Abstract
Telomeres comprise long tracts of double-stranded TTAGGG repeats that extend for 9–15 kb in humans. Telomere length is maintained by telomerase, a specialized ribonucleoprotein that prevents the natural ends of linear chromosomes from undergoing inappropriate repair, which could otherwise lead to deleterious chromosomal fusions. During the development of cardiovascular tissues, telomerase activity is strong but diminishes with age in adult hearts. Dysfunction of telomerase is associated with the impairment of tissue repair or regeneration in several pathologic conditions, including heart failure and infarction. Under both physiologic and pathophysiologic conditions, telomerase interacts with promyogenic nuclear transcription factors (e.g. myocardin, serum response factor) to augment the potency of cardiovascular cells during growth, survival, and differentiation. We review recent findings on the biologic function of telomerase and its potential for clinical application in cardiovascular development and repair. Understanding the roles of telomerase and its associated proteins in the functional regulation of cardiovascular cells and their progenitors may lead to new strategies for cardiovascular tissue repair and regeneration.
Keywords: Telomerase, Gene expression, Myogenesis, Differentiation, Proliferation, Apoptosis, Myocardin
Stem cell growth, quiescence and differentiation
Stem cells play a key role in both foetal cardiac development and post-injury repair or regeneration in adult cardiac tissue. Cardiac stem cells of intracardiac origin or from extracardiac sources, such as bone marrow,1 represent a group of undifferentiated cardiac cells that can give rise to mature cardiac cells while maintaining a strong capacity for self-renewal or proliferation. Increasing evidence indicates that low telomerase activity and telomere shortening are the core components that drive the senescent or apoptotic depletion of tissue stem cell reserves and age-related tissue degeneration, thus impairing the regenerative ability of various organs and tissue, including the heart, after embryonic development. These cellular checkpoint mechanisms have been known for years to contribute to the functional decline of highly proliferative tissue. However, it is largely unknown how these cellular mechanisms adversely affect more quiescent tissue—such as that in the heart—that is equally ravaged by the ageing or disease processes.
Our bodies possess a remarkable ability for extensive and sustained tissue renewal throughout a lifetime. The continuous self-renewal capacity of tissue, even in non-rapidly proliferating organs, is maintained by reservoirs of somatic tissue stem cells.2,3 These tissue stem cells have attracted the attention of investigators working in the field of regenerative research, given that there is accumulating evidence that age-associated physiologic decline, particularly in highly proliferative organs, parallels the blunted proliferative responses and misdirected differentiation of resident tissue stem cells.
Human tissue has widely varying levels of baseline proliferative activity and regenerative potential. In high-turnover tissues, resident stem cells have been shown to generate large numbers of specialized cell progeny, thereby maintaining tissue cellularity and functionality over a lifetime. In tissue with lower proliferative or regenerative capacity, such as the heart, the identification of stem cells has been more difficult, although not completely undocumented.1,4,5 Therefore, it seems logical to hypothesize that preserving an adequate pool of tissue stem cells with robust potential for renewal would be important for maintaining organ function after injury and for allowing cardiac repair. Knowledge in the field of regenerative biology will expand by understanding the role of telomeres and telomere-associated proteins.
Telomeres, ageing, and disease
Telomeres are specialized structures, or nucleoprotein caps, that adorn the ends of human chromosomes. Early studies have shown the essential role of telomeres in maintaining the integrity of chromosomes.6–8 Telomeres are maintained by the enzyme telomerase.9–11 The importance of adequate telomerase activity and maintenance of telomere length for both replicative potential in culture and ageing in organisms was initially inferred from studies of primary human fibroblasts. In culture, the division of fibroblasts results in progressive telomere attrition, culminating in a state of proliferative arrest, or cellular senescence, after a finite number of cell divisions—a barrier known as the Hayflick limit.12 Proliferation beyond this limit drives further telomere erosion, ultimately triggering rampant chromosomal instability driven by chromosome breakage-fusion-breakage events.13
Forced expression of telomerase reverse transcriptase (TERT), the catalytic subunit of telomerase, in cultured human fibroblasts stabilizes telomere length and endows the cells with unlimited replicative potential without engendering malignant properties.14,15 The remarkable capacity of experimentally induced telomerase activity to circumvent senescence and allow indefinite growth has been documented in many other human cell types. Compelling cell culture studies and complementary studies in telomerase knockout mice16 have since inspired significant efforts to determine whether telomere dynamics play any role in the processes of ageing or of various degenerative diseases in humans. Human population studies have correlated decreased telomere length in peripheral blood leukocytes with higher mortality rates in individuals older than 60 years, and a recent large cohort study reported a positive link between telomere length and years of healthy life.17,18 A recent study in centenarians and their offspring found a positive link between telomere length and longevity; in particular, those with longer telomeres had an overall improved health profile (with decreased age-associated disease and better cognitive function and lipid profiles) relative to that of control patients.19
Similarly, studies have shown that impaired telomere length and lower telomerase activity are related to risk factors for ageing and to the highest levels of oxidative stress in peripheral blood leukocytes,20 possibly through the activation of the autonomic and neuroendocrine systems and the subsequent glucocorticoid-driven increase in reactive oxygen species (ROS).21,22 Damaged telomeres may not be repaired efficiently because of low levels of telomerase activity and an inherent shielding of the telomeres from DNA repair machinery. As such, damaged telomeres may provide a reservoir of persistent DNA damage signals and consequent sustained p53 activation with senescent sequelae.
Evidence from the study of a wide range of human degenerative diseases, both inherited and acquired, points to the limiting of telomeres as a key pathogenetic element that drives degenerative pathologies, increases cancer risk, and shortens lifespan. In this light, the sizes of telomere reserves and their level of persistent damage or, better, measurements of their capping status, may prove useful as biomarkers of disease progression and may offer new opportunities for proactive therapeutic interventions involving transient somatic activation of endogenous telomerase to replenish or repair telomeres.16
Telomerase structure, biologic function, and canonical roles in cardiovascular development
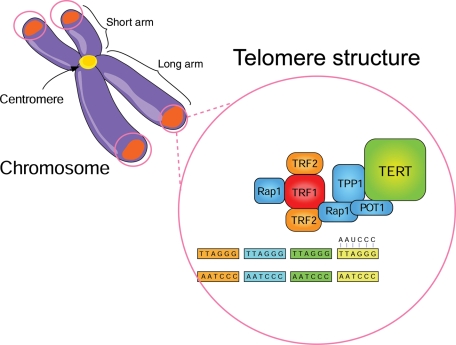
Telomerase is a ribonucleoprotein complex (Figure 1) that catalyzes the addition of oligonucleotide (TTAGGG) repeats to telomeres.11,23 Tissue restriction fragment (TRF)-1 and TRF-2 help in regulating telomere length and in maintaining the correct structure of telomeres, either by acting as inhibitors of telomerase or as activators of telomere degradation.24 Initially, TRF-1 was isolated as a double-stranded TTAGGG repeat-binding protein from HeLa cells.25 It binds to telomeres as a homodimer with a C-terminal helix-turn-helix motif and acts as an inhibitor of telomerase-dependent elongation of telomeres by limiting the access of telomerase to the telomere.26 TRF-2 differs from TRF-1 in that its N-terminus is basic rather than acidic.27 It binds telomeres and stabilizes the G-rich strand overhang, thereby inhibiting telomere–telomere fusion. Overexpression of TRF-2 in somatic cells leads to the progressive shortening of telomeres.28 Suppression of TRF-2 function in cultured cardiac myocytes provokes telomere erosion, and downregulation of this protein occurs in end-stage heart failure in humans.29
Figure 1.
Structure and function of telomerase. Mammalian telomeres comprise (TTAGGG)n DNA sequences and telomerase. Telomerase comprises three major subunits: (1) the RNA template telomerase component 1 [TLC1 or telomerase RNA component (TERC); (AAUCCC)n], which provides the sequence information used by telomerase to direct DNA synthesis; (2) the catalytic protein subunit [ever shorter telomeres 2 protein (Est2p) in Saccharomyces cerevisiae and telomerase reverse transcriptase (TERT) in other organisms], which catalyzes the addition of oligonucleotide (TTAGGG) repeats to telomeres, the repetitive DNA structures residing at the ends of linear chromosomes106; and (3) the telomerase-associated multiprotein complex known as ‘shelterin', which comprises six factors [telomere restriction fragment (TRF)-1, TRF-2, telomere-associated protein 1 (TPP1), protector of telomeres 1 (POT1), TRF1-interacting protein 2 (TIN2), and human protein repression and activation protein 1 (hRap1)] and assures proper telomere length regulation and telomere protection.107 kb, kilobase.
Telomerase plays a key role in maintaining the elongation of the 3′ DNA template at the end of the chromosome, thus compensating for the telomere shortening that occurs in the absence of this activity. The length of telomeric DNA in human somatic cells is heterogeneous among individuals, ranging from 3 to 5 kb and up to 20 kb, according to age, organ, and the proliferative activity of cells.30 Telomerase is expressed abundantly in embryonic or undifferentiated cells, but its expression and activity weaken with age in mature somatic cells, leading to gradual telomere shortening (Figure 1).
In differentiating embryonic stem cells, expression and activity of TERT is inhibited by histone deacetylation and DNA methylation of the TERT gene.31 In contrast, TERT is activated in cells that undergo rapid expansion (e.g. committed haematopoietic progenitor cells, activated lymphocytes, or keratinocytes), even in tissues with a low cell turnover, such as in the brain.32 In normal mice, telomerase activity has been detected in the brain and heart muscle, as well as in the colon, liver, ovaries, and testes.9 Telomerase activity is more readily detectable in mouse cells and tissues that generally contain longer telomeres than do human cells.33 The remarkable difference in telomerase activity among tissue and animal species may reflect the different regulatory mechanisms of telomerase expression, which may underlie tissue-specific features of differentiation and proliferation.
In most adult somatic cells, telomeres progressively shorten with each cell-division cycle because of the inability of replicative DNA polymerases to complete replication of the 3′ end of linear DNA molecules and because of the nucleolytic processing of telomeres.34 With continuous shortening, telomeres reach a critical length (critically short telomeres) when they become recognized by the cell as double-stranded DNA breaks, triggering the irreversible cell-cycle arrest known as cellular senescence.35
To date, the best-characterized activity of telomerase is the elongation and maintenance of telomeres. However, increasing evidence is emerging to indicate that in addition to maintenance of telomere length, telomerase may have other functions independent of telomere maintenance, including regulation of gene expression, cell differentiation, apoptosis, and proliferation. The precise molecular mechanism of the telomerase functions that are independent of telomere maintenance is unclear. However, the construction of genetically modified mice for TERT components has provided the opportunity to learn more about each component of the telomerase complex and their corresponding roles.
The first model of mTERT knockout mice was reported in 1997 by two independent laboratories.36,37 There was no telomerase activity or telomere shortening in the later generations of these mice, nor were there any major phenotypic abnormalities in early aged mice of the first generation. Also in 1997, Blasco et al.38 established an alternative model of telomerase knockout mice (TERC−/− mice) by knocking out the RNA template of telomerase. In earlier generations of these mice, there were telomere shortening and severe structural abnormalities on a cellular and macroscopic level; later generations showed aneuploidy and chromosomal end-to-end fusions, hair loss, infertility, testicular and spleen atrophy, and bone marrow proliferative defects.39,40 Interestingly, together with a reduced angiogenic potential, which was suggested as the explanation for the above-mentioned abnormalities, TERC−/− mice showed remarkable and progressive abnormalities of cardiac myocyte size, number, and proliferative potential between the first (G1) and the fifth (G5) generations.39,40 The G5 TERC−/− mice developed severe left ventricular failure (characterized by increased end-diastolic left ventricular pressure and decreased left ventricular pressure) and systolic and diastolic dysfunction, suggesting that, in the absence of telomerase, pathologic cardiac remodelling occurs spontaneously in TERC mice and that telomerase function is essential for normal cardiovascular development.39,40
Telomerase, cell growth, oxidative stress, and apoptosis
Several studies indicate that telomerase has a regulatory role in gene expression, independent of telomere maintenance and in the absence of telomerase RNA. For example, Flores et al.41 showed that telomerase overexpression activates epidermal stem cells (ESCs) and promotes ESC mobilization, hair growth, and stem cell proliferation. More recently, it has been shown that TERT mutants lacking reverse transcriptase function maintain their activity in enhancing keratinocyte proliferation in the mouse skin.42 These studies demonstrate that TERT has its own activity in stem cell proliferation and differentiation, independent of telomerase activity and telomere maintenance. In addition, a microarray analysis of 7000 genes showed that TERT induces expression of a wide spectrum of mitogenic and angiogenic genes, such as fibroblast growth factor,43 epidermal growth factor,44 and vascular endothelial growth factor.45
Conversely, TERT represses expression of tumor necrosis factor-related apoptosis-inducing ligand, tumor necrosis factor-related apoptotic genes,46 B-cell lymphoma 2 (BCL2),47 and p53.48 In a further analysis of 19 000 genes in bovine adrenocortical cells ectopically expressing TERT, 284 genes were found to be either positively or negatively affected by changes in the TERT activity.49 Interestingly, most of the genes affected were involved in cell-cycle regulation, cell signalling, and metabolism.49,50 Results of these studies show that TERT may contribute to proliferation not only by stabilizing telomeres but also by regulating expression of growth-related and apoptosis-related genes. It has been shown that TERT interacts directly with nuclear factor (NF)-κB p65 component, and this interaction mediates the nuclear translocation of telomerase.51 Tumor necrosis factor has been shown to modulate telomerase activity by inducing the translocation of the complex TERT-NF-κB p65 from the cytoplasm to the nucleus. It is not known whether the complex of TERT-NF-κB p65 can be recruited to the promoter of genes regulated by TERT.
Normally, telomerase is located within the nucleus and the cytoplasm, with the active form in the nucleus. However, recent observations indicate that telomerase is also localized in the mitochondria, suggesting that it has a function in protecting the mitochondria and, consequently, cells under stress. TERT is targeted to the mitrochondria by an import sequence at its N-terminus.52 Through this sequence, TERT can shuttle from the nucleus to the mitochondria upon oxidative challenge and drug treatment and bind to mitochondrial DNA. However, the biological significance of this binding is unclear. Observations from independent laboratories show that mitochondrial telomerase reduces the production of ROS and protects mitochondrial DNA from damage.53,54 In addition, the mitochondria in hearts of TERT knockout mice had less efficient respiration when compared with those of wild-type mice.54 However, other researchers have shown that mitochondrial telomerase actually plays a role in driving stem cells toward apoptosis by increasing the oxidative damage of the mitochondria.52 In contrast, the results of other studies suggest that telomerase has an anti-apoptotic role in that it blocks both the mitochondrial55 and the death receptor pathways46 of apoptosis through a mechanism that is independent of the enzymatic activity of telomerase.56
The exact mechanism for how TERT protects mitochondrial DNA remains unclear, but a potential pathway may include decreased mitochondrial ROS generation due to improved coupling or more effective respiration; direct binding to and protection of the mitochondrial DNA; or improved DNA repair or accelerated degradation of the mitochondria that harbour damaged DNA. Translocation of telomerase into the mitochondria upon oxidative stress can occur in less than 3 h under H2O2 treatment, which essentially excludes de novo synthesis as the source of the mitochondrial TERT protein pool.53,57
Myocardin-telomerase co-expression in cardiac stem cells: a checkpoint for growth and differentiation in the regulation of cardiovascular myocyte development
Myocardin belongs to the SAF-A/B, acinus, PIAS (SAP) domain family of nuclear proteins that regulate diverse aspects of chromatin remodelling and transcription.58,59 Myocardin activates gene expression through its interaction with CCA/T6GG (CArG) boxes, also referred to as serum-response elements. Myocardin activation of such elements is dependent on its interaction with the ‘MADS' box domain of serum-response factor (SRF) (Figure 2). The name MADS box derives from the proteins MCM1 (a yeast protein important in mating type determination), agamous from Arabidopsia thaliana and deficiens (plant proteins important in homeotic flower development), and SRF. Serum-response factor is a widely expressed transcription factor (molecular weight of 67 kD) required for the expression of several genes, including cardiac α-actin, α-actinin, skeletal α-actin, muscle creatine kinase, smooth muscle myosin heavy chain, smooth muscle α-actin, and smooth muscle calponin (Figure 2).60 It has also been shown to play a critical role in regulating early response genes, such as c-Fos and early growth response protein 1 (egr-1),61–63 and survival genes, such as B-cell lymphoma 2 (BCL2) and myeloid cell leukaemia sequence 1 (Figure 2).64
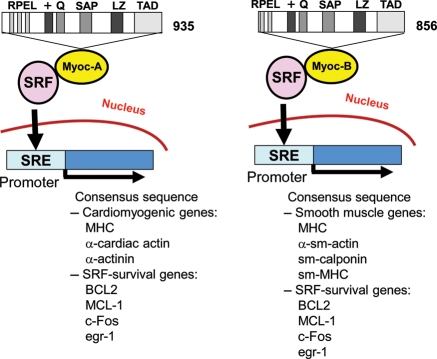
Figure 2.
Structure and function of myocardin. A schematic representation of the structure of myocardin-A (Myoc-A) and B (Myoc-B) and the mechanism of serum-response factor (SRF) activation. The myocardin gene encodes 935- and 856-amino acid protein isoforms (Myoc-A and Myoc-B, respectively) generated by alternative splicing. The 935-amino acid isoform is expressed predominantly in the heart, and the 856-aminoacid isoform is expressed predominantly in smooth muscle cells (SMCs). The domain structures of the myocardin proteins are shown [+, basic region; Q, glutamine-rich region; RPEL, consensus RPxxxEL sequence; LZ, leucine zipper; TAD, transcriptional activation domain; SAP (SAF-A/B, acinus, PIAS)]. BCL2, B-cell lymphoma 2; egr-1, early growth response protein 1; MCL-1, myeloid cell leukaemia sequence 1; MHC, myosin heavy chain; SM, smooth muscle; SRE, serum-response elements.
The human myocardin gene, located at chromosome 17p11.2, spans approximately 92 kb of genomic DNA.65 Myocardin mRNA, which contains 13 exons, is approximately 9.5 kb in size and encodes two alternatively spliced protein isoforms of 935 (myocardin-A) and 856 (myocardin-B) amino acids, respectively.66 The 935-amino acid isoform is expressed predominantly in the heart, whereas the 856-amino acid isoform is expressed predominantly in smooth muscle cells (SMCs) (Figure 2).66 Only the 935-amino acid protein physically associates with myocyte enhancer factor-2 (MEF2) and transactivates MEF2-dependent promoters, providing a potential mechanism to differentiate function and expression of myocardin in the heart. Myocardin contains a conserved N-terminal domain composed of RPXXXEL (RPEL) repeats that have been implicated in the Rho-dependent nuclear translocation of myocardin-related transcription factors (MRTFs). The basic domain of myocardin has been shown to mediate nuclear localization and SRF interaction. The leucine zipper-like domain mediates myocardin homodimerization and heterodimerization. The transcriptional activity of myocardin is mediated by the transcriptional activation domain at its C-terminus (Figure 2). The 35-amino acid SAP domain plays a role in the interaction of myocardin with genomic DNA. The SAP domain is required for the association of myocardin with A/T-rich DNA.
In embryonic cardiac tissue, myocardin is initially synthesized in the cardiac crescent at the time of cardiogenic specification and is maintained throughout the atrial and ventricular chambers of the heart during later development.59 In embryonic vascular SMCs, myocardin is expressed within the cardiac outflow tract and aortic arch arteries and in visceral SMCs of the respiratory, gastrointestinal, and genitourinary tracts.67 Subsequently, during late foetal and postnatal development, the myocardin gene is expressed abundantly in cardiac myocytes and visceral SMCs, but it is expressed at very low or undetectable levels in the coronary vasculature, dorsal aorta, and skeletal muscle cells of adults.67
A particularly interesting observation is that TERT is involved in the regulation of stem cell proliferation and differentiation. In the heart, telomerase activity is associated with myogenic cell survival, growth, and differentiation.29 Telomerase activity declines rapidly after birth, becoming almost undetectable within 3 weeks.68 The disappearance of telomerase activity at the time that cardiomyocytes become terminally differentiated suggests that telomerase downregulation is important in the permanent withdrawal of cardiomyocytes from the cell cycle. However, other groups have found that the expression of TERT does not affect differentiation in embryonic stem cells,69 adult stem cells,70,71 and leukaemic cells.72 Equal expression of differentiation markers such as ECMA-7, α-fetoprotein, (T/A)GATA(A/G)-binding proteins 4 (GATA-4), and paired box gene 6 (Pax6) was observed both in vector-transfected and TERT-overexpressing embryonic stem cell clones treated with retinoic acid or other methods of differentiation, such as dimethyl sulphoxide.69 In human bone marrow stromal cells, there is evidence of high and stable expression of TERT, together with increased expression of the Nurr-1 gene, a neuron marker, in neuronally differentiated cells.71
Recently, we have demonstrated the role of telomerase in the maintenance of a ‘myogenic stemness' in adipose tissue-derived mesenchymal stem cells (MSCs), i.e. the potency of these cells to undergo myogenic commitment while maintaining the ability to proliferate.73 In particular, we have observed that myocardin-A co-localizes with telomerase in the nucleus of MSCs where they exist in their active form, thus suggesting a relationship between telomerase and myocardin-A in actively proliferating adult tissues, such as adipose tissue.73 We have observed that the two proteins appear to co-exist and form complexes in the nuclei of adipose-derived myogenic stem cells, but that they carry out different functions—telomerase regulates the cell senescence and myocardin controls myogenesis.73 We have also observed that dually positive MSCs from the stromal compartment of adipose tissue show great potency for self-renewal and myogenic development when cultured in vitro. It is likely that the two molecules interact in the regulation of MSC growth and myogenesis. Our finding of increased expression of TERT and myocardin-A in these myogenic MSCs suggests that TERT has a role in the maintenance of MSCs in an intermediate step or a ‘biological window' in which an undifferentiated, uncommitted stem cell evolves into commitment toward myogenic development while maintaining potency for proliferation or quiescence.73 These data reinforce the concept that telomerase may have bioactivities not reported previously, including cell-cycle regulation and stem cell proliferation and differentiation.
Role of telomerase and its associated proteins in cardiovascular tissue repair and regeneration
Compelling evidence illustrates a role for telomerase and its associated proteins in the development of certain cardiovascular diseases, including atherosclerotic coronary disease74–76 and heart failure.29,77–79 Table 1 summarizes human studies that show an association between telomere dysfunction and cardiovascular disease. Furthermore, telomerase and its associated proteins may be potential therapeutic targets in the treatment of these diseases.
Table 1.
Human studies showing an association between telomere dysfunction and cardiovascular disease
| Cardiovascular risk factor or disease | Main findings | References |
|---|---|---|
| Gender | Adult men display lower telomerase activity. | 92–94 |
| Hypertension | Systo-diastolic hypertension inversely correlates with telomere length. | 92,93,100 |
| Type 1 and type 2 diabetes mellitus, insulin resistance and obesity | Diabetic and/or insulin resistant patients and obese subjects have shorter telomeres. | 78,92,95,98,99 |
| Smoking | Sex- and age-adjusted telomere length is shorter in smokers. | 95–97 |
| Atherosclerosis | Age-dependent telomere shortening characterizes fibrous cap and endothelial cells from iliac, thoracic, carotid, and coronary arteries. | 74,76,79,86 |
| Ischaemic and non-ischaemic heart failure | Telomere shortening, TRF-2 downregulation, and apoptosis accelerate end-stage heart failure. | 29,88 |
| Cardiac hyperthrophy | Telomere shortening and low telomerase activity worsen the development of pathologic cardiac remodelling with fibrosis after pressure and volume cardiac overload. | 77,89 |
| Premature myocardial infarction | Shorter telomere length and low telomerase activity increase the risk of premature myocardial infarction. | 75 |
TRF-2, telomere restriction fragment 2.
Recently, several gain-of-function and loss-of-function studies have shown that high telomerase activity and long telomeres are necessary for maintaining the proliferative potential and viability of endothelial cells,80–84 SMCs,85,86 and cardiomyocytes.29,87 In TERC−/− mice, an increased incidence of hypertension, ventricular dilation, thinning of myocardium, cardiac dysfunction, and sudden cardiac death has suggested that maintaining normal telomerase function is essential for cardiovascular development.39,40
Accelerated apoptosis and replicative senescence of cardiac stem cells are both among the possible mechanisms by which telomere dysfunction may impair the heart's regenerative capability and contribute to cardiac dysfunction. After ischaemic injury (coronary ligation), apoptosis is attenuated in the heart of transgenic mice that overexpress TERT specifically in the cardiac tissues,29,87 which correlates with findings of reduced infarct size, a less fibrous area, and preservation of systolic function.29,87 In addition, patients with myocardial infarction and heart failure show weakening of the cardiac-cell growth response after injury because of decreased telomerase activity and severe telomeric shortening in the heart's resident pool of cardiac progenitor cells, which leads to their replicative senescence.29,88 Without sufficient telomerase activity, cardiac stem cells become unable to support myocardial regeneration of the adult heart after myocardial infarction.5,88 In addition, telomere shortening and dysfunction may contribute to the development of pathologic cardiac remodelling by facilitating collagen deposition in hearts undergoing pressure and volume overload.89 Implications of the diminished proliferative capacity of the cardiovascular system might also include the depletion of endothelial progenitor cells, consequently impairing their neovasculogenic potential and repair capacity.90 As a result, neovascularization in ischaemic heart disease and vascular regeneration in atherosclerotic disease could be limited. The exhaustion of endothelial progenitor cells and telomere shortening have been causally linked to the exposure to cardiovascular risk factors91 such as gender,92–94 smoking,95–97 insulin resistance and obesity,78,95,98 type 1 and type 2 diabetes,92,99 and hypertension.100
Telomere dysfunction may also contribute to the pathogenesis of atherosclerosis. An association has been reported between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease.101 Endothelial cells and SMCs derived from atherosclerotic plaque have shorter telomeres than do endothelial cells derived from non-atherosclerotic areas of the same individual.76,86 In patients with atherosclerosis, shorter telomeres have been reported in the fibrous cap and in lymphocytes that infiltrate unstable atherosclerotic plaque.76,86
Telomeres and associated proteins as therapeutic targets for cardiovascular disease
Basic scientists and clinical investigators have been actively exploring the potential of telomere modifying agents to treat or prevent cardiovascular disorders, particularly age-associated adverse events. In this context, one possible strategy would be to shorten the telomere attrition rate associated with senescence or disease by increasing telomerase activity. Telomerase enzymatic activity can be regulated at multiple levels, including TERT transcription, alternative splicing, chaperone-mediated folding, phosphorylation, and nuclear translocation. The main control mechanism of human TERT activity, however, seems to be the regulation of TERT expression. Studies in human adult cells or human MSCs have indicated the possibility of using TERT gene transfer to blunt telomere shortening and associated senescence.14,71 Importantly, exogenous TERT can be genetically introduced into human MSCs ex vivo without interfering with their potential for cardiomyogenic differentiation.71 The final goal of this approach would be to re-introduce genetically manipulated MSCs in vivo, where they could provide therapeutically relevant levels of TERT and serve as a useful source of cardiomyocytes or cardiomyogenic stem cells.
Another approach to increasing TERT activity would be to pharmacologically manipulate its activity with antioxidant drugs. The rationale for using an antioxidant treatment to lessen stress-induced senescence and increase telomerase-induced telomere stabilization comes from the idea that oxidative stress is associated with ageing and risk factors for cardiovascular diseases102 and may induce DNA damage,103,104 accelerate reparative cell division, promote telomere instability and shortening, and lead to premature stress-induced senescence.86,101,104 Endothelial cells that were isolated from arterial segments of patients with severe coronary artery disease and that were chronically treated in culture with N acetyl-cystein (NAC, a sulphydryl group donor and an indirect antioxidant agent with the property of increasing intracellular glutathione and decreasing intracellular ROS)105displayed decreased lipid peroxidation, decreased DNA and cellular damage, elongated telomere length, and senescence delay.105 In this study, it was shown that the mechanism of NAC-induced telomere elongation involves inducing the translocation of TERT into the nucleus where it becomes activated.105
Conclusions
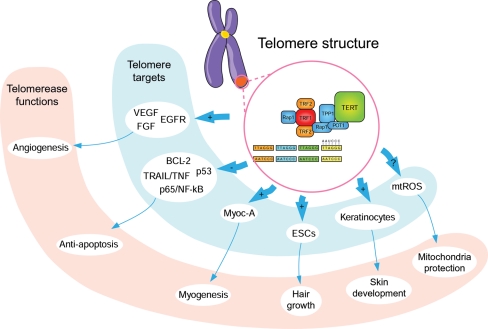
Telomerase localizes in and influences a wide range of cell types and tissue through a variety of activities that, although not fully characterized, go beyond the eponymous function of telomere maintenance and involve cell growth and differentiation. It is possible that telomerase works as a transcription co-factor or as part of a pathway leading to modifications of other transcription factors that regulate the expression of genes involved in the regulation of cell growth, apoptosis, and differentiation (Figure 3). Increased telomerase expression and function occur in the heart undergoing post-infarct repair and hypertrophy, suggesting that is has a role in cardiac tissue repair and regeneration. The recent discovery of a telomerase-myocardin interaction in adult myogenic stem cells points to the involvement of telomerase in the myocardin/SRF promyogenic pathway, which triggers the cardiomyogenic differentiation of multipotent stem cells. An understanding of how telomerase affects gene expression may lead to new therapies for heart failure aimed at promoting stem cell proliferation and differentiation and protecting stem cells from cell death and apoptosis. Further clinical investigation of telomerase and myocardin is warranted for exploring their therapeutic potential in the treatment of patients with myocardial infarction and heart failure.
Figure 3.
Non-canonical functions and targets of telomerase. Telomerase maintains telomere length and integrity by forming a complex of protein (TERT) and RNA (TERC) components. Additionally, telomerase may have a regulatory role in gene expression and the cellular response to oxidative stress and pro-apoptotic stimuli, collectively contributing to cell growth and differentiation. BCL2, B-cell lymphoma 2; EGFR, epidermal growth factor receptor; ESCs, epidermal stem cells; FGF, fibroblast growth factor; Myoc-A, myocardin-A; mtROS, mitochondrial reactive oxygen species; NF-κB, nuclear factor-κB; TNF-α, tumor necrosis factor-α; TRAIL, TNF-related apoptosis-inducing ligand; VEGF, vascular endothelial growth factor. +, induction; –, inhibition.
Funding
This work was supported by grants from the National Institutes of Health (R01HL59249 and R01HL69509 to Y.J.G.) and the Texas State Higher Education Coordinating Board ATP/TDP program to Y.J.G. and from the Istituto Nazionale Ricerche Cardiovascolari to R.D.C.
Conflict of interest: none declared.
Acknowledgements
Figure development, under direction of the authors, was provided by Yang James Jiang.
References
- 1.Barile L, Messina E, Giacomello A, Marban E. Endogenous cardiac stem cells. Prog Cardiovasc Dis. 2007;50:31–48. doi: 10.1016/j.pcad.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 4.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marban E. Big cells, little cells, stem cells: agents of cardiac plasticity. Circ Res. 2007;100:445–446. doi: 10.1161/01.RES.0000260271.33215.9b. [DOI] [PubMed] [Google Scholar]
- 6.McClintock B. The behavior in successive nuclear divisions of a chromosome broken at meiosis. Proc Natl Acad Sci U S A. 1939;25:405–416. doi: 10.1073/pnas.25.8.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shampay J, Szostak JW, Blackburn EH. DNA sequences of telomeres maintained in yeast. Nature. 1984;310:154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- 8.Szostak JW, Blackburn EH. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982;29:245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- 9.Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 10.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 11.Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- 12.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 13.Maser RS, DePinho RA. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- 14.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 15.Counter CM, Hahn WC, Wei W, Caddle SD, Beijersbergen RL, Lansdorp PM, Sedivy JM, Weinberg RA. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci U S A. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 18.Njajou OT, Hsueh WC, Blackburn EH, Newman AB, Wu SH, Li R, Simonsick EM, Harris TM, Cummings SR, Cawthon RM. Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64:860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atzmon G, Cho M, Cawthon RM, Budagov T, Katz M, Yang X, Siegel G, Bergman A, Huffman DM, Schechter CB, Wright WE, Shay JW, Barzilai N, Govindaraju DR, Suh Y. Evolution in health and medicine Sackler colloquium: genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc Natl Acad Sci U S A. 2010;107(Suppl. 1):1710–1717. doi: 10.1073/pnas.0906191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Dolbier C, Mendes WB, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Oexle K, Zwirner A. Advanced telomere shortening in respiratory chain disorders. Hum Mol Genet. 1997;6:905–908. doi: 10.1093/hmg/6.6.905. [DOI] [PubMed] [Google Scholar]
- 22.Passos JF, von Zglinicki T. Mitochondria, telomeres and cell senescence. Exp Gerontol. 2005;40:466–472. doi: 10.1016/j.exger.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Blackburn EH, Greider CW, Henderson E, Lee MS, Shampay J, Shippen-Lentz D. Recognition and elongation of telomeres by telomerase. Genome. 1989;31:553–560. doi: 10.1139/g89-104. [DOI] [PubMed] [Google Scholar]
- 24.Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G, de Lange T. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol. 2000;20:1659–1668. doi: 10.1128/mcb.20.5.1659-1668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chong L, van Steensel B, Broccoli D, Erdjument-Bromage H, Hanish J, Tempst P, de Lange T. A human telomeric protein. Science. 1995;270:1663–1667. doi: 10.1126/science.270.5242.1663. [DOI] [PubMed] [Google Scholar]
- 26.van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- 27.Broccoli D, Smogorzewska A, Chong L, de Lange T. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2. Nat Genet. 1997;17:231–235. doi: 10.1038/ng1097-231. [DOI] [PubMed] [Google Scholar]
- 28.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401–413. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 29.Oh H, Wang SC, Prahash A, Sano M, Moravec CS, Taffet GE, Michael LH, Youker KA, Entman ML, Schneider MD. Telomere attrition and Chk2 activation in human heart failure. Proc Natl Acad Sci U S A. 2003;100:5378–5383. doi: 10.1073/pnas.0836098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright WE, Shay JW. Telomere biology in aging and cancer. J Am Geriatr Soc. 2005;53:S292–294. doi: 10.1111/j.1532-5415.2005.53492.x. [DOI] [PubMed] [Google Scholar]
- 31.Lopatina NG, Poole JC, Saldanha SN, Hansen NJ, Key JS, Pita MA, Andrews LG, Tollefsbol TO. Control mechanisms in the regulation of telomerase reverse transcriptase expression in differentiating human teratocarcinoma cells. Biochem Biophys Res Commun. 2003;306:650–659. doi: 10.1016/s0006-291x(03)01033-7. [DOI] [PubMed] [Google Scholar]
- 32.Haik S, Gauthier LR, Granotier C, Peyrin JM, Lages CS, Dormont D, Boussin FD. Fibroblast growth factor 2 up regulates telomerase activity in neural precursor cells. Oncogene. 2000;19:2957–2966. doi: 10.1038/sj.onc.1203596. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Rivera L, Herrera E, Albar JP, Blasco MA. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc Natl Acad Sci U S A. 1998;95:10471–10476. doi: 10.1073/pnas.95.18.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hug N, Lingner J. Telomere length homeostasis. Chromosoma. 2006;115:413–425. doi: 10.1007/s00412-006-0067-3. [DOI] [PubMed] [Google Scholar]
- 35.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 36.Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- 37.Yuan X, Ishibashi S, Hatakeyama S, Saito M, Nakayama J, Nikaido R, Haruyama T, Watanabe Y, Iwata H, Iida M, Sugimura H, Yamada N, Ishikawa F. Presence of telomeric G-strand tails in the telomerase catalytic subunit TERT knockout mice. Genes Cells. 1999;4:563–572. doi: 10.1046/j.1365-2443.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- 38.Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 39.Herrera E, Samper E, Martin-Caballero J, Flores JM, Lee HW, Blasco MA. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 41.Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309:1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- 42.Middleman EJ, Choi J, Venteicher AS, Cheung P, Artandi SE. Regulation of cellular immortalization and steady-state levels of the telomerase reverse transcriptase through its carboxy-terminal domain. Mol Cell Biol. 2006;26:2146–2159. doi: 10.1128/MCB.26.6.2146-2159.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith LL, Coller HA, Roberts JM. Telomerase modulates expression of growth-controlling genes and enhances cell proliferation. Nat Cell Biol. 2003;5:474–479. doi: 10.1038/ncb985. [DOI] [PubMed] [Google Scholar]
- 44.Kim H, Muller WJ. The role of the epidermal growth factor receptor family in mammary tumorigenesis and metastasis. Exp Cell Res. 1999;253:78–87. doi: 10.1006/excr.1999.4706. [DOI] [PubMed] [Google Scholar]
- 45.Zaccagnini G, Gaetano C, Della Pietra L, Nanni S, Grasselli A, Mangoni A, Benvenuto R, Fabrizi M, Truffa S, Germani A, Moretti F, Pontecorvi A, Sacchi A, Bacchetti S, Capogrossi MC, Farsetti A. Telomerase mediates vascular endothelial growth factor-dependent responsiveness in a rat model of hind limb ischemia. J Biol Chem. 2005;280:14790–14798. doi: 10.1074/jbc.M414644200. [DOI] [PubMed] [Google Scholar]
- 46.Dudognon C, Pendino F, Hillion J, Saumet A, Lanotte M, Segal-Bendirdjian E. Death receptor signaling regulatory function for telomerase: hTERT abolishes TRAIL-induced apoptosis, independently of telomere maintenance. Oncogene. 2004;23:7469–7474. doi: 10.1038/sj.onc.1208029. [DOI] [PubMed] [Google Scholar]
- 47.Del Bufalo D, Rizzo A, Trisciuoglio D, Cardinali G, Torrisi MR, Zangemeister-Wittke U, Zupi G, Biroccio A. Involvement of hTERT in apoptosis induced by interference with Bcl-2 expression and function. Cell Death Differ. 2005;12:1429–1438. doi: 10.1038/sj.cdd.4401670. [DOI] [PubMed] [Google Scholar]
- 48.Rahman R, Latonen L, Wiman KG. hTERT antagonizes p53-induced apoptosis independently of telomerase activity. Oncogene. 2005;24:1320–1327. doi: 10.1038/sj.onc.1208232. [DOI] [PubMed] [Google Scholar]
- 49.Perrault SD, Hornsby PJ, Betts DH. Global gene expression response to telomerase in bovine adrenocortical cells. Biochem Biophys Res Commun. 2005;335:925–936. doi: 10.1016/j.bbrc.2005.07.156. [DOI] [PubMed] [Google Scholar]
- 50.Lai SR, Cunningham AP, Huynh VQ, Andrews LG, Tollefsbol TO. Evidence of extra-telomeric effects of hTERT and its regulation involving a feedback loop. Exp Cell Res. 2007;313:322–330. doi: 10.1016/j.yexcr.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akiyama M, Hideshima T, Hayashi T, Tai YT, Mitsiades CS, Mitsiades N, Chauhan D, Richardson P, Munshi NC, Anderson KC. Nuclear factor-kappaB p65 mediates tumor necrosis factor alpha-induced nuclear translocation of telomerase reverse transcriptase protein. Cancer Res. 2003;63:18–21. [PubMed] [Google Scholar]
- 52.Santos JH, Meyer JN, Skorvaga M, Annab LA, Van Houten B. Mitochondrial hTERT exacerbates free-radical-mediated mtDNA damage. Aging Cell. 2004;3:399–411. doi: 10.1111/j.1474-9728.2004.00124.x. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci. 2008;121:1046–1053. doi: 10.1242/jcs.019372. [DOI] [PubMed] [Google Scholar]
- 54.Haendeler J, Drose S, Buchner N, Jakob S, Altschmied J, Goy C, Spyridopoulos I, Zeiher AM, Brandt U, Dimmeler S. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. Arterioscler Thromb Vasc Biol. 2009;29:929–935. doi: 10.1161/ATVBAHA.109.185546. [DOI] [PubMed] [Google Scholar]
- 55.Xi L, Chen G, Zhou J, Xu G, Wang S, Wu P, Zhu T, Zhang A, Yang W, Xu Q, Lu Y, Ma D. Inhibition of telomerase enhances apoptosis induced by sodium butyrate via mitochondrial pathway. Apoptosis. 2006;11:789–798. doi: 10.1007/s10495-006-5701-2. [DOI] [PubMed] [Google Scholar]
- 56.Cao Y, Li H, Deb S, Liu JP. TERT regulates cell survival independent of telomerase enzymatic activity. Oncogene. 2002;21:3130–3138. doi: 10.1038/sj.onc.1205419. [DOI] [PubMed] [Google Scholar]
- 57.Saretzki G. Telomerase, mitochondria and oxidative stress. Exp Gerontol. 2009;44:485–492. doi: 10.1016/j.exger.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Aravind L, Koonin EV. SAP—a putative DNA-binding motif involved in chromosomal organization. Trends Biochem Sci. 2000;25:112–114. doi: 10.1016/s0968-0004(99)01537-6. [DOI] [PubMed] [Google Scholar]
- 59.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- 60.Norman C, Runswick M, Pollock R, Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988;55:989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 61.Christy B, Nathans D. Functional serum response elements upstream of the growth factor-inducible gene zif268. Mol Cell Biol. 1989;9:4889–4895. doi: 10.1128/mcb.9.11.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greenberg ME, Siegfried Z, Ziff EB. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987;7:1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5′ element and c-fos 3′ sequences. Cell. 1985;42:889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- 64.Townsend KJ, Zhou P, Qian L, Bieszczad CK, Lowrey CH, Yen A, Craig RW. Regulation of MCL1 through a serum response factor/Elk-1-mediated mechanism links expression of a viability-promoting member of the BCL2 family to the induction of hematopoietic cell differentiation. J Biol Chem. 1999;274:1801–1813. doi: 10.1074/jbc.274.3.1801. [DOI] [PubMed] [Google Scholar]
- 65.Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol. 2003;23:2425–2437. doi: 10.1128/MCB.23.7.2425-2437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Creemers EE, Sutherland LB, Oh J, Barbosa AC, Olson EN. Coactivation of MEF2 by the SAP domain proteins myocardin and MASTR. Mol Cell. 2006;23:83–96. doi: 10.1016/j.molcel.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A. 2003;100:7129–7134. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borges A, Liew CC. Telomerase activity during cardiac development. J Mol Cell Cardiol. 1997;29:2717–2724. doi: 10.1006/jmcc.1997.0503. [DOI] [PubMed] [Google Scholar]
- 69.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, II, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 70.Kobune M, Kawano Y, Ito Y, Chiba H, Nakamura K, Tsuda H, Sasaki K, Dehari H, Uchida H, Honmou O, Takahashi S, Bizen A, Takimoto R, Matsunaga T, Kato J, Kato K, Houkin K, Niitsu Y, Hamada H. Telomerized human multipotent mesenchymal cells can differentiate into hematopoietic and cobblestone area-supporting cells. Exp Hematol. 2003;31:715–722. doi: 10.1016/s0301-472x(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 71.Takeda Y, Mori T, Imabayashi H, Kiyono T, Gojo S, Miyoshi S, Hida N, Ita M, Segawa K, Ogawa S, Sakamoto M, Nakamura S, Umezawa A. Can the life span of human marrow stromal cells be prolonged by bmi-1, E6, E7, and/or telomerase without affecting cardiomyogenic differentiation? J Gene Med. 2004;6:833–845. doi: 10.1002/jgm.583. [DOI] [PubMed] [Google Scholar]
- 72.Yamada O, Akiyama M, Kawauchi K, Adachi T, Yamada H, Kanda N, Aikawa E. Overexpression of telomerase confers a survival advantage through suppression of TRF1 gene expression while maintaining differentiation characteristics in K562 cells. Cell Transplant. 2003;12:365–377. doi: 10.3727/000000003108746911. [DOI] [PubMed] [Google Scholar]
- 73.Madonna R, Willerson JT, Geng YJ. Myocardin A enhances telomerase activities in adipose tissue mesenchymal cells and embryonic stem cells undergoing cardiovascular myogenic differentiation. Stem Cells. 2008;26:202–211. doi: 10.1634/stemcells.2007-0490. [DOI] [PubMed] [Google Scholar]
- 74.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 75.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 76.Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, Hai E, Shirai N, Ehara S, Komatsu R, Naruko T, Ueda M. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol. 2004;24:546–550. doi: 10.1161/01.ATV.0000117200.46938.e7. [DOI] [PubMed] [Google Scholar]
- 77.Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–613. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 78.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 79.van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ, van Gilst WH, van Veldhuisen DJ. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 80.Yang J, Chang E, Cherry AM, Bangs CD, Oei Y, Bodnar A, Bronstein A, Chiu CP, Herron GS. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999;274:26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- 81.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 82.Murasawa S, Llevadot J, Silver M, Isner JM, Losordo DW, Asahara T. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation. 2002;106:1133–1139. doi: 10.1161/01.cir.0000027584.85865.b4. [DOI] [PubMed] [Google Scholar]
- 83.Kurz DJ, Hong Y, Trivier E, Huang HL, Decary S, Zang GH, Luscher TF, Erusalimsky JD. Fibroblast growth factor-2, but not vascular endothelial growth factor, upregulates telomerase activity in human endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:748–754. doi: 10.1161/01.ATV.0000069624.55424.61. [DOI] [PubMed] [Google Scholar]
- 84.Hastings R, Qureshi M, Verma R, Lacy PS, Williams B. Telomere attrition and accumulation of senescent cells in cultured human endothelial cells. Cell Prolif. 2004;37:317–324. doi: 10.1111/j.1365-2184.2004.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Minamino T, Kourembanas S. Mechanisms of telomerase induction during vascular smooth muscle cell proliferation. Circ Res. 2001;89:237–243. doi: 10.1161/hh1501.094267. [DOI] [PubMed] [Google Scholar]
- 86.Matthews C, Gorenne I, Scott S, Figg N, Kirkpatrick P, Ritchie A, Goddard M, Bennett M. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ Res. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 87.Oh H, Taffet GE, Youker KA, Entman ML, Overbeek PA, Michael LH, Schneider MD. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc Natl Acad Sci U S A. 2001;98:10308–10313. doi: 10.1073/pnas.191169098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:10440–10445. doi: 10.1073/pnas.1832855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van der Harst P, van Veldhuisen DJ, Samani NJ. Expanding the concept of telomere dysfunction in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2008;28:807–808. doi: 10.1161/ATVBAHA.108.164434. [DOI] [PubMed] [Google Scholar]
- 91.Edo MD, Andres V. Aging, telomeres, and atherosclerosis. Cardiovasc Res. 2005;66:213–221. doi: 10.1016/j.cardiores.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 92.Jeanclos E, Krolewski A, Skurnick J, Kimura M, Aviv H, Warram JH, Aviv A. Shortened telomere length in white blood cells of patients with IDDM. Diabetes. 1998;47:482–486. doi: 10.2337/diabetes.47.3.482. [DOI] [PubMed] [Google Scholar]
- 93.Benetos A, Okuda K, Lajemi M, Kimura M, Thomas F, Skurnick J, Labat C, Bean K, Aviv A. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37:381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 94.Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004;363:507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- 95.Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 96.Morla M, Busquets X, Pons J, Sauleda J, MacNee W, Agusti AG. Telomere shortening in smokers with and without COPD. Eur Respir J. 2006;27:525–528. doi: 10.1183/09031936.06.00087005. [DOI] [PubMed] [Google Scholar]
- 97.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16:815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 98.Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 99.Adaikalakoteswari A, Balasubramanyam M, Mohan V. Telomere shortening occurs in Asian Indian Type 2 diabetic patients. Diabet Med. 2005;22:1151–1156. doi: 10.1111/j.1464-5491.2005.01574.x. [DOI] [PubMed] [Google Scholar]
- 100.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Kimura M, Larson MG, Meigs JB, Keaney JF, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–330. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 101.Satoh M, Ishikawa Y, Takahashi Y, Itoh T, Minami Y, Nakamura M. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2008;198:347–353. doi: 10.1016/j.atherosclerosis.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 102.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 103.Lorenz M, Saretzki G, Sitte N, Metzkow S, von Zglinicki T. BJ fibroblasts display high antioxidant capacity and slow telomere shortening independent of hTERT transfection. Free Radic Biol Med. 2001;31:824–831. doi: 10.1016/s0891-5849(01)00664-5. [DOI] [PubMed] [Google Scholar]
- 104.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 105.Voghel G, Thorin-Trescases N, Farhat N, Mamarbachi AM, Villeneuve L, Fortier A, Perrault LP, Carrier M, Thorin E. Chronic treatment with N-acetyl-cystein delays cellular senescence in endothelial cells isolated from a subgroup of atherosclerotic patients. Mech Ageing Dev. 2008;129:261–270. doi: 10.1016/j.mad.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chan SW, Blackburn EH. New ways not to make ends meet: telomerase, DNA damage proteins and heterochromatin. Oncogene. 2002;21:553–563. doi: 10.1038/sj.onc.1205082. [DOI] [PubMed] [Google Scholar]
- 107.de Lange TS. The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]