Abstract
Despite the evidence for a communicative function of rodent scent marks and ultrasonic vocalizations, relatively little is known about the impact of social factors on these two forms of communication. Here, we tested the effects of two important social factors, prior exposure to a female and freshness of female urine, on male scent marks and ultrasonic vocalizations elicited by female urine. We also asked whether a recently reported strain difference between the highly social strain C57BL/6J (B6) and the mouse model of autism BTBR T+tf/J (BTBR) herein is specifically seen in response to female urine or also detectable in response to male urine traces. Results show that the emission of female urine-elicited ultrasonic vocalizations was dependent on previous female experience, while scent-marking behavior was not affected. A positive correlation was detected between scent-marking behavior and ultrasonic calling in the most biologically relevant context, male mice exposed to fresh female urine after female experience. Correlations were less prominent or missing in less biologically relevant contexts, e.g. in male mice exposed to fresh female urine without previous female experience, indicating that previous female experience is affecting both the emission of female urine-elicited ultrasonic vocalizations and the correlation between olfactory and acoustic communication. The strain difference in scent-marking behavior and ultrasonic calling between B6 and BTBR appears to be specific to female urine-elicited behavior as it was not seen in response to male urine traces, highlighting the relevance of the social context in which mouse communication is evaluated.
Keywords: Olfaction, Scent-marking, Ultrasonic vocalization, Communication, Mice
1. Introduction
Rodents communicate primarily via olfactory and acoustic signals. A major source of olfactory communicative signals is contained in scent marks. Scent-marking, the deposition of urine traces in strategic environmental locations, is used by mice to demarcate territories, orchestrate aggressive behavior, recognize individuals, maintain family organization, communicate danger, and to attract mates [1–4]. Male mice scent marks serve to indicate territorial boundaries [5,6], advertising the mouse’s ability to dominate it and hence the likelihood to maintain the territory against intruders [7,8,9] and aid in the orchestration of aggressive behavior [10,12–15]. They also allow the recognition of individuals and hence maintenance of family organization [16,17]. Male scent marks attract female mice [18–24]. Furthermore, they induce estrous [25] and accelerate the onset of puberty [26]. Scent marks from unfamiliar males can interrupt the establishment of pregnancy in female mice [27].
In addition to the deposition of scent marks, rodents emit ultrasonic vocalizations as acoustic communicative signals [28–32]. Dependent on species, age, sex and affective state, different types of ultrasonic vocalizations can be detected that appear to serve different communicative purposes. Pup isolation-induced ultrasonic vocalizations elicit maternal retrieval behavior in mice and rats [33–36]. Anxiety-induced ultrasonic vocalizations serve an alarm function and elicit freezing behavior in rats [37–40]. Interaction-induced ultrasonic vocalizations elicit social approach behavior in mice and rats [39,41]. Males exposed to females or female urine emit ultrasonic vocalizations that facilitate mating behavior in mice and rats [42–50]. In mice, ultrasonic calling is specifically seen in males exposed to female urine as male urine is ineffective [51–57].
Despite the evidence for a communicative function of rodent scent marks and ultrasonic vocalizations, relatively little is known about the impact of social factors on scent-marking and ultrasonic vocalizations. One of the best known phenomena of social modulation of male scent-marking in response to female scents is its dependence on social status. Dominant males scent mark more than subordinate males [11,19,58,59] and social defeat leads to a reduction in male scent-marking [60]. Importantly, females prefer scent marks deposited by dominant males [19,21]. Relatively little, however, is known about the social status for the production of ultrasonic vocalizations in response to female scents [61,62]. Here, one of the best known phenomena of social modulation is the dependence on previous female experience [51–53,63–66]. Typically, male mice do not emit ultrasonic vocalizations when exposed to female urine if they have not been in contact with females previously. However, some findings appear to be inconsistent with a critical familiarity or learning component. In some reports, male mice that had no previous female experience reliably emitted ultrasonic vocalizations to female urine, although mostly to a lower extent [46,57,64,65]. These inconsistencies in male ultrasonic calling might be due to a second modulating factor, freshness of the female urine [64–66]. For both factors, however, little is known about their effect on the time course of the male’s ultrasonic vocalization response.
The first aim of the present study was amore detailed characterization of the effects of these two factors, prior exposure to a female and freshness of the female urine, on male ultrasonic vocalizations elicited by female urine. The second aim was to test whether those two factors affect olfactory communication as well as acoustic responses. The third aim was to test the hypothesis that vocalization and scent-marking responses to female urine are linked. We recently reported that mice who deposit high numbers of scent marks in response to female urine emit more ultrasonic vocalizations than mice who deposit low numbers of scent marks [67]. This link between the two systems was seen under optimal conditions, namely male mice with previous female experience exposed to fresh female urine. The present experiments were designed to understand whether this link holds true under less optimal conditions.
The fourth aim of the present study addressed social cues mediating scent-marking between two inbred strains of mice which differ on sociability. We reported that scent-marking as well as the emission of ultrasonic vocalizations by male mice in response to female urine is dependent on the sociability of the strain [67]. C57BL/6J (B6) is a highly social strain of mice, while BTBR T+tf/J (BTBR) is a low sociability strain that may represent a mouse model of autism [68–74].We found that adult male BTBR deposited fewer scent marks and emitted extremely low numbers of ultrasonic vocalizations as compared to adult male B6. The question addressed in the present study is whether this strain difference is specific to exposure to female urine, relevant to sexual mating [18–24]. Male/male scent countermarking represents a different domain of mouse communication, relevant to territoriality [5,7–15] and dominance hierarchies [12,58–60]. We therefore tested whether a similar strain difference is seen in scent countermarking in response to male urine.
2. Materials and methods
2.1. Animals and housing
Subjects were adult male C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice. For the male subject + female urine spot experiment, N= 45 male B6 mice were used. For the male subject + male urine scent marks experiment, N= 40 male B6 and N=40 male BTBR mice were used. Breeding pairs were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and bred at the National Institute of Mental Health in Bethesda, MD, USA. About 2 weeks after pairing for breeding, the females were individually housed and subsequently inspected daily for pregnancy and delivery. After weaning on postnatal day 21, mice were socially housed in groups of 2–4 with same-sex, same-strain cagemates. All mice were housed in polycarbonate Makrolon cages (369 mm × 156 mm × 132 mm, 435 cm2; 1145T; Tecniplast, Milan, Italy). Bedding, paper strips, a nestlet square and a cardboard tube were provided in each cage. Standard rodent chow and water were available ad libitum. The colony room was maintained on a 12:12 light/dark cycle with lights on at 06:00 h, at 20 °C temperature and 55% humidity.
All procedures were conducted in strict compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the National Institute of Mental Health Animal Care and Use Committee.
2.2. Experimental design: male subject + female urine spot
To determine the optimal settings for eliciting scent-marking behavior and ultrasonic vocalizations in male B6 mice, 3 different conditions were evaluated, where both measures were determined in parallel: (1) scent-marking response to a spot of fresh B6 female urine, by male mice who previously had a brief episode of social interaction with a female mouse; (2) scent-marking response to a spot of fresh B6 female urine, by male mice who had no previous interactive experience with a female mouse; (3) scent-marking response to a spot of non-fresh B6 female urine, by male mice who had previous experience with a female mouse. Independent groups of N= 15 mice were tested in each condition. Each mouse was used only once. Subject mice were 14 ± 1 (mean ± standard errors of the mean) weeks old at the start of behavioral testing.
Previous female experience: the interactive experience with a female was conducted 7–10 days before the beginning of the scent-marking experiment. The experienced B6 male subject mouse was placed in a clean cage (369 mm × 156 mm × 132 mm, 425 cm2; 1145T; Techniplast) with fresh bedding together with a B6 female, about 2–3 months old, for a 5 min session. At the end of the 5 min session, subject males were placed back in their home cage.
Female urine collection: a B6 female, about 2–3 months old, was gently taken out of the home cage inside the vivarium, and held by the base of the tail on the home cage lid. Gentle pressure was applied to lift the back and expose the genital area, to determine the phase of the estrus cycle. The female was scored as in estrus when the vaginal area appeared wide, opened and red. Only estrus females were used to collect urine. Usually during this examination period, while held by the tail with pressure placed on the back, the female urinated spontaneously. Fresh urine was immediately collected in a 1.0 ml Eppendorf tube. Non-fresh urine was collected in the same condition as the fresh urine but was left for 1–2 h at room temperature in the closed Eppendorf tube.
2.3. Experimental design: male subject +male urine scent marks
To test whether male scent marks previously deposited in an open field elicit scent-marking behavior in subject B6 male mice, and whether such as response is also seen in male BTBR mice, scent counter marking behavior in response to fresh male scent marks was assessed. Each combination of B6 and BTBR males was tested: (a) B6 Male 1 scent-marking + B6 Male 2 countermarking; (b) B6 Male 1 scent-marking + BTBR Male 2 countermarking; (c) BTBR Male 1 scent-marking + B6 Male 2 countermarking; (d) BTBR Male 1 scent-marking + BTBR Male 2 countermarking. Independent groups of N= 10 mice of each strain were tested in each condition. Each mouse was used only once. Subject mice were 20 ± 1 (mean ± standard errors of the mean) weeks old at the start of behavioral testing.
2.4. Test setting
Test setting was described previously [67]. Briefly, adult male mice were exposed to urine samples in an Accuscan open field (40 cm × 40 cm × 30 cm), equipped with a Versamax animal activity monitor (AccuScan Instruments, Inc., Columbus, OH, USA). A sheet of specialized paper (Strathmore Drawing Paper Permium, recycled, microperforated, 400 series; Strathmore Artist Papers, Neenah, WI, USA) was placed on the floor of the open field.
2.5. Test procedure
The adult male mouse was habituated for 60 min to the clean open field, lined with the Strathmore paper and containing some of his own home cage bedding in one corner of the arena. At the end of the habituation period, the mouse was placed back in a clean polycarbonate Makrolon cage (369 mm × 156 mm × 132 mm, 435 cm2; 1145T; Tecniplast) with fresh bedding. The home cage bedding and any feces deposited by the mouse were removed from the open field. Scent marks deposited on the paper during habituation were visualized under ultraviolet (UV) light using a UV lamp (Sleeklook Super 18” Black Light-eParty unlimited; Can You Imagine, Chatsworth, CA, USA). Visualized scent marks were outlined using a pencil.
In the male subject + female urine spot experiment, a 15 µl sample of fresh (less than 5 min old) or non-fresh (1–2 h at room temperature) urine from an estrus female was manually pipetted from the Eppendorf tube into the center of a clean sheet of Strathmore paper placed in the open field. The same subject male was immediately replaced in the arena for a 5 min test session. The male mouse was then removed from the open field and placed back in his home cage. Male scent marks elicited by the female urine sample were visualized under the UV lamp and outlined with a blue colored pen.
In the male subject + male urine scent marks experiment, Male 1, which had a previous interaction with a female, was placed in a clean open field containing some of his own home cage bedding in one corner of the arena. Male 1 was left undisturbed for 1 h, then was removed, along with the home bedding and any deposited feces. The scent marks left by Male 1 were visualized under the UV lamp and outlined with a pencil on the Strathmore paper, without removing the paper from the open field. A second male (Male 2) was then placed in the arena for 15 min. At the end of the 15 min countermarking period, the scent countermarks left by male 2 were visualized under the UV lamp and outlined with a blue colored pen.
Prior to each session with a new subject mouse, the open field was cleaned with a 70% ethanol solution, followed by water and drying with paper towels. Behavioral testing was conducted between 09:00 and 17:00 h during the light phase of the circadian cycle.
2.6. Locomotor activity
Locomotor activity in the Accuscan open field was automatically recorded by the Accuscan software (AccuScan Instruments, Inc.) as previously described [67]. Exploratory locomotion was recorded for each subject. In one experiment, male subjects with previous female experience exposed to fresh female urine, unavoidable data loss resulted inN= 11 scored for locomotion during the habituation session but only N= 8 scored for locomotion during the test session.
2.7. Scent-marking
At the end of each female urine-elicited scent-marking and male/male countermarking session, the marked sheets of Strathmore paper were treated with ninhydrin spray (LC-NIN-16; TritechForensics Inc., Southport, NC, USA) and left to dry for at about 12 h, which allowed the visualization of the urine traces as purple spots (Fig. 1), in which spots circled with pencil represented the subject male’s scent marks deposited during habituation to the empty open field, while spots circled in blue pen representing the subject male’s scent marks in the presence of female urine or male scent-marking. Number of scent marks were quantitated as previously described [67,75]. A transparent plastic grid (40 cm2), divided into 1 cm2 squares, was placed on the top of the sheet of Strathmore paper. Every square containing a trace was counted as one scent mark unit. The total number of scent marks and the number of scent marks within an area of 10 cm2 around the female urine spot were counted. For the number of scent marks left in the proximal area, the grid was placed such as one of the 1 cm2 squares of the grid contained the female urine sample. Four blocks of 5 cm2 were defined around this “central” 1 cm2 square to determine the proximal area. High scent-marking was defined as the presence of at least one male scent mark in each of at least 3 out of the 4 blocks, 5 cm2 per block, constituting 10 cm2 surrounding the female mouse urine spot. Low scent-marking was defined by the absence of this pattern.
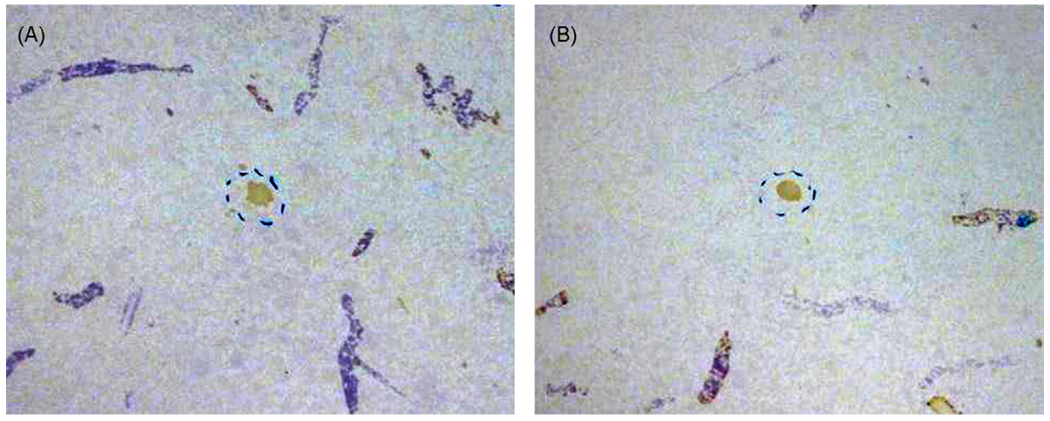
Fig. 1.
Two representative examples of scent marks left by adult male C57BL/6J (B6) mice with previous female experience exposed to fresh female urine. Male scent marks were visualized by ninhydrin spray and appear purple. The fresh female urine spot in the center is highlighted by a dashed circle.
2.8. Ultrasonic vocalizations
Ultrasonic vocalizations were recorded in the male subject + female urine spot experiment as previously described [67]. Briefly, vocal emissions during the 5 min female urine exposure were recorded by an UltraSoundGate Condenser Microphone (CM16; Avisoft Bioacoustics, Berlin, Germany), connected via an Avisoft UltraSoundGate 416 USB Audio device (Avisoft Bioacoustics) to a personal computer, where acoustic data were recorded with a sampling rate of 300,000 Hz in 16 bit format. For acoustical analysis, recordings were transferred to SASLab Pro (version 4.50; Avisoft Bioacoustics) and a fast Fourier transform was conducted (512 FFT length, 100% frame, Hamming window and 75% time window overlap). An experienced observer counted the number of ultrasonic vocalizations emitted during the 5 min female urine exposure. In addition, the number of ultrasonic vocalizations emitted during the first 3 min of female urine exposure as well as their numbers in 10 s time bins was determined, in order to visualize the time course of the ultrasonic vocalization response.
2.9. Statistical analysis
For the male subject + female urine spot experiment, comparisons of the three experimental conditions were statistically analyzed using One-Way ANOVAs with experimental condition as the between-subject-factor as independent groups of mice were used in each condition. Dependent parameters were locomotor activity, scent marks, ultrasonic vocalizations, and the time spent within a distance of 10 cm surrounding the female mouse urine spot. To analyze the time course of the ultrasonic vocalization responses between experimental conditions, a Two-Way ANOVA for Repeated Measures was calculated, using a within-subject-factor of session time and a between-subject-factor of experimental condition. When a significant ANOVA was detected, Bonferroni post hoc tests were conducted to compare individual means. To test whether the emission of ultrasonic vocalizations was dependent on whether mice displayed high versus low levels of female urine-elicited scent-marking, unpaired Student’s t-tests were used. Pearson’s product moment statistics were used for correlation analyses between numbers of scent marks and numbers of ultrasonic vocalizations. For the male subject + male urine scent marks experiment, inbred strain comparisons of number of scent marks deposited by Male 1 in the clean open field and locomotor activity were statistically analyzed using unpaired Student’s t-test. Numbers of Male 2 countermarks to Male 1 scent marks were compared between the four groups using a One-Way ANOVA followed by a Bonferroni post hoc test.Ap-value of <0.05 was considered statistically significant.
3. Results
3.1. Male subject + female urine spot: ultrasonic vocalizations
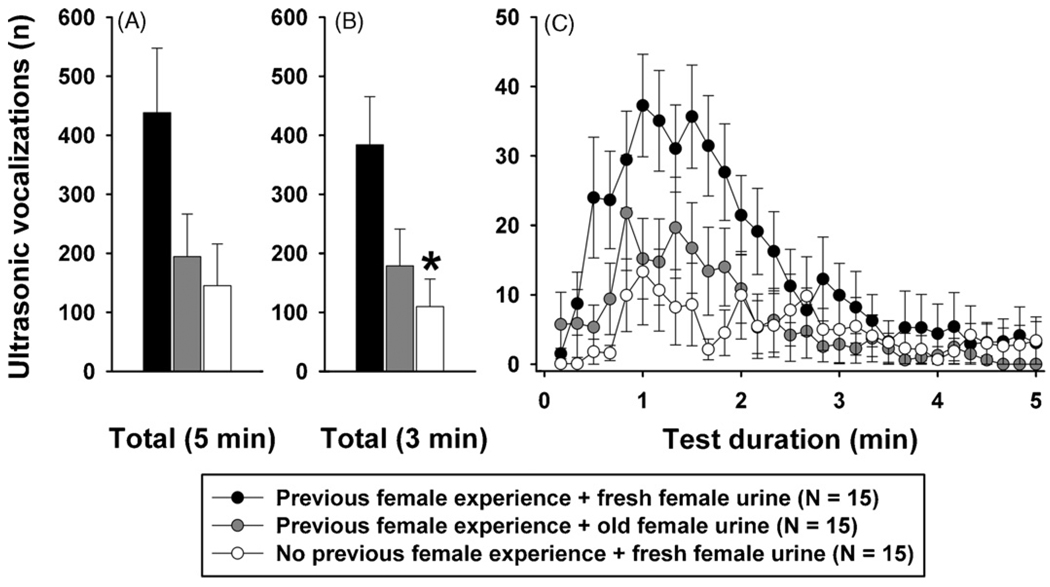
As shown in Fig. 2, emission of ultrasonic vocalizations was dependent on experimental condition in male B6 mice. When comparing the total number of ultrasonic vocalizations emitted during the entire 5 min of female B6 urine exposure, a significant effect of experimental condition was detected by One-Way ANOVA (F2,44 = 3.336, p = 0.045; Fig. 2A). Males with previous female experience exposed to fresh female urine emitted 438.33 ± 109.39 calls, while much lower call numbers were detected in the two other experimental conditions. Males with previous female experience exposed to 1–2 h old female urine emitted considerably fewer calls, 194.40 ± 72.13. Males without previous female experience exposed to fresh female urine emitted only 145.13 ± 70.68 calls. Despite these large differences in means between experimental conditions, however, Bonferroni post hoc tests failed to detect significant differences when comparing individual experimental conditions (all p-values > 0.05). The absence of significant Bonferroni post hoc tests was due to the time course of the ultrasonic vocalization responses. When comparing the total number of ultrasonic vocalizations emitted only during the first 3 min of female urine exposure, a significant effect of experimental condition was detected by One-Way ANOVA (F2,44 = 4.786, p = 0.013; Fig. 2B) and Bonferroni post hoc tests detected a significant difference between males that were exposed to fresh female urine dependent on whether they had previous female experience or no previous female experience (p = 0.015). Males with previous female experience emitted 383.73 ± 81.85 calls, while males without previous females experience emitted only 109.53 ± 46.62 calls. Comparisons of other individual experimental conditions were not significant (all Bonferroni post hoc test p-values > 0.05).
Fig. 2.
Ultrasonic vocalizations emitted by adult male C57BL/6J (B6) mice in the presence of female urine. Total number of ultrasonic vocalizations emitted during the 5 min exposure to female urine (A), number of ultrasonic vocalizations emitted during the first 3 min of the exposure to female urine (B), and time course for the number of ultrasonic vocalizations emitted during the 5 min exposure to female urine (C). The time course panel (C) illustrates the test sessions in 10 s time blocks. Black bar: B6 with previous female experience exposed to fresh female urine. Grey bar: B6 with previous female experience exposed to 1–2 h old female urine. White bar: B6 without previous female experience exposed to fresh female urine. Data are presented as means + standard errors of the mean. *p < 0.05 for the comparisons between B6 males with previous female experience exposed to fresh female urine and B6 males without previous female experience exposed to fresh female urine.
The importance of the vocalization time course was seen in greater detail by performing a Two-Way ANOVA for Repeated Measures (Fig. 2C). A significant effect of session time (F29,1218 = 10.227, p < 0.001) and a significant interaction between experimental condition and session time were detected (F58,1218 = 2.177, p < 0.001). Males with previous female experience started to emit calls almost immediately after being exposed to fresh female urine. They emitted a maximum of approximately 35 calls per 10 s between 1 and 2 min after the start of the session. Throughout the remaining 3 min of testing, call emission slowly declined.
In contrast, males without previous female experience displayed a longer latency before initiating ultrasonic vocalizations when exposed to fresh female urine, producing a significant difference in the number of ultrasonic vocalizations as compared to males with previous female experience at the time points of 20–40 s (all Bonferroni post hoc test p-values < 0.05). Males without previous female experience emitted a maximum of approximately 15 calls per 10 s between 1 and 2 min after the start of the session, significantly different as compared to males with previous female experience at the time points of 60–110 s (all Bonferroni post hoc test p-values < 0.05). Number of calls slowly declined throughout the remaining 3 min of testing, with no further significant differences detected (all Bonferroni post hoc test p-values > 0.05). Males with previous female experience exposed to 1–2 h old female urine displayed an intermediate ultrasonic vocalization response as compared to ultrasonic vocalizations by males exposed to fresh female urine (all Bonferroni post hoc test p-values > 0.05).
Differences in call emission were not primarily due to a difference in number of mice vocalizing, since the number of mice vocalizing was roughly similar in all three experimental conditions. More than 5 calls per subject were emitted by 11 out of 15 males with previous female experience exposed to fresh female urine, 9 out of 15 males with previous female experience exposed to 1–2 h old female urine, and 7 out of 15 males without previous female experience exposed to fresh female urine.
3.2. Male subject + female urine spot: scent-marking
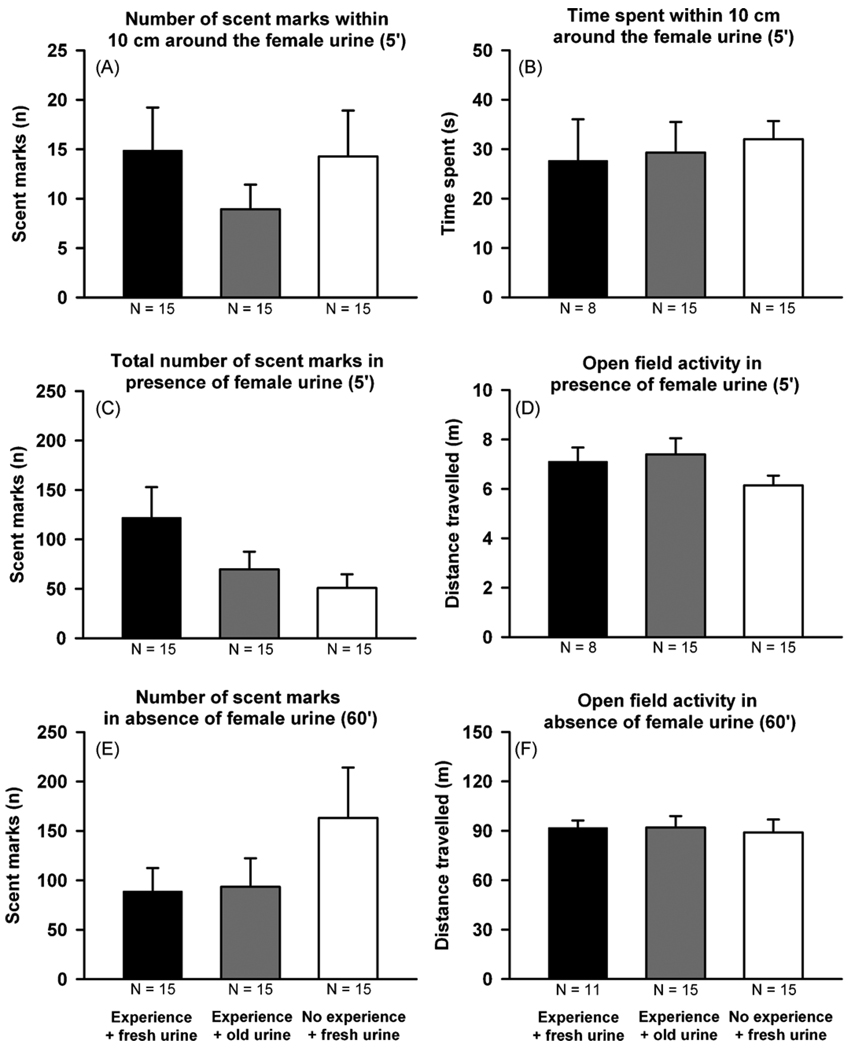
Fig. 3 illustrates scent-marking behavior by B6 male mice in response to a female urine spot in the open field arena. There was no significant difference in the number of scent marks left by B6 male mice within a distance of 10 cm around the 15 µl female B6 urine sample among the three conditions used to elicit the scent-marking behavior: (1) previous experience with a female and presentation of fresh female urine, (2) previous experience with a female and non-fresh female urine, and (3) no previous experience with a female and fresh female urine (F2,44 = 0.686, p = 0.509, Fig. 3A). Similarly, the three groups of male mice did not differ on time spent within a distance of 10 cm around the female urine spot (F2,44 = 0.065, p = 0.937, Fig. 3B). Total number of scent marks deposited in the entire open field in response to female urine was not significantly different among the male groups with or without previous experience with a female, and to fresh or 1–2 h old female urine, but a trend was indicated (F2,44 = 2.783, p = 0.073, Fig. 3C). Locomotor activity (total distance traveled) in the open field during this 5 min session was not different between the groups (F2,38 = 1.557, p = 0.225, Fig. 3D). In the absence of female urine, the three groups of male mice did not differ in their total number of scent marks deposited (F2,44 = 1.300, p = 0.283, Fig. 3E), or in their locomotor activity in the clean open field during the 60 min habituation session (F2,39 = 0.086, p = 0.918, Fig. 3F).
Fig. 3.
Scent-marking and locomotor activity in adult male C57BL/6J (B6) mice in the presence and absence of female urine. Number of scent marks deposited in proximity to the female urine spot (10 cm2) (A), time spent in proximity to the female urine spot (10 cm2) (B), total number of scent marks deposited throughout the entire open field (C), and open field activity (D) during the 5 min exposure to female urine. Total number of scent marks deposited in the entire open field (E) and open field activity (F) during the 60 min habituation to the clean open field. Black bar: B6 with previous female experience exposed to fresh female urine. Grey bar: B6 with previous female experience exposed to 1–2 h old female urine. White bar: B6 without previous female experience exposed to fresh female urine. Data are presented as means + standard errors of the mean.
As described in Table 1, call emission by males with previous female experience exposed to fresh female urine was positively correlated with female urine-elicited scent-marking within an area of 10 cm2 surrounding the female urine spot as recently reported (r = 0.571, p = 0.026 [67]). The present experiments show that call emissions by males with previous female experience exposed to 1–2 h old female urine and males without previous female experience exposed to fresh female urine were not significantly correlated with scent-marking within 10 cm2 of the female urine spot (r = 0.427, p = 0.112 and r = 0.122, p = 0.666, respectively). For the entire open field arena area, total call emission and total number of scent marks in the presence of female urine were correlated in males with previous female experience exposed to fresh female urine as recently reported (r = 0.552, p = 0.033 [67]). Correlations were similarly significant in males without previous female experience exposed to fresh female urine (r = 0.535, p = 0.040), but not in males with previous female experience exposed to 1–2 h old female urine (r =−0.165, p = 0.557).
Table 1.
Correlations (r value and * significance) between number of scent marks and ultrasonic vocalizations (USV) elicited by female urine.
| Experimental condition | Correlation between total number of scent marks and USV |
Correlation between number of scent marks within 10 cm of the female urine spot and USV |
|---|---|---|
| Previous female experience + fresh female urine [67] | 0.552* | 0.571* |
| Previous female experience + 1–2 h old female urine | −0.165 | 0.427 |
| No previous female experience + fresh female urine | 0.535* | 0.122 |
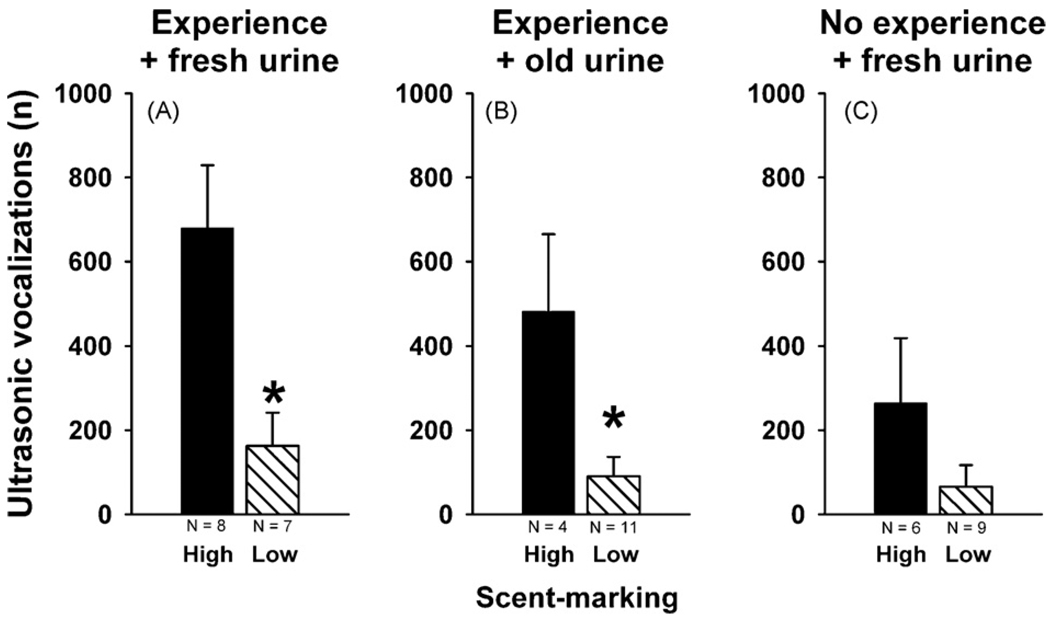
Fig. 4 shows the comparison of ultrasonic vocalizations by males that displayed high versus low levels of female urine-elicited scent-marking. High scent-marking was defined as the presence of at least one male scent mark in each of at least 3 out of the 4 blocks, 5 cm2 per block, constituting an area of 10 cm2 surrounding the female mouse urine spot. Low scent-marking was defined by the absence of this spatial pattern. Males displaying high scent-marking vocalized significantly more than males displaying low scent-marking. Differences were significant in males with previous female experience exposed to fresh female urine (t13 =−2.924, p = 0.012, Fig. 4A). A similar difference was detected in males with previous female experience exposed to 1–2 h old female urine (t13 =−3.006, p = 0.010, Fig. 4B). Again, males displaying high scent-marking vocalized significantly more than males displaying low scent-marking. Such a difference was not detected in males without previous female experience exposed to fresh female urine (t13 =−1.436, p = 0.175, Fig. 4C).
Fig. 4.
Ultrasonic vocalizations emitted by low and high scent-marking subgroups of adult male C57BL/6J (B6) mice in the presence of female urine. Total number of ultrasonic vocalizations emitted by subgroups of B6 subjects that deposited high versus low amounts of scent marks in the vicinity of the female urine spot during the 5 min test session. B6 males with previous female experience exposed to fresh female urine (A). B6 males with previous female experience exposed to 1–2 h old female urine (B). B6 males without previous female experience exposed to fresh female urine (C). Black bar: B6 that showed high levels of female urine-elicited scent-marking. Striped bar: B6 that showed low levels of female urine-elicited scent-marking. Data are presented as means + standard errors of the mean. *p < 0.05 for the high versus low comparison.
3.3. Male subject +male urine scent marks: scent-marking in B6 and BTBR
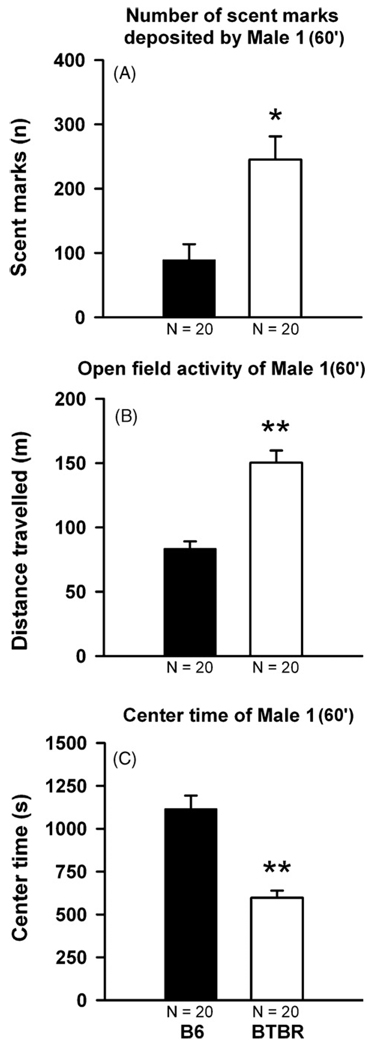
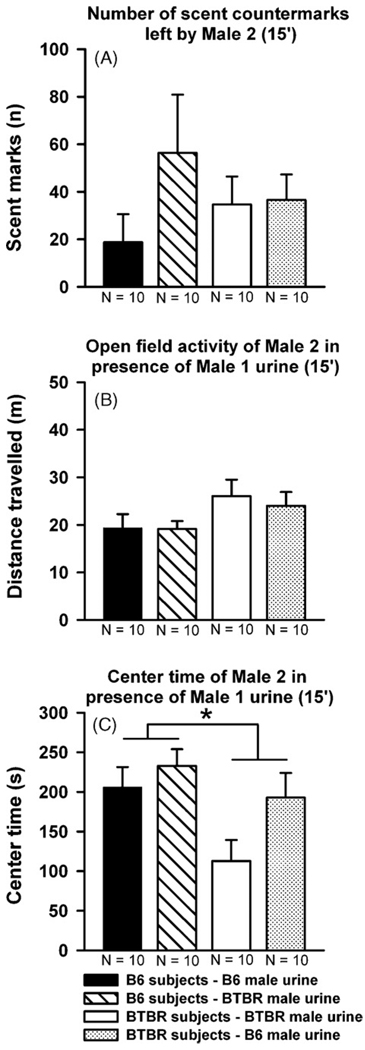
During the 60 min habituation session in the clean open field, BTBR Male 1 deposited more scent marks (t38 =−3.578, p = 0.001, Fig. 5A), displayed higher locomotor activity (t38 =−6.027, p < 0.001, Fig. 5B), and spent less time in the center of the open field as compared to B6 Male 1 (t38 = 5.893, p < 0.001, Fig. 5C). However, BTBR and B6 did not differ on the number of countermarks deposited by subject Male 2 to the scent marks deposited by Male 1 (F1,39 = 0.015, p = 0.902, Fig. 6A). BTBR Male 2 tended to show higher levels of locomotor activity during the 15 min test session than B6 Male 2 (F1,36 = 4.144, p = 0.05, Fig. 6B). In addition, BTBR and B6 again differed in the time they spent in the center of the open field (F1,36 = 5.829, p = 0.022, Fig. 6C). Furthermore, the number of countermarks deposited by subject Male 2 was not dependent on whether scent marks deposited by a BTBR or B6 Male 1 (F1,39 = 1.290, p = 0.264). Locomotor activity displayed by subject Male 2 and the time they spent in the center of the open field was not dependent on the strain of Male 1 (F1,36 = 0.043, p = 0.838 and F1,36 = 0.920, p = 0.345, respectively). No interaction between strain of subject Male 1 and strain of Male 2 was found for number of countermarks deposited and locomotor activity (F1,39 = 1.579, p = 0.217 and F1,36 = 0.077, p = 0.784, respectively), while there was a trend for center time (F1,36 = 3.742, p = 0.062). There was no significant correlation between number of scent marks deposited by Male 1 and scent counter marks left by subject Male 2 (under all conditions: p-values > 0.10).
Fig. 5.
Scent-marking and open field activity in adult male C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice in the absence of male urine. Total number of scent marks deposited in the entire open field (A), open field activity (B), and time spent in the center (C) during the 60 min habituation session in the clean open field. Black bar: B6 Male 1 subjects. White bar: BTBR Male 1 subjects. Data are presented as means + standard errors of the mean. *p < 0.05 and **p < 0.001 for the B6 versus BTBR comparison.
Fig. 6.
Scent-marking and open field activity in adult male C57BL/6J (B6) and BTBR T+tf/J (BTBR) mice in the presence of male urine: total number of scent countermarks deposited in the entire open field (A), open field activity (B), and time spent in the center (C) during the 15 min test session. Black bar: B6 Male 2 subjects exposed to B6 Male 1 scent marks. Striped bar: B6 Male 2 subjects exposed to BTBR Male 1 scent marks. White bar: BTBR Male 2 subjects exposed to BTBR Male 1 scent marks. Dotted bar: BTBR Male 2 subjects exposed to B6 Male 1 scent marks. Data are presented as means + standard errors of the mean. *p < 0.05 for the B6 versus BTBR comparison.
4. Discussion
Emission of female urine-elicited ultrasonic vocalizations in adult male mice was dependent on previous female experience. Male mice with a previous 5 min exposure to an adult female mouse approximately 1 week before the test session displayed an overall higher ultrasonic vocalization response than male mice with no previous exposure to an adult female mouse. Further, the latency to vocalize was shorter and the rate of vocalizations was more sustained in males with previous exposure to a female. Freshness of the female urine may be a second important factor in eliciting ultrasonic vocalizations. Call emission tended to be higher in response to fresh female urine as compared to non-fresh urine, presumably because volatile pheromones in the urine evaporated over the course of an hour or two at room temperature.
Our finding that mice without previous female experience emitted less vocalizations than mice with previous female experience is consistent with the existing literature on the effects of social experience on female urine-elicited ultrasonic calling [51,53,54,57,63–65]. These studies indicate that the signal value of female urine is at least in part learned during female experience by classical conditioning. Normally ineffective odors, such as commercial perfumes, can gain signal value when consistently paired with females after puberty, i.e. in a sexual context [76]. However, some findings appear to be inconsistent with a critical learning component. Mice that had no female experience reliably emitted ultrasonic vocalizations to female urine, although to a lower extent [57,64,65].More recent studies indicate that male mice emit ultrasonic vocalizations to at least two chemosignals present in female urine [66]. One chemosignal, which is present in freshly voided female urine, acts as an unconditioned stimulus. The second chemosignal, which remains after the first has disappeared, acts as a conditioned stimulus, eliciting responses to urine that remained at room temperature for 30 days [54]. Typically, ultrasonic vocalizations were detected in male mice without female experience only when fresh urine was used and ultrasonic vocalization responses to non-fresh female urine are weaker than in response to fresh urine [64–66]. These observations are in accordance with the present results, as mice tended to emit fewer vocalizations in response to non-fresh urine than in response to fresh urine.
In contrast, our results suggest that previous female experience and freshness of urine play a less prominent role in scent-marking to estrus female urine by adult male mice. Countermarking behavior has been shown to be dependent on non-volatile components [5] and male mice scent-marking is enhanced when the subjects are in direct contact with an unfamiliar male urine sample [77]. In our experiment, the male mice were in direct contact with the female urine sample, which could have induced a strong scent-marking response in the proximal zone around the female urine regardless of the experimental condition. However, the results for total number of scent mark in the entire arena appear to be similar to the results for ultrasonic vocalizations. Again, fresh female urine tended to be more effective in males with previous female experience. Since the difference in the production in ultrasonic vocalizations was significant only when the time course was taken into account, it may be interesting to focus the analysis of scent-marking behavior on time-dependent parameters.
The present studies explored the correlation between female urine-elicited scent-marking behavior and call emission. When considering the total number of scent marks deposited in the entire open field, a significant positive correlation was detected in mice exposed to fresh female urine after female experience. Similarly, such a positive correlation was seen in mice exposed to fresh female urine without previous female experience. However, in mice exposed to 1–2 h old female urine no such positive correlation was seen, despite the fact that they had previous female experience. Importantly, however, when considering only scent marks deposited in proximity to the female urine spot, a positive correlation between scent marks and ultrasonic vocalizations was only seen in the most biologically relevant context, namely when mice were exposed to fresh female urine after having being in contact with a female.
Results of the correlational analysis were confirmed by a comparison of number of ultrasonic vocalizations emitted between high and low scent-marking males. High scent-marking was defined as the presence of at least one male scent mark in each of at least 3 out of the 4 blocks constituting an area of 10 cm2 surrounding the female mouse urine spot. Low scent-marking was defined by the absence of this pattern. In support of a link between scent marks and ultrasonic vocalizations under certain conditions, high scent-marking mice vocalized more than low scent-marking mice when they were exposed to fresh female urine after female experience. Without female experience, no such association was found, while evidence for such an association was even found in mice exposed to 1–2 h old urine after female experience. Overall, these findings show that there is a clear link between scent-marking and the emission of ultrasonic vocalizations in mice exposed to fresh female urine after female experience. In mice exposed to 1–2 h old urine or without female experience, such an association is less prominent or missing. In total, correlational analyses and the comparison between high and low scent-marking mice indicate that scent-marking responses proximal to female urine could be an important measure that may convey information about the abilities of the subject to detect and respond to social cues, as compared to scent-marking at distal regions of the open field.
The lack of significant correlations between call emission and female urine-elicited scent-marking behavior in the latter experimental conditions is unlikely due to lower levels of call production or scent-marking behavior. In fact, no such correlation was seen in B6 mice exposed to fresh female BTBR urine, a condition that was found to be highly effective in eliciting ultrasonic vocalizations and scent-marking [67]. These findings indicate therefore that call emission and female urine-elicited scent-marking behavior are positively correlated only in the most biologically relevant context, male mice exposed to fresh female urine after female experience. This means that previous female experience is not only affecting the emission of female urine-elicited ultrasonic vocalizations, but also the correlations between olfactory and acoustic signaling.
The present evidence for an association of scent marks and ultrasonic vocalizations in response to female urine is in accordance with studies showing that both scent-marking behavior and the emission of ultrasonic vocalizations are androgen-dependent [60,78,79]. Furthermore, repeated social defeats suppress scent-marking and ultrasonic calling [60], probably by reducing androgen levels [80]. Importantly, however, to our knowledge, our data offer the first clear evidence for a modulation of such an association by a social factor, namely previous female experience, consisting of a 5 min exposure to an adult female mouse approximately 1 week before the test session.
Recently we reported that scent-marking as well as the emission of ultrasonic vocalizations by male mice in response to female urine is dependent on the sociability of the strain [67]. The highly social strain B6 deposited more scent marks and emitted more ultrasonic vocalizations in response to a female urine spot than BTBR. The BTBR inbred mouse strain is characterized by low sociability on reciprocal social interaction and social approach tasks, and may represent a mouse model of autism [68–74]. While it is known that male mice do not vocalize in response to male urine scent marks [53–57]), it is well established that male mice deposit scent countermarks in response to male urine traces [12,24,58,81,82].
The question addressed in the present study was therefore whether this strain difference is specific to exposure to female urine or also seen in response to male urine traces. In support of a stimulus specificity of this strain difference, B6 and BTBR did not differ on the number of scent countermarks deposited by subject Male 2 to the scent marks deposited by Male 1. Lack of a strain difference in scent countermarking during exposure of male urine traces, while there was a strain difference in scent-marking during habituation, may indicate an inhibitory effect of male urine traces on scent countermarking behavior in BTBR. However, the similarity between B6 and BTBR in scent countermarking behavior could also be due to the shorter duration of the test session as compared to the habituation session. Strain differences in locomotor activity and time spent in the center of the open field appear to be independent of exposure to male urine traces, as the same strain differences were detected during habituation. Furthermore, number of scent countermarks deposited by subject Male 2 as well as its locomotor behavior was not dependent on whether scent marks were deposited by a BTBR or B6 Male 1. Overall, these findings indicate that there is no prominent effect of strain on male/male scent countermarking behavior when comparing B6 and BTBR. These data contrast with the recently reported strain difference in scent-marking behavior in response to a female urine spot [67] for two reasons. Firstly, while higher levels of scent-marking during habituation were detected in BTBR than in B6 in the present study, no such difference was seen in the earlier study [67]. The difference might be due to differences in social experience, locomotor activity, or to the age of the mice tested in the present study (20 ± 1 weeks versus 13 ± 1 weeks in [67]) as those parameters have been shown to influence the scent-marking behavior in B6 male mice [75]. Secondly, while no strain difference in male urine-elicited scent countermarking was detected in the present study, a strain difference was detected in female urine-elicited scent-marking in the earlier study [67]. One of the most likely explanations for this contrast is the context-specificity of the communicative function of scent-marking. While female urine-elicited scent marks are relevant to sexual mating [18–24], male/male scent-marking represents a different domain of mouse communication, relevant to territoriality [5,7–15] and dominance hierarchies [12,58–60].
To summarize, emission of female urine-elicited ultrasonic vocalizations in adult male mice was dependent on previous female experience. Male mice with a previous 5 min exposure to an adult female mouse approximately 1 week before the test session displayed an overall higher ultrasonic vocalization response to female urine than male mice with no previous exposure to an adult female mouse. Further, the latency to vocalize was shorter, and the rate of vocalizations was more sustained, in males with previous exposure to a female. In contrast, our results suggest that previous female experience plays a less prominent role in scent-marking to estrus female urine by adult male mice. There was a positive correlation between female urine-elicited scent-marking behavior and ultrasonic calling in the most biologically relevant context, male mice exposed to fresh female urine after female experience. This association was less prominent or missing in less biologically relevant contexts, e.g. in male mice exposed to fresh female urine without previous female experience, indicating that previous female experience is not only affecting the emission of female urine-elicited ultrasonic vocalizations, but also the synchronization of the acoustic and olfactory communicatory pathways. In light of our recently reported strain difference in scent-marking behavior and ultrasonic calling between the highly social strain B6 and the autism mouse model BTBR [67], the strain difference between B6 and BTBR appears to be specific to female urine-elicited behavior, as it was not seen in response to male urine traces, highlighting the relevance of the social context in which mouse communication is evaluated.
Acknowledgment
Supported by the National Institute of Mental Health Intramural Research Program.
References
- 1.Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev. 2008;32:1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan PA, Kendrick KM. Mammalian social odours: attraction and individual recognition. Phil Trans Roy Soc Lond. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts SC. Scent marking. In: Wolff JO, Sherman PW, editors. Rodent societies. An ecological and evolutionary perspective. Chicago: The University of Chicago Press; 2007. pp. 255–266. [Google Scholar]
- 4.Wyatt TD. Communication by taste and smell. Cambridge: Cambridge University Press; 2003. Pheromones and animal behaviour. [Google Scholar]
- 5.Humphries RE, Robertson DHL, Beynon RJ, Hurst JL. Unravelling the chemical basis of competitive scent marking in house mouse. Anim Behav. 1999;58:1177–1190. doi: 10.1006/anbe.1999.1252. [DOI] [PubMed] [Google Scholar]
- 6.Ralls K. Mammalian scent marking. Science. 1971;171:443–449. doi: 10.1126/science.171.3970.443. [DOI] [PubMed] [Google Scholar]
- 7.Gosling LM, Roberts SC, Thornton EA, Andrew MJ. Life history costs of olfactory status signaling in mice. Behav Ecol Sociobiol. 2000;48:328–332. [Google Scholar]
- 8.Jones RB, Nowell NW. The coagulating glands as a source of averse and aggression-inhibiting pheromone(s) in the male albino mouse. Physiol Behav. 1973;10:221–223. doi: 10.1016/0031-9384(73)90031-0. [DOI] [PubMed] [Google Scholar]
- 9.Jones RB, Nowell NW. Aversive potency of urine from dominant and subordinate male laboratory mice (Mus musculus): resolution of a conflict. Aggress Behav. 1989;15:291–296. [Google Scholar]
- 10.Archer JE. The effect of strange male odor on aggressive behavior in male mice. J Mammal. 1968;49:572–575. [PubMed] [Google Scholar]
- 11.Dixon AK, Mackintosh JH. Effects of female urine upon the social behavior of adult male mice. Anim Behav. 1971;19:138–140. [Google Scholar]
- 12.Hurst JL. Urine marking in populations of wild house mice (Mus domesticus Rutty). I. Communication between males. Anim Behav. 1990;40:209–222. [Google Scholar]
- 13.Lacey JC, Beynon RJ, Hurst JL. The importance of exposure to other male scents in determining competitive behaviour among inbred male mice. Appl Anim Behav Sci. 2007;104:130–142. [Google Scholar]
- 14.Mucignat-Caretta C, Cavaggioni A, Caretta A. Male urinary chemosignals differentially affect aggressive behavior in male mice. J Chem Ecol. 2004;30:777–791. doi: 10.1023/b:joec.0000028431.29484.d7. [DOI] [PubMed] [Google Scholar]
- 15.Mugford RA, Nowell NW. Pheromones and their effect on aggression in mice. Nature. 1970;22:967–968. doi: 10.1038/226967a0. [DOI] [PubMed] [Google Scholar]
- 16.Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice. Behav Brain Res. 2008;190:97–104. doi: 10.1016/j.bbr.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowers JM, Alexander BK. Mice: individual recognition by olfactory cues. Science. 1967;158:1208–1210. doi: 10.1126/science.158.3805.1208. [DOI] [PubMed] [Google Scholar]
- 18.Caroom D, Bronson FH. Responsiveness of female mice to perpetual attractant: effects of sexual experience and ovarian hormones. Physiol Behav. 1971;7:659–662. doi: 10.1016/0031-9384(71)90126-0. [DOI] [PubMed] [Google Scholar]
- 19.Drickamer LC. Oestrous female house mice discriminate dominant from subordinate males and sons of dominant from sons of subordinate males by odour cues. Anim Behav. 1992;43:868–870. [Google Scholar]
- 20.Hurst JL. Urine marking in populations of wild house mice (Mus domesticus Rutty). III. Communication between the sexes. Anim Behav. 1990;40:233–243. [Google Scholar]
- 21.Jones RB, Nowell NW. A comparison of the aversive and female attractant properties of urine from dominant and subordinate male mice. Anim Learn Behav. 1974;2:141–144. doi: 10.3758/bf03199141. [DOI] [PubMed] [Google Scholar]
- 22.Scott JW, Pfaff DW. Behavioral and electrophysiological responses of female mice to male urine odors. Physiol Behav. 1970;5:407–411. doi: 10.1016/0031-9384(70)90243-x. [DOI] [PubMed] [Google Scholar]
- 23.Zala SM, Potts WK, Penn DJ. Scent-marking displays provide honest signals of health and infection. Behav Ecol. 2004;15:338–344. [Google Scholar]
- 24.Rich TJ, Hurst JL. The competing countermarks hypothesis: reliable assessment of competitive ability by potential mates. Anim Behav. 1999;58:1027–1037. doi: 10.1006/anbe.1999.1217. [DOI] [PubMed] [Google Scholar]
- 25.Whitten WK. Effect of exteroceptive factors on the oestrous cycle of mice. Nature. 1957;180:1436. doi: 10.1038/1801436a0. [DOI] [PubMed] [Google Scholar]
- 26.Vandenbergh JG. Male odor accelerates female sexual maturation in mice. Endocrinology. 1969;84:658–660. doi: 10.1210/endo-84-3-658. [DOI] [PubMed] [Google Scholar]
- 27.Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- 28.Costantini F, D’Amato FR. Ultrasonic vocalizations in mice and rats: social contexts and functions. Acta Zool Sin. 2006;52:619–633. [Google Scholar]
- 29.Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;28:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- 30.Sales GD, Pye D. Ultrasonic communication by animals. NewYork: Wiley; 1974. [Google Scholar]
- 31.Wöhr M, Schwarting RKW. Activation of limbic system structures by replay of ultrasonic vocalization in rats, Chapter 4.2. In: Brudzynski SM, editor. Handbook of mammalian vocalization. An integrative neuroscience approach. Amsterdam: Academic Press–Elsevier; 2010. pp. 113–124. [Google Scholar]
- 32.Wöhr M, Oddi D, D’Amato FR. Effect of altricial pup ultrasonic vocalization on maternal behavior, Chapter 5.2. In: Brudzynski SM, editor. Handbook of mammalian vocalization. An integrative neuroscience approach. Amsterdam: Academic Press–Elsevier; 2010. pp. 159–166. [Google Scholar]
- 33.Ehret G, Haack B. Ultrasound recognition in house mice: key-stimulus configuration and recognition mechanisms. J Comp Physiol. 1982;148:245–251. [Google Scholar]
- 34.Sewell GD. Ultrasonic communication in rodents. Nature. 1970;227:410. doi: 10.1038/227410a0. [DOI] [PubMed] [Google Scholar]
- 35.Wöhr M, Schwarting RKW. Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behav Neurosci. 2008;122:310–330. doi: 10.1037/0735-7044.122.2.310. [DOI] [PubMed] [Google Scholar]
- 36.Wöhr M, Dahlhoff M, Wolf E, Holsboer F, Schwarting RK, Wotjak CT. Effects of genetic background, gender, and early environmental factors on isolation-induced ultrasonic calling in mouse pups: an embryo-transfer study. Behav Genet. 2008;38:579–595. doi: 10.1007/s10519-008-9221-4. [DOI] [PubMed] [Google Scholar]
- 37.Brudzynski SM, Chiu EMC. Behavioural responses of laboratory rats to playback of 22 kHz ultrasonic calls. Physiol Behav. 1995;57:1039–1044. doi: 10.1016/0031-9384(95)00003-2. [DOI] [PubMed] [Google Scholar]
- 38.Sales GD. The effect of 22 kHz calls and artificial 38 kHz signals on activity in rats. Behav Proc. 1991;24:83–93. doi: 10.1016/0376-6357(91)90001-G. [DOI] [PubMed] [Google Scholar]
- 39.Wöhr M, Schwarting RKW. Ultrasonic communication in rats: can playback of 50-kHz calls induce approach behavior? PLoS One. 2007;2:e1365. doi: 10.1371/journal.pone.0001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wöhr M, Schwarting RKW. Ultrasonic calling during fear conditioning in the rat: no evidence for an audience effect. Anim Behav. 2008;76:749–760. [Google Scholar]
- 41.Wöhr M, Moles A, Schwarting RKW, D’Amato FR. A lack of social exploratory activation in male μ-opioid receptor KO mice in response to playback of female ultrasonic vocalizations. Soc Neurosci. doi: 10.1080/17470911003765560. in press. [DOI] [PubMed] [Google Scholar]
- 42.Geyer LA, Barfield RJ. Influence of gonadal hormones and sexual behavior on ultrasonic vocalization in rats. I. Treatment of females. J Comp Physiol Psychol. 1978;92:436–446. doi: 10.1037/h0077480. [DOI] [PubMed] [Google Scholar]
- 43.Geyer LA, McIntosh TK, Barfield RJ. Effects of ultrasonic vocalizations and male’s urine on female rat readiness to mate. J Comp Physiol Psychol. 1978;92:457–462. [Google Scholar]
- 44.Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic ‘songs’ with approach behaviour. Biol Lett. 2009;5:589–592. doi: 10.1098/rsbl.2009.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McIntosh TK, Barfield RJ, Geyer LA. Ultrasonic vocalisations facilitate sexual behaviour of female rats. Nature. 1978;272:163–164. doi: 10.1038/272163a0. [DOI] [PubMed] [Google Scholar]
- 46.Musolf K, Hoffmann F, Penn DJ. Ultrasonic courtship vocalizations in wild house mice, Mus musculus musculus. Anim Behav. 2010;79:757–764. [Google Scholar]
- 47.Pomerantz SM, Nunez AA, Bean NJ. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiol Behav. 1983;31:91–96. doi: 10.1016/0031-9384(83)90101-4. [DOI] [PubMed] [Google Scholar]
- 48.Thomas DA, Talalas L, Barfield RJ. Effects of devocalization of the male on mating behavior in rats. J Comp Physiol Psychol. 1981;95:630–637. [Google Scholar]
- 49.White NR, Barfield RJ. Effects of male pre-ejaculatory vocalizations on female receptive behavior in the rat (Rattus norvegicus) J Comp Psychol. 1990;104:140–146. doi: 10.1037/0735-7036.104.2.140. [DOI] [PubMed] [Google Scholar]
- 50.Thomas DA, Howard SB, Barfield RJ. Male-produced postejaculatory vocalizations and the mating behaviour of estrous female rats. Behav Neural Biol. 1982;36:403–410. doi: 10.1016/s0163-1047(82)90802-0. [DOI] [PubMed] [Google Scholar]
- 51.Dizinno G, Whitney G, Nyby J. Ultrasonic vocalizations by male mice (Mus musculus) to female sex pheromone: experimental determinants. Behav Biol. 1978;22:104–113. [Google Scholar]
- 52.Guo Z, Holy TE. Sex selectivity of mouse ultrasonic songs. Chem Sens. 2007;23:463–473. doi: 10.1093/chemse/bjm015. [DOI] [PubMed] [Google Scholar]
- 53.Nyby J, Wysocki CJ, Whitney G, Dizinno G. Pheromonal regulation of male mouse ultrasonic courtship (Mus musculus) Anim Behav. 1977;25:333–341. doi: 10.1016/0003-3472(77)90009-4. [DOI] [PubMed] [Google Scholar]
- 54.Nyby J, Zakeski D. Elicitation of male mouse ultrasounds: bladder urine and aged urine from females. Physiol Behav. 1980;24:737–740. doi: 10.1016/0031-9384(80)90405-9. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2,M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitney G, Alpern M, Dizinno G, Horowitz G. Female odors evoke ultrasounds from male mice. Anim Learn Behav. 1974;2:13–18. doi: 10.3758/bf03199109. [DOI] [PubMed] [Google Scholar]
- 57.Nyby J, Bigelow J, Kerchner M, Barbehenn F. Male mouse (Mus musculus) ultrasonic vocalizations to female urine: why is heterosexual experience necessary? Behav Neural Biol. 1983;38:32–46. doi: 10.1016/s0163-1047(83)90354-0. [DOI] [PubMed] [Google Scholar]
- 58.Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science. 1973;182:939–941. doi: 10.1126/science.182.4115.939. [DOI] [PubMed] [Google Scholar]
- 59.Drickamer LC. Urine marking and social dominance in male house mice (Mus musculus domesticus) Behav Proc. 2001;53:113–120. doi: 10.1016/s0376-6357(00)00152-2. [DOI] [PubMed] [Google Scholar]
- 60.Lumley LA, Sipos ML, Charles RC, Charles RF, Meyerhoff JL. Social stress effects on territorial marking and ultrasonic vocalizations in mice. Physiol Behav. 1999;67:769–775. doi: 10.1016/s0031-9384(99)00131-6. [DOI] [PubMed] [Google Scholar]
- 61.D’Amato FR. Courtship ultrasonic vocalizations and social status in mice. Anim Behav. 1991;41:875–885. [Google Scholar]
- 62.Nyby J, Dizinno GA, Whitney G. Social status and ultrasonic vocalizations of male mice. Behav Biol. 1976;18:285–289. doi: 10.1016/s0091-6773(76)92198-2. [DOI] [PubMed] [Google Scholar]
- 63.Maggio JC, Maggio JH, Whitney G. Experience-based vocalization of male mice to female chemosignals. Physiol Behav. 1983;31:269–272. doi: 10.1016/0031-9384(83)90186-5. [DOI] [PubMed] [Google Scholar]
- 64.Sipos ML, Kerchner M, Nyby JG. An ephemeral sex pheromone in the urine of female house mice (Mus domesticus) Behav Neural Biol. 1992;58:138–143. doi: 10.1016/0163-1047(92)90375-e. [DOI] [PubMed] [Google Scholar]
- 65.Sipos ML, Wysocki CJ, Nyby JG, Wysocki L, Nemura TA. An ephemeral pheromone of female house mice: perception via the main and accessory olfactory systems. Physiol Behav. 1995;58:529–534. doi: 10.1016/0031-9384(95)00089-2. [DOI] [PubMed] [Google Scholar]
- 66.Sipos ML, Nyby JG, Serran MF. An ephemeral sex pheromone in the urine of female house mice (Mus domesticus): pheromone fade-out time. Physiol Behav. 1993;54:171–174. doi: 10.1016/0031-9384(93)90061-j. [DOI] [PubMed] [Google Scholar]
- 67.Wöhr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. doi: 10.1111/j.1601-183X.2010.00582.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behaviour in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 70.Moy SS, Nadler JJ, Young NB, Perez AP, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, et al. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57BL/6J mice: sexual and developmental determination. Behav Brain Res. 2007;182:73–79. doi: 10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nyby J, Whitney G, Schmitz S, Dizinno G. Postpubertal experience establishes signal value of mammalian sex odor. Behav Biol. 1978;22:545–552. doi: 10.1016/s0091-6773(78)92745-1. [DOI] [PubMed] [Google Scholar]
- 77.Nevison CM, Armstrong S, Beynon RJ, Humphries RE, Hurst JL. The ownership signature in the mouse scent marks is involatile. Proc Biol Sci. 2003;270(1527):1957–1963. doi: 10.1098/rspb.2003.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nyby J, Matochik JA, Barfield RJ. Intracranial androgenic and estrogenic stimulation of male-typical behaviors in house mice (Mus domesticus) Horm Behav. 1992;26:24–45. doi: 10.1016/0018-506x(92)90029-u. [DOI] [PubMed] [Google Scholar]
- 79.Sipos ML, Nyby JG. Intracranial androgenic activation of male-typical behaviours in house mice: concurrent stimulation of the medial preoptic area and medial nucleus of the amygdale. J Neuroendocrinol. 1998;10:577–586. doi: 10.1046/j.1365-2826.1998.00215.x. [DOI] [PubMed] [Google Scholar]
- 80.Bronson FH, Desjardins C. Relationships between scent marking by male mice and the pheromone-induced secretion of the gonadotropic and ovarian hormones that accompany puberty in female mice. In: Montagna W, Sadler WA, editors. Reproductive behavior. New York: Plenum Press; 1974. pp. 157–178. [DOI] [PubMed] [Google Scholar]
- 81.Arakawa H, Arakawa K, Blanchard C, Blanchard RJ. Social features of scent-donor mice modulate scent marking of C57BL/6J recipient males. Behav Brain Res. 2009;205:138–145. doi: 10.1016/j.bbr.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maruniak JA, Owen K, Bronson FH, Desjardins C. Urinary marking in male house mice: responses to novel environmental and social stimuli. Physiol Behav. 1974;12:1035–1039. doi: 10.1016/0031-9384(74)90151-6. [DOI] [PubMed] [Google Scholar]