binding sites in Salmonella ZntB.
(A) The three Zn2+ (orange) binding sites in the monomer (green) within the soluble domain pentamer are labeled 1, 3 and 4. Other monomers are in gray. (B) The anomalous difference map contoured at 3σ is shown for site 4, where Zn2+ is coordinated by two neighboring His41 residues related by crystal packing. (C) Site 1 on the outside surface of the funnel where Zn2+ is coordinated by C94 and H159 of the same monomer and (D) in site 3 Zn2+ is bound inside the interior wall of the funnel by H168 and C246 of the same monomer. See also Supplementary Figure 3.
ITC measurement of Zn2+ binding to StZntB
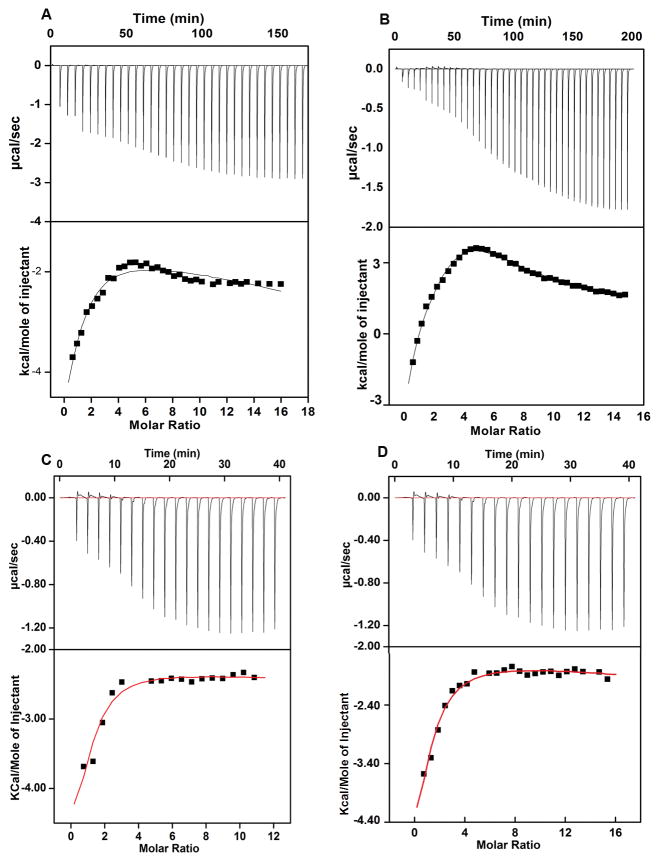
Zn2+ binding to StZntB was further studied by ITC. When measuring the blank, Zn2+ was injected into the sample cell filled with buffer. Initial injections showed small endothermic heat changes, while the later injections showed increasing exothermic heat changes probably due to the heat of dilution. A similar titration experiment using the soluble domain of StZntB resulted in large exothermic heat changes in the first few injections indicative of binding isotherms (Figure 4A). The heat changes at various molar ratios of Zn2+ added to the soluble domain of ZntB could be best fitted by a 2 site sequential binding model that gave an apparent dissociation constant of 15 μM for the first site and 775 μM for the second site. For the first binding site a high negative enthalpic change relative to entropy indicates that the binding reaction is predominantly enthalpically driven. Since we did not observe the complete saturation at very high concentrations of Zn2+, the second binding site is weak and dynamic in nature.
Figure 4. ITC profiles of Zn2+ binding to the soluble and full-length StZntB.
The binding isotherms were obtained as described in Methods with correction for heat of dilution at 25°C. Zn2+ binding to the soluble domain and full-length StZntB was derived from the non-linear least square fit of the isotherms. The isotherm of the soluble domain could be best fitted to the two site sequential binding model while the full-length StZntB was best fitted to a three site binding model. See also Supplementary Figure 4.
The ITC profile for full-length StZntB (Figure 4B) is significantly different compared to the soluble domain of StZntB. Such differences in ITC profiles may be due to differences in binding and/or Zn2+-induced conformational changes. The isotherm profile of heat change versus molar ratio of Zn2+ could be best fitted with a 3-site sequential binding model involving largely enthalpic with smaller entropic contributions. The apparent dissociation constants for the high affinity binding sites of full-length StZntB are 6 μM and 66 μM, while the lower affinity binding site has an apparent dissociation constant of 215 μM. Thermodynamic parameters for the soluble domain and full-length StZntB are shown in Table 4. The additional Zn2+ binding site is likely at a conserved Cys residue within the membrane domain (C307).
Table 4.
Thermodynamic parameters of Zn2+ binding to StZntB and its mutants measured by ITC at 25 °C.
| Protein | Fit model | Binding constant | Affinity (μM) | ΔG (Kcalmol−1) | ΔH (Kcalmol−1) | −TΔS (Kcalmol−1) | Putative assignment |
|---|---|---|---|---|---|---|---|
| Soluble domain StZntB | 2-site sequential | Kd1 | 15 ± 0.1 | −6.5 | −9.8 ± 0.6 | 3.3 | C94 |
| binding model | Kd3 | 775 ± 10 | −4.1 | nda | nda | C246 | |
|
| |||||||
| Full length StZntB | Kd1 | 6.0 ± 0.45 | −7.0 | 11 ± 1.0 | −4.0 | C94 | |
| 3-site sequential | Kd2 | 66 ± 5.0 | −5.6 | −10.4 ± 1.2 | 4.8 | C307 | |
| binding model | Kd3 | 215 ± 4.0 | −4.9 | nda | nda | C246 | |
|
| |||||||
| C94S StZntB | 2-site sequential | Kd2 | 59 ± 4 | −5.7 | −8 ± 0.73 | 2.3 | C307 |
| binding model | Kd3 | 877 ± 10 | −4.1 | 0.8 | −3.3 | C246 | |
|
| |||||||
| C307S StZntB | 2-site sequential | Kd1 | 19 ± 2 | −6.4 | −7.6 ± 0.73 | 2.3 | C94 |
| binding model | Kd3 | 840 ± 10 | −4.1 | nda | nda | C246 | |
|
| |||||||
| C246S StZntB | Kd1 | 5.8 ± 0.6 | −7.1 | −9 | 1.9 | C94 | |
| 3 –site sequential | Kd2 | 59 ± 4 | −5.7 | −7.5 | 1.8 | C307 | |
| binding model | Kd3 | 1275 ± 9 | nda | nda | nda | C246 | |
ΔH and -TΔS were not determined in the cases indicated because complete saturation of the ITC profiles was not observed. Hence, ΔH calculated from fitted parameters would be overestimated.
To understand the role of Zn2+ binding of StZntB, we substituted Ser for Cys at the proposed three Zn2+ binding sites, C307 located in the transmembrane domain, and C94 or C246 located on the cytoplasmic domain. The C94S, C246S and C307S single mutants of the full length protein were tested for Zn2+ binding using ITC. For the C307S mutant heat changes at various molar ratios of Zn2+ could be best fitted to a 2 site sequential binding model that yielded a dissociation constant of 19 μM for the first site and 840 μM for the second site (Figure 4C). A high negative enthalpy suggests that the enthalpy is the major driving force for the Zn2+ binding to C307S StZntB (Table 4). The isotherm profile of heat changes versus molar ratio of Zn2+ for the C94S mutant could also be best fitted to a 2-site sequential binding model (Figure 4D). The binding is again predominantly driven by enthalpy as it is evident from a high negative enthalpic value with small entropic contribution. The apparent dissociation constant for the high and low affinity binding sites are 59 μM and 877 μM, respectively. The isotherm profile for the C246S StZntB mutant best fits to a three sequential binding site model with Kd= 5.8 μM, 59 μM and 1275 μM. Zn2+ binding to C246S StZntB is also predominantly driven by enthalpy (Table 4 and Figure S7).
DISCUSSION
The available structures of the CorA Mg2+ channel (Lunin et al., 2006; Payandeh et al., 2006; Eshaghi et al., 2006) are apparently of a closed form. The structure of the soluble domain in CorA closely resembles the VpZntB soluble domain (Supplementary Figure S2), suggesting that the VpZntB structure (Tan et al., 2009) may also represent a “closed” or inactive form of the ZntB transporter. In contrast, the StZntB soluble domain structure presented here clearly represents a different conformation, possibly an outwardly open form of the transporter. In the StZntB structure, the large diameter of the funnel pore results from parallel α7 helices while retaining the relatively compact pentameric structure. Since TM1 is a continuation of the α7 helix, this suggests that, at least near the cytosolic membrane face, the five TM1 helices of StZntB would also be parallel and perpendicular to the membrane.
The CorA pentamer has been proposed to rotate like an iris in the plane of the membrane, expanding the funnel outward and widening the pore. Recent molecular dynamics simulations support this mechanism (Chakrabarti et al., 2010). Rotation would necessarily greatly expand the volume of the pentameric soluble domain. The StZntB structure suggests however that a large diameter pore at the cytosolic membrane face might be achieved by this protein scaffold without rotation and volume expansion of the soluble domain. Such distinctly different movements of ZntB and CorA would seem possible however, given the different associations between monomers in CorA and ZntB. The CorA homopentamer is held together by a few salt-bridges, several hydrophobic interactions plus a substantial contribution from 5 Mg2+ ions bound to D89 of one monomer and D253 of the adjacent monomer and possibly a second metal binding site. The Mg2+ sites are proposed to act as Mg2+ sensors (Lunin et al., 2006; Payandeh et al., 2006; Eshaghi et al., 2006; Chakrabarti et al., 2010), their dissociation freeing the monomers to rotate away from each other, expanding the structure and opening the pore. In ZntB, such a concerted movement would not be required. Zn2+ does not help hold the pentamer together, and the pore does not have to remain continuously open, as it must in an ion channel such as CorA that maintains a high Mg2+ conductance (Schindl et al., 2007). The Zn2+ binding sites observed in StZntB are thus unlikely to act as sensors.
In this regard, the ions bound to the various ZntB structures must be considered. The VpZntB structure shows no Zn2+ but instead has 5 Cl− ions bound per monomer, 4 external and 1 internal to the funnel. StZntB retained Zn2+ but no Cl−. Tan et al. attempted to incorporate Zn2+ into the VpZntB crystals without success (Tan et al., 2009). Likewise, we attempted to incorporate Br− into the StZntB crystals, again without success. The physiological relevance of the Cl− sites is unknown. The Cl− could represent an environmental adaptation since Vibrio sp. are marine organisms and are exposed to high salt. The cytosolic Cl− concentration in Vibrio sp. is unknown, although many bacteria and archaea living in a high salt environment maintain a very high cytosolic Cl− concentration (Mevarech et al., 2000; Ginzburg et al., 1970).
To investigate whether the bound Zn2+ atoms in the crystal structure of the StZntB soluble domain were physiologically relevant, we conducted ITC measurements. The site formed by H41 between crystallographically related monomers would not be present in the ITC experiments. In agreement with this expectation, ITC results show only two Zn2+ binding sites for the soluble domain with Kd1 = 15 μM and Kd3 = 775 μM (Figure 4A and Table 4). The low μM range Kd1 may be similar to Zn2+ binding reported for other prokaryotic Zn2+ transporters (Anton et al., 2004; Lu and Fu, 2007; Nies and Silver, 1995).
For the full-length StZntB, the ITC data is best fitted to a three binding-site model. We also observe that the affinities for Zn2+ binding are slightly higher for the full-length (Kd1 = 6 μM, Kd2 = 66 μM, and Kd3 = 216 μM) compared to the soluble domain (Kd1= 15 μM and Kd3= 775 μM). A net change in the thermodynamic parameters of binding as observed between the soluble and full-length StZntB denotes a change in Zn2+ binding and a possible conformational change. From the crystal structure and the ITC data for the soluble domain, two of these three sites are located in the soluble domain. Based on crystal structures of other Zn2+ transporters (Gao et al., 2010; Gao et al., 2009; Shimamura et al., 2010; Huang et al., 2003; Abramson et al., 2009; Krishnamurthy et al., 2009; Lu et al., 2009), there should be at least one additional Zn2+ binding site within the transmembrane domain, which would account for the additional site seen in the full-length StZntB. Thus, the Zn2+ binding site within the transmembrane domain is represented by one of the two sites with a Kd in the low μM range. Residue C307 within the membrane domain of StZntB is highly conserved in ZntB transporters. Analogous sites are seen in other structurally unrelated Zn2+ transport systems (Krishna et al., 2003; Anton et al., 2004; Lu et al., 2007; Lu et al., 2009; Ohana et al., 2009). C307 is in transmembrane domain 2 (TM2) of the full-length ZntB (Caldwell et al., 2003). Metal ion transport presumably requires a relative translocation of the α-helices in both TM1 and TM2 upon binding and releasing of the metal (Shimamura et al., 2010; Abramson et al., 2009; Krishnamurthy et al., 2009; Lu et al., 2009).
To further define the role of Zn2+ binding to StZntB, we mutated C94, C246 and C307 to Ser. ITC data for the C94S and C307S single mutants fits best to a 2-site sequential binding model with one high affinity and one low affinity binding site (Table 4). For the C246S mutant, a 3 site model fits best, although the third site had a Kd in the mM range. We suggest that three Zn2+ sites in the full length StZntB can be assigned as follows: Kd1 represents C94 (site 1 in Figure 3), Kd2 represents C307 and Kd3 represents C246 (site 3 in Figure 3). In the C94S mutant, the highest affinity site is absent. In the C307S mutant, the middle affinity site appears missing. In contrast, in the C246 site, both the high and middle affinity sites are present. The Kd3 site at ~1.3 mM could be spurious binding but most likely represents a weak interaction of Zn2+ with Ser246 and His168 rather than with Cys246 and His168 in the wild type StZntB. While the ITC results support these assignments, even rather conservative mutations can markedly affect protein conformation. Thus, it is possible that the C94 and C307 assignments might be reversed, with C94 being the middle affinity site and C307 the high affinity site.
A simple model for ZntB-mediated Zn2+ transport must take into account the finding that cytosolic Zn2+ is in the low femtomolar range (Hitomi et al., 2001; Outten et al., 2001). This translates to much less than one atom of free Zn2+ in the entire cell. This concentration is 9 orders of magnitude below the Kd values of the observed Zn2+ binding sites. Thus, none of these sites would actually by occupied in an intact cell. How then does ZntB obtain Zn2+ for transport out of the cell? Cu+ concentration is also in the femtomolar range, and a model analogous to that in some Cu transport systems may apply for Zn2+ (Changela et al., 2003; Rosen, 2002; Magnani et al., 2005; Lu et al., 2002; Kaplan et al., 2009). In these systems, a selective Cu binding protein docks with the transporter and directly transfers Cu. A similar Zn2+ selective binding protein would complex with ZntB, releasing Zn2+ to bind most likely at site 3 (C246-H168) within the funnel. We cannot exclude however, that the binding protein might deliver Zn2+ to site 1 (C94-H159), which could act as a transient Zn2+ storage site. Even though site 3 has a poor affinity, the relative concentration of Zn2+ within the funnel would be rather high. Thus, the H168-C246 site might be a transient binding site for Zn2+ for delivery to the pore despite its weaker affinity (Figure 3D). Interaction of ZntB with the binding protein and/or binding of Zn2+ to site 1 and/or site 3 might promote adoption of an open conformation. Subsequently, Zn2+ would move from within the funnel to site 2 at C307, located within the transmembrane domain. Zn2+ binding to site 2 might trigger movement within the TM domain, releasing Zn2+ to the periplasm. Finally, the entry of the counterion from the periplasm, presumably H+, would allow the protein to shift back to its original closed conformation, a conformation similar to that seen with VpZntB and CorA.
The structures of StZntB reported here, coupled with the VpZntB (Tan et al., 2009) and CorA structures (Lunin et al., 2006; Payandeh et al., 2006; Eshaghi et al., 2006), provide a striking example of the evolutionary use of the same structure for transport of different substrates in different directions. In the CorA superfamily of ion transport systems, the CorA subfamily forms Mg2+-selective ion channels (Schindl et al., 2007), mediating the influx of Mg2+ into the cell down its electrochemical gradient. In contrast, ZntB mediates the efflux of a different cation, Zn2+, against its electrochemical gradient. In the former, the membrane potential creates a large electrochemical gradient for Mg2+, thus providing the energy for ion movement. With ZntB, the energy for Zn2+ efflux is presumably provided by anti-transport of another ion using that ion’s electrochemical gradient; for ZntB, this is most likely H+ (B. Gorzelle and M.E. Maguire, unpublished observations). Thus, the CorA superfamily (Maguire, 2006b; Maguire, 2006a; Knoop et al., 2005) contains both channels and transporters, similar to other families such as the ClC family of chloride transport systems (Mindell, 2008; Mindell et al., 2001).
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
The DNA sequences of the cytoplasmic domain and full-length ZntB of Salmonella enterica serovar Typhimurium was amplified by PCR from their genomic DNA using high fidelity Pfu polymerase (Strategene). NdeI and BamH1 restriction sites were added to the 5′ and 3′ ends of the PCR product, respectively. The digested PCR product was inserted directionally between the corresponding sites of a modified pET-15b (Novagen) T7 polymerase expression vector with a Tobacco Etch Virus (TEV) protease recognition site (ENLYFQ^G) instead of the original thrombin cleavage site. The recombinant protein has an N-terminal 6X-His tag followed by a TEV protease site (Lunin et al., 2006). To construct three expression plasmids that contain the mutations, C94S, C246S and C307S, individually, the WT expression plasmid was mutated using the QuickChange site-directed mutagenesis kit (Strategene, La Jolla, CA) as we did previously (Gupta et al., 2004).
E. coli BL21(DE3) cells (Invitrogen) carrying the expression plasmid were grown in Luria Broth with 50 mg/ml ampicillin at 37°C until OD600nm = 0.6–0.8. After cooling to 16°C, 0.6 mM IPTG was added to induce protein expression. After 12 hr induction, the cells were harvested by centrifugation at 5,000 × g and frozen at −80°C. Ten g of frozen cells were suspended in 50 ml of cold lysis buffer (50 mM HEPES, pH 8.0, 500 mM NaCl, 5% (v/v) glycerol, 10 mM imidazole, 5 mM β-mercaptoethanol, 2.5 units/ml Benzonase, 0.1 mM PMSF and complete protease inhibitor cocktail (Roche)).
When purifying the cytoplasmic domain ZntB, the above suspended cells were lysed by French Press (ThermoSpectronic) at 1000 psi, and the lysate was centrifuged at 25,000 × g for 1 hr. The supernatant pH was adjusted to pH 8.0. Ni2+-NTA resin (5 ml) was pre-equilibrated with lysis buffer before mixing with supernatant at 4°C for 1 hr. The resin was loaded on a 3 × 10 cm column and washed with 100 ml of wash buffer (50 mM HEPES pH 8.0, 500 mM NaCl, 5% glycerol, 30 mM imidazole and 5 mM β-mercaptoethanol). Bound protein was recovered with elution buffer (50mM HEPES pH 8.0, 500 mM NaCl, 5% (v/v) glycerol, 300 mM imidazole and 5 mM β-mercaptoethanol). TEV protease (3% (w/w)) was added to remove the N-terminal 6X His tag during dialysis against 2 L of dialysis buffer (25 mM HEPES pH 8.0, 500 mM NaCl, 10% (v/v) glycerol, 5 mM β-mercaptoethanol and 1 mM ZnSO4) overnight at 4°C. Purified protein was then further dialyzed against 2 L of buffer (50 mM HEPES, pH 7.6, 150 mM NaCl, 5% (v/v) glycerol, 10 mM imidazole and 5 mM β-mercaptoethanol). The sample was concentrated to 15 mg/ml and aliquoted to be 20 μl per aliquot. Finally, the sample was flash frozen in liquid nitrogen and stored at −80°C.
When purifying the full-length ZntB, the suspended cells containing the expressed protein was lysed by French press and centrifuged only at 5,000 x g for half an hour. The supernatant containing the periplasmic debris was further centrifuged at 210,000 × g for 60 minutes. The membrane pellet was resuspended to 1.5 mg/ml protein (measured by Bradford assay) in lysis buffer (50 mM HEPES, 350 mM NaCl, 5% (v/v) glycerol, 10 mM imidazole, pH 8.0). This suspended solution was titrated dropwise using the solubilization buffer (10% (w/v) n-dodecyl-β-D-maltoside (DDM, Anatrace), 50mM HEPES, 350 mM NaCl, 5% (v/v) glycerol, 10 mM imidazole, pH 8.0) to reach a final concentration of 1% DDM, and was gently agitated at 4°C for 1 hr until the cell membrane was fully solubilized. This solution was further centrifuged at 210,000 × g for 1 hr. The protein in the supernatant was purified by the Ni2+-NTA column and the gel-filtration column as described before for the cytoplasmic domain purification except that all the buffers used here containing 0.01% DDM.
The three mutated full-length ZntBs (C94S, C246S and C307S) were expressed and purified as described for the WT ZntB.
The cytoplasmic domain of Selenomethionine (Se-Met)-labeled ZntB was expressed in E. coli B834(DE3), a methionine auxotroph (Novagen). During growth in M9 minimal media, Se-Met (~1 mM) was added as the sole source to replace methionine (Hendrickson et al., 1990). Se-Met ZntB was purified identically to wild type protein. The percentage of Se-Met incorporation was ~98% based on mass spectrometric analysis (data not shown).
Size Exclusion Chromatography
The oligomeric states of the full-length ZntB or its cytoplasmic domain were analyzed using size exclusion chromatography. A ProSec 300S column (Varian) was pre-equilibrated with the running buffer (50 mM HEPES, pH7.6, 5% (v/v) glycerol, 5 mM β-mercaptoethanol, 100–200 mM NaCl and 1 mM ZnSO4). About 50 μl of ~50 μM protein was loaded into the column. The molecular weights of the protein components were derived using a protein standard curve. The protein standards were purchased from (Bio-Rad). The absorption was monitored at 280 nm.
Isothermal Titration Calorimetry
Zn2+ binding was performed by ITC using a VP-ITC instrument (Microcal Inc., Northampton, MA USA). For soluble domain StZntB titration experiment, 2.5 μl aliquots of 3 mM ZnSO4 in 50 mM HEPES buffer, pH7.6, containing 5 mM β-mercaptoethanol, 150 mM NaCl and 5% (v/v) glycerol (Buffer A) were injected into the cell containing 13 μM StZntB protein (0.4 mg/ml) in buffer A at 25 °C. For the full-length StZntB titration experiment, 2.5 μl aliquots of 3 mM ZnSO4 in 50 mM HEPES buffer, pH 7.6 containing 5 mM β-mercaptoethanol, 150 mM NaCl, 5% (v/v) glycerol and 0.01% DDM (Buffer B) were injected into the cell containing 13 μM StZntB (0.4 mg/ml) in the buffer B at 25 °C. Blank titrations for both soluble domain and full-length StZntB were conducted in the presence of 3mM ZnSO4 in buffer A and buffer B, respectively. The integrated heat of injection, after subtracting the blank, was used to fit a two site sequential model to the soluble domain and a three site sequential binding model for the full-length StZntB using Microcal Origin 7.0 (Microcal Inc., Northampton, MA USA). The observed binding constants were used to calculate the Gibbs free energy relationship (ΔG) using ΔG = −RTln K (obs), and ΔS was calculated from ΔG using the Gibbs free energy equation ΔG = ΔH - TΔS.
Crystallization, data collection and data processing
Initial crystallization conditions for the StZntB soluble domain were found using the Hampton Index HT screen at 4°C by the hanging drop method at an initial protein concentration of 11 mg/ml in the presence of 1mM ZnSO4. After optimization, plate-like crystals were obtained at 4°C with 30–35% PEG 3350, 0.1 M Tris, pH 8.5 and 0.2 M (NH4)2SO4. The addition of 500 mM NaCl under these same conditions gave rod clusters after incubation at 20°C.
Data for StZntB crystals belonging to space groups P1 and P21 were collected at the GMCA ID-B and ID-D beam lines at the Advanced Photon Source. The data of the native, Se-Met and Zn2+-bound crystals were collected at wavelengths of 1.0 Å, 0.9793 Å (Se peak absorption edge) and 1.283–1.284 Å (Zn peak absorption edge), respectively. Data for StZntB crystals belonging to the space group C2 were collected at the BioCARS ID-B beam line at APS at a wavelength of 1.282 Å. All data were collected at 100°K and were integrated and scaled using the program HKL2000 (Otwinowski et al., 1997).
Structure determination, refinement, and analysis
The structure of the Se-Met labeled StZntB crystal at 2.7 Å resolution was solved by the combination of single-wavelength anomalous dispersion and molecular replacement (MR) technique (Schuermann et al., 2003). The MR model used was PDB ID 3CK6 (Tan et al., 2009), which has a sequence identity of 23% with StZntB. Side chains of non-conserved residues were pruned using Chainsaw (Stein, 2008). The initial MR model was obtained using Phaser (McCoy et al., 2007). The initial MR model and the single-wavelength anomalous dispersion data were used in phase recombination with the program Solve (Terwilliger et al., 1999), and the electron density map was traced using Resolve (Terwilliger, 2003) implemented in the Phenix software suite (Adams et al., 2010), giving about 70% of the structure. This partial structure was used as the template to solve the wild type 2.3 Å resolution MR structure using Phaser. The complete model was built by Resolve interspersed with manual building using Coot (Emsley et al., 2004). The structure was refined using Phenix (Adams et al., 2010). One monomer from the 2.3 Å resolution StZntB dimeric structure was used as the search model to solve the pentameric form using the MR method. Model building was interspersed with refinement using Coot and Phenix, respectively. Figures were generated using Pymol (Delano, 2002).
Structures were aligned using O (Jones et al., 1991). Pore sizes and shapes of the cytoplasmic domains of StZntB, VpZntB and T. maritima CorA were analyzed with HOLE (Smart et al., 1996). Contacts between neighboring monomers of the ZntB and CorA homopentamers were analyzed by Contact in CCP4i (Potterton et al., 2004; Krissinel et al., 2004). Sequence alignment was performed using Blast (Altschul et al., 1997). Electrostatic and desolvation free energies were calculated by the FastContact server to estimate the stability of the protein assembly (Champ et al., 2007).
Supplementary Material
Acknowledgments
We thank members of the GMCA-CAT, NE-CAT and BIOCARS beam-lines at the Advanced Photon Source for assistance with data collection. This research was supported by NIH grant GM39447 (M.E.M). QW, MFA and CD were funded by NIH grants CA100827 and CA100827-S1 and LDRD from the DOE.
Footnotes
ACCESSION NUMBERS
Atomic coordinates and structures factors have been deposited in the Protein Data Bank under the ID codes: 3NVO and 3NWI.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramson J, Wright EM. Structure and function of Na+-symporters with inverted repeats. Curr Opin Struct Biol. 2009;19:425–432. doi: 10.1016/j.sbi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola S, Pasquali P, Pistoia C, Petrucci P, Petrarca P, Rotilio G, Battistoni A. High-Affinity Zn2+ Uptake System ZnuABC Is Required for Bacterial Zinc Homeostasis in Intracellular Environments and Contributes to the Virulence of Salmonella enterica. Infect Immun. 2007;75:5867–5876. doi: 10.1128/IAI.00559-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton A, Weltrowski A, Haney CJ, Franke S, Grass G, Rensing C, Nies DH. Characteristics of zinc transport by two bacterial cation diffusion facilitators from Ralstonia metallidurans CH34 and Escherichia coli. J Bacteriol. 2004;186:7499–7507. doi: 10.1128/JB.186.22.7499-7507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe DK, Morby AP. Zn(II) metabolism in prokaryotes. FEMS Microbiol Rev. 2003;27:291–311. doi: 10.1016/S0168-6445(03)00041-X. [DOI] [PubMed] [Google Scholar]
- Caldwell AM, Smith RL. Membrane topology of the ZntB efflux system of Salmonella enterica serovar Typhimurium. J Bacteriol. 2003;185:374–376. doi: 10.1128/JB.185.1.374-376.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti N, Neale C, Payandeh J, Pai EF, Pomès R. An Iris-Like Mechanism of Pore Dilation in the CorA Magnesium Transport System. Biophysical Journal. 2010;98:784–792. doi: 10.1016/j.bpj.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champ PC, Camacho CJ. FastContact: a free energy scoring tool for protein-protein complex structures. Nucleic Acids Res. 2007;35:W556–W560. doi: 10.1093/nar/gkm326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changela A, Chen K, Xue Y, Holschen J, Outten CE, O’Halloran TV, Mondragon A. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science. 2003;301:1383–1387. doi: 10.1126/science.1085950. [DOI] [PubMed] [Google Scholar]
- Chao Y, Fu D. Kinetic study of the antiport mechanism of an Escherichia coli zinc transporter, ZitB. J Biol Chem. 2004a;279:12043–12050. doi: 10.1074/jbc.M313510200. [DOI] [PubMed] [Google Scholar]
- Chao Y, Fu D. Thermodynamic studies of the mechanism of metal binding to the Escherichia coli zinc transporter YiiP. J Biol Chem. 2004b;279:17173–17180. doi: 10.1074/jbc.M400208200. [DOI] [PubMed] [Google Scholar]
- Delano WL. The PyMOL Molecular Graphics System. [0.98] Palo Alto, CA, USA: DeLano Scientific; 2002. [Google Scholar]
- Devirgiliis C, Zalewski PD, Perozzi G, Murgia C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat Res. 2007;622:84–93. doi: 10.1016/j.mrfmmm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Eshaghi S, Niegowski D, Kohl A, Martinez MD, Lesley SA, Nordlund P. Crystal structure of a divalent metal ion transporter CorA at 2.9 angstrom resolution. Science. 2006;313:354–357. doi: 10.1126/science.1127121. [DOI] [PubMed] [Google Scholar]
- Gao X, Lu F, Zhou L, Dang S, Sun L, Li X, Wang J, Shi Y. Structure and mechanism of an amino acid antiporter. Science. 2009;324:1565–1568. doi: 10.1126/science.1173654. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhou L, Jiao X, Lu F, Yan C, Zeng X, Wang J, Shi Y. Mechanism of substrate recognition and transport by an amino acid antiporter. Nature. 2010;463:828–832. doi: 10.1038/nature08741. [DOI] [PubMed] [Google Scholar]
- Ginzburg M, Sachs L, Ginzburg BZ. Ion metabolism in a Halobacterium. I. Influence of age of culture on intracellular concentrations. J Gen Physiol. 1970;55:187–207. doi: 10.1085/jgp.55.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass G, Franke S, Taudte N, Nies DH, Kucharski LM, Maguire ME, Rensing C. The metal permease ZupT from Escherichia coli is a transporter with a broad substrate spectrum. J Bacteriol. 2005;187:1604–1611. doi: 10.1128/JB.187.5.1604-1611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Peterson CB, Dice LT, Uchiki T, Racca J, Guo JT, Xu Y, Hettich R, Zhao X, Rothstein R, Dealwis CG. Sml1p is a dimer in solution: characterization of denaturation and renaturation of recombinant Sml1p. Biochemistry. 2004;43:8568–8578. doi: 10.1021/bi0361721. [DOI] [PubMed] [Google Scholar]
- Hantke K. Bacterial zinc uptake and regulators. Curr Opin Microbiol. 2005;8:196–202. doi: 10.1016/j.mib.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Hitomi Y, Outten CE, O’Halloran TV. Extreme zinc-binding thermodynamics of the metal sensor/regulator protein, ZntR. J Am Chem Soc. 2001;123:8614–8615. doi: 10.1021/ja016146v. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowman SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47:110–116. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kaplan JH, Lutsenko S. Copper transport in mammalian cells: special care for a metal with special needs. J Biol Chem. 2009;284:25461–25465. doi: 10.1074/jbc.R109.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop V, Groth-Malonek M, Gebert M, Eifler K, Weyand K. Transport of magnesium and other divalent cations: evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol Genet Genomics. 2005;274:205–216. doi: 10.1007/s00438-005-0011-x. [DOI] [PubMed] [Google Scholar]
- Krishna SS, Majumdar I, Grishin NV. Structural classification of zinc fingers: survey and summary. Nucleic Acids Res. 2003;31:532–550. doi: 10.1093/nar/gkg161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Piscitelli CL, Gouaux E. Unlocking the molecular secrets of sodium-coupled transporters. Nature. 2009;459:347–355. doi: 10.1038/nature08143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel EB, Winn MD, Ballard CC, Ashton AW, Patel P, Potterton EA, McNicholas SJ, Cowtan KD, Emsley P. The new CCP4 Coordinate Library as a toolkit for the design of coordinate-related applications in protein crystallography. Acta Crystallogr D Biol Crystallogr. 2004;60:2250–2255. doi: 10.1107/S0907444904027167. [DOI] [PubMed] [Google Scholar]
- Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- Lu M, Chai J, Fu D. Structural basis for autoregulation of the zinc transporter YiiP. Nat Struct Mol Biol. 2009;16:1063–1067. doi: 10.1038/nsmb.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Fu D. Structure of the zinc transporter YiiP. Science. 2007;317:1746–1748. doi: 10.1126/science.1143748. [DOI] [PubMed] [Google Scholar]
- Lu ZH, Solioz M. Bacterial copper transport. Adv Protein Chem. 2002;60:93–121. doi: 10.1016/s0065-3233(02)60052-x. [DOI] [PubMed] [Google Scholar]
- Lunin VV, Dobrovetsky E, Khutoreskaya G, Zhang R, Joachimiak A, Doyle DA, Bochkarev A, Maguire ME, Edwards AM, Koth CM. Crystal structure of the CorA Mg2+ transporter. Nature. 2006;440:833–837. doi: 10.1038/nature04642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani D, Solioz M. Copper chaperone cycling and degradation in the regulation of the cop operon of Enterococcus hirae. Biometals. 2005;18:407–412. doi: 10.1007/s10534-005-3715-9. [DOI] [PubMed] [Google Scholar]
- Maguire ME. Magnesium transporters: properties, regulation and structure. Front Biosci. 2006a;11:3149–3163. doi: 10.2741/2039. [DOI] [PubMed] [Google Scholar]
- Maguire ME. The Structure of the CorA Magnesium Transporter, a Divalent Cation Channel. Curr Opin Struct Biol. 2006b;4:432–438. doi: 10.1016/j.sbi.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Maguire ME. The CorA Mg2+ Channel. In: Messerschmidt A, Huber R, Wieghardt K, Poulos T, editors. Handbook of Metalloproteins. New York: Wiley; 2008. [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M, Frolow F, Gloss LM. Halophilic enzymes: proteins with a grain of salt. Biophys Chem. 2000;86:155–164. doi: 10.1016/s0301-4622(00)00126-5. [DOI] [PubMed] [Google Scholar]
- Mindell JA. The chloride channel’s appendix. Nat Struct Mol Biol. 2008;15:781–783. doi: 10.1038/nsmb0808-781. [DOI] [PubMed] [Google Scholar]
- Mindell JA, Maduke M. ClC chloride channels. Genome Biol. 2001;2:3003.1–3003.6. doi: 10.1186/gb-2001-2-2-reviews3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moomaw AS, Maguire ME. The Unique Nature of Mg2+ Channels. Physiology. 2008;23:275–285. doi: 10.1152/physiol.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Hoch E, Keasar C, Kambe T, Yifrach O, Hershfinkel M, Sekler I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J Biol Chem. 2009;284:17677–17686. doi: 10.1074/jbc.M109.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. In: Carter CW, Sweet RM, Simon MI, editors. Macromolecular Crystallography part A. San Diego: Academic Press; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- Outten CE, O’Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- Papp-Wallace KM, Maguire ME. Bacterial homologs of eukaryotic membrane proteins: the 2-TM-GxN family of Mg2+ transporters. Mol Membr Biol. 2007;24:351–356. doi: 10.1080/09687680701441883. [DOI] [PubMed] [Google Scholar]
- Pasquali P, Ammendola S, Pistoia C, Petrucci P, Tarantino M, Valente C, Marenzoni ML, Rotilio G, Battistoni A. Attenuated Salmonella enterica serovar Typhimurium lacking the ZnuABC transporter confers immune-based protection against challenge infections in mice. Vaccine. 2008;26:3421–3426. doi: 10.1016/j.vaccine.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Patzer SI, Hantke K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol. 1998;28:1199–1210. doi: 10.1046/j.1365-2958.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- Payandeh J, Pai EF. A structural basis for Mg2+ homeostasis and the CorA translocation cycle. EMBO J. 2006;25:3762–3773. doi: 10.1038/sj.emboj.7601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potterton L, McNicholas S, Krissinel E, Gruber J, Cowtan K, Emsley P, Murshudov GN, Cohen S, Perrakis A, Noble M. Developments in the CCP4 molecular-graphics project. Acta Crystallogr D Biol Crystallogr. 2004;60:2288–2294. doi: 10.1107/S0907444904023716. [DOI] [PubMed] [Google Scholar]
- Rosen BP. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:689–693. doi: 10.1016/s1095-6433(02)00201-5. [DOI] [PubMed] [Google Scholar]
- Schindl R, Weghuber J, Romanin C, Schweyen RJ. Mrs2p forms a high conductance Mg2+ selective channel in mitochondria. Biophys J. 2007;93:3872–3883. doi: 10.1529/biophysj.107.112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuermann JP, Tanner JJ. MRSAD: using anomalous dispersion from S atoms collected at Cu Kα wavelength in molecular-replacement structure determination. Acta Crystallogr D Biol Crystallogr. 2003;59:1731–1736. doi: 10.1107/s0907444903015725. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010;328:470–473. doi: 10.1126/science.1186303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart OS, Neduvelil JG, Wang X, Wallace BA, Sansom MS. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J Mol Graph. 1996;14:354–60. 376. doi: 10.1016/s0263-7855(97)00009-x. [DOI] [PubMed] [Google Scholar]
- Stein N. CHAINSAW: A program for mutating pdb files used as templates in molecular replacement. J Appl Crys. 2008;41:641–643. [Google Scholar]
- Tan K, Sather A, Robertson JL, Moy S, Roux B, Joachimiak A. Structure and electrostatic property of cytoplasmic domain of ZntB transporter. Protein Sci. 2009;18:2043–2052. doi: 10.1002/pro.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC. Automated main-chain model building by template matching and iterative fragment extension. Acta Crystallogr D Biol Crystallogr. 2003;59:38–44. doi: 10.1107/S0907444902018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger TC, Berendzen J. Automated MAD and MIR structure solution. Acta Crystallogr D Biol Crystallogr. 1999;55:849–861. doi: 10.1107/S0907444999000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusnady GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics. 2001;17:849–850. doi: 10.1093/bioinformatics/17.9.849. [DOI] [PubMed] [Google Scholar]
- Worlock AJ, Smith RL. ZntB Is a Novel Zn2+ Transporter in Salmonella enterica Serovar Typhimurium. J Bacteriol. 2002;184:4369–4373. doi: 10.1128/JB.184.16.4369-4373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunyk LA, Easton JA, Kim LR, Sugarbaker SA, Bennett B, Breece RM, Vorontsov II, Tierney DL, Crowder MW, Rosenzweig AC. Structure and metal binding properties of ZnuA, a periplasmic zinc transporter from Escherichia coli. J Biol Inorg Chem. 2008;13:271–288. doi: 10.1007/s00775-007-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.