Abstract
The analysis of oral pathologies is routinely a part of bioarchaeological and paleopathological investigations. Oral health, while certainly interesting by itself, is also potentially informative about general or systemic health. Numerous studies within modern populations have shown associations between oral pathologies and other diseases, such as cardiovascular disease, certain types of cancer, and pulmonary infections. This paper addresses the question of how oral health was associated with general health in past populations by examining the relationship between two oral pathologies (periodontal disease and dental caries) and the risk of mortality in a cemetery sample from medieval England. The effects of periodontitis and dental caries on risk of death were assessed using a sample of 190 individuals from the St. Mary Graces, London cemetery dating to approximately A.D. 1350–1538. The results suggest that the oral pathologies are associated with elevated risks of mortality in the St. Mary Graces cemetery, such that individuals with periodontitis and dental caries were more likely to die than their peers without such pathologies. The results shown here suggest that these oral pathologies can be used as informative indicators of general health in past populations.
Keywords: frailty, selective mortality, oral health, paleodemography
INTRODUCTION
Various oral and dental pathologies are frequently analyzed as part of bioarchaeological and paleopathological investigations of the skeletal remains of past populations. These pathologies include dental caries (i.e. cavities), enamel hypoplasia, periodontal disease, and antemortem tooth loss. Many biological anthropologists are interested in dental and oral health (from here subsumed under the phrase “oral health”) per se and how they might reflect more general levels of health. Of particular interest to anthropologists are the ways in which oral health varies within a population according to sex, age, and social status, how diet affects oral health, and how oral health has varied over time in human populations (Moore and Corbett, 1971; Moore and Corbett, 1973; Corbett and Moore, 1976; Cohen and Armelagos, 1984; Goodman et al., 1984; Goodman et al., 1987; Hodges, 1987; Kerr, 1991; Sledzik and Moore-Jansen, 1991; Beckett and Lovell, 1994; Danforth et al., 1994; Sutter, 1995; Lukacs, 1996; Sakashita et al., 1997; Danforth, 1999; Scaronlaus, 2000; Robb et al., 2001; Steckel et al., 2002; Schollmeyer and Ii, 2004; Wols and Baker, 2004; Eshed et al., 2006; Lukacs and Largaespada, 2006; Oxenham and Tayles, 2006; Oztunc et al., 2006; Phillips, 2006; Boldsen, 2007; Lieverse et al., 2007; Paine et al., 2007; e.g. Starling and Stock, 2007; Temple and Larsen, 2007; Keenleyside, 2008; Rose and Vieira, 2008; Watson, 2008).
The advantage of oral health indicators for bioarchaeological studies is that, given the highly mineralized nature of teeth, they provide a relatively durable record of the patterns of health within past populations (Roberts and Cox, 2003). Many anthropological studies examine oral pathologies as a proxy measure for general health. Several studies have demonstrated that enamel hypoplasias, enamel defects that reflect interruptions in enamel formation as a result of physiological stress, are reflective of general levels of health (e.g. Goodman and Rose, 1990; Boldsen, 2007), and many studies use enamel hypoplasia as a non-specific indicator of stress or health in skeletal samples. But are other oral pathologies, which are also routinely examined in anthropological studies, also informative about more than just oral health? Are such oral pathologies, in fact, useful markers for patterns of systemic health? The focus of this paper is the relationship between two types of oral pathology, periodontal disease and dental caries, and general health in past human populations. The analyses presented here address the question of whether certain oral pathologies are indicative of frailty (Vaupel et al., 1979), an individual’s age standardized relative risk of death, in past populations. This paper focuses on periodontal disease and dental caries in particular because they are the most common oral pathologies in modern populations (Rose and Vieira, 2008), and there is therefore a huge body of literature about their associations with general health (see examples below).
Periodontal Disease
Periodontal disease (periodontitis) is characterized by a bacterial infection that causes inflammation and destruction of gum tissue (gingiva), the periodontal ligament, root cementum, and alveolar bone. Periodontitis can be caused by a variety of pathogenic infectious agents that are found in oral biofilms (dental plaque), complex ecosystems including the oral microorganisms themselves and their by-products surrounded by an extracellular matrix; these infectious agents include, among others, the bacteria Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Treponema denticola and various herpes viruses (van Winkelhoff and Slots, 1999; Li et al., 2000; Slots, 2004). Many of the pathogens that cause periodontal disease are found in the mouths of healthy people, albeit at lower levels than are found in individuals with periodontitis (Meyer et al., 2008). Periodontal disease is very common in modern populations; for example, mild forms of periodontal disease may affect up to 90 percent of individuals worldwide, and moderate periodontitis affects approximately 30 percent of adults in the United States (Slots, 2004; Pihlstrom et al., 2005). Many studies have established relationships between periodontal disease and risks of morbidity and mortality from other diseases in modern human populations. One study has found that among adults in the United States, periodontitis is associated with a 46 percent increased risk of all-cause mortality (DeStefano et al., 1993), and hundreds of studies have shown that periodontitis is a risk factor for such diseases as cardiovascular diseases, respiratory infections, certain types of cancer, Alzheimer’s disease, diabetes mellitus, renal disease, necrotizing fasciitis, osteoporosis, and rheumatoid arthritis (for example, see the following reviews: Hollister and Weintraub, 1993; Li et al., 2000; Kowolik et al., 2001; Slots, 2004; Pihlstrom et al., 2005; Johnson et al., 2006; Irwin et al., 2008; Williams et al., 2008).
Perhaps the best studied association between oral health and systemic disease is that existing between periodontal disease and cardiovascular diseases, including atherosclerosis and associated complications such as myocardial infarction and ischemic stroke (e.g. Joshipura et al., 1996; Dorn et al., 2000; Hujoel et al., 2000; Desvarieux et al., 2003; Joshipura et al., 2003; Grau et al., 2004; Pussinen et al., 2004a; Beck and Offenbacher, 2005; Mattila et al., 2005; Demmer and Desvarieux, 2006; Spahr et al., 2006). Cardiovascular diseases are the most common causes of death in industrialized nations, and given that both periodontal disease and cardiovascular diseases are very common throughout the world, understanding the association between the two is important from a public health perspective (Hujoel et al., 2000; Desvarieux et al., 2004). Studies of the association between periodontitis and systemic disease have attempted to determine the exact mechanism by which oral health is related to general health, i.e. whether periodontitis in some direct or indirect way causes or is caused by diseases in other areas of the body, or whether all of these diseases are caused by certain common underlying risk factors or are associated with immune response and are thus coincidentally associated. Several studies have found significant associations between periodontitis and cardiovascular disease, even after controlling for established cardiovascular risk factors that are potential confounders (e.g. age, sex, smoking, body mass, diet, and alcohol consumption) (Spahr et al., 2006). This suggests, to some, that periodontitis and cardiovascular disease are causally related (Beck and Offenbacher, 2005).
Periodontitis can cause chronic systemic infection or inflammation, and this might influence cardiovascular disease. The pathogens that cause periodontitis, or components of those pathogens such as endotoxins and outer membrane proteins, can enter the bloodstream through pockets of ulcerated gingival tissue that form as the disease progresses (Loos, 2005; Amabile et al., 2008). The body responds to the presence of such bacteria and bacterial antigens in the oral cavity and in the bloodstream through the production of proinflammatory immune cytokines, which can have systemic or distant effects (Loos, 2005; Spahr et al., 2006). Inflammatory cytokines can increase platelet aggregation in blood vessels, leading to or accelerating the progression of atherogenesis (the formation of plaques within arteries) (Amabile et al., 2008; Kamer et al., 2008; Watts et al., 2008). The oral pathogens themselves, once disseminated from the oral cavity, may also mediate coagulation directly or invade arterial walls and initiate or exacerbate the inflammatory response of atherosclerosis (Dorn et al., 2000; Demmer and Desvarieux, 2006). Chronic periodontitis may also influence lipoprotein levels and thereby favor atherogenesis (Katz et al., 2002; Pussinen et al., 2004b; Mattila et al., 2005). Periodontal pathogens have been found in coronary artery atherosclerotic plaques, which suggests that these pathogens played a direct role in the formation of such plaques (Pucar et al., 2007).
Periodontal disease appears to be a risk factor for pulmonary disease, including pneumonia and chronic obstructive pulmonary disease (COPD, which encompasses chronic bronchitis and emphysema) (Hayes et al., 1998; Terpenning, 2001; Terpenning et al., 2001; Scannapieco et al., 2003; Scannapieco and Rethman, 2003). Awano et al. (2008) found that in elderly individuals, periodontitis significantly increases the risk of mortality from bacterial pneumonia; improved oral care, including the use of antiseptic rinses and mechanical cleaning, both of which lower levels of bacteria in the oral cavity, has been shown in some studies to reduce the incidence of and mortality from pneumonia among patients in long-term care facilities (Yoneyama et al., 2002; Paju and Scannapieco, 2007; Pan et al., 2009). Dental plaque provides a reservoir of both oral and respiratory bacteria which, in addition to causing periodontitis, can be shed into the saliva and then aspirated into the lungs, subsequently causing pulmonary infection. Pulmonary infection might also arise by spread of bacteria from sites of periodontitis in the oral cavity to the lungs via the bloodstream (i.e. bacteremia) (Paju and Scannapieco, 2007). If aspirated into the lungs, periodontal pathogens may facilitate the invasion of airway epithelia cells by respiratory pathogens, thereby assisting in an essential step in the infection process. As described above for cardiovascular disease, periodontal pathogens may also stimulate an inflammatory response which facilitates the onset or progression of respiratory disease (Pan et al., 2009).
Periodontal disease is also associated with increased risks of certain types of cancer, including oral, esophageal, upper gastrointestinal, gastric, pancreatic, lung, and kidney cancers (Zheng et al., 1990; Stolzenberg-Solomon et al., 2003; Abnet et al., 2005; Meyer et al., 2008). For example, Michaud et al. (2008) found that periodontal disease is significantly associated with increased risks of lung, kidney, pancreatic, and haematological cancers (after controlling for smoking, diet, body mass, and other potential confounders). The observed association between periodontitis and cancers might reflect an impaired immune system that is unable to clear an infection and also insufficiently monitors and controls tumor growth (Michaud et al., 2008). Alternatively, the inflammatory markers produced as a result of chronic periodontitis might affect cell growth control and lead to carcinogenesis (Meyer et al., 2008).
Several studies have also demonstrated associations between periodontitis and the following systemic diseases: Alzheimer’s disease, obesity, diabetes, and kidney disease (Saremi et al., 2005; Khader et al., 2006; Shultis et al., 2007; Taylor and Borgnakke, 2008; Khader et al., 2009; Kshirsagar et al., 2009). As is true with respect to the diseases described in detail above, there is an ongoing effort to more clearly understand the mechanism by which periodontitis is linked to these systemic diseases. Of particular concern to many researchers is demonstrating how treatment of periodontitis might lower the risks of morbidity and mortality associated with these other diseases (Kamer et al., 2008; Watts et al., 2008).
In addition to the above diseases, periodontitis has also been linked to adverse pregnancy outcomes, including preeclampsia, spontaneous preterm birth, low birth weight, and stillbirth (Holmstrup et al., 2003; Goepfert et al., 2004; Boggess, 2005; Boggess et al., 2006; Mobeen et al., 2008; Ruma et al., 2008; Polyzos et al., 2009). According to some researchers, the association between periodontitis and adverse pregnancy outcomes is likely the result of a maternal systemic inflammatory response initiated by chronic periodontitis; this inflammatory response results in abnormal placental or fetal growth (Boggess et al., 2006; Ruma et al., 2008).
Dental Caries
Dental caries is characterized by the localized demineralization of the hard tissues of the teeth (enamel, dentine, and cementum) by weak organic acids that are the byproducts resulting from bacterial fermentation of dietary carbohydrates (Larsen, 1997; Selwitz et al., 2007). The oral bacteria ultimately responsible for caries formation (primarily Streptococcus mutans, Streptococcus sobrinus, and Lactobacillus spp) reside in the oral biofilm, and carious lesions form when the biofilm remains on the surface of a tooth for an extended period of time (Selwitz et al., 2007). Dental caries can affect both the crowns (coronal caries) and roots (root caries) of teeth. Dental caries begins as subsurface demineralization of the hard dental tissues beneath the biofilm, which, if unchecked, can cause cavity formation (cavitation) (Selwitz et al., 2007). Dental caries is one of the most common chronic diseases in modern populations (Selwitz et al., 2007), and according to Misra et al. (2007), it is the most common chronic disease in children worldwide.
As is true of periodontitis, several studies have found an association between dental caries and general health status (e.g. Acs et al., 1999; Joshipura et al., 2006; Ylostalo et al., 2006; Misra et al., 2007). Infection can spread from dental caries to other tissues in the mouth and elsewhere in the body, and these infections can potentially be life-threatening (Ngoenwiwatkul and Leela-Adisorn, 2009). As with periodontitis, caries can lead to a systemic inflammatory response with adverse health outcomes. For example, coronal caries can lead to inflammation of the dental pulp which, in turn, can lead to a systemic inflammatory response which may increase the risk of coronary heart disease (Joshipura et al., 2006). The development of caries might be the result of the body’s inability to control the proliferation of cariogenic oral bacteria; as with periodontitis, the link between caries and systemic disease might reflect general inadequacies of the body’s immune response (Acs et al., 1999). Some studies have shown an association between nutritional status and early childhood caries, such that underweight or obese children are more likely to have severe dental caries than children of normal weight (Miller et al., 1982; Ayhan et al., 1996; Acs et al., 1999; Thomas and Primosch, 2002; Willershausen et al., 2004; Sheiham, 2006; Marshall et al., 2007; Willershausen et al., 2007; Alm, 2008; Gerdin et al., 2008; Oliveira et al., 2008; Ngoenwiwatkul and Leela-Adisorn, 2009). Acs et al. (1999) found that following comprehensive dental treatment, children with early childhood caries experienced catch-up growth, which suggests that caries negatively affects growth. Dental caries can adversely affect growth and development by interfering with normal food consumption; dental caries can cause toothaches which negatively affect food intake and thereby contribute to low weight gain in children (Acs et al., 1999). Furthermore, the pain associated with dental caries can disrupt sleep, which can affect the production of glucosteroids and therefore adversely affect growth (Sheiham, 2006). The link between dental caries and obesity might reflect a form of malnutrition whereby individuals consume an abundance of foods that are cariogenic and full of “empty calories” but do not provide sufficient nutrients (Miller et al., 1982). Given that obesity is a risk factor for several diseases, the link observed in some studies between dental caries and obesity might also mean that caries is linked to the negative outcomes associated with obesity.
Periodontitis and Dental Caries in Past Population
The observed association between oral pathology and systemic health in modern populations raises the following question: how was oral health associated with general health in past populations? Were periodontitis and dental caries associated with other diseases and elevated risks of mortality in past populations, as they are in populations today? To address this question, this paper examines the relationships between periodontitis and dental caries and the risk of mortality in a cemetery sample from medieval England. The goal is to address the question of whether these oral pathologies are indicative of frailty (an individual’s age-adjusted relative risk of death) in past populations and can thus be used informatively in investigations of general heath in the past (Vaupel et al., 1979).
Numerous studies have examined patterns of periodontitis and dental caries in historic populations, and there are abundant data regarding the frequencies of these oral pathologies in various samples. Moore and Corbett examined patterns of dental caries in Britain from the Iron Age through the 19th century (1971; 1973; 1975; 1976) and found that for much of the 2000-year period between the Iron Age and the end of the Medieval period, overall caries prevalence was low (less than 20 percent of teeth, on average, were carious). Studies of various medieval populations have found frequencies of individuals with caries that range from 5 to nearly 50 percent (Kerr et al., 1988; Kerr, 1990; Šlaus, 2002; Vodanovic et al., 2005; Caglar et al., 2007) and percentages of teeth that are carious that range from less than 5 to nearly 25 percent (Lunt, 1974; Kerr et al., 1988; Kerr, 1990; O'Sullivan et al., 1993; Watt et al., 1997; Esclassan et al., 2009). Investigations of dental caries in more recent populations, such as 18th century England (O'Sullivan et al., 1993; Whittaker and Molleson, 1996), late 19th/early 20th century Portugal (Wasterlain et al., 2009), 19th century Canada (Saunders et al., 1997), and 19th and early 20th century U.S. (Sledzik and Moore-Jansen, 1991; Sutter, 1995; Winchell et al., 1995; Davidson et al., 2002; Higgins et al., 2002; Wols and Baker, 2004; Phillips, 2006) found frequencies of individuals with caries ranging from 27 to nearly 90 percent and frequencies of carious teeth ranging from 11 to 70 percent. Investigations of periodontitis in samples from 4th century BC Turkey (Oztunc et al., 2006), medieval France and Scotland (Kerr, 1991; Chazel et al., 2005), 18th century England (Kerr, 1994), and the late 19th century U.S. (Rose and Vieira, 2008) have found frequencies of periodontitis ranging from 18 to 100 percent. The above summary is not exhaustive, but even this sample of studies demonstrates the variation that has been observed with respect to dental caries and periodontitis. The possible reasons for such variation are described in the Discussion below.
These previous studies provide invaluable data regarding the patterns of oral health in past populations and the relationships between oral health and diet, oral hygiene, dental care, and other factors. But they do not explicitly address the relationship between oral pathologies and risk of death. By incorporating a model of health and mortality, this study goes beyond looking at frequencies of certain dental pathology and determines how those pathologies actually affect risks of mortality. This study examines a single population to determine the effect of these oral pathologies on risk of death, thereby providing a clearer picture of the association between oral health and general health in the past than is possible when comparing different populations.
MATERIALS AND METHODS
Skeletal sample
St. Mary Graces Cemetery
The St. Mary Graces cemetery in London was associated with the Cistercian Abbey of St. Mary Graces, which was established shortly after the Black Death ended in London in 1350 (Grainger and Hawkins, 1988). The Abbey of St. Mary Graces was established on land north-east of the Tower of London (Grainger et al., 2008). The Abbey of St. Mary Graces was in use until the Reformation in 1538. Monks and important lay people were buried within the Abbey’s church and chapels, but the cemetery itself was used for the general population (Grainger and Hawkins, 1988; Rogers and Waldron, 2001). The St. Mary Graces cemetery provides a good sample of the late Medieval population of London, as it contains individuals of all ages, both sexes, and both higher and lower socioeconomic statuses. St. Mary Graces was excavated by the Museum of London Department of Greater London Archaeology (now the Museum of London Archaeology Service) in the 1980s as part of the Royal Mint site which also revealed the East Smithfield Black Death cemetery (c. 1348 – 1350) and a Royal naval victualling yard (c. 1560 – 1785) (Hawkins, 1990). Excavation of St. Mary Graces revealed 133 skeletons from within the Abbey church and chapels and 310 within the larger lay cemetery (Grainger and Hawkins, 1988).
A sample of 190 individuals from St. Mary Graces was used in these analyses. This sample represents all the excavated individuals in the cemetery who were preserved well enough to be scored for skeletal indicators of age and sex (in adults) and the presence of periodontitis and dental caries.
Age and Sex Estimation
Age estimation
Sub-adult ages (i.e. ages less than 18 years) were estimated using the diaphyseal lengths of major long bones (only for fetal and neonatal remains between ages 10–50 gestational weeks), epiphyseal fusion, and dental development and eruption (Moorrees et al., 1969; Gustafson and Koch, 1974; Scheuer et al., 1980; Smith, 1991; Scheuer and Black, 2000). Adult ages (i.e. ages 18 years or older) were estimated based on tooth wear (Brothwell, 1981), and age-related changes of the pubic symphysis (Brooks and Suchey, 1990), iliac auricular surface (Lovejoy et al., 1985; Buckberry and Chamberlain, 2002), and sternal rib ends (Iscan et al., 1984; Iscan et al., 1985).
Sex Estimation
Sex was estimated using standard methods based on sexually dimorphic features of the skull and pelvis (Phenice, 1969; Brothwell, 1981; Bass, 1987; Buikstra and Ubelaker, 1994).
Oral Pathology
This study examines the relationships between risk of death and each of the following oral pathologies: periodontitis (periodontal disease) and dental caries. Periodontitis is caused by a chronic oral bacterial infection resulting in gingival inflammation and the gradual destruction of periodontal tissues and alveolar bone (Irfan et al., 2001). In skeletal material, periodontitis can be identified by the loss of alveolar bone which exposes the underlying trabecular bone and thereby produces porosity (Clarke and Hirsch, 1991; Larsen, 1997) or causes the alveolar crest (AC) to recede relative to the cementoenamel junction (CEJ) of the associated dentition (Larsen, 1997). For the current study, periodontitis was scored as present if the alveolar bone displayed porosity or if the distance between the CEJ and AC was greater than 2 mm (Figure 1). The alveolar bone surrounding each tooth was scored individually. However, the analyses for the current study incorporated pooled mandibular scores and pooled maxillary scores to assess periodontitis at a broader scale. The use of the distance between the CEJ and the AC to indicate periodontitis is potentially problematic, given that non-pathological processes can increase the distance beyond 2 mm (Costa, 1982; Clarke et al., 1986; Clarke, 1990; Clarke and Hirsch, 1991; Hildebolt and Molnar, 1991; Varrela et al., 1995; Hillson, 1996). For example, normal dental attrition that occurs with age can be compensated for by the continuing eruption of teeth throughout life to maintain lower facial height and the occlusal level of teeth (Clarke and Hirsch, 1991; Hildebolt and Molnar, 1991). Without simultaneous growth of the alveolar crest, this supereruption of teeth results in an increase in the CEJ-AC distance (Whittaker et al., 1990; Clarke and Hirsch, 1991; Hildebolt and Molnar, 1991; Varrela et al., 1995). Similarly, continued growth of the facial skeleton in adulthood also results in continued eruption of the teeth to maintain dental articulation (Clarke and Hirsch, 1991; Hildebolt and Molnar, 1991). In both cases, the increased distance between the CEJ and AC occurs without any pathological destruction of the alveolar bone. Use of the “greater than 2mm” criterion to diagnose periodontitis can lead to the overestimation of the prevalence of periodontitis in past populations, as individuals with a CEJ-AC distance greater than 2mm caused by non-pathological processes would be incorrectly diagnosed with periodontitis. This is potentially a problem for the current study because the inclusion of such false positives for periodontitis in the sample would result in an underestimation of the risk of death associated with periodontitis. The possible effect of this potential inclusion of false positives on the results of this study is described below in the Discussion section.
Figure 1.
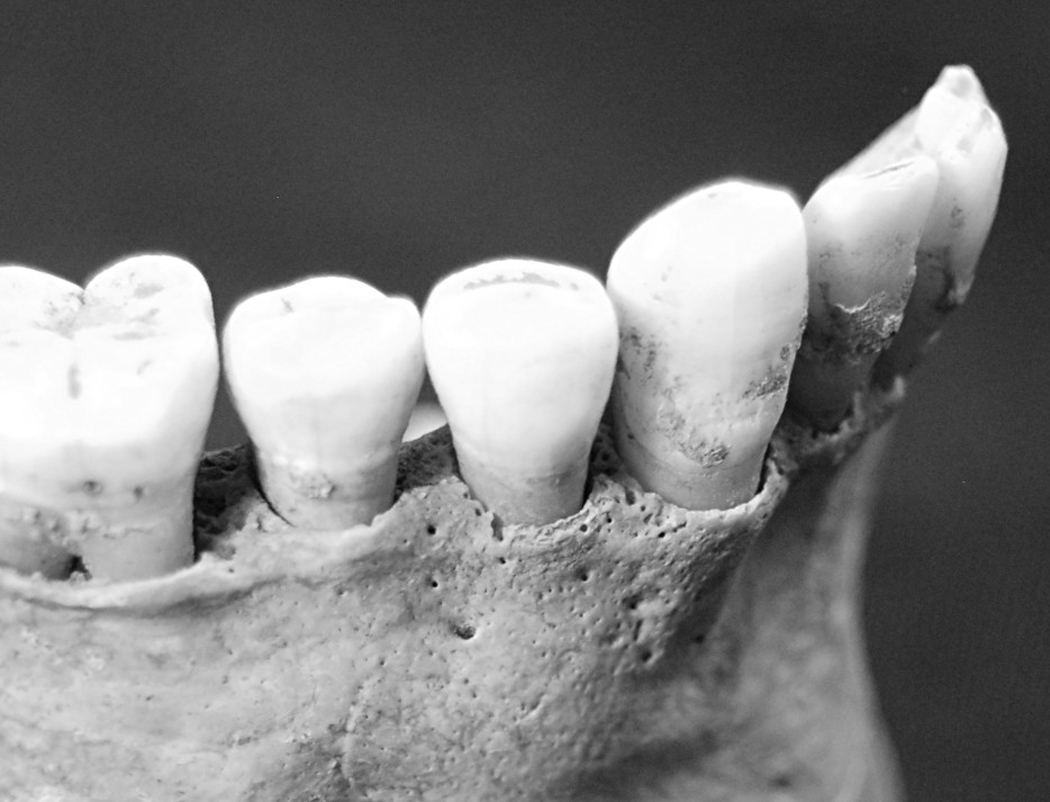
Example of periodontitis on the right mandible. Destruction of cortical bone has exposed the underlying trabecular bone along the alveolar margin, producing a porous appearance. © Museum of London.
Dental caries is characterized by the localized demineralization of the hard tissues of the teeth and can be initially indentified as white spots on the surface of the tooth and later as destruction of the enamel or dentine. For this study, caries presence was assessed for the premolars and molars; carious lesions were scored as present if destruction of enamel or dentine was visible to the naked eye (Figure 2 shows a rather extreme example). For this sample, the severity of caries ranged from small areas involving only the destruction of enamel, destruction of dentine with or without exposure of the underlying pulp chamber, to gross destruction (the crown mostly destroyed); though data on level of severity are available for this sample, the analyses described here incorporated only presence/absence data.
Figure 2.
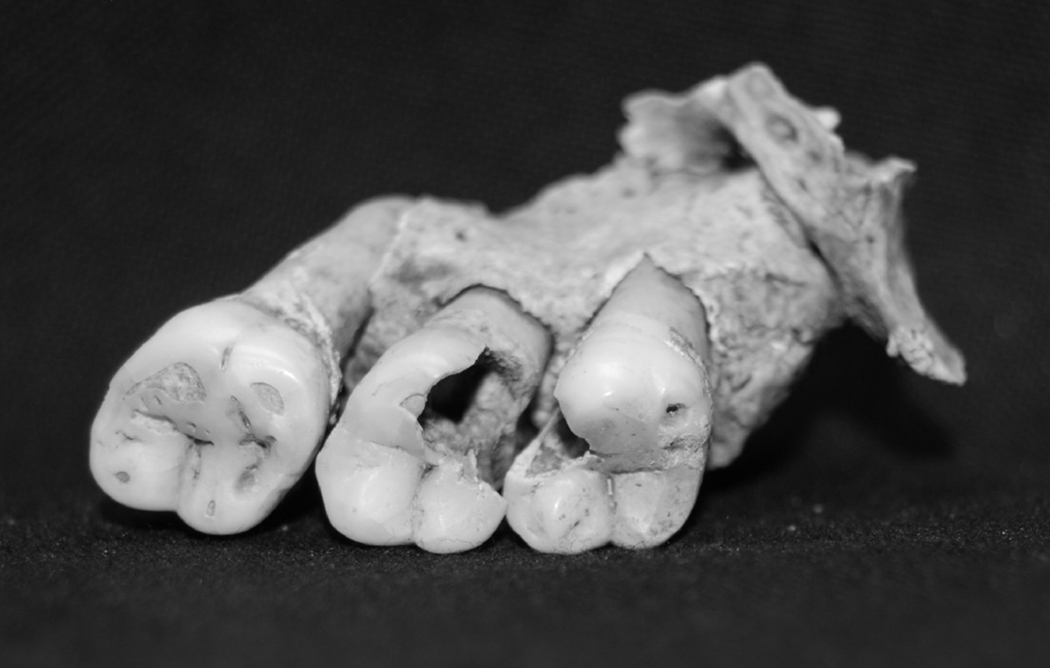
Example of dental caries on the left maxillary second and third molars. © Museum of London.
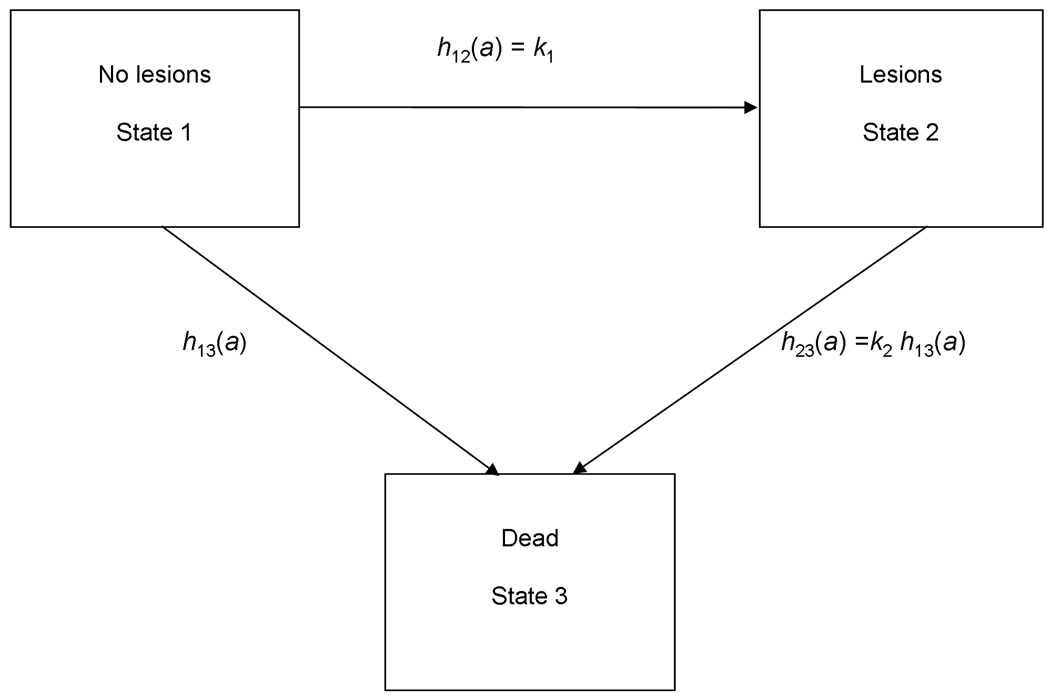
Model
The risk of death associated with oral pathology within St. Mary Graces was analyzed using a multistate model of morbidity and mortality that was developed for paleodemography (Usher, 2000). In the three state-state version of Usher’s more general model, individuals can be in one of three non-overlapping states: State 1 includes those individuals without detectable skeletal or oral lesions, State 2 includes those with detectable lesions, and State 3 is death (Figure 3). In the Usher model, individuals can only be in one state at a time, and all individuals in a sample are in one of three states at a given time. Transitions between stages occur at age-specific hazards rates, and the hazards for dying from State 1 and State 2 can differ. By allowing variation in the transition rates between each of the two living states and death, the model can be used to estimate the differential risk of death associated with the living states. The model therefore allows one to investigate selective mortality with respect to skeletal lesions – that is whether individuals with lesions are at higher risks of dying than their peers without lesions. If the risk of mortality is higher for any one state, the model can help resolve one aspect of the “osteological paradox” (Wood et al., 1992), i.e. whether an individual with a certain lesion is more or less healthy than an individual without that lesion. Some researchers (e.g. Ortner, 1991; Wood et al., 1992) have suggested that skeletal lesions might sometimes actually indicate a relatively healthy individual. This is based on the fact that visible skeletal lesions do not form immediately in response to trauma or disease, but rather take some time to form to a point at which they are detectable. Individuals with skeletal lesions might therefore have been healthier than their peers without lesions, given that they were able to survive malnutrition, trauma, or disease long enough for the skeletal lesions to form. Absence of a certain skeletal lesion might, in this scenario, indicate relatively poor health, as individuals without lesions were in such poor health that they succumbed to illness, trauma, or malnutrition and died before lesions ever formed. For the current study, no assumption is made about whether skeletal lesions indicate good or bad health. Rather, the difference in risk of death between individuals with and without lesions was estimated by using the Usher model to evaluate what the lesions used in this study indicate about health in the St. Mary Graces population.
Figure 3.
Three-state model of morbidity and mortality. Individuals are born into State 1. The transitions between states occur at age-specific hazard rates h(a), where a is age in years. The transition between States 1 and 3 follows the baseline Siler hazard function h13(α)=α1 exp (−β1α)+α2+α3 exp (β3α), and the relative risk of death (frailty) associated with a given lesion type, k2, acts to modify this baseline rate. The hazard of developing a detectable bony lesion, h12(a), is set equal to the constant.k1 (Redrawn from Usher 2000.)
As is true for any study using skeletal samples, all individuals in this study are observed in State 3, and data on age at death and presence of lesions allows for the estimation of all the parameters in the Usher model.
The baseline risk of death from State 1 is specified as a Siler mortality function h13 (a) = α1e−β1a + α2 + α3eβ3a, a parsimonious parametric model of mortality that fits a wide variety of human mortality patterns (Gage, 1991; Wood et al., 2002). The Siler model has three components. The first component, α1e−β1a, describes the juvenile risk that declines exponentially with age, where α1 is the risk of death at birth caused by immaturity and β1 is the rate at which this risk decreases with age a. The second component of the Siler model,α2, is the constant age-independent or “baseline” risk that everyone within the population faces. The third component of the model, α3eβ3a, is the exponentially increasing senescent risk, where α3 is the risk of death associated with senescence at the moment of birth and β3 is the rate at which this risk increases with age (Gage, 1988). The three components of the Siler model are independent, so surviving one component of mortality has no effect on risk of death from another component of mortality (e.g. surviving the juvenile component neither increases nor decreases an individual’s risk of either the age-independent or senescent components of mortality) (Wood et al., 2002). The Siler model represents an advantage over typical life-table approaches to investigating mortality patterns because it requires the estimation of only five parameters (Gage, 1990).
In the Usher multistate model, the hazard of developing lesions, h12(a), is estimated as a constant k1, as the age of onset of conditions resulting in lesions is generally unknown in paleopathological studies. That is, for most skeletal lesions, one does not know the age at which an individual became ill or suffered some other physiological stress, nor does one know the timing of the development of the lesion. For simplicity, in this study, the age of onset of lesions is an exponential random variable. The hazard of dying from State 2, h23(a), is modeled as proportional to the baseline age-specific risk of dying from State 1. Under this specification, k2 is a proportional term on the Siler function and is thus independent of age; it indicates the proportional difference in risk of death between individuals with and without lesions. When k2 is significantly larger than one, individuals with lesions faced an elevated risk of dying compared to similarly aged individuals without lesions. Because the model compares the risk of death between similarly aged individuals with and without lesions, it avoids overestimating the risk of death associated with age-progressive pathologies that are more likely to be found in older individuals. When k2 is significantly lower than one, individuals with lesions faced a decreased risk of death compared to their age-peers without lesions. If k2 is equal to one, individuals with and without lesions were at the same risk of death. Because of the error associated with age estimation (which was not taken into account when estimating the Usher model in this study), the k2 parameter should be viewed as a general, qualitative measure of excess mortality associated with lesions. That is, the k2 parameter estimates associated with various lesions are more important in terms of the patterns they reveal than their actual numerical values.
Model parameters were estimated using maximum likelihood analysis with Holman’s program mle (2005), using multiple start values to avoid local maxima. The model was fit separately to data on the presence of periodontitis and to data on the presence of caries. A likelihood ratio test (LRT) was used to assess the fit of the full model compared to a reduced model in which the value of the k2 parameter was set equal to 1 (H0: k2 =1); the LRT therefore tests the null hypothesis that lesions are not associated with elevated or decreased risks of death such that individuals with and without oral pathologies were at the same risk of death. The LRT was computed as follows: LRT = −2[ln(Lreduced) – ln(Lfull)], where LRT approximates a χ2 distribution with df=1.
RESULTS
The frequencies of dental caries and periodontitis within the St. Mary Graces cemetery are shown in Table 1.
Table 1.
Frequencies of oral pathologies in the St. Mary Graces Cemetery. Sample sizes refer to the number of individuals with appropriate elements preserved well enough to allow for scoring the presence of the corresponding pathologies.
| Pathology | Location | n | % with pathology |
|---|---|---|---|
| Periodontitis | Mandible | 182 | 37% |
| Maxilla | 186 | 43% | |
| Dental Caries | Mandibular Premolars | 144 | 15% |
| Maxillary Premolars | 118 | 20% | |
| Mandibular Molars | 125 | 42% | |
| Maxillary Molars | 97 | 55% | |
The maximum likelihood estimates of the excess mortality k2 associated with periodontitis and premolar and molar dental caries, along with the standard errors associated with these estimates and results from the likelihood ratio tests, are shown in Table 2. These results indicate that these particular oral pathologies are, in fact, associated with increased risks of death in the St. Mary Graces cemetery. It should be noted that these estimates have relatively large standard errors associated with them as shown in Table 2, and these standard errors are probably underestimated. To estimate the parameters of the model used in this study, point estimates of age were used without their corresponding errors. Because the errors associated with ages were not incorporated when the model was fit, the reported standard error might be underestimated to an unknown degree; readers should therefore view the standard error estimates with caution. However, it is encouraging that there is a consistent pattern among the results, as the k2 estimates for all oral pathologies are greater than one. The k2 estimates suggest that individuals with periodontitis or dental caries were at elevated risks of dying compared to similarly aged individuals without such oral pathologies. The consistent pattern of elevated risks of mortality suggests that these oral pathologies are indicative of higher frailty; however, the numerical value of the k2 parameter should not necessarily be taken at face value given the uncertainty associated with the standard error estimates. The results of the likelihood ratio tests further suggest that these oral pathologies affected risk of death in St. Mary Graces, as they indicate that including the k2 covariate improved the fit of the model with respect to all of the pathologies considered (though the results of the likelihood ratio tests were not significant for the models applied to periodontitis). These results suggest that oral health, as indicated by the observable oral pathologies examined here, is associated with frailty in the St. Mary Graces population.
Table 2.
Maximum likelihood estimates of k2 with standard errors (s.e.) and likelihood ratio tests of H0: k2=1
| Pathology | k̂2 (s.e.) | LRT |
|---|---|---|
| Periodontitis: Mandible | 1.2 (0.3) | 0.6 |
| Periodontitis: Mandible | 1.3 (0.3) | 0.5 |
| Dental Caries: Premolars | 1.2 (0.3) | 7.9* |
| Dental Caries: Molars | 2.05 (0.7) | 9.8* |
(indicates significant at α = 0.05).
DISCUSSION
The results of this study suggest moderate levels of dental caries and periodontitis within the St. Mary Graces sample. As shown in Table 1, the frequency of premolar caries in the sample ranges from 15 to 20 percent and for molars the frequencies range from 42 to 55 percent. Most of these frequencies fall within the range of dental caries frequencies observed for other medieval skeletal samples. In a 10–11th century Croatian sample, nearly 47 percent of individuals had caries (Vodanovic et al., 2005). Another study of a collection of sites in Croatia dating from 1050–1500 AD, found that the frequency of caries ranged from 5 to 20 percent (Šlaus, 2002). Caglar et al. (2007) found in a 13th-century AD Byzantine population that approximately 14 percent of individuals had caries. Kerr et al. (1990) found that 44 percent of a medieval Scottish sample, from Linlithgow near Edinburgh, had caries; in another late medieval Scottish sample (1300–1600 AD) from Aberdeen, nearly 30 percent had caries (Kerr et al., 1988). Roberts and Cox (2003) summarize the findings from several sites in medieval Britain, and the overall prevalence of dental caries for these sites is 53 percent. The frequencies of caries observed in the current study are not directly comparable to the results of several other investigations of dental caries in medieval populations because of differences in the reporting of data. Table 1 reports the proportions of individuals with teeth present who had at least one carious lesion, whereas numerous other studies report the percentage of carious teeth among all the teeth present (e.g. Moore and Corbett, 1973; Lunt, 1974; Moore and Corbett, 1975; O'Sullivan et al., 1993; Watt et al., 1997; Esclassan et al., 2009). Moore and Corbett (1975) found that in a medieval British sample, 0 to 20 percent of premolars and molars had caries, depending on age. Of the deciduous molars present in their sample from late medieval Britain (13–15th century AD), O’Sullivan et al. (1993) found that 23 percent had caries. Nearly 18 percent of teeth in Esclassan et al.’s (2009) sample from medieval France were carious. Only about 6 percent of teeth in two different medieval Scottish samples studied by Lunt (1974) and Watt et al. (1997) were carious. It is clear from all these studies, regardless of reporting method, that there was variation among these medieval samples with respect to frequencies of dental caries. The differences in caries frequencies might be the result of dietary differences; for example, higher caries frequencies are observed among individuals with diets containing more refined carbohydrates or less abrasive foods (i.e. some studies have shown that dental attrition has a negative relationship with caries) (Moore and Corbett, 1973; Maat and Van der Velde, 1987; Kerr et al., 1990; Whittaker and Molleson, 1996; Vodanovic et al., 2005; Esclassan et al., 2009). Differences in caries frequencies might also reflect differences in oral hygiene (Whittaker and Molleson, 1996; Phillips, 2006), differences in susceptibility to caries (Kerr et al., 1990), or differences in fluoride levels in the local water (Roberts and Cox, 2003). It is possible that the variation in caries might at least partly reflect sampling issues rather than real differences within past populations. For example, the observed variation might be the result of differences in the age distributions of the samples studied given that dental caries is an age-progressive process (i.e. samples with a greater proportion of older adults might have higher frequencies of dental caries) (Kerr et al., 1990; Sledzik and Moore-Jansen, 1991; Sutter, 1995; Saunders et al., 1997). Variations in the sex composition of samples might also affect the observed frequencies of caries; several studies have found higher caries rates in females when controlling for age (Whittaker and Molleson, 1996; Saunders et al., 1997; Lukacs, 2008; Wasterlain et al., 2009).
As shown in Table 1, the frequencies of periodontitis within St. Mary Graces range from 37 to 43 percent. These frequencies are higher than those observed by Clarke and colleagues in various samples from premodern populations (Clarke et al., 1986; Clarke and Hirsch, 1991); in those samples, frequencies of periodontitis ranged from 8 to 11 percent. Clarke et al. (1986) argue that diagnosing periodontitis based on distances between the CEJ and AC greater than 2mm will overestimate the frequency of the pathology (for reasons provided above), so they recommend only scoring oral lesions that are more likely to be specific to periodontitis. The higher frequencies of periodontitis observed in the St. Mary Graces sample might suggest that the method for detecting periodontitis used for this study is capturing individuals who did not actually have periodontal disease. However, the frequencies of periodontitis observed in the current study are lower than those observed in a roughly contemporaneous sample from medieval Scotland. Kerr (1991) scored bone destruction on the interdental septa, a method he argues is more discriminating than using distances between the CEJ and AC. He found that in a sample from medieval Scotland (900–1600 AD), 72 percent of individuals over the age of 16 years and 91 percent of individuals over the age of 26 years had signs of destructive periodontitis. These results are similar to those found in a later English sample; in a predominantly 18th century skeletal sample from London, Kerr (1994) found that the frequency of periodontitis was 73 percent and higher for individuals over the age of 16 years and that all individuals above the age of 35 in the sample had periodontitis. The frequencies observed in St. Mary Graces are also lower than those observed by Chazel et al. (2005) in a sample from medieval France; more than 60 percent of the French sample had periapical inflammation indicative of periodontitis. The variation in observed frequencies of periodontitis might result from variation in methods for identifying the pathology, differences in the age composition of the samples examined (i.e. as with caries, the frequency of periodontitis increases with age), variation in oral hygiene (Kerr, 1991; Kerr, 1994; Oztunc et al., 2006), or some other factor.
As described above, the St. Mary Graces cemetery provides a sample of the general population of London from the mid-14th century until the Reformation in the 16th century. At this time, London was the largest city in Britain and one of the largest cities in Europe (Rappaport, 1989; Harding, 2002). During the fifteenth and sixteenth centuries, London experienced population growth as a result of immigration into the city. Without people moving into London, the population actually would have decreased given that mortality in the city exceeded the birth rate (Rappaport, 1989). The 14th-century Black Death reduced the population of London to below 50,000 people, but by the end of the 16th century, the population had tripled in size (Rappaport, 1989). The thousands of people living within an area not much larger than a square mile created conditions that were deleterious to health (Roberts and Cox, 2003), and, according to Rappaport (1989), mortality levels were higher in London than in other parts of England by the 16th century. However, this period in London’s history was also one of relatively high standards of living. Because of the devastating mortality of the 14th century Black Death, the demand for labor in England exceeded the supply, and wages therefore increased; there was also a decrease in the price of food and rent following the epidemic (Rappaport, 1989; Dyer, 2002; Stone, 2006; Woolgar, 2006).
Despite rising standards of living, there were differences in the diets of lower and higher status individuals in London (Moore and Corbett, 1973; Dyer, 1983), all of whom are represented in the St. Mary Graces cemetery. For individuals of all status levels, grains (primarily wheat, rye, and barley) provided the largest component of the diet and were consumed in the forms of bread, ale, and pottage. Before the Black Death, wealthy individuals in England consumed higher quality breads made from fine wheat flour while poorer individuals were generally limited to lower quality, coarser breads (Moore and Corbett, 1973; Stone, 2006). However, following the Black Death, because of lower grain prices, individuals of all status levels consumed greater quantities of higher quality wheat bread (Stone, 2006). Following the Black Death, there was also an increase in the amount of meat and fish in the diet, though higher status individuals consumed a greater amount and variety of both (Sykes, 2006; Woolgar, 2006). Fruits, vegetables, and dairy products were consumed by all classes (Dyer, 1983; Woolgar, 2006). Wealthy individuals ate more imported luxuries such as honey, dried fruits, spices, wine, and cane sugar (Moore and Corbett, 1973; Dyer, 1983). Because they could afford such luxuries, wealthy individuals had more sucrose in their diets, but they did not consume large quantities of it (Moore and Corbett, 1973; Whittaker and Molleson, 1996; Roberts and Cox, 2003). The late medieval English diet, regardless of status, was thus high in carbohydrates and protein, but generally did not contain the large quantities of refined carbohydrates seen in more recent populations (Moore and Corbett, 1975).
It is perhaps not surprising to find moderate frequencies of oral pathologies in a medieval English sample given that oral hygiene and dental care in medieval Europe were rudimentary at best (Newman, 2001; Anderson, 2004). According to Anderson (2004), medieval medical texts indicate that dental care in England was primarily based on herbal remedies, amulets, and charms, and though surgeries were performed occasionally to treat oral cancers and wounds, dental care was mostly non-invasive. Dental caries were thought to be caused by “tooth-worms” and methods believed to kill the worms, such as treatment with acid or covering the affected tooth with wax, were sometimes used (Roberts and Cox, 2003). Individuals with toothaches had the offending teeth removed, and some people rinsed their mouths with vinegar and cleaned their teeth and gums with clothes or soft sticks, but methods for removing plaque and filling caries were not widely practiced (Newman, 2001; Roberts and Cox, 2003; Anderson, 2004).
The results shown in Table 2 suggest that the oral pathologies analyzed here are associated with frailty in the St. Mary Graces sample such that individuals with periodontitis and premolar and molar dental caries were at elevated risks of death compared to their peers without such pathologies. If dental caries and periodontitis really are indicative of frailty in the St. Mary Graces medieval population, the question remains whether the relationship between oral health and systemic health in past populations was similar to the relationship existing between the two in modern populations. As described above, studies of oral health in modern populations have suggested that periodontitis and dental caries are associated with diseases elsewhere in the body, and these associations might occur because of the spread of infection from the mouth to other areas of the body, chronic inflammation initiated by an oral pathology, an underlying problem with the immune system, or some other common risk factor (or perhaps some combination thereof). It is possible that periodontitis and dental caries were associated with other systemic health problems in past populations because of these same mechanisms.
Both periodontitis and dental caries might have elevated the risks of mortality within the St. Mary Graces population by directly causing disease elsewhere in the body. For example, in some individuals infections might have spread from the localized infections in the mouth to other tissues of the body and thereby caused infections that ultimately resulted in death, as apparently occurs in some cases of bacterial pneumonia. Periodontitis or dental caries might also have caused chronic systemic infection or inflammation which subsequently influenced the development or severity of other life-threatening diseases within St. Mary Graces.
The oral pathologies might reflect preexisting high frailty among individuals in St. Mary Graces. As has been suggested with respect to the periodontitis and dental caries in modern populations, perhaps the oral pathologies did not directly cause systemic infection or inflammation in individuals in the St. Mary Graces population, but rather they reflect inadequate immune functioning or deleteriously exaggerated immune responses which increased an individual’s risk of developing oral pathologies and a variety of other diseases that ultimately caused death.
Compromised immune functioning in the St. Mary Graces population could have been the result of suboptimal nutritional status. According to Dyer (1983) typical diets in medieval England, even of higher status individuals, would have been deficient nutritionally given their minimal consumption of dairy products and fresh fruits and vegetables. As described above, some studies of modern populations have found that dental caries is more common among underweight and obese children compared to children of normal weight; that is, dental caries is possibly associated with malnutrition in modern populations. The pain of severe caries might interfere with the ability to eat and thereby contribute to abnormally low weight, and obesity can be the result of a diet composed of an abundance of highly cariogenic foods with low nutritional value. In both cases, malnutrition might result in lowered immune competence. Obesity might not have been a significant problem for the St. Mary Graces population, as some argue that obesity was not highly prevalent in populations until the 20th century (Ulijaszek and Lofink, 2006). However, perhaps in the St. Mary Graces population, individuals with painful dental caries were consequently undernourished and therefore suffered reduced immune competence and were more highly susceptible to various causes of morbidity and mortality.
It is also possible that dental caries in the St. Mary Graces population was caused by, rather than contributed to, poor immune functioning, as it has been suggested that in modern populations, the development of caries might be the result of the immune system’s inability to control the proliferation of cariogenic oral bacteria (Acs et al., 1999). Similarly, periodontitis in St. Mary Graces might reflect preexisting diseases or genetic factors which affected immune function. Studies have shown that in individuals whose immune systems become compromised by such factors as HIV infection or chemotherapy for treatment of cancer, the severity of periodontitis increases (Beck et al., 1996). Beck et al. (1996), who study the association between periodontitis and cardiovascular disease, have hypothesized the existence of individuals with a hyperinflammatory trait who therefore exhibit abnormally high inflammatory responses to pathogens and are thus at high risk of both developing periodontal disease and cardiovascular disease. Watts et al. (2008) suggest that genetic polymorphisms for genes involved in the inflammatory process might explain the link between periodontitis and Alzheimer’s disease. If, as these and other studies suggest, it is reasonable to suspect that the associations between oral health and systemic diseases in modern populations are at least partly determined by variation in genes influencing the immune system or by infection with diseases that affect immune response, such mechanisms could also have been at work in past populations. In the St. Mary Graces population, there might have been some individuals who were genetically predisposed to developing dental caries, periodontitis and other diseases or who had immune systems weakened by disease and were thus more highly susceptible to oral pathologies and other causes of morbidity and mortality.
As described above, periodontitis in modern populations is associated with elevated risks of mortality and is a risk factor for several diseases, including cardiovascular disease, certain types of cancer, pulmonary infection, and diabetes. Given that the results of the current study suggest that periodontitis was also associated with elevated risks of death in past populations, periodontitis might also have been associated with other systemic diseases in the past. This study does not address specifically what systemic diseases periodontitis might have been associated with in the St. Mary Graces population. It is not necessarily the case that in past populations, periodontitis was associated with the same systemic diseases to which it is linked in modern populations. In fact, given that many of the diseases associated with periodontitis today are degenerative diseases such as cardiovascular disease and cancer, it is perhaps more likely that periodontitis was not associated with the same suite of diseases as is observed today. There is evidence that in industrialized nations, such as England, the prevalence of degenerative diseases has increased over the last few centuries, such that degenerative diseases have replaced infectious diseases as the most common causes of death (Omran, 1977; Gage, 2005). This would suggest that within the St. Mary Graces population of the 14th – 16th centuries, if periodontitis was in fact associated with systemic diseases, those diseases would have been predominantly infectious rather than degenerative diseases. For anthropologists interested in general health patterns in past populations, the actual causes of morbidity and mortality with which periodontitis was associated in the past might not be as important as the apparent association between the oral pathology and elevated risk of death in the St. Mary Graces population shown here. These results suggest that, regardless of the ultimate cause of death, periodontal disease is associated with increased risks of mortality and thus is potentially informative about general health in past populations. Health indices, such as that proposed by Steckel and Rose (2002) which incorporates information on length of life and the presence of certain skeletal pathologies to assess levels of health in past populations (and already includes dental caries), might benefit from the inclusion of periodontitis.
As described above, one of the criteria used in this study to identify periodontitis was a distance between the cemento-enamel junction (CEJ) and the alveolar crest (AC) greater than 2 mm, a criterion which might not always indicate periodontitis. The other criterion used in this study to diagnose periodontitis, the exposure of cancellous bone as a result of the destruction of overlying cortical bone (i.e. porous alveolar bone), is one that is recommended by numerous researchers who criticize the “greater than 2mm” criterion (Clarke et al., 1986; Clarke and Hirsch, 1991). So we are confident that the periodontitis sample used in this study does include individuals who suffered from the oral pathology. However, non-pathological processes, such as continued eruption of teeth to compensate for attrition, can result in an increase in the CEJ-AC distance beyond 2 mm (Costa, 1982; Clarke et al., 1986; Clarke, 1990; Clarke and Hirsch, 1991; Hildebolt and Molnar, 1991; Varrela et al., 1995; Hillson, 1996). It is possible that individuals with a CEJ-AC distance greater than 2mm caused by non-pathological processes were incorrectly diagnosed with periodontitis for the current study. This is potentially a problem for the current study because the inclusion of such false positives for periodontitis in the sample would result in an underestimation of the risk of death associated with periodontitis. It is therefore reassuring that an elevated risk of death with the periodontitis (i.e. a k2 estimate of greater than 1) was detected despite the possible inclusion of false positives in the sample. This result suggests that periodontitis is associated with elevated risks of mortality. The possible inclusion of false positives suggests that the true risk of mortality associated with periodontitis might have been even higher than that suggested by the results of this study. In light of the possible inclusion of false positives in the sample, in addition to the errors associated with age estimation mentioned above, the estimated value of the k2 parameter for periodontitis shown here should not be taken at face value, but rather should be interpreted as a general indicator of an elevated risk of mortality.
CONCLUSION
The results of these analyses suggest that periodontitis and dental caries were associated with increased risks of death in the St. Mary Graces population. These pathologies are associated with systemic health problems in modern populations. However, no attempt is made here to determine which particular diseases or causes of death periodontitis or dental caries were associated with in the St. Mary Graces population. Future work might fruitfully examine the skeletal pathologies which are associated with periodontitis and dental caries within St. Mary Graces or other skeletal samples to address this particular question. The results of this study suggest that periodontitis and dental caries can be used as informative indicators of general health in past populations.
ACKNOWLEDGEMENTS
We would like to thank Christopher Ruff, two anonymous reviewers, Eric Jones, Barney Sloane, Dorothy DeWitte, and Gordon DeWitte for their very helpful and insightful comments on an earlier version of this manuscript. We would like to thank Bill White at the Museum of London Centre for Human Bioarchaeology for providing access to the St. Mary Graces skeletons and for providing the physical facilities for this work. Funding was provided by the University at Albany Center for Social and Demographic Analysis and the University at Albany Research Foundation.
Grant Sponsors: University at Albany Center for Social and Demographic Analysis (CSDA), University at Albany Research Foundation.
LITERATURE CITED
- Abnet CC, Kamangar F, Dawsey SM, Stolzenberg-Solomon RZ, Albanes D, Pietinen P, Virtamo J, Taylor PR. Tooth loss is associated with increased risk of gastric non-cardia adenocarcinoma in a cohort of Finnish smokers. Scand J Gastroenterol. 2005;40(6):681–687. doi: 10.1080/00365520510015430. [DOI] [PubMed] [Google Scholar]
- Acs G, Shulman R, Ng MW, Chussid S. The effect of dental rehabilitation on the body weight of children with early childhood caries. Pediatr Dent. 1999;21(2):109–113. [PubMed] [Google Scholar]
- Alm A. On dental caries and caries-related factors in children and teenagers. Swed Dent J. 2008;(195) Suppl:7–63. 61p preceding table of contents. [PubMed] [Google Scholar]
- Amabile N, Susini G, Pettenati-Soubayroux I, Bonello L, Gil JM, Arques S, Bonfil JJ, Paganelli F. Severity of periodontal disease correlates to inflammatory systemic status and independently predicts the presence and angiographic extent of stable coronary artery disease. J Intern Med. 2008;263(6):644–652. doi: 10.1111/j.1365-2796.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Anderson T. Dental treatment in medieval England. Br Dent J. 2004;197:419–425. doi: 10.1038/sj.bdj.4811723. [DOI] [PubMed] [Google Scholar]
- Awano S, Ansai T, Takata Y, Soh I, Akifusa S, Hamasaki T, Yoshida A, Sonoki K, Fujisawa K, Takehara T. Oral health and mortality risk from pneumonia in the elderly. J Dent Res. 2008;87(4):334–339. doi: 10.1177/154405910808700418. [DOI] [PubMed] [Google Scholar]
- Ayhan H, Suskan E, Yildirim S. The effect of nursing or rampant caries on height, body weight and head circumference. J Clin Pediatr Dent. 1996;20(3):209–212. [PubMed] [Google Scholar]
- Bass WM. Human osteology: a laboratory and field manual. Columbia, Mo: Missouri Archaeological Society; 1987. [Google Scholar]
- Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67(10 Suppl):1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76(11 Suppl):2089–2100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- Beckett S, Lovell NC. Dental disease evidence for agricultural intensification in the Nubian C-Group. International Journal of Osteoarchaeology. 1994;4(3):223–239. [Google Scholar]
- Boggess KA. Pathogenicity of periodontal pathogens during pregnancy. Am J Obstet Gynecol. 2005;193(2):311–312. doi: 10.1016/j.ajog.2005.04.056. [DOI] [PubMed] [Google Scholar]
- Boggess KA, Beck JD, Murtha AP, Moss K, Offenbacher S. Maternal periodontal disease in early pregnancy and risk for a small-for-gestational-age infant. Am J Obstet Gynecol. 2006;194(5):1316–1322. doi: 10.1016/j.ajog.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Boldsen JL. Early childhood stress and adult age mortality-A study of dental enamel hypoplasia in the medieval Danish village of Tirup. Am J Phys Anthropol. 2007;132(1):59–66. doi: 10.1002/ajpa.20467. [DOI] [PubMed] [Google Scholar]
- Brooks S, Suchey J. Skeletal age determination based on the os pubis: a comparison of the Ascadi-Nemeskéri and Suchey-Brooks methods. Human Evo. 1990;5:227–238. [Google Scholar]
- Brothwell DR. Digging up bones : the excavation, treatment, and study of human skeletal remains. Ithaca, N.Y: Cornell University Press; 1981. [Google Scholar]
- Buckberry JL, Chamberlain AT. Age estimation from the auricular surface of the ilium: a revised method. Am J Phys Anthropol. 2002;119(3):231–239. doi: 10.1002/ajpa.10130. [DOI] [PubMed] [Google Scholar]
- Buikstra JE, Ubelaker DH, editors. Standards for data collection from human skeletal remains: Proceedings of a seminar at the Field Museum of Natural History (Arkansas Archaeology Research Series 44) Fayetteville, Ark: Arkansas Archeological Survey Press; 1994. [Google Scholar]
- Caglar E, Kuscu OO, Sandalli N, Ari I. Prevalence of dental caries and tooth wear in a Byzantine population (13th c. A.D.) from northwest Turkey. Arch Oral Biol. 2007;52(12):1136–1145. doi: 10.1016/j.archoralbio.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Chazel JC, Tramini P, Valcarcel J, Pélissier B. A comparative analysis of periapical health based on historic and current data. Int Endod J. 2005;38(5):277–284. doi: 10.1111/j.1365-2591.2005.00905.x. [DOI] [PubMed] [Google Scholar]
- Clarke NG. Periodontal defects of pulpal origin: Evidence in early man. Am J Phys Anthropol. 1990;82(3):371–376. doi: 10.1002/ajpa.1330820312. [DOI] [PubMed] [Google Scholar]
- Clarke NG, Carey SE, Srikandi W, Hirsch RS, Leppard PI. Periodontal disease in ancient populations. Am J Phys Anthropol. 1986;71(2):173–183. doi: 10.1002/ajpa.1330710205. [DOI] [PubMed] [Google Scholar]
- Clarke NG, Hirsch RS. Physiological, pulpal, and periodontal factors influencing alveolar bone. In: Kelly A, Larsen CS, editors. Advances in Dental Anthropology. New York: Wiley-Liss; 1991. pp. 241–266. [Google Scholar]
- Cohen MN, Armelagos GJ, editors. Paleopathology at the origins of agriculture. New York: Academic Press; 1984. [Google Scholar]
- Corbett ME, Moore WJ. Distribution of dental caries in ancient British populations. IV. The 19th century. Caries Res. 1976;10(6):401–414. doi: 10.1159/000260233. [DOI] [PubMed] [Google Scholar]
- Costa RL., Jr Periodontal disease in the prehistoric Ipiutak and Tigara skeletal remains from Point Hope, Alaska. Am J Phys Anthropol. 1982;59(1):97–110. doi: 10.1002/ajpa.1330590109. [DOI] [PubMed] [Google Scholar]
- Danforth ME. Nutrition and Politics in Prehistory. Annual Review of Anthropology. 1999;28:1–25. [Google Scholar]
- Danforth ME, Cook DC, Iii SGK. The Human Remains from Carter Ranch Pueblo, Arizona: Health in Isolation. American Antiquity. 1994;59(1):88–101. [Google Scholar]
- Davidson JM, Rose JC, Gutmann MP, Haines MR, Condon K, Condon C. The quality of African-American life in the old Southwest near the turn of the twentieth century. In: Steckel RH, Rose JC, editors. The backbone of history: Health and nutrition in the Western Hemisphere. Cambridge: Cambridge Univ Press; 2002. pp. 226–277. [Google Scholar]
- Demmer RT, Desvarieux M. Periodontal infections and cardiovascular disease: the heart of the matter. J Am Dent Assoc. 2006;137 Suppl:14S–20S. doi: 10.14219/jada.archive.2006.0402. quiz 38S. [DOI] [PubMed] [Google Scholar]
- DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306(6879):688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Jr, Papapanou PN, Sacco RL. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Stroke. 2003;34(9):2120–2125. doi: 10.1161/01.STR.0000085086.50957.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Schwahn C, Volzke H, Demmer RT, Ludemann J, Kessler C, Jacobs DR, Jr, John U, Kocher T. Gender differences in the relationship between periodontal disease, tooth loss, and atherosclerosis. Stroke. 2004;35(9):2029–2035. doi: 10.1161/01.STR.0000136767.71518.36. [DOI] [PubMed] [Google Scholar]
- Dorn BR, Burks JN, Seifert KN, Progulske-Fox A. Invasion of endothelial and epithelial cells by strains of Porphyromonas gingivalis. FEMS Microbiol Lett. 2000;187(2):139–144. doi: 10.1111/j.1574-6968.2000.tb09150.x. [DOI] [PubMed] [Google Scholar]
- Dyer C. English diet in the later middle ages. In: Aston TH, Coss PR, Dyer C, Thirsk J, editors. Social relations and ideas: essays in honour of RH Hilton. Cambridge: Cambridge University Press; 1983. pp. 191–216. [Google Scholar]
- Dyer C. Making a living in the middle ages : the people of Britain 850–1520. New Haven, CT: Yale University Press; 2002. [Google Scholar]
- Esclassan R, Grimoud AM, Ruas MP, Donat R, Sevin A, Astie F, Lucas S, Crubezy E. Dental caries, tooth wear and diet in an adult medieval (12th–14th century) population from Mediterranean France. Arch Oral Biol. 2009;54(3):287–297. doi: 10.1016/j.archoralbio.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Eshed V, Gopher A, Hershkovitz I. Tooth wear and dental pathology at the advent of agriculture: new evidence from the Levant. Am J Phys Anthropol. 2006;130(2):145–159. doi: 10.1002/ajpa.20362. [DOI] [PubMed] [Google Scholar]
- Gage TB. Mathematical hazard models of mortality: an alternative to model life tables. Am J Phys Anthropol. 1988;76(4):429–441. doi: 10.1002/ajpa.1330760403. [DOI] [PubMed] [Google Scholar]
- Gage TB. Variation and classification of human age patterns of mortality: analysis using competing hazards models. Hum Biol. 1990;62:589–617. [PubMed] [Google Scholar]
- Gage TB. Causes of death and the components of mortality: Testing the biological interpretations of a competing hazards model. Hum Biol. 1991;3:289–300. doi: 10.1002/ajhb.1310030308. [DOI] [PubMed] [Google Scholar]
- Gage TB. Are modern environments really bad for us?: revisiting the demographic and epidemiologic transitions. Am J Phys Anthropol Suppl. 2005;41:96–117. doi: 10.1002/ajpa.20353. [DOI] [PubMed] [Google Scholar]
- Gerdin EW, Angbratt M, Aronsson K, Eriksson E, Johansson I. Dental caries and body mass index by socio-economic status in Swedish children. Community Dent Oral Epidemiol. 2008;36(5):459–465. doi: 10.1111/j.1600-0528.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- Goepfert AR, Jeffcoat MK, Andrews WW, Faye-Petersen O, Cliver SP, Goldenberg RL, Hauth JC. Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet Gynecol. 2004;104(4):777–783. doi: 10.1097/01.AOG.0000139836.47777.6d. [DOI] [PubMed] [Google Scholar]
- Goodman AH, Allen LH, Hernandez GP, Amador A, Arriola LV, Chavez A, Pelto GH. Prevalence and age at development of enamel hypoplasias in Mexican children. Am J Phys Anthropol. 1987;72(1):7–19. doi: 10.1002/ajpa.1330720103. [DOI] [PubMed] [Google Scholar]
- Goodman AH, Armelagos GJ, Rose JC. The chronological distribution of enamel hypoplasias from prehistoric Dickson Mounds populations. Am J Phys Anthropol. 1984;65(3):259–266. doi: 10.1002/ajpa.1330650305. [DOI] [PubMed] [Google Scholar]
- Goodman AH, Rose JC. Assessment of systemic physiological perturbations from dental enamel hypoplasias and associated histological structures. Yearbook of Physical Anthropology. 1990;33:59–110. [Google Scholar]
- Grainger I, Hawkins D. Excavations at the Royal Mint site 1986–1988. The London Archaeologist. 1988;5:429–436. [Google Scholar]
- Grainger I, Hawkins D, Cowal L, Mikulski R. Museum of London Archaeology Service Monograph 43. London: Museum of London Archaeology Service; 2008. The Black Death cemetery, East Smithfield, London. [Google Scholar]
- Grau AJ, Becher H, Ziegler CM, Lichy C, Buggle F, Kaiser C, Lutz R, Bultmann S, Preusch M, Dorfer CE. Periodontal disease as a risk factor for ischemic stroke. Stroke. 2004;35(2):496–501. doi: 10.1161/01.STR.0000110789.20526.9D. [DOI] [PubMed] [Google Scholar]
- Gustafson G, Koch G. Age estimation up to 16 years of age based on dental development. Odontol Revy. 1974;25(3):297–306. [PubMed] [Google Scholar]
- Harding V. The dead and the living in Paris and London, 1500–1670. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Hawkins D. Black Death and the new London cemeteries of 1348. Antiquity. 1990;64:637–642. [Google Scholar]
- Hayes C, Sparrow D, Cohen M, Vokonas PS, Garcia RI. The association between alveolar bone loss and pulmonary function: the VA Dental Longitudinal Study. Ann Periodontol. 1998;3(1):257–261. doi: 10.1902/annals.1998.3.1.257. [DOI] [PubMed] [Google Scholar]
- Higgins RL, Haines MR, Walsh L, Sirianni JE. The Poor in the Mid-Nineteenth-Century Northeastern United States. In: Steckel RH, Rose JC, editors. The backbone of history: Health and nutrition in the Western Hemisphere. Cambridge: Cambridge University Press; 2002. pp. 162–184. [Google Scholar]
- Hildebolt CF, Molnar S. Measurement and description of periodontal disease in anthropological studies. In: Kelly A, Larsen CS, editors. Advances in Dental Anthropology. New York: Wiley-Liss; 1991. pp. 225–240. [Google Scholar]
- Hillson S. Dental anthropology. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Hodges DC. Health and agricultural intensification in the prehistoric Valley of Oaxaca, Mexico. Am J Phys Anthropol. 1987;73(3):323–332. doi: 10.1002/ajpa.1330730305. [DOI] [PubMed] [Google Scholar]
- Hollister MC, Weintraub JA. The association of oral status with systemic health, quality of life, and economic productivity. J Dent Educ. 1993;57(12):901–912. [PubMed] [Google Scholar]
- Holman DJ. mle: A programming language for building likelihood models. Version 2.1 ed. Seattle, WA: 2005. [Google Scholar]
- Holmstrup P, Poulsen AH, Andersen L, Skuldbol T, Fiehn NE. Oral infections and systemic diseases. Dent Clin North Am. 2003;47(3):575–598. doi: 10.1016/s0011-8532(03)00023-5. [DOI] [PubMed] [Google Scholar]
- Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontal disease and coronary heart disease risk. JAMA. 2000;284(11):1406–1410. doi: 10.1001/jama.284.11.1406. [DOI] [PubMed] [Google Scholar]
- Irfan UM, Dawson DV, Bissada NF. Epidemiology of periodontal disease: a review and clinical perspectives. J Int Acad Periodontol. 2001;3(1):14–21. [PubMed] [Google Scholar]
- Irwin C, Mullally B, Ziada H, Byrne PJ, Allen E. Periodontics: 9. Periodontitis and systemic conditions--is there a link? Dent Update. 2008;35(2):92–94. 97–98, 100–101. doi: 10.12968/denu.2008.35.2.92. [DOI] [PubMed] [Google Scholar]
- Iscan MY, Loth SR, Wright RK. Age estimation from the rib by phase analysis: white males. J Forensic Sci. 1984;29(4):1094–1104. [PubMed] [Google Scholar]
- Iscan MY, Loth SR, Wright RK. Age estimation from the rib by phase analysis: white females. J Forensic Sci. 1985;30(3):853–863. [PubMed] [Google Scholar]
- Johnson NW, Glick M, Mbuguye TN. (A2) Oral health and general health. Adv Dent Res. 2006;19(1):118–121. doi: 10.1177/154407370601900122. [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34(1):47–52. doi: 10.1161/01.str.0000052974.79428.0c. [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Pitiphat W, Hung HC, Willett WC, Colditz GA, Douglass CW. Pulpal inflammation and incidence of coronary heart disease. J Endod. 2006;32(2):99–103. doi: 10.1016/j.joen.2005.10.039. [DOI] [PubMed] [Google Scholar]
- Joshipura KJ, Rimm EB, Douglass CW, Trichopoulos D, Ascherio A, Willett WC. Poor oral health and coronary heart disease. J Dent Res. 1996;75(9):1631–1636. doi: 10.1177/00220345960750090301. [DOI] [PubMed] [Google Scholar]
- Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer's disease: possible role of periodontal diseases. Alzheimers Dement. 2008;4(4):242–250. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Katz J, Flugelman MY, Goldberg A, Heft M. Association between periodontal pockets and elevated cholesterol and low density lipoprotein cholesterol levels. J Periodontol. 2002;73(5):494–500. doi: 10.1902/jop.2002.73.5.494. [DOI] [PubMed] [Google Scholar]
- Keenleyside A. Dental pathology and diet at Apollonia, a Greek colony on the Black Sea. International Journal of Osteoarchaeology. 2008;18(3):262–279. [Google Scholar]
- Kerr NW. The prevalence and pattern of distribution of root caries in a Scottish medieval population. J Dent Res. 1990;69(3):857–860. doi: 10.1177/00220345900690030501. [DOI] [PubMed] [Google Scholar]
- Kerr NW. Prevalence and natural history of periodontal disease in Scotland--the medieval period (900–1600 A.D.) J Periodontal Res. 1991;26(4):346–354. doi: 10.1111/j.1600-0765.1991.tb02073.x. [DOI] [PubMed] [Google Scholar]
- Kerr NW. Prevalence and natural history of periodontal disease in a London, Spitalfields, population (1645–1852 AD) Arch Oral Biol. 1994;39(7):581–588. doi: 10.1016/0003-9969(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Kerr NW, Bruce MF, Cross JF. Caries experience in the permanent dentition of late mediaeval Scots (1300–1600 a.d.) Arch Oral Biol. 1988;33(3):143–148. doi: 10.1016/0003-9969(88)90038-6. [DOI] [PubMed] [Google Scholar]
- Kerr NW, Bruce MF, Cross JF. Caries experience in Mediaeval Scots. Am J Phys Anthropol. 1990;83(1):69–76. doi: 10.1002/ajpa.1330830108. [DOI] [PubMed] [Google Scholar]
- Khader YS, Bawadi HA, Haroun TF, Alomari M, Tayyem RF. The association between periodontal disease and obesity among adults in Jordan. J Clin Periodontol. 2009;36(1):18–24. doi: 10.1111/j.1600-051X.2008.01345.x. [DOI] [PubMed] [Google Scholar]
- Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. 2006;20(1):59–68. doi: 10.1016/j.jdiacomp.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Kowolik MJ, Dowsett SA, Rodriguez J, De La Rosa RM, Eckert GJ. Systemic neutrophil response resulting from dental plaque accumulation. J Periodontol. 2001;72(2):146–151. doi: 10.1902/jop.2001.72.2.146. [DOI] [PubMed] [Google Scholar]
- Kshirsagar AV, Craig RG, Moss KL, Beck JD, Offenbacher S, Kotanko P, Klemmer PJ, Yoshino M, Levin NW, Yip JK others. Periodontal disease adversely affects the survival of patients with end-stage renal disease. Kidney Int. 2009;75(7):746–751. doi: 10.1038/ki.2008.660. [DOI] [PubMed] [Google Scholar]
- Larsen CS. Bioarchaeology: interpereting behavior from the human skeleton. New York: Cambridge University Press; 1997. [Google Scholar]
- Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13(4):547–558. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieverse AR, Link DW, Bazaliiskiy VI, Goriunova OI, Weber AW. Dental health indicators of hunter-gatherer adaptation and cultural change in Siberia's Cis-Baikal. Am J Phys Anthropol. 2007;134(3):323–339. doi: 10.1002/ajpa.20672. [DOI] [PubMed] [Google Scholar]
- Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(11 Suppl):2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO, Meindl RS, Mensforth RP, Barton TJ. Multifactorial determination of skeletal age at death: a method and blind tests of its accuracy. Am J Phys Anthropol. 1985;68(1):1–14. doi: 10.1002/ajpa.1330680102. [DOI] [PubMed] [Google Scholar]
- Lukacs JR. Sex Differences in Dental Caries Rates With the Origin of Agriculture in South Asia. Current Anthropology. 1996;37(1):147–153. [Google Scholar]
- Lukacs John R. Fertility and Agriculture Accentuate Sex Differences in Dental Caries Rates. Current Anthropology. 2008;49(5):901–914. [Google Scholar]
- Lukacs JR, Largaespada LL. Explaining sex differences in dental caries prevalence: Saliva, hormones, and ldquolife-historyrdquo etiologies. American Journal of Human Biology. 2006;18(4):540–555. doi: 10.1002/ajhb.20530. [DOI] [PubMed] [Google Scholar]
- Lunt DA. The prevalence of dental caries in the permanent dentition of Scottish prehistoric and mediaeval populations. Arch Oral Biol. 1974;19:431–437. doi: 10.1016/0003-9969(74)90148-4. [DOI] [PubMed] [Google Scholar]
- Maat GJR, Van der Velde EA. The caries-attrition competition. International Journal of Anthropology. 1987;2:281–292. [Google Scholar]
- Marshall TA, Eichenberger-Gilmore JM, Broffitt BA, Warren JJ, Levy SM. Dental caries and childhood obesity: roles of diet and socioeconomic status. Community Dent Oral Epidemiol. 2007;35(6):449–458. doi: 10.1111/j.1600-0528.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- Mattila KJ, Pussinen PJ, Paju S. Dental infections and cardiovascular diseases: a review. J Periodontol. 2005;76(11 Suppl):2085–2088. doi: 10.1902/jop.2005.76.11-S.2085. [DOI] [PubMed] [Google Scholar]
- Meyer MS, Joshipura K, Giovannucci E, Michaud DS. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control. 2008;19(9):895–907. doi: 10.1007/s10552-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud DS, Liu Y, Meyer M, Giovannucci E, Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol. 2008;9(6):550–558. doi: 10.1016/S1470-2045(08)70106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J, Vaughan-Williams E, Furlong R, Harrison L. Dental caries and children's weights. J Epidemiol Community Health. 1982;36(1):49–52. doi: 10.1136/jech.36.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Tahmassebi JF, Brosnan M. Early childhood caries--a review. Dent Update. 2007;34(9):556–558. 561–552, 564. doi: 10.12968/denu.2007.34.9.556. [DOI] [PubMed] [Google Scholar]
- Mobeen N, Jehan I, Banday N, Moore J, McClure EM, Pasha O, Wright LL, Goldenberg RL. Periodontal disease and adverse birth outcomes: a study from Pakistan. Am J Obstet Gynecol. 2008;198(5):514.e511–514.e518. doi: 10.1016/j.ajog.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WJ, Corbett E. The distribution of dental caries in ancient British populations. II. Iron Age, Romano-British and Mediaeval periods. Caries Res. 1973;7(2):139–153. doi: 10.1159/000259838. [DOI] [PubMed] [Google Scholar]
- Moore WJ, Corbett ME. The distribution of dental caries in ancient British populations. 1. Anglo-saxon period. Caries Res. 1971;5(2):151–168. doi: 10.1159/000259743. [DOI] [PubMed] [Google Scholar]
- Moore WJ, Corbett ME. Distribution of dental caries in ancient British populations. III. The 17th century. Caries Res. 1975;9(2):163–175. doi: 10.1159/000260155. [DOI] [PubMed] [Google Scholar]
- Moorrees CF, Gron AM, Lebret LM, Yen PK, Frohlich FJ. Growth studies of the dentition: a review. Am J Orthod. 1969;55(6):600–616. doi: 10.1016/0002-9416(69)90037-2. [DOI] [PubMed] [Google Scholar]
- Newman PB. Daily life in the Middle Ages. Jefferson, N.C: McFarland & Co; 2001. [Google Scholar]
- Ngoenwiwatkul Y, Leela-Adisorn N. Effects of dental caries on nutritional status among first-grade primary school children. Asia Pac J Public Health. 2009;21(2):177–183. doi: 10.1177/1010539509331787. [DOI] [PubMed] [Google Scholar]
- O'Sullivan EA, Williams SA, Wakefield RC, Cape JE, Curzon ME. Prevalence and site characteristics of dental caries in primary molar teeth from prehistoric times to the 18th century in England. Caries Res. 1993;27(2):147–153. doi: 10.1159/000261533. [DOI] [PubMed] [Google Scholar]
- Oliveira LB, Sheiham A, Bonecker M. Exploring the association of dental caries with social factors and nutritional status in Brazilian preschool children. Eur J Oral Sci. 2008;116(1):37–43. doi: 10.1111/j.1600-0722.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- Omran AR. Epidemiologic transition in the United States: the health factor in population change. Popul Bull. 1977;32(2):1–42. [PubMed] [Google Scholar]
- Ortner DJ. Theoretical and methodological issues in paleopathology. In: Ortner DJ, Aufderheide AC, editors. Human paleopathology: current syntheses and future options. Washington, DC: Smithsonian Institution Press; 1991. pp. 5–11. [Google Scholar]
- Oxenham MF, Tayles N, editors. Bioarchaeology of Southeast Asia. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Oztunc H, Yoldas O, Nalbantoglu E. The periodontal disease status of the historical population of Assos. International Journal of Osteoarchaeology. 2006;16(1):76–81. [Google Scholar]
- Paine RR, Vargiu R, Coppa A, Morselli C, Schneider EE. A health assessment of high status Christian burials recovered from the Roman-Byzantine archeological site of Elaiussa Sebaste, Turkey. Homo. 2007;58(2):173–190. doi: 10.1016/j.jchb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007;13(6):508–512. doi: 10.1111/j.1601-0825.2007.1410a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Teng D, Burke AC, Haase EM, Scannapieco FA. Oral bacteria modulate invasion and induction of apoptosis in HEp-2 cells by Pseudomonas aeruginosa. Microb Pathog. 2009;46(2):73–79. doi: 10.1016/j.micpath.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Phenice TW. A newly developed visual method of sexing the os pubis. Am J Phys Anthropol. 1969;30(2):297–301. doi: 10.1002/ajpa.1330300214. [DOI] [PubMed] [Google Scholar]
- Phillips SM. Diet and Oral Hygiene: Oral Health in a Nineteenth Century Asylum for the Mental Ill. International Journal of Dental Anthropology. 2006;8:10–21. [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Polyzos NP, Polyzos IP, Mauri D, Tzioras S, Tsappi M, Cortinovis I, Casazza G. Effect of periodontal disease treatment during pregnancy on preterm birth incidence: a metaanalysis of randomized trials. Am J Obstet Gynecol. 2009;200(3):225–232. doi: 10.1016/j.ajog.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Pucar A, Milasin J, Lekovic V, Vukadinovic M, Ristic M, Putnik S, Kenney EB. Correlation between atherosclerosis and periodontal putative pathogenic bacterial infections in coronary and internal mammary arteries. J Periodontol. 2007;78(4):677–682. doi: 10.1902/jop.2007.060062. [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Alfthan G, Rissanen H, Reunanen A, Asikainen S, Knekt P. Antibodies to periodontal pathogens and stroke risk. Stroke. 2004a;35(9):2020–2023. doi: 10.1161/01.STR.0000136148.29490.fe. [DOI] [PubMed] [Google Scholar]
- Pussinen PJ, Jauhiainen M, Vilkuna-Rautiainen T, Sundvall J, Vesanen M, Mattila K, Palosuo T, Alfthan G, Asikainen S. Periodontitis decreases the antiatherogenic potency of high density lipoprotein. J Lipid Res. 2004b;45(1):139–147. doi: 10.1194/jlr.M300250-JLR200. [DOI] [PubMed] [Google Scholar]
- Rappaport SL. Worlds within worlds: structures of life in sixteenth-century London. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Robb J, Bigazzi R, Lazzarini L, Scarsini C, Sonego F. Social "status" and biological "status": a comparison of grave goods and skeletal indicators from Pontecagnano. Am J Phys Anthropol. 2001;115(3):213–222. doi: 10.1002/ajpa.1076. [DOI] [PubMed] [Google Scholar]
- Roberts CA, Cox M. Health and disease in Britain from Prehistory to the Present Day. Stroud: Sutton Publishing Ltd; 2003. [Google Scholar]
- Rogers J, Waldron T. DISH and the monastic way of life. Int J Osteoarch. 2001;11:357–365. [Google Scholar]
- Rose EK, Vieira AR. Caries and periodontal disease: insights from two U.S. populations living a century apart. Oral Health Prev Dent. 2008;6(1):23–28. [PubMed] [Google Scholar]
- Ruma M, Boggess K, Moss K, Jared H, Murtha A, Beck J, Offenbacher S. Maternal periodontal disease, systemic inflammation, and risk for preeclampsia. Am J Obstet Gynecol. 2008;198(4):389.e381–389.e385. doi: 10.1016/j.ajog.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Sakashita R, Inoue M, Inoue N, Pan Q, Zhu H. Dental disease in the Chinese Yin-Shang period with respect to relationships between citizens and slaves. Am J Phys Anthropol. 1997;103(3):401–408. doi: 10.1002/(SICI)1096-8644(199707)103:3<401::AID-AJPA9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Saremi A, Nelson RG, Tulloch-Reid M, Hanson RL, Sievers ML, Taylor GW, Shlossman M, Bennett PH, Genco R, Knowler WC. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28(1):27–32. doi: 10.2337/diacare.28.1.27. [DOI] [PubMed] [Google Scholar]
- Saunders SR, Vito CD, Katzenberg MA. Dental caries in nineteenth century Upper Canada. Am J Phys Anthropol. 1997;104(1):71–87. doi: 10.1002/(SICI)1096-8644(199709)104:1<71::AID-AJPA5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 2003;8(1):54–69. doi: 10.1902/annals.2003.8.1.54. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Rethman MP. The relationship between periodontal diseases and respiratory diseases. Dent Today. 2003;22(8):79–83. [PubMed] [Google Scholar]
- Scaronlaus M. Biocultural analysis of sex differences in mortality profiles and stress levels in the late medieval population from Nova Rača, Croatia. Am J Phys Anthropol. 2000;111(2):193–209. doi: 10.1002/(SICI)1096-8644(200002)111:2<193::AID-AJPA6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Scheuer JL, Musgrave JH, Evans SP. The estimation of late fetal and perinatal age from limb bone length by linear and logarithmic regression. Ann Hum Biol. 1980;7(3):257–265. doi: 10.1080/03014468000004301. [DOI] [PubMed] [Google Scholar]
- Scheuer L, Black SM. Developmental juvenile osteology. San Diego, CA: Academic Press; 2000. [Google Scholar]
- Schollmeyer KG, Ii CGT. Dental Caries, Prehistoric Diet, and the Pithouse-to-Pueblo Transition in Southwestern Colorado. American Antiquity. 2004;69(3):569–582. [Google Scholar]
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. The Lancet. 2007;369(9555):51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- Sheiham A. Dental caries affects body weight, growth and quality of life in pre-school children. Br Dent J. 2006;201(10):625–626. doi: 10.1038/sj.bdj.4814259. [DOI] [PubMed] [Google Scholar]
- Shultis WA, Weil EJ, Looker HC, Curtis JM, Shlossman M, Genco RJ, Knowler WC, Nelson RG. Effect of periodontitis on overt nephropathy and end-stage renal disease in type 2 diabetes. Diabetes Care. 2007;30(2):306–311. doi: 10.2337/dc06-1184. [DOI] [PubMed] [Google Scholar]
- Šlaus M. The Bioarchaeaology of Continental Croatia. Oxford: Archaeopress; 2002. [Google Scholar]
- Sledzik PS, Moore-Jansen PH. Dental disease in nineteenth century military skeletal samples. In: Kelley MA, Larsen CS, editors. Advances in dental anthropology. New York: Wiley-Liss; 1991. pp. 215–224. [Google Scholar]
- Slots J. Update on human cytomegalovirus in destructive periodontal disease. Oral Microbiol Immunol. 2004;19(4):217–223. doi: 10.1111/j.1399-302X.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- Smith BH. Standards of human tooth formation and dental age assessment. In: Kelley M, Larsen CS, editors. Advances in Dental Anthropology. New York: Wiley-Liss; 1991. pp. 143–168. [Google Scholar]
- Spahr A, Klein E, Khuseyinova N, Boeckh C, Muche R, Kunze M, Rothenbacher D, Pezeshki G, Hoffmeister A, Koenig W. Periodontal infections and coronary heart disease: role of periodontal bacteria and importance of total pathogen burden in the Coronary Event and Periodontal Disease (CORODONT) study. Arch Intern Med. 2006;166(5):554–559. doi: 10.1001/archinte.166.5.554. [DOI] [PubMed] [Google Scholar]
- Starling AP, Stock JT. Dental indicators of health and stress in early Egyptian and Nubian agriculturalists: a difficult transition and gradual recovery. Am J Phys Anthropol. 2007;134(4):520–528. doi: 10.1002/ajpa.20700. [DOI] [PubMed] [Google Scholar]
- Steckel RH, Rose JC, editors. The backbone of history: Health and nutrition in the Western Hemisphere. New York: Cambridge University Press; 2002. [Google Scholar]
- Steckel RH, Sciulli PW, Rose JC. A Health Index from Skeletal Remains. In: Steckel RH, Rose JC, editors. The backbone of history: Health and nutrition in the Western Hemisphere. Cambridge: Cambridge University Press; 2002. pp. 61–93. [Google Scholar]
- Stolzenberg-Solomon RZ, Dodd KW, Blaser MJ, Virtamo J, Taylor PR, Albanes D. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am J Clin Nutr. 2003;78(1):176–181. doi: 10.1093/ajcn/78.1.176. [DOI] [PubMed] [Google Scholar]
- Stone DJ. The consumption of field crops in late medieval England. In: Woolgar CM, Serjeantson D, Waldron T, editors. Food in medieval England: diet and nutrition. Oxford: Oxford University Press; 2006. pp. 11–26. [Google Scholar]
- Sutter R. Dental pathologies among inmates of the Monroe County poorhouse. In: Grauer AL, editor. Bodies of evidence: reconstructing history through skeletal analysis. New York: Wiley-Liss; 1995. pp. 185–196. [Google Scholar]
- Sykes NJ. From Cu and Sceap to Beffe and Motton. In: Woolgar CM, Serjeantson D, Waldron T, editors. Food in medieval England : diet and nutrition. Oxford: Oxford University Press; 2006. pp. 56–71. [Google Scholar]
- Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. 2008;14(3):191–203. doi: 10.1111/j.1601-0825.2008.01442.x. [DOI] [PubMed] [Google Scholar]
- Temple DH, Larsen CS. Dental caries prevalence as evidence for agriculture and subsistence variation during the Yayoi period in prehistoric Japan: biocultural interpretations of an economy in transition. Am J Phys Anthropol. 2007;134(4):501–512. doi: 10.1002/ajpa.20694. [DOI] [PubMed] [Google Scholar]
- Terpenning MS. The relationship between infections and chronic respiratory diseases: an overview. Ann Periodontol. 2001;6(1):66–70. doi: 10.1902/annals.2001.6.1.66. [DOI] [PubMed] [Google Scholar]
- Terpenning MS, Taylor GW, Lopatin DE, Kerr CK, Dominguez BL, Loesche WJ. Aspiration pneumonia: dental and oral risk factors in an older veteran population. J Am Geriatr Soc. 2001;49(5):557–563. doi: 10.1046/j.1532-5415.2001.49113.x. [DOI] [PubMed] [Google Scholar]
- Thomas CW, Primosch RE. Changes in incremental weight and well-being of children with rampant caries following complete dental rehabilitation. Pediatr Dent. 2002;24(2):109–113. [PubMed] [Google Scholar]
- Ulijaszek SJ, Lofink H. Obesity in Biocultural Perspective. Annual Review of Anthropology. 2006;35(1):337–360. [Google Scholar]
- Usher BM. A multistate model of health and mortality for paleodemography : Tirup cemetery [Dissertation] Pennsylvania State University; 2000. [Google Scholar]
- van Winkelhoff AJ, Slots J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontology. 1999;20(1):122–135. doi: 10.1111/j.1600-0757.1999.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Varrela TM, Paunio K, Wouters FR, Tiekso J, Söder PÖ. The relation between tooth eruption and alveolar crest height in a human skeletal sample. Arch Oral Biol. 1995;40(3):175–180. doi: 10.1016/0003-9969(95)98805-9. [DOI] [PubMed] [Google Scholar]
- Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16:439–454. [PubMed] [Google Scholar]
- Vodanovic M, Brkic H, Slaus M, Demo Z. The frequency and distribution of caries in the mediaeval population of Bijelo Brdo in Croatia (10th–11th century) Arch Oral Biol. 2005;50(7):669–680. doi: 10.1016/j.archoralbio.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Wasterlain SN, Hillson S, Cunha E. Am J Phys Anthropol. NA: 2009. Dental caries in a Portuguese identified skeletal sample from the late 19th and early 20th centuries. In press. [DOI] [PubMed] [Google Scholar]
- Watson JT. Prehistoric dental disease and the dietary shift from cactus to cultigens in northwest Mexico. International Journal of Osteoarchaeology. 2008;18(2):202–212. [Google Scholar]
- Watt ME, Lunt DA, Gilmour WH. Caries prevalence in the permanent dentition of a mediaeval population from the south-west of Scotland. Arch Oral Biol. 1997;42(9):601–620. doi: 10.1016/s0003-9969(97)00061-7. [DOI] [PubMed] [Google Scholar]
- Watts A, Crimmins EM, Gatz M. Inflammation as a potential mediator for the association between periodontal disease and Alzheimer's disease. Neuropsychiatr Dis Treat. 2008;4(5):865–876. doi: 10.2147/ndt.s3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker DK, Griffiths S, Robson A, Roger-Davies P, Thomas G, Molleson T. Continuing tooth eruption and alveolar crest height in an eighteenth-century population from Spitalfields, East London. Arch Oral Biol. 1990;35(2):81–85. doi: 10.1016/0003-9969(90)90167-9. [DOI] [PubMed] [Google Scholar]
- Whittaker DK, Molleson T. Caries prevalence in the dentition of a late eighteenth century population. Arch Oral Biol. 1996;41:55–61. doi: 10.1016/0003-9969(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Willershausen B, Haas G, Krummenauer F, Hohenfellner K. Relationship between high weight and caries frequency in German elementary school children. Eur J Med Res. 2004;9(8):400–404. [PubMed] [Google Scholar]
- Willershausen B, Moschos D, Azrak B, Blettner M. Correlation between oral health and body mass index (BMI) in 2071 primary school pupils. Eur J Med Res. 2007;12(7):295–299. [PubMed] [Google Scholar]
- Williams RC, Barnett AH, Claffey N, Davis M, Gadsby R, Kellett M, Lip GY, Thackray S. The potential impact of periodontal disease on general health: a consensus view. Curr Med Res Opin. 2008;24(6):1635–1643. doi: 10.1185/03007990802131215. [DOI] [PubMed] [Google Scholar]
- Winchell F, Rose JC, Moir RW. Health and hard times: a case study from the middle to late nineteenth century in Eastern Texas. In: Grauer AL, editor. Bodies of evidence: reconstructing history through skeletal analysis. New York: Wiley-Liss; 1995. pp. 161–172. [Google Scholar]
- Wols HD, Baker JE. Dental health of elderly confederate veterans: Evidence from the Texas State Cemetery. Am J Phys Anthropol. 2004;124(1):59–72. doi: 10.1002/ajpa.10334. [DOI] [PubMed] [Google Scholar]
- Wood JW, Holman DJ, O’Connor KA, Ferrell RJ. Mortality models for paleodemography. In: Hoppa RD, Vaupel JW, editors. Paleodemography: Age distributions from skeletal samples. Cambridge: Cambridge University Press; 2002. pp. 129–168. [Google Scholar]
- Wood JW, Milner GR, Harpending HC, Weiss KM. The Osteological Paradox: Problems of Inferring Prehistoric Health from Skeletal Samples. Current Anthropology. 1992;33:343–370. [Google Scholar]
- Woolgar CM. Meat and dairy products in late medieval England. Food in medieval England: diet and nutrition. Oxford: Oxford University Press; 2006. pp. 88–101. [Google Scholar]
- Ylostalo PV, Jarvelin MR, Laitinen J, Knuuttila ML. Gingivitis, dental caries and tooth loss: risk factors for cardiovascular diseases or indicators of elevated health risks. J Clin Periodontol. 2006;33(2):92–101. doi: 10.1111/j.1600-051X.2005.00875.x. [DOI] [PubMed] [Google Scholar]
- Yoneyama T, Yoshida M, Ohrui T, Mukaiyama H, Okamoto H, Hoshiba K, Ihara S, Yanagisawa S, Ariumi S, Morita T others. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc. 2002;50(3):430–433. doi: 10.1046/j.1532-5415.2002.50106.x. [DOI] [PubMed] [Google Scholar]
- Zheng TZ, Boyle P, Hu HF, Duan J, Jian PJ, Ma DQ, Shui LP, Niu SR, Scully C, MacMahon B. Dentition, oral hygiene, and risk of oral cancer: a case-control study in Beijing, People's Republic of China. Cancer Causes Control. 1990;1(3):235–241. doi: 10.1007/BF00117475. [DOI] [PubMed] [Google Scholar]