Abstract
The expression of the nicotinic acetylcholine receptor alpha 2 subunit gene is highly restricted to the Spiriform lateralis nucleus of the Chick diencephalon. As a first step toward understanding the molecular mechanism underlying this regulation, we have investigated the structural and regulatory properties of the 5' sequence of this gene. A strategy based on the ligation of an oligonucleotide to the first strand of the cDNA (SLIC) followed by PCR amplification was used. A new exon was found approximately 3kb upstream from the first coding exon, and multiple transcription start sites of the gene were mapped. Analysis of the flanking region shows many consensus sequences for the binding of nuclear proteins, suggesting that the 1 kb flanking region contains at least a portion of the promoter of the gene. We have analysed the negative regulatory elements present within this region and found that a silencer region located between nucleotide -144 and +76 is active in fibroblasts as well as in neurons. This silencer is composed of six tandem repeat Oct-like motifs (CCCCATGCAAT), but does not bind any member of the Oct family. Moreover these motifs were found to act as a silencer only when they were tandemly repeated. When two, four or five motifs were deleted, the silencer activity of the motifs unexpectedly became an enhancer activity in all cells we have tested.
Full text
PDF



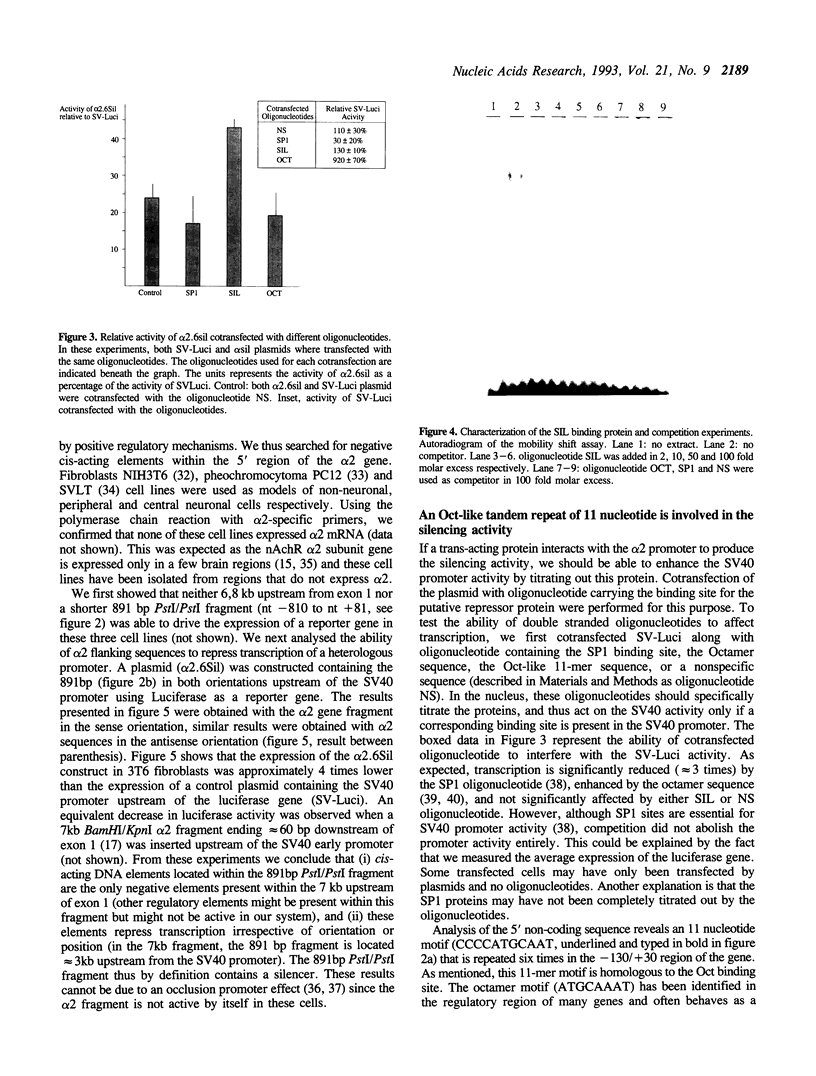
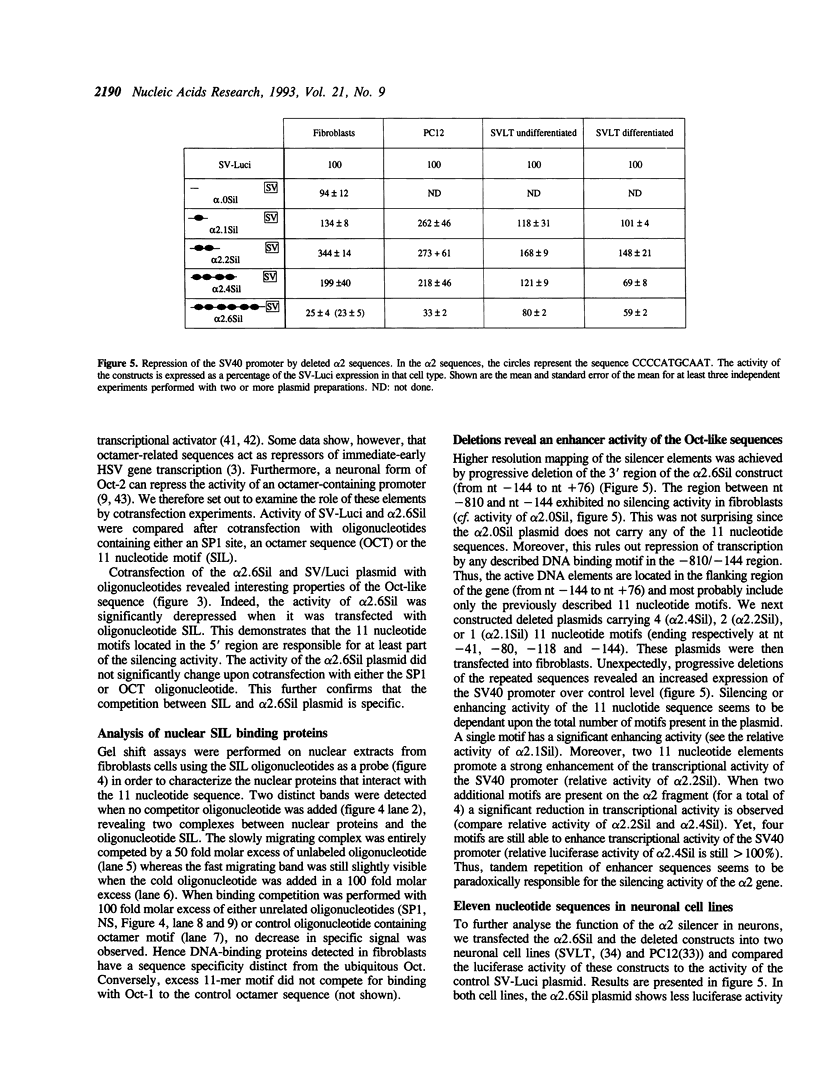


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrera-Saldana H., Takahashi K., Vigneron M., Wildeman A., Davidson I., Chambon P. All six GC-motifs of the SV40 early upstream element contribute to promoter activity in vivo and in vitro. EMBO J. 1985 Dec 30;4(13B):3839–3849. doi: 10.1002/j.1460-2075.1985.tb04156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy B., Ballivet M., Spierer P. Conservation of neural nicotinic acetylcholine receptors from Drosophila to vertebrate central nervous systems. EMBO J. 1988 Mar;7(3):611–618. doi: 10.1002/j.1460-2075.1988.tb02854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Boyce F. M., Beggs A. H., Feener C., Kunkel L. M. Dystrophin is transcribed in brain from a distant upstream promoter. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1276–1280. doi: 10.1073/pnas.88.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A., Mudd J., Merlie J. P. Isolation and characterization of the beta and epsilon subunit genes of mouse muscle acetylcholine receptor. J Biol Chem. 1989 May 5;264(13):7611–7616. [PubMed] [Google Scholar]
- Changeux J. P., Babinet C., Bessereau J. L., Bessis A., Cartaud A., Cartaud J., Daubas P., Devillers-Thiéry A., Duclert A., Hill J. A. Compartmentalization of acetylcholine receptor gene expression during development of the neuromuscular junction. Cold Spring Harb Symp Quant Biol. 1990;55:381–396. doi: 10.1101/sqb.1990.055.01.039. [DOI] [PubMed] [Google Scholar]
- Chelly J., Hamard G., Koulakoff A., Kaplan J. C., Kahn A., Berwald-Netter Y. Dystrophin gene transcribed from different promoters in neuronal and glial cells. Nature. 1990 Mar 1;344(6261):64–65. doi: 10.1038/344064a0. [DOI] [PubMed] [Google Scholar]
- Couturier S., Bertrand D., Matter J. M., Hernandez M. C., Bertrand S., Millar N., Valera S., Barkas T., Ballivet M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron. 1990 Dec;5(6):847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- Daubas P., Devillers-Thiéry A., Geoffroy B., Martinez S., Bessis A., Changeux J. P. Differential expression of the neuronal acetylcholine receptor alpha 2 subunit gene during chick brain development. Neuron. 1990 Jul;5(1):49–60. doi: 10.1016/0896-6273(90)90032-b. [DOI] [PubMed] [Google Scholar]
- Dent C. L., Lillycrop K. A., Estridge J. K., Thomas N. S., Latchman D. S. The B-cell and neuronal forms of the octamer-binding protein Oct-2 differ in DNA-binding specificity and functional activity. Mol Cell Biol. 1991 Aug;11(8):3925–3930. doi: 10.1128/mcb.11.8.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. B., Delort J., Mallet J. Oligodeoxyribonucleotide ligation to single-stranded cDNAs: a new tool for cloning 5' ends of mRNAs and for constructing cDNA libraries by in vitro amplification. Nucleic Acids Res. 1991 Oct 11;19(19):5227–5232. doi: 10.1093/nar/19.19.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard C., Borde I., Marin P., Galiana E., Prémont J., Gros F., Rouget P. Immortalization of bipotential and plastic glio-neuronal precursor cells. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3062–3066. doi: 10.1073/pnas.87.8.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forss-Petter S., Danielson P. E., Catsicas S., Battenberg E., Price J., Nerenberg M., Sutcliffe J. G. Transgenic mice expressing beta-galactosidase in mature neurons under neuron-specific enolase promoter control. Neuron. 1990 Aug;5(2):187–197. doi: 10.1016/0896-6273(90)90308-3. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Yaniv M. Nuclear oncogenes. Curr Opin Cell Biol. 1991 Jun;3(3):484–491. doi: 10.1016/0955-0674(91)90077-c. [DOI] [PubMed] [Google Scholar]
- Gruda M. C., Alwine J. C. Simian virus 40 (SV40) T-antigen transcriptional activation mediated through the Oct/SPH region of the SV40 late promoter. J Virol. 1991 Jul;65(7):3553–3558. doi: 10.1128/jvi.65.7.3553-3558.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman A., Wasylyk B. Nuclear targets for transcription regulation by oncogenes. Trends Genet. 1991 Feb;7(2):49–54. doi: 10.1016/0168-9525(91)90231-E. [DOI] [PubMed] [Google Scholar]
- He X., Rosenfeld M. G. Mechanisms of complex transcriptional regulation: implications for brain development. Neuron. 1991 Aug;7(2):183–196. doi: 10.1016/0896-6273(91)90257-z. [DOI] [PubMed] [Google Scholar]
- Hirsch M. R., Gaugler L., Deagostini-Bazin H., Bally-Cuif L., Goridis C. Identification of positive and negative regulatory elements governing cell-type-specific expression of the neural cell adhesion molecule gene. Mol Cell Biol. 1990 May;10(5):1959–1968. doi: 10.1128/mcb.10.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. F., Lüscher B., Admon A., Mermod N., Tjian R. Transcription factor AP-4 contains multiple dimerization domains that regulate dimer specificity. Genes Dev. 1990 Oct;4(10):1741–1752. doi: 10.1101/gad.4.10.1741. [DOI] [PubMed] [Google Scholar]
- Hurst H. C., Totty N. F., Jones N. C. Identification and functional characterisation of the cellular activating transcription factor 43 (ATF-43) protein. Nucleic Acids Res. 1991 Sep 11;19(17):4601–4609. doi: 10.1093/nar/19.17.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi R., Yamada T., Yoshikai S., Sasaki H., Hattori M., Sakaki Y. Positive and negative regulatory elements for the expression of the Alzheimer's disease amyloid precursor-encoding gene in mouse. Gene. 1992 Mar 15;112(2):189–195. doi: 10.1016/0378-1119(92)90375-y. [DOI] [PubMed] [Google Scholar]
- Jackson I. J. A reappraisal of non-consensus mRNA splice sites. Nucleic Acids Res. 1991 Jul 25;19(14):3795–3798. doi: 10.1093/nar/19.14.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadesch T., Berg P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single plasmid. Mol Cell Biol. 1986 Jul;6(7):2593–2601. doi: 10.1128/mcb.6.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda N., Sasaoka T., Kobayashi K., Kiuchi K., Nagatsu I., Kurosawa Y., Fujita K., Yokoyama M., Nomura T., Katsuki M. Tissue-specific and high-level expression of the human tyrosine hydroxylase gene in transgenic mice. Neuron. 1991 Apr;6(4):583–594. doi: 10.1016/0896-6273(91)90061-4. [DOI] [PubMed] [Google Scholar]
- Kemler I., Bucher E., Seipel K., Müller-Immerglück M. M., Schaffner W. Promoters with the octamer DNA motif (ATGCAAAT) can be ubiquitous or cell type-specific depending on binding affinity of the octamer site and Oct-factor concentration. Nucleic Acids Res. 1991 Jan 25;19(2):237–242. doi: 10.1093/nar/19.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp L. M., Dent C. L., Latchman D. S. Octamer motif mediates transcriptional repression of HSV immediate-early genes and octamer-containing cellular promoters in neuronal cells. Neuron. 1990 Feb;4(2):215–222. doi: 10.1016/0896-6273(90)90096-x. [DOI] [PubMed] [Google Scholar]
- Korner M., Rattner A., Mauxion F., Sen R., Citri Y. A brain-specific transcription activator. Neuron. 1989 Nov;3(5):563–572. doi: 10.1016/0896-6273(89)90266-3. [DOI] [PubMed] [Google Scholar]
- Kraner S. D., Chong J. A., Tsay H. J., Mandel G. Silencing the type II sodium channel gene: a model for neural-specific gene regulation. Neuron. 1992 Jul;9(1):37–44. doi: 10.1016/0896-6273(92)90218-3. [DOI] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Tourkine N., Jeanteur P., Blanchard J. M. Demonstration in living cells of an intragenic negative regulatory element within the rodent c-fos gene. Cell. 1990 May 4;61(3):485–496. doi: 10.1016/0092-8674(90)90530-r. [DOI] [PubMed] [Google Scholar]
- Lillycrop K. A., Dent C. L., Wheatley S. C., Beech M. N., Ninkina N. N., Wood J. N., Latchman D. S. The octamer-binding protein Oct-2 represses HSV immediate-early genes in cell lines derived from latently infectable sensory neurons. Neuron. 1991 Sep;7(3):381–390. doi: 10.1016/0896-6273(91)90290-g. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part I. Myc. Genes Dev. 1990 Dec;4(12A):2025–2035. doi: 10.1101/gad.4.12a.2025. [DOI] [PubMed] [Google Scholar]
- Matter-Sadzinski L., Hernandez M. C., Roztocil T., Ballivet M., Matter J. M. Neuronal specificity of the alpha 7 nicotinic acetylcholine receptor promoter develops during morphogenesis of the central nervous system. EMBO J. 1992 Dec;11(12):4529–4538. doi: 10.1002/j.1460-2075.1992.tb05554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maue R. A., Kraner S. D., Goodman R. H., Mandel G. Neuron-specific expression of the rat brain type II sodium channel gene is directed by upstream regulatory elements. Neuron. 1990 Feb;4(2):223–231. doi: 10.1016/0896-6273(90)90097-y. [DOI] [PubMed] [Google Scholar]
- Messing A., Behringer R. R., Hammang J. P., Palmiter R. D., Brinster R. L., Lemke G. P0 promoter directs expression of reporter and toxin genes to Schwann cells of transgenic mice. Neuron. 1992 Mar;8(3):507–520. doi: 10.1016/0896-6273(92)90279-m. [DOI] [PubMed] [Google Scholar]
- Miskimins R., Knapp L., Dewey M. J., Zhang X. Cell and tissue-specific expression of a heterologous gene under control of the myelin basic protein gene promoter in transgenic mice. Brain Res Dev Brain Res. 1992 Feb 21;65(2):217–221. doi: 10.1016/0165-3806(92)90182-v. [DOI] [PubMed] [Google Scholar]
- Mori N., Schoenherr C., Vandenbergh D. J., Anderson D. J. A common silencer element in the SCG10 and type II Na+ channel genes binds a factor present in nonneuronal cells but not in neuronal cells. Neuron. 1992 Jul;9(1):45–54. doi: 10.1016/0896-6273(92)90219-4. [DOI] [PubMed] [Google Scholar]
- Mori N., Stein R., Sigmund O., Anderson D. J. A cell type-preferred silencer element that controls the neural-specific expression of the SCG10 gene. Neuron. 1990 Apr;4(4):583–594. doi: 10.1016/0896-6273(90)90116-w. [DOI] [PubMed] [Google Scholar]
- Morris B. J., Hicks A. A., Wisden W., Darlison M. G., Hunt S. P., Barnard E. A. Distinct regional expression of nicotinic acetylcholine receptor genes in chick brain. Brain Res Mol Brain Res. 1990 May;7(4):305–315. doi: 10.1016/0169-328x(90)90081-n. [DOI] [PubMed] [Google Scholar]
- Nef P., Oneyser C., Alliod C., Couturier S., Ballivet M. Genes expressed in the brain define three distinct neuronal nicotinic acetylcholine receptors. EMBO J. 1988 Mar;7(3):595–601. doi: 10.1002/j.1460-2075.1988.tb02852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondek B., Herr W. Stable growth of simian virus 40 recombinants containing multimerized enhancers. J Virol. 1991 Mar;65(3):1596–1599. doi: 10.1128/jvi.65.3.1596-1599.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986 Aug 7;322(6079):562–565. doi: 10.1038/322562a0. [DOI] [PubMed] [Google Scholar]
- Sauerwald A., Hoesche C., Oschwald R., Kilimann M. W. The 5'-flanking region of the synapsin I gene. A G+C-rich, TATA- and CAAT-less, phylogenetically conserved sequence with cell type-specific promoter function. J Biol Chem. 1990 Sep 5;265(25):14932–14937. [PubMed] [Google Scholar]
- Savagner P., Miyashita T., Yamada Y. Two silencers regulate the tissue-specific expression of the collagen II gene. J Biol Chem. 1990 Apr 25;265(12):6669–6674. [PubMed] [Google Scholar]
- Sawruk E., Hermans-Borgmeyer I., Betz H., Gundelfinger E. D. Characterization of an invertebrate nicotinic acetylcholine receptor gene: the ard gene of Drosophila melanogaster. FEBS Lett. 1988 Aug 1;235(1-2):40–46. doi: 10.1016/0014-5793(88)81230-4. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A., Patil N., Chao M. A constitutive promoter directs expression of the nerve growth factor receptor gene. Mol Cell Biol. 1988 Aug;8(8):3160–3167. doi: 10.1128/mcb.8.8.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneidman P. S., Bruce J., Schwartz M. L., Schlaepfer W. W. Negative regulatory regions are present upstream in the three mouse neurofilament genes. Brain Res Mol Brain Res. 1992 Mar;13(1-2):127–138. doi: 10.1016/0169-328x(92)90052-d. [DOI] [PubMed] [Google Scholar]
- Struhl K. Mechanisms for diversity in gene expression patterns. Neuron. 1991 Aug;7(2):177–181. doi: 10.1016/0896-6273(91)90256-y. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Mikoshiba K. Demonstration of a transcription element in vitro between the capping site and translation initiation site of the mouse myelin basic protein gene. FEBS Lett. 1991 Mar 11;280(1):75–78. doi: 10.1016/0014-5793(91)80207-j. [DOI] [PubMed] [Google Scholar]
- Vandaele S., Nordquist D. T., Feddersen R. M., Tretjakoff I., Peterson A. C., Orr H. T. Purkinje cell protein-2 regulatory regions and transgene expression in cerebellar compartments. Genes Dev. 1991 Jul;5(7):1136–1148. doi: 10.1101/gad.5.7.1136. [DOI] [PubMed] [Google Scholar]
- Vidal M., Morris R., Grosveld F., Spanopoulou E. Tissue-specific control elements of the Thy-1 gene. EMBO J. 1990 Mar;9(3):833–840. doi: 10.1002/j.1460-2075.1990.tb08180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E., Wada K., Boulter J., Deneris E., Heinemann S., Patrick J., Swanson L. W. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jun 8;284(2):314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wada K., Ballivet M., Boulter J., Connolly J., Wada E., Deneris E. S., Swanson L. W., Heinemann S., Patrick J. Functional expression of a new pharmacological subtype of brain nicotinic acetylcholine receptor. Science. 1988 Apr 15;240(4850):330–334. doi: 10.1126/science.2832952. [DOI] [PubMed] [Google Scholar]
- Wirak D. O., Bayney R., Kundel C. A., Lee A., Scangos G. A., Trapp B. D., Unterbeck A. J. Regulatory region of human amyloid precursor protein (APP) gene promotes neuron-specific gene expression in the CNS of transgenic mice. EMBO J. 1991 Feb;10(2):289–296. doi: 10.1002/j.1460-2075.1991.tb07949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuenschell C. W., Mori N., Anderson D. J. Analysis of SCG10 gene expression in transgenic mice reveals that neural specificity is achieved through selective derepression. Neuron. 1990 Apr;4(4):595–602. doi: 10.1016/0896-6273(90)90117-x. [DOI] [PubMed] [Google Scholar]
- Yoon S. O., Chikaraishi D. M. Tissue-specific transcription of the rat tyrosine hydroxylase gene requires synergy between an AP-1 motif and an overlapping E box-containing dyad. Neuron. 1992 Jul;9(1):55–67. doi: 10.1016/0896-6273(92)90220-8. [DOI] [PubMed] [Google Scholar]
- Zopf D., Dineva B., Betz H., Gundelfinger E. D. Isolation of the chicken middle-molecular weight neurofilament (NF-M) gene and characterization of its promoter. Nucleic Acids Res. 1990 Feb 11;18(3):521–529. doi: 10.1093/nar/18.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]




