Abstract
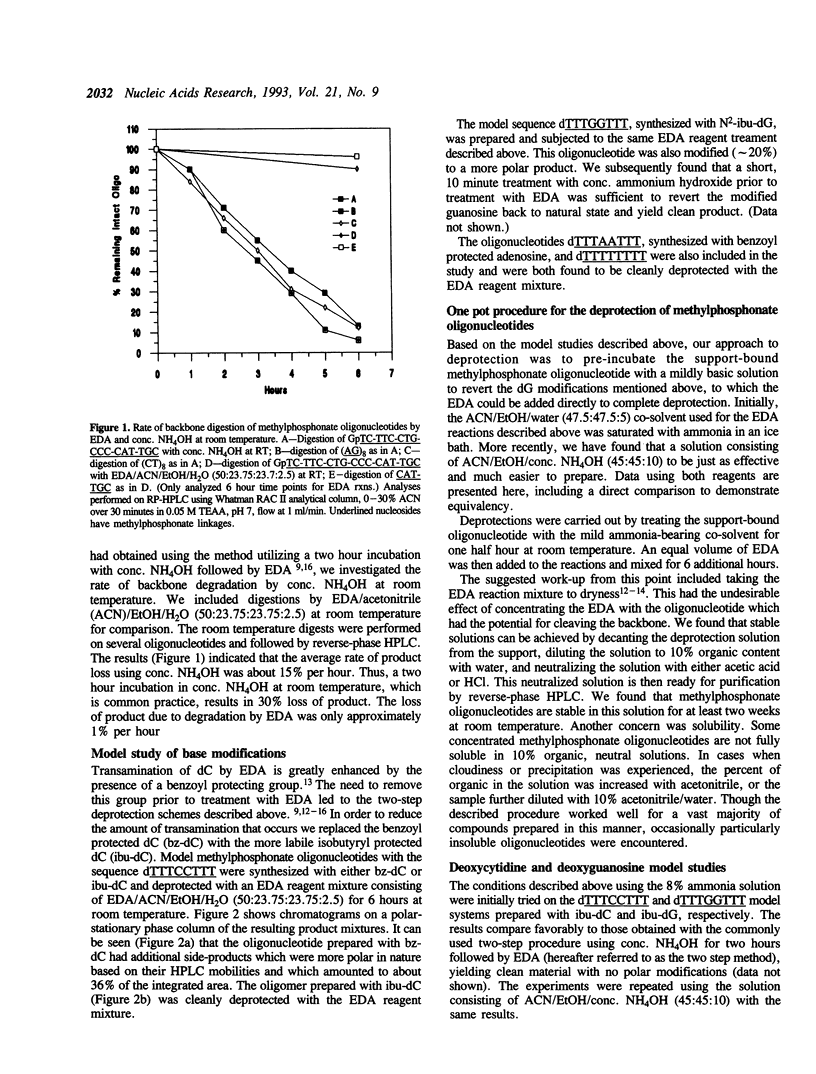
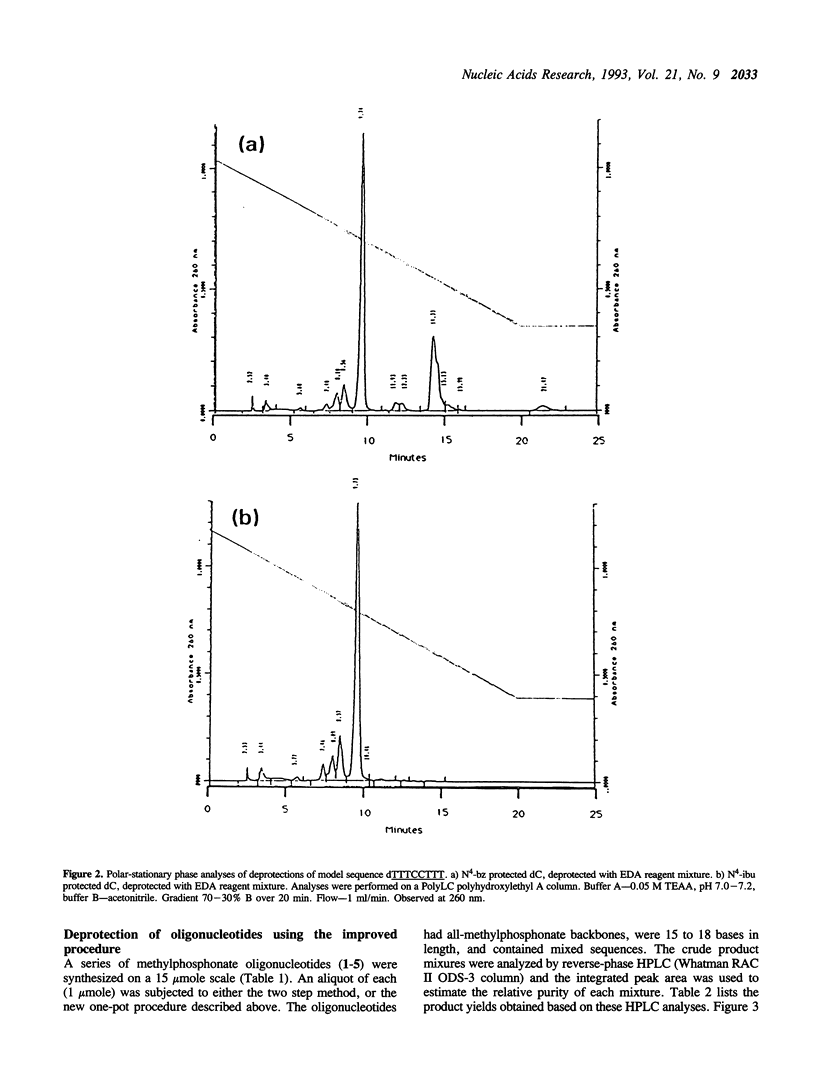
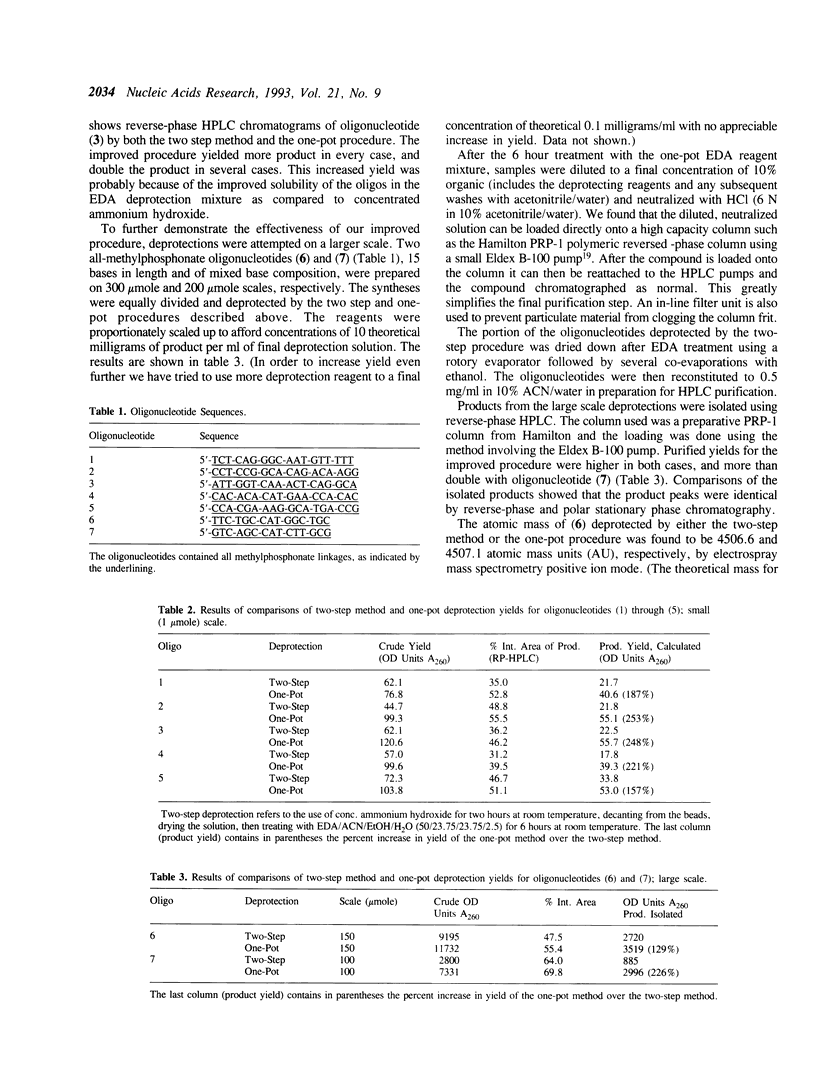
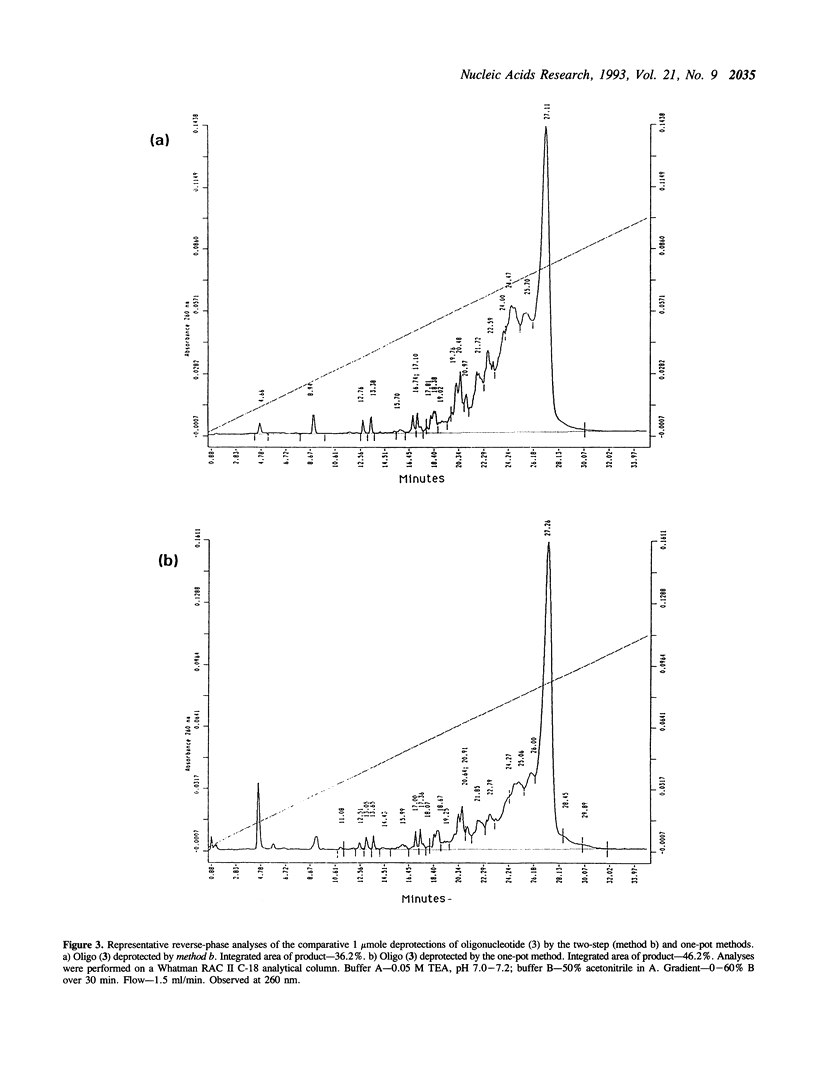
Deprotection of methylphosphonate oligonucleotides with ethylenediamine was evaluated in a model system. Methylphosphonate sequences of the form 5'-TTTNNTTT, where N was either N4-bz-dC, N4-ibu-dC, N2-ibu-O6-DPC-dG, N2-ibu-dG, N6-bz-dA, or T, were used to determine the extent of modifications that occur during deprotection. Up to 15% of N4-bz-dC was found to transaminate at the C4 position when treated with ethylenediamine. A similar displacement reaction with ethylenediamine was observed at the O6 position of N2-ibu-O6-DPC-dG, and to a much lesser extent of N2-ibu-dG. Side reactions were not observed when oligonucleotides containing N4-ibu-dC, N6-bz-dA, or T were treated with ethylenediamine. A novel method of deprotecting methylphosphonate oligonucleotides was developed from these studies. The method incorporates a brief treatment with dilute ammonia for 30 minutes followed by addition of ethylenediamine for 6 hours at room temperature to complete deprotection in a one-pot format. The solution is then diluted and neutralized to stop the reaction and prepare the crude product for chromatographic purification. This method was used to successfully deprotect a series of oligonucleotides at the 1, 100, and 150 mumole scales. These deprotection results were compared to a commonly used two-step method and found to be superior in yield of product by as much as 250%.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Riftina F. Synthesis and enzymatic properties of deoxyribooligonucleotides containing methyl and phenylphosphonate linkages. Nucleic Acids Res. 1979 Jul 11;6(9):3009–3024. doi: 10.1093/nar/6.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Miller P. S., Cushman C., Devadas K., Pirollo K. F., Ts'o P. O., Yu Z. P. Antisense inhibition of ras p21 expression that is sensitive to a point mutation. Biochemistry. 1991 Aug 27;30(34):8283–8286. doi: 10.1021/bi00098a001. [DOI] [PubMed] [Google Scholar]
- Cohen J. S. Antisense oligodeoxynucleotides as antiviral agents. Antiviral Res. 1991 Sep;16(2):121–133. doi: 10.1016/0166-3542(91)90019-n. [DOI] [PubMed] [Google Scholar]
- Dolnick B. J. Antisense agents in cancer research and therapeutics. Cancer Invest. 1991;9(2):185–194. doi: 10.3109/07357909109044229. [DOI] [PubMed] [Google Scholar]
- Kulka M., Smith C. C., Aurelian L., Fishelevich R., Meade K., Miller P., Ts'o P. O. Site specificity of the inhibitory effects of oligo(nucleoside methylphosphonate)s complementary to the acceptor splice junction of herpes simplex virus type 1 immediate early mRNA 4. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6868–6872. doi: 10.1073/pnas.86.18.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Comparative hybrid arrest by tandem antisense oligodeoxyribonucleotides or oligodeoxyribonucleoside methylphosphonates in a cell-free system. Nucleic Acids Res. 1988 Apr 25;16(8):3341–3358. doi: 10.1093/nar/16.8.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S., Agris C. H., Murakami A., Reddy P. M., Spitz S. A., Ts'o P. O. Preparation of oligodeoxyribonucleoside methylphosphonates on a polystyrene support. Nucleic Acids Res. 1983 Sep 24;11(18):6225–6242. doi: 10.1093/nar/11.18.6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. S. Oligonucleoside methylphosphonates as antisense reagents. Biotechnology (N Y) 1991 Apr;9(4):358–362. doi: 10.1038/nbt0491-358. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Reddy M. P., Murakami A., Blake K. R., Lin S. B., Agris C. H. Solid-phase syntheses of oligodeoxyribonucleoside methylphosphonates. Biochemistry. 1986 Sep 9;25(18):5092–5097. doi: 10.1021/bi00366a017. [DOI] [PubMed] [Google Scholar]
- Miller P. S., Yano J., Yano E., Carroll C., Jayaraman K., Ts'o P. O. Nonionic nucleic acid analogues. Synthesis and characterization of dideoxyribonucleoside methylphosphonates. Biochemistry. 1979 Nov 13;18(23):5134–5143. doi: 10.1021/bi00590a017. [DOI] [PubMed] [Google Scholar]
- Sarin P. S., Agrawal S., Civeira M. P., Goodchild J., Ikeuchi T., Zamecnik P. C. Inhibition of acquired immunodeficiency syndrome virus by oligodeoxynucleoside methylphosphonates. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7448–7451. doi: 10.1073/pnas.85.20.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulhof J. C., Molko D., Teoule R. The final deprotection step in oligonucleotide synthesis is reduced to a mild and rapid ammonia treatment by using labile base-protecting groups. Nucleic Acids Res. 1987 Jan 26;15(2):397–416. doi: 10.1093/nar/15.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. C., Aurelian L., Reddy M. P., Miller P. S., Ts'o P. O. Antiviral effect of an oligo(nucleoside methylphosphonate) complementary to the splice junction of herpes simplex virus type 1 immediate early pre-mRNAs 4 and 5. Proc Natl Acad Sci U S A. 1986 May;83(9):2787–2791. doi: 10.1073/pnas.83.9.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidd D. M. Synthetic oligonucleotides as therapeutic agents. Br J Cancer. 1991 Jan;63(1):6–8. doi: 10.1038/bjc.1991.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar G., Ahmed N. K. Modulation of drug resistance in a daunorubicin resistant subline with oligonucleoside methylphosphonates. Cancer Commun. 1989;1(4):225–232. [PubMed] [Google Scholar]
- Zaia J. A., Rossi J. J., Murakawa G. J., Spallone P. A., Stephens D. A., Kaplan B. E., Eritja R., Wallace R. B., Cantin E. M. Inhibition of human immunodeficiency virus by using an oligonucleoside methylphosphonate targeted to the tat-3 gene. J Virol. 1988 Oct;62(10):3914–3917. doi: 10.1128/jvi.62.10.3914-3917.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


