Abstract
Background
Metal ions released from arthroplasty devices are largely cleared in urine, leading to high exposure in renal tissues. Validated early markers of renal damage are routinely used to monitor workers in heavy metal industries, and renal risk can be quantified in these industries. It is unclear if the ion levels in patients with metal-on-metal hips are sufficient to cause renal damage.
Question
Does metal-on-metal (MOM) bearing use over a 10-year period lead to elevation of early renal markers compared with the levels expected in subjects with no metal exposure?
Methods
We retrospectively reviewed 31 patients who underwent MOM hip resurfacings 10 years earlier. Whole blood specimens were collected for metal ion analysis, serum for creatinine estimation, and urine for timed metal ion output and renal markers. The renal marker levels of 30 age- and gender-matched subjects with no metal exposure and no known renal problems or diabetes mellitus were used as controls for renal markers.
Results
Median serum creatinine level in the MOM group was 1.1 mg/dL (interquartile range, 1.0–1.2 mg/dL) and median creatinine clearance was 79.2 mL/min. In this cohort, the number of patients with markers of renal damage above the reference range was comparable to the controls. None of the renal markers were associated with metal levels.
Conclusion
The absence of elevation of renal markers in this cohort 10 years after MOM bearing implantation is reassuring. However, we believe surveillance through further longer-term, large-scale controlled trials are needed to monitor this arthroplasty-induced low-intensity (but long-term) trace element exposure to rule out potential nephrotoxicity.
Level of Evidence
Level III, retrospective comparative study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The reintroduction of metal-on-metal (MOM) bearings in the management of hip arthritis has led to their increased use in young, active patients [27]. Release and dissemination of metal particles and ions is an unavoidable consequence of MOM use [8, 17]. Metal components used in conventional hip and knee arthroplasties (stems and socket carriers) also release metal particles and ions through wear and corrosion [18, 25]. An extensive review of orthopaedic metal toxicity [17] identified the renal system as a key area needing investigation. That review and another study [17, 20] noted chromium and cobalt are excreted by the kidney and have the potential to induce tubular necrosis. The authors suggest the incidence of metal-induced toxicity in the kidney must be “clarified by renal monitoring of arthroplasty patients.”
Chromate-induced tubular necrosis was reported after hexavalent chromium intake in other settings [24, 29, 30]. In the heavy metal industry, biologic markers of early renal dysfunction are routinely used [2, 4, 13, 22, 23, 28, 32] to monitor exposure. Renal marker assessment such as retinol binding protein (RBP) and Brush Border antigen (BBA) revealed doubling of marker levels in chromate workers with 7 years exposure when compared with controls [14]. Furthermore, in workers with elevated chromium levels (greater than 15 μg/L) [14], one-third had marker levels above the reference range compared with one in 30 in the controls. A 5-year study of smelter workers [12] showed a 50% elevation of β2-microglobulin (β2M) and reduction of glomerular filtration, denoting reduced kidney function. A study of patients with nephrotic syndrome and normal baseline renal function demonstrated N-acetyl-β-D-glucosaminidase (NAG) was highly predictive of both remission and progression depending on whether NAG was below or above the reference range [3]. In patients with sickle cell/β thalassemia, in which progressive renal failure is a known complication, the current renal markers β2M and NAG were 10 times more sensitive (70%–75%) than serum creatinine (7%) as predictors of eventual renal impairment [31].
These findings demonstrate renal markers are elevated after exposure to nephrotoxic substances and are reliable early predictors of reduced renal function. However, transient changes in renal markers can be produced by several physiological processes, including the hour of the day, posture, physical activity, protein intake, and hydration [22]. One positive test at one time point does not necessarily indicate impending renal disease. Such variations also are found in unexposed patients. Although workers in the heavy metal industries are at risk for renal damage, it is unclear whether the levels of ions released in MOM hips is sufficient to cause renal damage.
Our primary purpose was therefore to determine whether renal marker levels differed in patients who underwent MOM resurfacing 10 years prior and in matched implant-free controls and whether there was an association between blood metal ion levels and renal markers in patients with MOM resurfacing.
Patients and Methods
This is a retrospective, cross-sectional, observational study of renal markers in a subgroup of patients who attended a routine 10-year followup of their MOM hip resurfacings. One hundred twenty-one of 128 patients with surviving implants who received McMinn hybrid hip resurfacings (Corin Group, Cirencester, UK) in 1996 under a single surgeon (DJWM) were reviewed clinicoradiologically during 2006 and 2007, the details of which were published earlier [7]. Seven who confirmed survival were unable to attend.
We used this 10-year review visit to also assess renal markers. By choosing the 10-year followup period, we allowed ample time for renal marker elevation to develop if it were to occur after an initial lag period. Thirty-five patients in this cohort were seen between December 2006 and March 2007. We excluded four of these 35 patients who had diabetes mellitus, leaving 31 patients for review. There were no other exclusion criteria. These 31 patients (24 men and seven women) had a mean age of 62 years (range, 34–76 years) and a mean BMI of 27.6 kg/m2 (range, 21–41 kg/m2). Twenty-six underwent unilateral resurfacings and five underwent bilateral resurfacings. Of the 26 unilateral McMinn resurfacings, one had a well-functioning contralateral Stanmore MOM THA (Stanmore, Middlesex, UK) implanted in 1969 (Table 1). Two others had a MOM resurfacing (Birmingham Hip Resurfacing; Smith and Nephew Orthopaedics, Warwick, UK) implanted in their contralateral hip in 2001 and 2006, respectively. Therefore, there were eight patients who had bilateral MOM bearing hips.
Table 1.
Types of metal-on-metal arthroplasties used in the study group
| Unilateral MOM devices (n = 23) | Unilateral McMinn hybrid resurfacings (Corin) | 23 |
| Bilateral MOM devices (n = 8) | Bilateral McMinn hybrid resurfacings (Corin) | 5 |
| Unilateral McMinn hybrid resurfacings (Corin) + Stanmore MOM THA | 1 | |
| Unilateral McMinn hybrid resurfacing + Birmingham hip resurfacing | 2 | |
| 31 | Total | 31 |
MOM = metal-on-metal.
Renal marker levels of 30 age- and gender-matched subjects with no metal exposure and no known renal problems or diabetes mellitus were used as controls. They included volunteer blood donors from one of the participating institutions and were composed of 24 men and seven women with a mean age of 62 years (range, 35–72 years). Through a standardized questionnaire, we ensured they fulfilled the following criteria: no renal or systemic diseases, no intake of potentially nephrotoxic drugs, and no exposure to other known or suspected nephrotoxins. Informed consent for participation was given. We did not perform metal ion assessment in these subjects because cobalt and chromium levels in a group with no arthroplasty device are always lower than those with MOM bearings.
There are four domains in which kidney function may be monitored (Fig. 1): (1) Global kidney function is assessed from glomerular filtration rate as measured from creatinine clearance. (2) Glomerular proteinuria: high-molecular-weight proteins such as albumin and globulin do not pass through the glomerular filter under normal conditions. Their leakage occurs as a result of increased glomerular permeability and signifies glomerular disease; their levels and ratios in urine can be used to distinguish disease. (3) Tubular proteinuria: low-molecular-weight proteins such as RBP and β2M are normally filtered by the glomerulus and extensively reabsorbed in the proximal convoluted tubule. Increased low-molecular-weight proteinuria indicates tubular dysfunction. (4) Excretion of the enzyme NAG or of BBA in urine is useful in assessing the presence of renal microtissue damage.
Fig. 1.
The relationship between different domains of renal dysfunction and the different renal markers. The presence of albumin in urine denotes glomerular dysfunction and the presence of low-molecular-weight (LMW) proteins (β2M [beta 2 microglobulin] and/or RBP [retinol binding protein]) indicate tubular dysfunction. NAG (N-acetyl-beta-d-glucosaminidase) and BBA (Brush Border antigen) signify renal microtissue damage. Reduced glomerular filtration as assessed from creatinine clearance denotes global kidney dysfunction.
The null hypothesis is that for each individual renal marker, the proportion of patients whose markers are high (above the reference range) is no different from the proportion of controls whose markers are high. Prior data [14] indicate that the probability of high marker levels is 0.03 among controls and 0.32 among patients if the metal ion exposure is at nephrotoxic levels. Performing a power analysis, we found we needed to study 26 patients and 26 control subjects to be able to reject the null hypothesis with a power of 0.8 and a Type I error probability of 0.05 using a chi-squared statistic.
Before the review appointment, each patient was sent instructions and a trace metal-free container to bring a 12-hour specimen of urine. They collected urine the previous night and brought it along for estimation of metal ion output. A 12-hour collection was used rather than a 24-hour collection, because patient compliance is better [1, 11] with a 12-hour collection. Twenty-seven of the 31 patients provided a 12-hour urine sample for metal ion analysis. The median creatinine clearance was estimated by the Cockcroft-Gault formula [6].
For each patient at the 10-year followup, the reviewing clinician noted the patient diagnosis, demographics, hip function, and radiographic appearances as well as general medical conditions, regular medications, smoking, and alcohol intake. No patient had a history of renal failure. The comorbidities recorded in this cohort included hypertension (three patients), rheumatoid arthritis (two patients), breast cancer (one patient), and a duplex kidney with previous renal infection (one patient).
An anteroposterior radiograph of the pelvis and a cross-table lateral radiograph of the index hip(s) were taken at review and evaluated as published earlier [7]. Radiographic assessment showed that the following adverse features were observed in this cohort: loose cups (three hips), osteolysis (two hips), lucent lines in all three zones (two hips), migration (three hips), and neck thinning of greater than 10% original width (three hips). A detailed clinical and radiographic assessment of the entire cohort of patients, of which this subgroup is a part, was published earlier [7].
Whole blood specimens were collected without contamination for metal ion analysis, and serum was obtained for estimation of creatinine. On the day of the clinic, a spot specimen of urine was collected directly in a 30-mL specimen bottle (Sarstedt Ltd, Leicester, UK) for renal marker assessment. High-resolution inductively coupled plasma mass spectrometry was used for metal ion analysis [10].
The test battery of urinary markers included albumin, RBP, β2M, fibronectin, BBA, and NAG. The techniques used and reference values are described in earlier publications [21, 22].
We used a chi-squared test to test the difference between the number of subjects above the reference range in the patient group and in the control. Spearman’s rank correlation (ρ) was used to assess the association between renal marker levels and daily output of metal ions. Nonoverlapping 95% confidence intervals on the box plots were used to demonstrate differences. Statistical calculations were performed using Microsoft Excel 2007 (Microsoft Inc, Redmond, WA) and MedCalc Version 9 (MedCalc Software, Mariakerke, Belgium).
Results
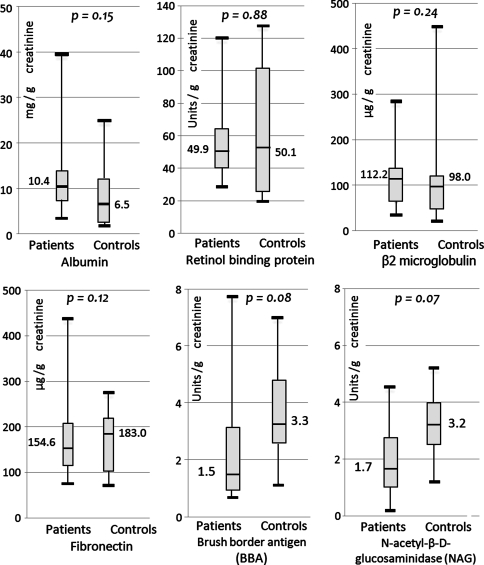
The number of subjects with renal markers above the upper reference limit in this cohort of patients was comparable to the control subjects (Table 2). The median and interquartile ranges of renal markers in the MOM cohort were within the upper reference limits for each marker. The median serum creatinine level in the MOM group was 1.1 mg/dL (interquartile range [IQR], 1.0–1.2 mg/dL) and the median creatinine clearance was 79.2 mL/min. The median urinary level of albumin in the MOM group was 10.4 mg/g of creatinine (Cn) (IQR, 7.6–13.9 mg/g), RBP was 49.9 μg/g of Cn (IQR, 40.9–63.9 μg/g), β2M 112 μg/g of Cn (IQR, 61.9–134.7 μg/g), fibronectin 154.6 μg/g of Cn (IQR, 115.6–207.6 μg/g), BBA 1.5 U/g of Cn (IQR, 0.98–3.1 U/g), and NAG 1.67 U/g of Cn (IQR, 1.1–2.7 U/g); and there were no differences between the renal marker values in the two groups (Fig. 2).
Table 2.
Comparison of renal marker elevations in patients with MOM bearings and matched subjects with no known exposure to metals
| Renal marker | Assay used for analysis | Upper reference limit (URL) | Number of subjects with values greater than URL (%) | p (chi square test) | |
|---|---|---|---|---|---|
| Subjects with MOM devices | Controls with no metal exposure | ||||
| In urine | |||||
| Albumin | Competitive ELISA | 20 mg/g creatinine | 3/31 (9.7%) | 3/30 (10.0%) | 0.7 |
| Retinol binding protein | “Sandwich” ELISA | 130 μg/g creatinine | 1/31 (3.2%) | 2/30 (6.7%) | 1 |
| β2 microglobulin | “Sandwich” ELISA | 300 μg/g creatinine | 2/31 (6.5%) | 3/30 (10.0%) | 1 |
| Fibronectin | “Sandwich” ELISA | 250 μg/g creatinine | 4/31 (12.9%) | 2/30 (6.7%) | 0.8 |
| Brush Border antigen | “Sandwich” ELISA | 8.3 U/g creatinine | 1/31 (3.2%) | 2/30 (6.7%) | 1 |
| N-acetyl-β-D glucosaminidase | Colorimetric | 5.0 U/g creatinine | 1/31 (3.2%) | 0/30 (0%) | 0.97 |
| In serum | |||||
| Creatinine | Colorimetric | 1.5 mg/dL | 0/31 (0%) | 0/30 (0%) | 1 |
MOM = metal-on-metal; ELISA = enzyme-linked immunosorbent assay.
Fig. 2.
Box plots showing the renal marker levels in patients and in controls. None of the differences were statistically significant.
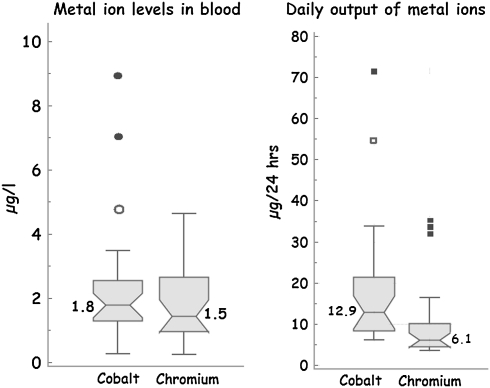
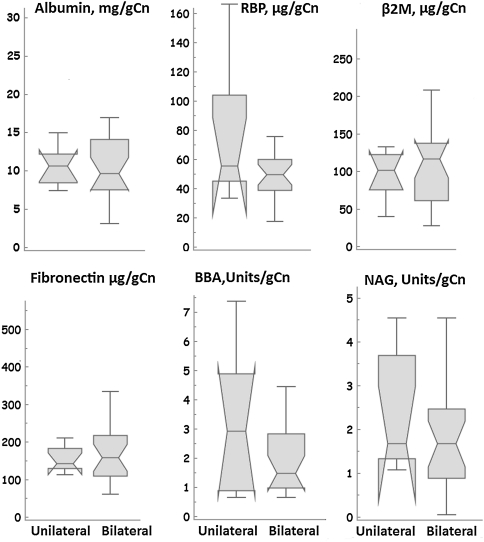
None of the renal markers was associated with the daily output of either cobalt or chromium (Table 3). The median 24-hour output of cobalt and chromium in urine of patients with MOM bearings was 12.9 μg (range, 6.1–71.5 μg) and 6.1 μg (range, 3.5–34.8 μg) and their whole blood levels were 1.8 μg/L (range, 0.3–9.0 μg/L) and 1.5 μg/L (range, 0.2–4.6 μg/L), respectively (Fig. 3). Median levels of renal markers in patients with unilateral MOM bearings were similar to those with bilateral MOM bearings (Fig. 4).
Table 3.
Association between daily output of metal ions and early renal markers
| Renal markers | Daily output of cobalt | Daily output of chromium | ||
|---|---|---|---|---|
| Spearman’s Rho (ρ) | p | Spearman’s Rho (ρ) | p | |
| Albumin | 0.2 | 0.31 | 0.2 | 0.32 |
| RBP | 0.22 | 0.28 | 0.13 | 0.53 |
| Beta2m | 0.2 | 0.31 | 0.03 | 0.88 |
| Fibronectin | 0.08 | 0.69 | 0.03 | 0.90 |
| BBA | −0.14 | 0.49 | −0.05 | 0.81 |
| NAG | 0.18 | 0.36 | 0.10 | 0.61 |
RBP = retinol binding protein; Beta2m = beta 2 microglobulin; BBA = Brush Border antigen; NAG = N-acetyl-β-D-glucosaminidase.
Fig. 3.
Box plot showing metal ion levels in whole blood and 24-hour output of metal ions in urine. The median metal ion levels in this cohort are within the expected range for metal-on-metal (MOM) bearing devices. This shows the present cohort is a representative sample of the MOM arthroplasty population. The inclusion of patients with poorly functioning devices explains the outliers in the group.
Fig. 4.
Box and whisker plots showing the median levels of renal markers in patients with unilateral metal-on-metal (MOM) hip resurfacings (n = 23) as compared with those with bilateral MOM artthroplasties (n = 8). There was no significant difference between the two groups as seen from the overlapping 95% confidence intervals of the medians (notches) indicating renal marker levels are not significantly affected by the higher ion level elevations associated with bilateral devices. RBP = retinol binding protein; β2M = beta 2 microglobulin; BBA = Brush Border antigen; NAG = N-acetyl-beta-d-glucosaminidase.
Discussion
With the increasing use of MOM bearings in the management of hip arthritis in young patients, it is necessary we investigate the possibility that adverse renal effects may follow long-term use of these devices. One study [19] found no elevation of serum creatinine and creatinine clearance in patients with MOM bearings at 10-year followup. Creatinine and its clearance are reliable measures of renal function, but these are late signs, which confirm renal damage rather than act as early warning signs. Biologic early markers are routinely used in the heavy metal industry to assess potential nephrotoxicity before the development of overt failure. Whether the ion level elevations seen in patients with MOM resurfacing are associated with nephrotoxicity is unknown. We therefore determined whether renal marker levels differed in patients who underwent MOM resurfacing 10 years prior and in matched implant-free controls and whether there was an association between blood metal ion levels and renal markers in patients with MOM resurfacing.
We recognize several limitations to our study. First, the presence of comorbidities in the study group may have affected the results. However, we decided not to exclude all possible comorbidities because these are expected in routine clinical practice, and it is necessary to study the effect of MOM bearings in the presence of these comorbidities. Because we found no differences between the renal markers in the study and control groups, the heterogeneity of the study group did not affect the results. However, diabetes mellitus was excluded because it is a known risk factor for loss of renal function, even in the absence of other nephrotoxic agents. Second, the number of patients is small compared with the tens of thousands who undergo these procedures every year. Although power and sample size calculation, based on prior data, has enabled us to study this subject using an adequately sized sample, larger scale studies would be desirable to provide a more powerful evidence base. The high cost of performing these tests can be a limiting factor. Third, young patients with these devices are likely to be exposed to elevated metal ion levels for several decades during their lifetime. Although it is generally believed that renal effects from potential toxins would manifest during the early years of exposure, monitoring at a longer-term followup is desirable if adverse effects manifest after a longer latency. Fourth, renal markers are indirect surrogate measures of early renal dysfunction. Only a renal biopsy provides the ultimate proof. However, such an invasive procedure is not justified in this assessment, and these batteries of markers are established, reliable predictors of eventual renal impairment.
We observed no difference in renal marker elevations in this cohort when compared with the controls that represent a general population with no known renal disease. Our results of both the patients and the controls compare well with the marker levels of controls published in the literature (Table 4). Franchini et al. [13] studied renal markers among cobalt workers and concluded that the kidney is not a target organ in these workers. We report similar renal marker levels of both the patients and the controls. Voskaridou et al. [31] studied urinary albumin levels in patients with HbS/β-thalassemia, and Idasiak-Piechocka et al. [16] studied fibronectin in patients with chronic glomerulonephritis; both reported marker levels higher than the levels in the patients and controls of this study. Both of these conditions are known to predispose to renal failure in later years.
Table 4.
Levels of renal markers in the present study compared with published literature
| Study and cohort | Serum creatinine | Urine albumin | RBP μg/g creatinine | β2M μg/g creatinine | Fibronectin μg/g creatinine | BBA U/g creatinine | NAG U/g creatinine | |
|---|---|---|---|---|---|---|---|---|
| Upper reference limit | 1.5 mg/dL | 20 mg/g creatinine | 130 μg/g creatinine | 300 μg/g creatinine | 250 μg/g creatinine | 8.3 U/g creatinine | 5.0 U/g creatinine | |
| Franchini et al. [13] | Cobalt-exposed workers, n = 26 | 4.45 mg/g creatinine | 42.1 μg/g creatinine | 120.8 μg/g creatinine | 2.44 U/g creatinine | |||
| Control workers, n = 35 | 4.01 mg/g creatinine | 45.8 μg/g creatinine | 85.8 μg/g creatinine | 2.64 U/g creatinine | ||||
| Voskaridou et al. [31] | Hbs/β-Thal patients, n = 87 | 0.8 | 454.5 mg/24 h proteinuria | 6.6 U/day | ||||
| Controls, n = 30 | 0.7 | 57.4 mg/24 h proteinuria | 2.0 U/day | |||||
| Mutti et al. [21] | Normal subjects, n = 300 | 5.1 mg/g creatinine | 38.1 μg/g creatinine | 98 μg/g creatinine | 3.5 U/g creatinine | 57.2 mmol/g creatinine | ||
| Idasiak-Piechocka et al. [16] | Chronic glomerulonephritis patients, n = 55 | 245.0 ng/mmol creatinine | ||||||
| Healthy subjects, n = 19 | 100.7 ng/mmol creatinine | |||||||
| Present study | Patients with MOM, n = 31 | 1.1 mg/dL | 10.4 mg/g creatinine | 49.9 μg/g creatinine | 112.0 μg/g creatinine | 154.6 μg/g creatinine | 1.5 U/g creatinine | 1.7 U/g creatinine |
| Controls, n = 30 | 5.2 mg/g creatinine | 50.1 μg/g creatinine | 98 μg/g creatinine | 183 μg/g creatinine | 3.3 U/g creatinine | 3.2 U/g creatinine | ||
RBP = retinol binding protein; β2M = beta 2 microglobulin; BBA = Brush Border antigen; NAG = N-acetyl-β-D-glucosaminidase; MOM = metal-on-metal.
The median and IQRs of renal markers in the MOM cohort were within the upper reference limits for each marker. The results of early renal markers in this study supplement the findings of Marker et al. [19], who reported that creatinine clearance is not compromised in patients with MOM bearings at 10 years followup. Their observations, reinforced by our findings, suggest that clinically relevant metal ion elevations after MOM hip arthroplasty do not lead to nephrotoxicity over a period of 10 years.
Furthermore, we did not find a correlation between the daily release of cobalt and chromium and the levels of renal markers. These findings agree with those reported by Franchini and Mutti [14], who studied workers exposed to hexavalent chromium in the chemical industry. They too reported that they did not find dose-effect or dose-response relationships between chromium levels and renal marker levels (RBP and BB50), although they found a higher percentage of workers with elevations of chromium had renal marker levels above the reference range.
Studies have shown serum cobalt levels are highly elevated in patients with MOM bearings who are also in renal failure [5, 15, 19]. Therefore, some authors consider renal failure to be a contraindication to MOM hip arthroplasty [5, 9, 26], and we agree with that view. None of our patients were in renal failure and our data do not address the problem of metal ion retention in patients with compromised renal function. Therefore, we cannot and do not advocate the use of MOM bearings in patients with a known history of renal failure.
The absence of elevation of renal markers in these patients 10 years after a MOM bearing implantation is reassuring. However, continued surveillance through longer-term large-scale prospective and retrospective controlled studies may be necessary to conclusively rule out the possibility of nephrotoxicity from arthroplasty-induced low-intensity exposure to these trace elements.
Acknowledgments
We acknowledge the technical advice and expertise of Dr Ilia Rodushkin and his team from ALS Scandinavia AB Laboratories, Luleå Sweden, in matters relating to metal ion assessment.
Footnotes
One of the authors (DJWM) is a paid consultant to Smith and Nephew Orthopaedics UK. In addition, the institution of one or more of the authors (JD, HZ, DJWM) receives royalties and institutional funding from Smith and Nephew Orthopaedics UK.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at The McMinn Centre, Birmingham, UK, and the University of Parma, Parma, Italy.
References
- 1.Alessio L, Berlin A, Dell’Orto A, Toffoletto F, Ghezzi I. Reliability of urinary creatinine as a parameter to adjust values of urinary biological indicators. Int Arch Occup Environ Health. 1985;55:99–106. doi: 10.1007/BF00378371. [DOI] [PubMed] [Google Scholar]
- 2.Barregård L, Svalander C, Schütz A, Westberg G, Sällsten G, Blohmé I, Mölne J, Attman PO, Haglind P. Cadmium, mercury, and lead in kidney cortex of the general Swedish population: a study of biopsies from living kidney donors. Environ Health Perspect. 1999;107:867–871. doi: 10.1289/ehp.99107867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazzi C, Petrini C, Rizza V, Arrigo G, Napodano P, Paparella M, D’Amico G. Urinary N-acetyl-beta-glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol Dial Transplant. 2002;17:1890–1896. doi: 10.1093/ndt/17.11.1890. [DOI] [PubMed] [Google Scholar]
- 4.Bonde JP, Vittinghus E. Urinary excretion of proteins among metal welders. Hum Exp Toxicol. 1996;15:1–4. doi: 10.1177/096032719601500101. [DOI] [PubMed] [Google Scholar]
- 5.Brodner W, Grohs J, Bitzan P. Serum cobalt and serum chromium levels in two patients with chronic renal failure and total hip arthroplasty with metal-on-metal articulations. Z Orthop. 2000;138:425–430. doi: 10.1055/s-2000-10172. [DOI] [PubMed] [Google Scholar]
- 6.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 7.Daniel J, Ziaee H, Kamali A, Pradhan C, Band T, McMinn DJ. Ten-year results of a double-heat-treated metal-on-metal hip resurfacing. J Bone Joint Surg Br. 2010;92:20–27. doi: 10.2106/JBJS.H.01821. [DOI] [PubMed] [Google Scholar]
- 8.Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study. J Bone Joint Surg Br. 2007;89:169–173. doi: 10.1302/0301-620X.89B2.18519. [DOI] [PubMed] [Google Scholar]
- 9.Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Renal clearance of cobalt in relation to the use of metal-on-metal bearings in hip arthroplasty. J Bone Joint Surg Am. 2010;92:840–845. doi: 10.2106/JBJS.H.01821. [DOI] [PubMed] [Google Scholar]
- 10.Daniel J, Ziaee H, Salama A, Pradhan C, McMinn DJ. The effect of the diameter of metal-on-metal bearings on systemic exposure to cobalt and chromium. J Bone Joint Surg Br. 2006;88:443–448. doi: 10.1302/0301-620X.88B4.17355. [DOI] [PubMed] [Google Scholar]
- 11.Elkins HB, Pagnotto LD, Smith HL. Concentration adjustment in urinalysis. Am Ind Hyg Assoc J. 1974;35:559–565. doi: 10.1080/0002889748507072. [DOI] [PubMed] [Google Scholar]
- 12.Franchini I, Alinovi R, Bergamaschi E, Mutti A. Contribution of studies on renal effects of heavy metals and selected organic compounds to our understanding of the progression of chronic nephropathies towards renal failure. Acta Biomed. 2005;76(Suppl 2):58–67. [PubMed] [Google Scholar]
- 13.Franchini I, Bocchi MC, Giaroli C, Ferdenzi O, Alinovi R, Bergamaschi E. Does occupational cobalt exposure determine early renal changes? Sci Total Environ. 1994;150:149–152. doi: 10.1016/0048-9697(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 14.Franchini I, Mutti A. Selected toxicological aspects of chromium (VI) compounds. Sci Total Environ. 1988;71:379–387. doi: 10.1016/0048-9697(88)90210-0. [DOI] [PubMed] [Google Scholar]
- 15.Hur CI, Yoon TR, Cho SG, Song EK, Seon JK. Serum ion level after metal-on-metal THA in patients with renal failure. Clin Orthop Relat Res. 2008;466:696–699. doi: 10.1007/s11999-007-0093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Idasiak-Piechocka I, Oko A, Pawliczak E, Kaczmarek E, Czekalski S. Elevated urinary fibronectin excretion predicts poor outcome in patients with primary chronic glomerulonephritis. Nephron Clin Pract. 2010;116:c47–c52. doi: 10.1159/000314550. [DOI] [PubMed] [Google Scholar]
- 17.Keegan GM, Learmonth ID, Case CP. Orthopaedic metals and their potential toxicity in the arthroplasty patient: a review of current knowledge and future strategies. J Bone Joint Surg Br. 2007;89:567–573. doi: 10.1302/0301-620X.89B5.18903. [DOI] [PubMed] [Google Scholar]
- 18.Luetzner J, Krummenauer F, Lengel AM, Ziegler J, Witzleb WC. Serum metal ion exposure after total knee arthroplasty. Clin Orthop Relat Res. 2007;461:136–142. doi: 10.1097/BLO.0b013e31806450ef. [DOI] [PubMed] [Google Scholar]
- 19.Marker M, Grübl A, Riedl O, Heinze G, Pohanka E, Kotz R. Metal-on-metal hip implants: do they impair renal function in the long-term? A 10-year followup study. Arch Orthop Trauma Surg. 2008;128:915–919. doi: 10.1007/s00402-007-0466-9. [DOI] [PubMed] [Google Scholar]
- 20.Merritt K, Brown SA. Distribution of cobalt chromium wear and corrosion products and biologic reactions. Clin Orthop Relat Res. 1996;329(Suppl):S233–S243. doi: 10.1097/00003086-199608001-00020. [DOI] [PubMed] [Google Scholar]
- 21.Mutti A, Alinovi R, Bergamaschi E, Biagini C, Cavazzini S, Franchini I, Lauwerys RR, Bernard AM, Roels H, Gelpi E, Rosello J, Ramis I, Price RG, Taylor SA, Broe M, Nuyts GD, Stolte H, Fels LM, Herbort C. Nephropathies and exposure to perchloroethylene in dry-cleaners. Lancet. 1992;340:189–193. doi: 10.1016/0140-6736(92)90463-D. [DOI] [PubMed] [Google Scholar]
- 22.Mutti A, Alinovi R, Bergamaschi E, Franchini I. Reference values for early markers of renal damage. Sci Total Environ. 1992;120:7–15. doi: 10.1016/0048-9697(92)90212-B. [DOI] [PubMed] [Google Scholar]
- 23.Mutti A, Lucertini S, Valcavi P, Neri TM, Fornari M, Alinovi R, Franchini I. Urinary excretion of brush-border antigen revealed by monoclonal antibody: early indicator of toxic nephropathy. Lancet. 1985;2:914–917. doi: 10.1016/S0140-6736(85)90850-5. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen RS, Morch PT. Chromic acid poisoning treated with acute hemodialysis. Nephron. 1978;22:592–595. doi: 10.1159/000181540. [DOI] [PubMed] [Google Scholar]
- 25.Rasquinha VJ, Ranawat CS, Weiskopf J, Rodriguez JA, Skipor AK, Jacobs JJ. Serum metal levels and bearing surfaces in total hip arthroplasty. J Arthroplasty. 2006;21(Suppl 2):47–52. doi: 10.1016/j.arth.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Shimmin A, Beaulé PE, Campbell P. Metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg Am. 2008;90:637–654. doi: 10.2106/JBJS.G.01012. [DOI] [PubMed] [Google Scholar]
- 27.Sixth Annual Report of the National Joint Registry of England and Wales. 2009. Available at: www.njrcentre.org.uk/NjrCentre/LinkClick.aspx?fileticket=EuUKuR4jPyc%3d&tabid=86&mid=523. Accessed July 9, 2010.
- 28.Suwazono Y, Sand S, Vahter M, Filipsson AF, Skerfving S, Lidfeldt J, Akesson A. Benchmark dose for cadmium-induced renal effects in humans. Environ Health Perspect. 2006;114:1072–1076. doi: 10.1289/ehp.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toxicological Profile for Chromium. Atlanta, GA: US Department of Health and Human Services, Public Health Service. Available at: www.atsdr.cdc.gov/toxprofiles/tp7.pdf. Accessed July 9, 2010.
- 30.Varma PP, Jha V, Ghosh AK, Joshi K, Sakhuja V. Acute renal failure in a case of fatal chromic acid poisoning. Ren Fail. 1994;16:653–657. doi: 10.3109/08860229409044893. [DOI] [PubMed] [Google Scholar]
- 31.Voskaridou E, Terpos E, Michail S, Hantzi E, Anagnostopoulos A, Margeli A, Simirloglou D, Loukopoulos D, Papassotiriou I. Early markers of renal dysfunction in patients with sickle cell/beta-thalassemia. Kidney Int. 2006;69:2037–2042. doi: 10.1038/sj.ki.5000248. [DOI] [PubMed] [Google Scholar]
- 32.Wedeen RP, Qian LF. Chromium-induced kidney disease. Environ Health Perspect. 1991;92:71–74. doi: 10.2307/3431139. [DOI] [PMC free article] [PubMed] [Google Scholar]