Abstract
The herpes simplex virus 1 (HSV-1) virion host shutoff protein (vhs) degrades viral and cellular mRNAs. Here, we demonstrate for the first time that vhs also boosts translation of viral true late mRNAs in a cell type-dependent manner and that this effect determines the viral growth phenotype in the respective cell type. Our study was prompted by the detection of stress granules, indicators of stalled translation initiation, in cells infected with vhs mutants but not in wild-type-virus-infected cells. Accumulation of true late-gene products gC and US11 was strongly reduced in the absence of vhs in HeLa cells and several other restrictive cell lines but not in Vero and other permissive cells and was independent of phosphorylation of the α subunit of eukaryotic initiation factor 2 (eIF2α). Polysome analysis showed that gC and US11 transcripts were poorly translated in vhs-null-virus-infected HeLa cells, while translation of a cellular mRNA was not affected. Interestingly, hippuristanol, an eIF4A inhibitor, produced a similar phenotype in HeLa cells infected with wild-type HSV-1, while Vero cells were much more resistant to the inhibitor. These results suggest that translation of true late-gene transcripts is particularly sensitive to conditions of limited access to translation factors and that vhs is able either to prevent the limiting conditions or to facilitate translation initiation under these conditions. The varied permissivity of cell lines to vhs-null infection may stem from differences in the resilience of the translation machinery or the ability to control the accumulation of mRNAs.
INTRODUCTION
Herpes simplex virus 1 (HSV-1), a large, enveloped DNA virus, is a master in subverting host cell functions for its own benefit. In particular, HSV-1 employs several mechanisms to block expression of cellular proteins and facilitate efficient synthesis of viral proteins (reviewed in references 29 and 55). The viral protein that executes the earliest attack on cellular gene expression is the virion host shutoff (vhs) protein (reviewed in reference 54).
The vhs protein, encoded by the UL41 gene, is part of the tegument and as such is delivered into the cytoplasm after fusion of the virion envelope with the host cell membrane (43). It is an endoribonuclease that degrades both cellular and viral mRNA (24, 32). The degradation of cellular mRNA presumably improves the access of viral mRNAs to the cellular translation machinery. The vhs-mediated increased turnover of viral mRNAs is thought to sharpen the transition between the successive expression of viral immediate-early (IE), early (E), and late (L) genes (32).
vhs is able to degrade all types of RNA in an in vitro system (10, 60, 61, 63); however, in infected cells vhs degrades only mRNA and spares other RNA species (24, 32). In an effort to explain this mRNA specificity, vhs has been shown to associate with the translation initiation complex eIF4F, which binds the 5′ cap structure of mRNAs (33). In particular, vhs interacts with the eIF4F component eIF4AII, an ATP-dependent RNA helicase and the helicase cofactors eIF4H and eIF4B (7, 11). These observations suggest that interactions with eIF4AII, eIF4H, and eIF4B target vhs to actively translating mRNAs. This model is underscored by the finding that knockdown of eIF4H prevented vhs-mediated mRNA decay in cell culture (47).
vhs-mediated shutoff of host protein synthesis occurs mainly in the immediate-early and early phases of infection. Later in infection, the nuclease activity of newly synthesized vhs appears to be dampened by the viral proteins VP16 and VP22 (22, 25, 48, 52). This is thought to be necessary to ensure sufficient accumulation of viral late-gene transcripts and efficient virus production.
Since vhs destabilizes most mRNAs, it reduces synthesis of proteins involved in the innate and adaptive immune responses. This vhs-mediated downregulation is most likely the reason for the capacity of vhs to dampen the type I interferon (IFN) system (8, 31, 35), block activation of dendritic cells (45), and reduce production of proinflammatory cytokines and chemokines (58). These factors might account at least in part for the crucial role of vhs in HSV-1 virulence and pathogenicity (reviewed in reference 54). For example, vhs mutants are strongly attenuated in the corneas and central nervous system of mice (26, 34, 36, 56, 57). Accordingly, replication and virulence of vhs mutants is enhanced in knockout mice lacking IFN receptors (8, 26) or STAT1 (34, 36). However, even in IFN-αβγ−/− and STAT−/− mice, the vhs mutant is still attenuated relative to the wild-type (WT) virus (26, 34, 36), suggesting that vhs contributes to efficient replication beyond its role as modulator of the immune response.
In cell culture, the attenuation of vhs-deficient HSV-1 is less pronounced than in mice. Similar to what was observed in the mouse model, the reduced virus yield in mouse embryonic fibroblasts (MEFs) is not completely due to IFN signaling, since virus titers of a vhs mutant in multicycle growth curve analyses were only partially restored in IFN-αβγR−/− MEFs (35). Knockout of the double-stranded-RNA (dsRNA)-dependent protein kinase R (PKR) protein did not alleviate this defect (35). vhs-deficient HSV-1 is also slightly attenuated in single-step growth curves in Vero cells, which lack IFN-α and IFN-β genes (39, 40, 53), suggesting an IFN-independent growth defect. The longstanding hypothesis advanced to explain this inherent growth defect is that the inappropriate overaccumulation of IE and E mRNAs might impair replication (32), but the exact mechanism by which this impairment might be achieved has not been thoroughly examined so far.
A potential clue to resolving this question came from two publications that point to a novel role for vhs in modulating translation. First, our group showed that vhs modulates translation in a bicistronic reporter gene assay without significantly altering the level of the respective mRNA (44). While expression of the 5′ cistron was inhibited by vhs, expression of the 3′ cistron was dependent on the sequence of the intergenic region. Expression driven by the encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES) was suppressed, whereas a mutant EMCV IRES and the cellular ApaF1, BiP, and DAP5 IRES elements facilitated increased expression in the presence of vhs. Interestingly, the 5′ untranslated region (UTR) of the HSV-1 mRNAs for UL12, thymidine kinase (TK), or glycoprotein C (gC) also mediated vhs-dependent expression of the 3′ cistron, suggesting that vhs may be involved in boosting translation of viral mRNAs during infection (44). Second, Esclatine and colleagues reported that vhs-deficient HSV-1 (F) induces formation stress granules (SGs) (9), which are considered diagnostic for the accumulation of stalled translation initiation intermediates (20, 21). SGs form as a response to various forms of stress (reviewed in reference 1). Depending on the type of stress, the serine/threonine kinases PKR, PKR-like endoplasmic reticulum kinase (PERK), general control nonrepressed 2 (GCN2), and heme-regulated inhibitor (HRI) are activated and phosphorylate the translation initiation factor eIF2α (reviewed in reference 46). This event stalls the initiation process at the point of loading the initiator tRNAiMet onto the small ribosomal subunit (reviewed in reference 16) and results in accumulation of 48S preinitiation complexes containing mRNA, the translation initiation complex eIF4F, poly(A) binding protein (PABP), eIF3, eIF1, eIF1A, and the small ribosomal subunit (19). The preinitiation complexes are then assembled into SGs by proteins such as TIA-1 and TIAR (20, 21). Notably, SG formation and inhibition of translation can also occur independent of eIF2α phosphorylation, for example, by blocking the activity of eIF4A with the novel compounds hippuristanol and pateamine (2, 3, 28). Since SGs are dynamic structures, mRNAs shuttle between SGs and polysomes to resume translation or are targeted to processing bodies for degradation (reviewed in reference 1).
Based on these observations, we hypothesized that inactivation of vhs may impair translation of viral proteins accounting for the reduced virus yield. To test this hypothesis, we examined the effect of vhs mutations on SG formation, viral replication, and viral gene expression in various cell types. We report here that infection with vhs-deficient HSV-1 led to SG formation, reduced virus yield, and decreased accumulation of true late-gene products in HeLa cells and several additional cell lines but not in Vero cells. Furthermore, we determined that the reduced late-gene expression was due to reduced translational activity on true late-gene transcripts in HeLa cells. This block in translation initiation was specific for viral transcripts, i.e., cellular mRNAs were not affected. Inhibitor studies implied that the resilience of the translational machinery of the respective cell type might be critical for translation of true late-gene transcript in the absence of vhs.
Taken together, these findings suggest that in many cell types vhs plays an important role in boosting translation of late-gene products and that the lack of this activity might account for the inherent growth defect of vhs mutants.
MATERIALS AND METHODS
Cells and viruses.
HeLa (cervical carcinoma), Vero (African green monkey kidney), U2OS (osteosarcoma), HEp2, and SK-N-SH (neuroblastoma) cells and HFF TEL 12 (telomerase immortalized human foreskin fibroblasts; kind gift from W. Bresnahan) were maintained in Dulbecco's modified Eagle Medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Human embryo lung (HEL) fibroblasts were cultured as described above except for the addition of 1 mM sodium pyruvate. The wild-type HSV-1 strains used in this study were KOS and its plaque-purified derivative KOS1.1 (17), and the vhs mutants were KOS-derived ΔSma, which contains a 588-nucleotide deletion in the UL41 gene (40), vhs1, which contains a point mutation in the UL41 gene (39), and GFP vhs−, in which all but the last 7 codons of the UL41 open reading frame (ORF) are deleted and replaced by green fluorescent protein (GFP), so that GFP is under the control of the vhs promoter (4). Viruses were propagated, and the titer in Vero cells was determined. Virus absorption, infection, and mock infection were carried out at 37°C at a multiplicity of 10 PFU/cell.
Drug treatments.
Phosphonoacetic acid (PAA; Sigma) was used at 300 μg/ml starting at 1 h postinfection (hpi) to inhibit viral DNA replication. Thapsigargin (Sigma) was used at 1 μM for 1 h prior to fixing to induce endoplasmic reticulum stress. Hippuristanol (a polyoxygenated streroid isolated from the coral Isis hippuris [3]) in dimethyl sulfoxide (DMSO) was used at concentrations ranging from 20 nM to 4,000 nM starting at 1 hpi to inhibit eIF4A activity. Corresponding amounts of DMSO were used as controls.
Immunofluorescence (IF) microscopy.
Cells grown on coverslips were mock infected or infected with the respective viruses at a multiplicity of infection (MOI) of 10. At the indicated time points, cells were washed with phosphate-buffered saline (PBS), fixed with 3.65% paraformaldehyde for 15 min, permeabilized with ice-cold methanol for 10 min, washed with PBS, and blocked with 5% fetal bovine serum (FBS) or 2.5% human serum. Proteins were stained with primary antibodies specific for HSV-1 ICP4 (mouse, P1101; Virusys Corporation) and cellular TIA-1 (goat, C-20; Santa Cruz), TIAR (goat, C-18; Santa Cruz), PABP (mouse, 10E10; Santa Cruz), G3BP (mouse, 611126; BD Transduction Laboratories), and eIF4A1 (rabbit, ab31217; Abcam). Primary antibodies were detected by suitable secondary antibodies coupled to Alexa 488, Alexa 555, or Alexa 647 (Invitrogen). Images were obtained using an Axiovert 200 M fluorescence microscope, an ApoTome optical sectioning device (Zeiss), and the AxioVision 4.5 program (Zeiss). All image processing was performed using Photoshop CS3 (Adobe).
Western blot analysis.
Mock-infected and infected cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (1% Igepal, 150 mM NaCl, 20 mM Tris-HCl [pH 7.5], 1 mM EDTA, 0.1% SDS, 0.25% sodium deoxycholate, 1× Roche protease inhibitor, 10 mM Na–β-glycerophosphate, 2 mM Na3VO4, 1 mM NaF). Cleared lysates were analyzed by 7.5% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a nitrocellulose membrane (Hybond ECL; Amersham), and immunoblotted with primary antibodies specific for the HSV-1 proteins gC (mouse, P1104; Virusys Corporation), gB (mouse, P1123; Virusys), ICP4 (mouse, P1101; Virusys Corporation), ICP27 (mouse, P1113; Virusys Corporation), ICP34.5 (rabbit; kind gift from Ian Mohr), US11 (mouse; kind gift from Bernard Roizman), UL47 (rabbit; kind gift from Gillian Elliott), thymidine kinase (TK) (rabbit; kind gift from William C. Summers), and VP16 (mouse, LP1; kind gift from Tony Minson) and the cellular proteins β-actin (mouse, A5441; Sigma), phospho-eIF2α (rabbit, 9721; Cell Signaling), and eIF2α (rabbit, 9722; Cell Signaling). Primary antibodies were detected by suitable secondary horseradish peroxidase (HRP)-conjugated antibodies using an enhanced chemiluminescence protocol (GE Healthcare) (see Fig. 3 and 7) or suitable secondary antibodies coupled to Alexa Fluor 680 (Invitrogen) or IRDye800 (Rockland), using the Odyssey infrared imaging system (LI-COR) (see Fig. S2 in the supplemental material).
Fig. 3.
Single-cycle growth curve analyses. HeLa and Vero cells (A), HEp2, HFF, and SK-N-SH cells (B), or HEL and U2OS cells (C) were infected with KOS1.1 or ΔSma at an MOI of 10. At the indicated time points, cells and supernatants were harvested. Viral titers were determined by a plaque assay for Vero cells. The graphs represent the averages of results from three independent experiments. Error bars indicate the standard deviations.
RNA extraction and Northern blot analysis.
Total RNA was isolated from cells in 60-mm culture dishes by use of TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA samples (10 μg) were subjected to 1.2% agarose-formaldehyde gel electrophoresis, visualized by SYBR gold staining, and transferred to a Genescreen membrane (NEN). Hybridizations of gC, US11, and p56 probes, radiolabeled with 32P by random priming, were performed using ExpressHyb (Clontech) according to the user's manual. The gC and US11 probes were generated by PCR amplification of viral DNA using the primers 5′ CCCAAACCCAAGAACAACAC and 5′ TGTTCGTCAGGACCTCCTCT (gC) or 5′ GGCGACCCAGATGGTTACTT and 5′ GCGAGCCGTACGTGGTTC (US11). The p56 probe has previously been described (30). γ-Actin transcripts were detected with a 32P-labeled oligonucleotide, 5′ AATGACCAGCGCGGCGATCTCTTCTTCCATT. Hybridization was carried out in modified Westneat solution (6.6% SDS, 250 mM MOPS [morpholinepropanesulfonic acid] [pH 7.0], 5× Denhardt's solution, 1 mM EDTA) at 53°C. Signals were analyzed with a Fujifilm FLA-5100 phosphorimager and Image Gauge version 4.22.
Polysome gradient.
HeLa or Vero cells from two 100-mm culture dishes were mock infected or infected with KOS1.1 or ΔSma at an MOI of 10. Cells were lysed at 12 hpi in lysis buffer (250 mM sucrose, 25 mM KCl, 5 mM MgCl2, 50 mM Tris-HCl [pH 7.4], 0.5% Triton X-100, 100 μg/ml cycloheximide, RNaseOUT). Cleared supernatant was incubated with MgCl2 (final concentration, 10 mM) or EDTA (final concentration, 25 mM) for 10 min on ice. Lysates were layered onto gradients of 10 to 50% sucrose solution in 50 mM Tris-HCl (pH 7.4), 25 mM KCl, 1.5 mM MgCl2, 100 μg/ml cycloheximide, 2 mM dithiothreitol (DTT), and RNaseOUT and centrifuged in an SW40 rotor for 105 min at 38,000 rpm and 4°C. Twenty fractions were collected from the top of the gradient, and their absorbance at 254 nm was measured. Samples were proteinase K treated, and RNA was phenol-chloroform extracted. Half of each RNA sample was subjected to Northern blot analysis as described above to determine the distribution of selected RNA species across the gradient.
RESULTS
Formation of stress granules in response to infection with vhs-deficient HSV-1 is cell type dependent.
Esclatine et al. showed that infection of HEp-2 and HFF cells with the ΔUL41 mutant virus but not wild-type HSV-1(F) led to the formation of cytoplasmic structures that stained positive for the SG marker antigens TIA-1/TIAR (9). We asked whether these cytoplasmic structures are true SGs and whether SG formation is a universal response to vhs-deficient HSV-1. Therefore, we infected cells with wild-type and vhs-deficient HSV-1 at an MOI of 10. Indirect immunofluorescence microscopy using antibodies against TIA-1 showed that ΔSma but not KOS1.1 infection induced formation of cytoplasmic granules in HeLa cells at 16 h postinfection (hpi) (Fig. 1A). As expected, at late time points postinfection ICP4 localized to viral replication compartments and DAPI (4′,6-diamidino-2-phenylindole) staining demonstrated chromatin marginalization. The cytoplasmic structures observed in ΔSma-infected HeLa cells also stained positive for a second key SG marker, TIAR (see Fig. S1a in the supplemental material). Furthermore, immunostaining against TIA-1 or TIAR demonstrated that the cytoplasmic structures were also induced in response to the additional vhs mutants vhs1 and GFPvhs− (Fig. S1B and C) but not in response to parental KOS (Fig. S1D), confirming that the observed phenotype is due to the loss of vhs function and not due to other derivative-specific differences. Costaining of ΔSma-infected HeLa cells with TIA-1/G3BP, G3BP/eIF4A, and PABP/eIF4A (Fig. 1B) revealed that the cytoplasmic structures contain G3BP, eIF4A, and PABP and hence are bona fide SGs. We also observed SG formation in ΔSma-infected HEp-2, SK-N-SH, HFF, U2OS, and HEL cells (Fig. 1C). However, SG formation was less prominent in U2OS and HEL cells than in HeLa, HEp-2, and SK-N-SH cells, and HFF cells showed an intermediate phenotype. Surprisingly, however, we were not able to detect any SGs in ΔSma-infected Vero cells (Fig. 1D). Although the TIA-1 signal in KOS1.1-infected Vero cells appeared somewhat speckled, these structures were mainly found in the nucleus and the cytoplasmic TIA signal was mostly dispersed. Therefore, this pattern clearly differed from SGs as seen in ΔSma-infected HeLa cells. We considered the possibility that Vero cells are inherently unable to form SGs. However, endoplasmic reticulum stress induced by thapsigargin led to strong SG formation (Fig. 1D). This experiment verified that Vero cells are capable of forming SGs and suggests that Vero cells may have either different mechanisms to deal with the stress induced by vhs-deficient HSV-1 or the level of stress may be below the specific threshold to induce SG formation. One distinguishing feature of Vero cells is that they do not express IFN-α/β (6). However, IFN-α/β treatment did not induce SGs in HeLa cells (data not shown), making it unlikely that IFN-α/β signaling is responsible for the difference between HeLa and Vero cells. As HeLa and Vero cells are two of the most widely used cell lines in HSV-1 research, we felt that the striking difference in SG formation in response to vhs-deficient HSV-1 warranted further investigation.
Fig. 1.
Characterization of stress granule (SG) formation in response to infection with wild-type or vhs-deficient HSV-1. (A) HeLa cells were mock treated or infected with HSV-1 KOS1.1 or ΔSma at an MOI of 10. At 16 hours postinfection (hpi), cells were stained for ICP4 and TIA-1. DNA was stained with DAPI. The merged images (upper row) show ICP4 (pink), TIA-1 (green), and nuclear DNA (blue), and the images in the lower row show the TIA-1 signal in white. (B) Costaining of ΔSma-infected HeLa cells against several SG marker proteins. HeLa cells were infected with ΔSma at an MOI of 10. At 16 hpi, cells were stained for TIA-1 (green) and G3BP (red), G3BP (green) and eIF4A (red), or PABP (green) and eIF4A (red). (C) HEp-2, SK-N-SH, HFF, U2OS, and HEL cells were infected with HSV-1 ΔSma and stained as described for panel A. (D) Vero cells were mock treated, infected with HSV-1 KOS1.1 or ΔSma at an MOI of 10, or treated with 1 μM thapsigargin for 1 h. At 16 hours postinfection (hpi), cells were stained as described for panel A.
SGs form late in infection and require viral DNA replication.
To assess when SGs form during infection, we infected HeLa cells with ΔSma at an MOI of 10 and stained for TIA-1 and the viral protein ICP4 at various time points. SGs formed as early as 8 hpi (Fig. 2A). The fraction of ICP4-positive SG-containing cells increased over time to greater than 50% at 16 hpi (Fig. 2A), suggesting that SG formation is triggered during the phase of late-gene expression. To further test this hypothesis, we treated ΔSma-infected HeLa cells with phosphonoacetic acid (PAA), which inhibits viral DNA replication and consequentially transcription of true late genes. This treatment efficiently blocked formation of SGs (Fig. 2B), indicating that one or more events linked to viral DNA replication, such as accumulation of viral true late-gene transcripts, triggers SG formation.
Fig. 2.
(A) Dependency of SGs formation on the stage of infection. HeLa cells were infected with HSV-1 ΔSma at an MOI of 10, fixed at the indicated time points postinfection, and stained for TIA-1 and ICP4. The percentage of cells containing SGs was calculated by counting ca. 300 ICP4-positive cells in several different fields. (B) HeLa cells were infected with HSV-1 ΔSma at an MOI of 10 and mock treated or treated with 300 μg/ml phosphonoacetic acid (PAA) starting at 1 hpi. At 16 hpi, cells were stained for TIA-1 (green), ICP4 (pink), and DNA (blue).
Attenuated virus production in the absence of vhs is cell type dependent.
In order to test whether the difference in SG formation in response to ΔSma is reflected in differences in viral replication between HeLa and Vero cells, we performed a single cycle growth curve analysis. HeLa and Vero cells were infected with HSV-1 KOS1.1 or ΔSma at an MOI of 10, and virus titers were determined at 1, 6, 12, 18, and 24 hpi. While KOS1.1 and ΔSma replicated to similar titers in Vero cells, ΔSma virus titers were reduced about 50-fold compared to the wild-type virus in HeLa cells (Fig. 3A). In HEp-2, HFF, and SK-N-SH cells, ΔSma showed a growth phenotype similar to that observed in HeLa cells, with a difference in viral titers of vhs-deficient and wild-type virus between 29- and 45-fold (Fig. 3B), whereas U2OS and HEL cells showed a phenotype similar to that of Vero cells, supporting ΔSma virus replication to titers about 2- or 6-fold lower than those for KOS1.1, respectively (Fig. 3C). We are confident that during this single-step replication cycle, the impact of IFN signaling was negligible, as demonstrated by the low to nondetectable mRNA levels of IFN-stimulated gene 56 under the conditions used as described below (see Fig. 5).
Fig. 5.
Induction of p56 mRNA. Vero, HeLa, HEp2, SK-N-SH, HFF, U2OS, and HEL cells were mock treated, infected with KOS1.1 or ΔSma at an MOI of 10, or treated with 1,000 U IFN-α 6 h prior to harvest. RNA was extracted at 6 and 12 hpi and analyzed by Northern blotting with probes specific for p56 and γ-actin mRNA.
vhs is necessary for efficient accumulation of true late gene products in HeLa and other restrictive cell lines but not in Vero and other permissive cells.
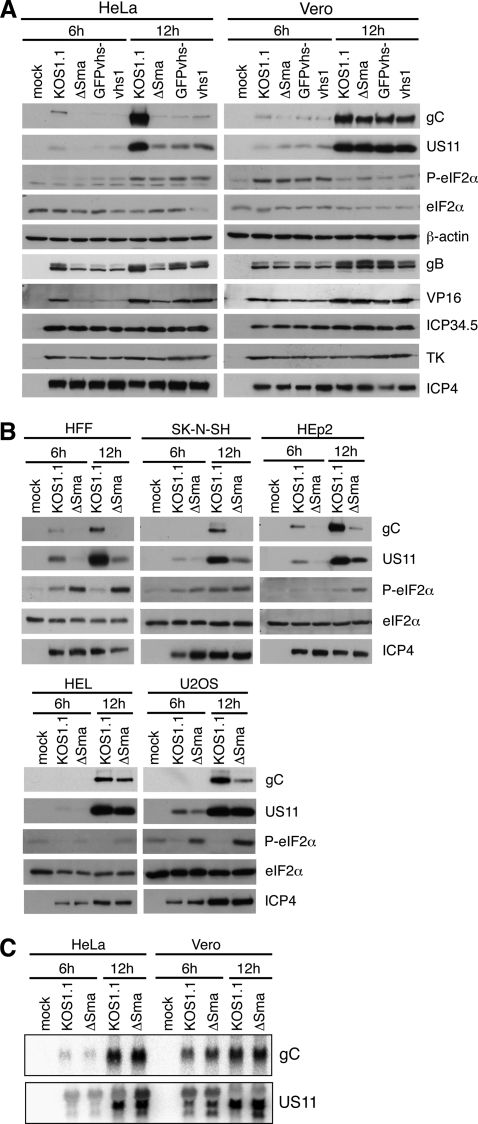
As vhs-deficient HSV-1 induces formation of SGs and formation of SGs is indicative of stalled translation initiation (reviewed in reference 1), we hypothesized that translation of viral transcripts might be reduced in the absence of vhs. To test this hypothesis, we examined lysates of infected HeLa or Vero cells by Western blot analysis. Detection of the viral proteins ICP4 (immediate-early), thymidine kinase (TK; early), and ICP34.5 (leaky late) showed that these proteins are expressed equally well in HeLa and Vero cells infected with KOS1.1 or the vhs mutant ΔSma, GFPvhs−, or vhs1 at 6 and 12 hpi (Fig. 4A). The amounts of the leaky-late gene products VP16 and glycoprotein B (gB) were slightly reduced in the absence of vhs in HeLa cells but not in Vero cells (Fig. 4A). The true late-gene products glycoprotein C (gC) and US11, however, were strongly reduced in ΔSma-infected HeLa cells compared to the level in KOS1.1-infected HeLa cells, while no difference in gC and US11 protein levels was observed in Vero cells (Fig. 4A). Lower gC and US11 protein levels in ΔSma-infected HeLa cells were not due to differences in the accumulation of the respective mRNAs, as shown by Northern blot analysis of RNA extracted from HeLa and Vero cells infected with KOS1.1 or ΔSma for 6 or 12 h (Fig. 4C). Given that both transcripts accumulate with true late kinetics, this observation furthermore excluded a potential defect in DNA replication of the vhs mutant. Reduced expression was also observed for another true late-gene product, UL47 (see Fig. S2 in the supplemental material). Figure S2 also shows that KOS displays the same phenotype as its plaque-purified derivative KOS1.1. Further Western blot analyses demonstrated that HFF, SK-N-SH, and HEp2 cells show a HeLa-like expression profile, with reduced expression of gC and US11 in the absence of vhs (Fig. 4B), whereas late-gene expression in U2OS and HEL cells was more similar to that in Vero cells, although the level of gC but not US11 protein was slightly reduced in U2OS cells (Fig. 4B). We also looked at ICP27 levels since gC expression strictly depends on ICP27 (38, 50). However, ICP27 was present in equal amounts in all samples (Fig. S2).
Fig. 4.
Analyses of protein profiles in different cell types infected with wild-type and vhs mutant HSV-1. (A) HeLa or Vero cells were mock treated or infected with KOS1.1 or the vhs mutant ΔSma, GFPvhs−, or vhs1. Lysates were prepared at 6 and 12 hpi and analyzed by immunoblotting with antibodies specific for gC, US11, phospho-eIF2α, total eIF2α, β-actin, gB, VP16, ICP34.5, TK, and ICP4. (B) HFF, SK-N-SH, HEp2, HEL, and U2OS cells were mock treated or infected with KOS1.1 or ΔSma. Lysates were prepared 6 and 12 hpi and analyzed by immunoblotting with antibodies specific for gC, US11, phospho-eIF2α, eIF2α, and ICP4. (C) HeLa or Vero cells were mock treated or infected with KOS1.1 or ΔSma. RNA was extracted at 6 and 12 hpi and analyzed by Northern blot with probes specific for gC or US11 mRNA.
eIF2α phosphorylation and p56 induction do not correlate with reduced late-gene expression.
Phosphorylation of the translation initiation factor eIF2α is a common cause for reduced protein expression in virus-infected cells and has been shown to be responsible for reduced late-gene expression with vhs-deficient HSV-2 (62). However, we found that eIF2α is phosphorylated to similar extents in the presence and absence of vhs in HeLa cells (Fig. 4A). Consistent with this observation, expression of ICP34.5, a viral cofactor of eIF2α dephosphorylation (14, 15), does not depend on the presence of vhs (Fig. 4A). It is possible that phosphorylation of eIF2α in ΔSma-infected U2OS and HFF cells contributes to the reduced expression of gC (Fig. 4B), despite efficient expression of ICP34.5 (data not shown). However, comparison of Western blot results from all cell types tested determined that the level of eIF2α phosphorylation did not correlate with reduced late-gene expression in HeLa, SK-N-SH, and Hep2 cells. We also tested a possible involvement of p56 (ISG56, or IFIT1) in the reduced late-gene expression during vhs-null infection. ISG56 can be induced directly by virus infection or by IFN-α/β signaling and has been shown to interfere with translation by binding to the translation initiation factor eIF3 (12, 18). RNA from cells treated with 1,000 U IFN-α, mock treated, or infected with KOS1.1 or ΔSma at an MOI of 10 was extracted at 6 and 12 hpi and analyzed by Northern blotting with probes specific for p56 and γ-actin (Fig. 5). As expected, γ-actin mRNA levels were reduced in the presence of wild-type vhs. Compared to the IFN-α treated cells, only a weak signal for p56 mRNA was detected in ΔSma-infected HeLa and Hep2 cells, whereas no p56 mRNA was detected in all other cell types tested. It is not clear whether these low levels of p56 affect translation, and we cannot rule out that p56 contributes slightly to the phenotype in HeLa and Hep2 cells. However, similar to eIF2α phosphorylation, p56 induction was not found to strictly correlate to the reduced late-gene expression phenotype when results from all cell types were compared.
vhs increases translation of late-gene transcripts in HeLa cells.
The experimental data gathered so far support the hypothesis that late-gene expression is blocked at the level of translation. Therefore, we directly assessed the translational activity on gC and US11 mRNAs. We mock treated or infected HeLa or Vero cells with KOS1.1 or ΔSma, prepared lysates at 12 hpi, and analyzed polysome profiles by sucrose density centrifugation. As a control, we also examined the sedimentation profiles of lysates that were treated with EDTA to dissociate the polysomes. RNA was extracted from fractions 1 to 20 (top to bottom) and subjected to agarose gel electrophoresis. Staining of rRNA with SYBR gold showed that 40S ribosomal subunits are found in fractions 3 and 4, 60S-80S complexes are found in fractions 5 to 7, and higher fractions represent polysomes (see Fig. S3A and S4A in the supplemental material). The RNA was further analyzed by Northern blotting with probes specific for gC and US11 mRNA and cellular γ-actin mRNA and detected by phosphorimaging (Fig. S3B, C, and D and S4B, C, and D). The distribution of the respective mRNA signal over the gradients is graphically represented in Fig. 6 as the percentage of total mRNA signal present in each fraction. As expected from the Western blot data, viral gC and US11 mRNA from both KOS1.1 and ΔSma-infected Vero cells sedimented in the fractions representing polysomes, indicating efficient translation initiation (Fig. 6D and E). Although the peak for gC mRNA from ΔSma-infected Vero cells was slightly shifted toward smaller fractions, the majority of the mRNA was still in the polysome fraction. The gC and US11 mRNA from KOS1.1-infected HeLa cells was also mainly found in polysome fractions, although with more of the mRNA signal at lower fractions than for KOS1.1-infected Vero cells (Fig. 6A and B). In contrast, gC and US11 mRNA from ΔSma-infected HeLa cells was shifted toward fractions representing monosomes or preinitiation complexes and overlapped with mRNAs from EDTA-dissociated polysomes (Fig. 6A and B). This result indicated that in the absence of vhs in HeLa cells, translation initiation on viral true late transcripts is strongly compromised, whereas vhs has little impact on translation efficiency in Vero cells.
Fig. 6.
Polysome analyses. HeLa cells (A, B, C) or Vero cells (D, E, F) were mock treated or infected with KOS1.1 or ΔSma at an MOI of 10. Cells were lysed 12 hpi, incubated with or without EDTA, and analyzed by sedimentation through a 10 to 50% sucrose gradient. Samples were taken from top to bottom, and extracted RNA was analyzed by Northern blotting for the viral mRNAs for gC (A, D) and US11 (B, E) or the cellular γ-actin mRNA (C, F). Bands were quantified by a phosphorimager, and the distribution of the mRNAs across the gradient is shown as a percentage of the total signal of the respective mRNA. The graphs represent one out of three comparable experiments.
Reduced translation is specific for viral transcripts.
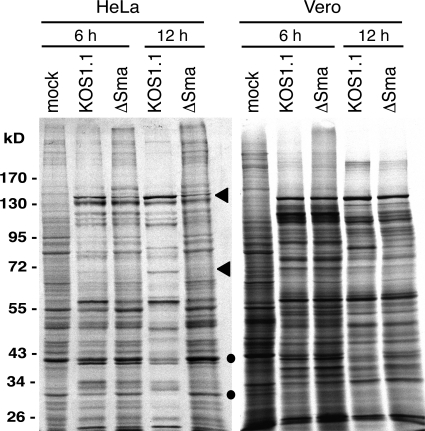
Interestingly, vhs is not required for the translation of cellular γ-actin mRNA, as the majority of the mRNA was found in polysome fractions in both mock-treated and ΔSma-infected HeLa and Vero cells (Fig. 6C and F). This finding was corroborated by metabolic labeling of infected cells (Fig. 7). HeLa cells and Vero cells were mock treated or infected with KOS1.1 or ΔSma at an MOI of 10 and labeled with [35S]methionine at 5 or 11 hpi. Lysates were prepared at 6 or 12 hpi and subjected to PAGE and autoradiography. In KOS1.1-infected HeLa cells, virus-specific protein bands were clearly visible at 6 and 12 hpi, whereas translation of cellular proteins was strongly reduced (Fig. 7). In ΔSma-infected HeLa cells, some virus-specific signals were weaker or not detectable at 12 hpi (Fig. 7, arrowheads), while cell specific bands were as strong as in mock-infected cells (Fig. 7, dots). In contrast, the translation patterns of KOS1.1 and ΔSma-infected Vero cells showed only minor differences (Fig. 7).
Fig. 7.
Metabolic labeling of proteins in virus-infected HeLa or Vero cells. Cells were either mock infected or infected with KOS1.1 or ΔSma at an MOI of 10. At 5 or 11 hpi, cells were metabolically labeled with [35S]methionine for 1 h. Cell extracts were prepared and analyzed by SDS-polyacrylamide gel electrophoresis and autoradiography. The positions of the molecular mass markers are indicated on the left. (●, cellular protein; ◀, viral protein).
Viral late-gene expression in Vero cells is more resistant to an eIF4A inhibitor than that in HeLa cells.
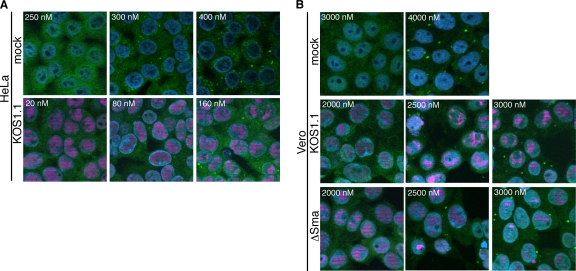
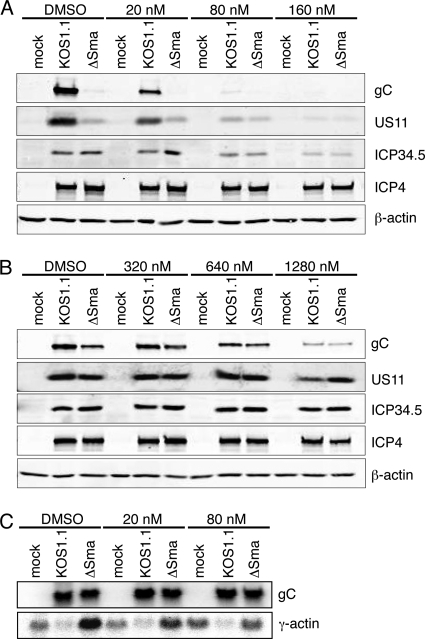
As demonstrated above, the SG formation and reduced late-gene expression observed during vhs-null infection do not correlate with enhanced eIF2α phosphorylation (Fig. 3). However, it was recently demonstrated that SGs can be induced independently of eIF2α phosphorylation by antagonizing the function of the RNA helicase eIF4A using inhibitors such as hippuristanol (3, 28). This observation, and the reported interactions of vhs with eIF4A and its cofactors eIF4B and eIF4H, raised the possibility that eIF4A activity is impaired in the absence of vhs, leading to the observed defect in late-gene expression. If so, then one might predict that hippuristanol treatment would mimic a vhs-null phenotype in KOS1.1-infected HeLa and Vero cells. Indirect immunofluorescence microscopy confirmed that treatment of uninfected HeLa and Vero cells with hippuristanol led to formation of SGs in both cell types, as expected. However, HeLa cells were much more sensitive than Vero cells, forming SGs in response to 300 nM hippuristanol, compared to 4,000 nM in Vero cells (Fig. 8A, top row, and B, top row). In addition, treatment of HeLa cells with as little as 80 nM hippuristanol sensitized cells so that infection with KOS1.1 resulted in formation of SGs (Fig. 8A, bottom row), whereas in Vero cells 2,500 nM hippuristanol was needed to drive cells to form SGs in response to KOS1.1 and ΔSma virus infection (Fig. 8B, middle and bottom rows). In accordance with the IF results, we found a prominent difference in how hippuristanol treatment affected translation of true late mRNAs in HeLa and Vero cells. Western blot analysis of cells infected with KOS1.1 at an MOI of 10 for 12 h showed that in HeLa cells as little as 20 nM hippuristanol decreased gC and US11 levels and 80 nM hippuristanol blocked accumulation of these proteins (Fig. 9A). At these concentrations, hippuristanol had only minor effects on accumulation of ICP34.5 and ICP4 and did not affect actin levels (Fig. 9A). Northern blot analysis confirmed that hippuristanol treatment did not affect gC or US11 mRNA levels (Fig. 9C), indicating that the reduced protein expression is due to the effect of hippuristanol on translation and not on transcription or mRNA stability. Furthermore, it has been reported that hippuristanol does not induce phosphorylation of eIF2α (28). Similar to what was observed in the induction of SGs, far larger amounts of hippuristanol were required in Vero cells (1,280 nM) to significantly reduce gC and US11 levels during KOS1.1 and ΔSma infection (Fig. 9B). These results suggest that translation in Vero cells is more resilient during conditions of reduced availability of translation factors than translation in HeLa cells. The apparently selective effect of low doses of hippuristanol on viral late- versus IE- and E-gene expression in HeLa cells further implies either that translation of late-gene transcripts is more sensitive to restrictive conditions than translation of IE and E mRNAs or that conditions become more restrictive during the late stages of infection.
Fig. 8.
Impact of hippuristanol on SG formation in HeLa and Vero cells. HeLa cells (A) or Vero cells (B) were mock treated or infected with KOS1.1 and ΔSma at an MOI of 10. Hippuristanol was added at 1 hpi. At 16 hpi, cells were stained for TIA-1 (green), ICP4 (pink), and DNA (DAPI; blue). Images shown represent the largest amount of hippuristanol without formation of SGs, the smallest amount of hippuristanol inducing SGs, and a larger amount of hippuristanol showing dose-dependent increase of SG formation.
Fig. 9.
Impact of hippuristanol treatment on expression of viral proteins in HeLa and Vero cells. HeLa cells (A) and Vero cells (B) were mock treated or infected with KOS1.1 or ΔSma at an MOI of 10. At 1 hpi, cells were treated with hippuristanol as indicated or treated with DMSO as a control. Lysates were prepared at 12 hpi and analyzed by immunoblotting with antibodies specific for gC, US11, ICP34.5, ICP4, and β-actin. (C) Hippuristanol treatment does not reduce the level of gC mRNA. HeLa cells were mock treated or infected with KOS1.1 or ΔSma at an MOI of 10. At 1 hpi, cells were treated with hippuristanol as indicated or treated with DMSO as a control. RNA was extracted at 12 hpi and analyzed by Northern blotting for the viral gC mRNA or the cellular γ-actin mRNA.
DISCUSSION
It is well established that vhs degrades viral and cellular mRNAs during the early stages of infection. However, there is mounting evidence that vhs also plays a role in translation. First, vhs associates with the translation initiation factors eIF4A, eIF4B, and eIF4H (7, 11). Furthermore, we have previously shown that vhs increases translation of the second cistron driven by several IRES elements or viral 5′ UTRs in a bicistronic reporter gene assay (44). Finally, infection with vhs-deficient HSV-1 leads to formation of TIA-1/TIAR-positive cytoplasmic foci, presumably SGs (9), which might indicate stalled translation initiation. In this study, we demonstrated that vhs boosts translation of viral late-gene products and promotes efficient virus production in a cell type-dependent manner and that the requirement for vhs might be determined by the resilience of the translation machinery in the respective cell type. This novel role of vhs during HSV-1 infection is supported by the following findings. (i) HSV-1 with mutated vhs or partial or complete deletion of the vhs ORF, but not wild-type viruses, triggered formation of SGs in all cell types tested except Vero cells. (ii) The vhs mutant ΔSma was attenuated in several cell lines, including HeLa cells, but not in Vero, HEL, or U2OS cells. (iii) Expression of true late genes was strongly reduced in the absence of functional vhs in HeLa and other restrictive cells but not in Vero, HEL, or U2OS cells, showing a strong correlation with the results of the growth curve. (iv) Reduced gene expression in ΔSma-infected HeLa cells was due to decreased translation initiation on the respective viral mRNAs. (v) This effect was specific for late viral transcripts since translation of cellular mRNAs was not affected in the absence of vhs. (vi) Treatment of HeLa and Vero cells with the eIF4A inhibitor hippuristanol induced SG formation and reduced late-gene expression during KOS1.1 infection, mimicking the phenotype of a ΔSma infection. The effective dose for HeLa cells was far lower than that for Vero cells, indicating that the resilience of the translational machinery differs between HeLa and Vero cells. This difference might be the critical factor for translation of true late-gene transcript in the absence of vhs and might determine the scale of the inherent growth defect of vhs-deficient HSV-1.
It is not yet clear whether SG formation directly impedes virus production or, alternatively, whether the SGs are only a readout of the stalled translation initiation. TIA-1−/− or TIAR−/− MEFs have been shown to support replication of HSV-1 (strain 129) to higher titers than WT MEFs (27), indicating that TIA-1 and TIAR have a negative effect on viral replication. On the other hand, Wylie and coworkers showed that knockout of TIA-1 or TIAR in MEFs did not restore replication of an HSV-2 vhs mutant to wild-type titers (62). However, they did not determine whether the knockouts resulted in an increase in virus production relative to that in wild-type MEFs and, if so, whether this increase was different for HSV-2 and the vhs mutant. While it will be interesting to study the ΔSma phenotype in the absence of TIA-1 or TIAR, we speculate that SG formation is more likely a readout than a cause of the ΔSma phenotype because the reduced gC and US11 protein levels are observed as early as 6 hpi, whereas SG formation starts between 8 and 10 hpi.
We suggest that the accumulation of high levels of viral late mRNAs following the onset of viral DNA replication overwhelms the capacity of the translational initiation machinery, leading to the observed translational defect in restrictive cells. The observation that hippuristanol generates a similar phenotype in cells infected with wild-type virus is consistent with this hypothesis and further suggests that eIF4A may be one of the limiting factors. In this context, it is interesting to note that the 5′ UTRs of many HSV mRNAs adopt quite stable secondary structures because of the overall GC-rich nature of the HSV genome. For example, the 5′ UTRs of ICP4, TK, ICP34.5, gC, and US11 mRNAs have predicted values of −125, −31, −103, −63, and −80 kcal/mole, respectively, as estimated by the Mfold algorithm. Most 5′ UTRs of well-translated cellular mRNAs have little or no secondary structure, with predicted free energies between 0 and −30 kcal/mole (5), whereas secondary structures with a free energy of −50 kcal/mole or lower impose a strong block on ribosome scanning (23, 37). For highly structured 5′ UTRs, optimal helicase activity is absolutely critical for initiation of translation (59). Thus, it seems quite plausible that eIF4A could become limiting due to the massive accumulation of highly structured viral mRNAs during the late stages of infection. As shown in Fig. 8 and 9, Vero cells can deal with over 10 times more hippuristanol than HeLa cells before the balance is tipped toward SG formation and a block in translation of viral late-gene transcripts. These observations suggest that translation in Vero cells is more resistant to reduced eIF4A availability than translation in HeLa cells. Therefore, vhs would be less critical for late-gene expression under these circumstances. It is also possible that Vero cells are more efficient at degrading excess mRNA to avoid overloading of the translation machinery.
Interestingly, the translational blockade observed in HeLa cells in the absence of vhs appears to selectively impair translation of viral true late mRNAs, sparing other mRNAs that are present in the infected cells. Thus, cellular γ-actin mRNA remains associated with large polysomes late during vhs-null infection (Fig. 6), and many other cellular and viral mRNAs are also translationally active as judged by [35S]methionine incorporation (Fig. 7, 12-hpi lanes). How might this selective blockade arise? One possibility is that some structural feature of late mRNAs places them at a disadvantage relative to other mRNAs. Alternatively, perhaps true late transcripts fail to be efficiently translated simply because they accumulate after translation initiation factors have become limiting. According to the latter model, the limiting factors are stably sequestered by the preexisting cellular and viral mRNAs and are not readily recycled in the absence of vhs. Further studies are required to distinguish between these and other possibilities.
There are several possibilities for how vhs boosts translation initiation on true late mRNAs in restrictive cells. The simplest is that vhs prevents “mRNA overload” during the late stages of infection by eliminating host mRNAs and promoting the decay of viral IE and E transcripts. A second (and related) possibility is that vhs promotes recycling of translation initiation factors by shortening the half-life of viral mRNAs, allowing transfer of factors from the viral IE, E, and leaky late mRNAs made before DNA replication onto true late mRNAs. A third possibility is that vhs plays a more active role beyond reducing mRNA levels and recycling translation initiation factors. eIF4B and eIF4H are cofactors of eIF4A that increase the helicase activity, and their requirement correlates with the degree of secondary structure in the 5′ UTRs of mRNAs (41, 42, 51). vhs has been shown to interact with eIF4A, eIF4B, and eIF4H (7, 11). Therefore, we speculate that vhs might act as an adaptor to bring helicase and cofactors together and thereby increase unwinding of the secondary structure. vhs has no defined RNA-binding capacity, so how would it assemble helicase and cofactors preferably on true late-gene transcripts? Although we cannot yet answer this question, it is possible that vhs is targeted to mRNAs as a complex with VP16 and VP22, of which the latter has been shown to bind to mRNAs (49). Furthermore, late in infection the RNase activity of vhs would be dampened in a complex with VP16 and VP22 (13, 22, 25, 48, 52).
These three models for how vhs boosts translation of true late-gene transcripts are not mutually exclusive, and we are currently investigating the potential role of eIF4A availability, RNA accumulation, 5′ UTR structure, and interaction of vhs with translation initiation factors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Holly Saffran for technical support.
This research was supported by operating grants from the Canadian Institutes for Health Research to J.R.S. (FRN 37995) and from the Canadian Cancer Society Research Institute to J.P. (20066). B.D. was supported by a postdoctoral fellowship from Alberta Innovates—Health Solutions, and J.R.S. is a Canada Research Chair in Molecular Virology.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 23 March 2011.
REFERENCES
- 1. Anderson P., Kedersha N. 2008. Stress granules: the Tao of RNA triage. Trends Biochem. Sci. 33:141–150 [DOI] [PubMed] [Google Scholar]
- 2. Bordeleau M. E., et al. 2006. RNA-mediated sequestration of the RNA helicase eIF4A by Pateamine A inhibits translation initiation. Chem. Biol. 13:1287–1295 [DOI] [PubMed] [Google Scholar]
- 3. Bordeleau M. E., et al. 2006. Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat. Chem. Biol. 2:213–220 [DOI] [PubMed] [Google Scholar]
- 4. Corcoran J. A., Hsu W. L., Smiley J. R. 2006. Herpes simplex virus ICP27 is required for virus-induced stabilization of the ARE-containing IEX-1 mRNA encoded by the human IER3 gene. J. Virol. 80:9720–9729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davuluri R. V., Suzuki Y., Sugano S., Zhang M. Q. 2000. CART classification of human 5′ UTR sequences. Genome Res. 10:1807–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diaz M. O., et al. 1988. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc. Natl. Acad. Sci. U. S. A. 85:5259–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doepker R. C., Hsu W. L., Saffran H. A., Smiley J. R. 2004. Herpes simplex virus virion host shutoff protein is stimulated by translation initiation factors eIF4B and eIF4H. J. Virol. 78:4684–4699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duerst R. J., Morrison L. A. 2004. Herpes simplex virus 2 virion host shutoff protein interferes with type I interferon production and responsiveness. Virology 322:158–167 [DOI] [PubMed] [Google Scholar]
- 9. Esclatine A., Taddeo B., Roizman B. 2004. Herpes simplex virus 1 induces cytoplasmic accumulation of TIA-1/TIAR and both synthesis and cytoplasmic accumulation of tristetraprolin, two cellular proteins that bind and destabilize AU-rich RNAs. J. Virol. 78:8582–8592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Everly D. N., Jr., Feng P., Mian I. S., Read G. S. 2002. mRNA degradation by the virion host shutoff (Vhs) protein of herpes simplex virus: genetic and biochemical evidence that Vhs is a nuclease. J. Virol. 76:8560–8571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng P., Everly D. N., Jr., Read G. S. 2005. mRNA decay during herpes simplex virus (HSV) infections: protein-protein interactions involving the HSV virion host shutoff protein and translation factors eIF4H and eIF4A. J. Virol. 79:9651–9664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo J., Hui D. J., Merrick W. C., Sen G. C. 2000. A new pathway of translational regulation mediated by eukaryotic initiation factor 3. EMBO J. 19:6891–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hafezi W., Bernard E., Cook R., Elliott G. 2005. Herpes simplex virus tegument protein VP22 contains an internal VP16 interaction domain and a C-terminal domain that are both required for VP22 assembly into the virus particle. J. Virol. 79:13082–13093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He B., Gross M., Roizman B. 1998. The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J. Biol. Chem. 273:20737–20743 [DOI] [PubMed] [Google Scholar]
- 15. He B., Gross M., Roizman B. 1997. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc. Natl. Acad. Sci. U. S. A. 94:843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hershey J. W. 1991. Translational control in mammalian cells. Annu. Rev. Biochem. 60:717–755 [DOI] [PubMed] [Google Scholar]
- 17. Hughes R. G., Jr., Munyon W. H. 1975. Temperature-sensitive mutants of herpes simplex virus type 1 defective in lysis but not in transformation. J. Virol. 16:275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hui D. J., Bhasker C. R., Merrick W. C., Sen G. C. 2003. Viral stress-inducible protein p56 inhibits translation by blocking the interaction of eIF3 with the ternary complex eIF2.GTP. Met-tRNAi. J. Biol. Chem. 278:39477–39482 [DOI] [PubMed] [Google Scholar]
- 19. Kedersha N., et al. 2002. Evidence that ternary complex (eIF2-GTP-tRNA(i) (Met))-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol. Biol. Cell 13:195–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kedersha N., et al. 2000. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151:1257–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kedersha N. L., Gupta M., Li W., Miller I., Anderson P. 1999. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J. Cell Biol. 147:1431–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knez J., Bilan P. T., Capone J. P. 2003. A single amino acid substitution in herpes simplex virus type 1 VP16 inhibits binding to the virion host shutoff protein and is incompatible with virus growth. J. Virol. 77:2892–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kozak M. 1989. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol. Cell. Biol. 9:5134–5142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kwong A. D., Frenkel N. 1987. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc. Natl. Acad. Sci. U. S. A. 84:1926–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lam Q., et al. 1996. Herpes simplex virus VP16 rescues viral mRNA from destruction by the virion host shutoff function. EMBO J. 15:2575–2581 [PMC free article] [PubMed] [Google Scholar]
- 26. Leib D. A., et al. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189:663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li W., et al. 2002. Cell proteins TIA-1 and TIAR interact with the 3′ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J. Virol. 76:11989–12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazroui R., et al. 2006. Inhibition of ribosome recruitment induces stress granule formation independently of eukaryotic initiation factor 2alpha phosphorylation. Mol. Biol. Cell 17:4212–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohr I. 2006. Phosphorylation and dephosphorylation events that regulate viral mRNA translation. Virus Res. 119:89–99 [DOI] [PubMed] [Google Scholar]
- 30. Mossman K. L., et al. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murphy J. A., Duerst R. J., Smith T. J., Morrison L. A. 2003. Herpes simplex virus type 2 virion host shutoff protein regulates alpha/beta interferon but not adaptive immune responses during primary infection in vivo. J. Virol. 77:9337–9345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oroskar A. A., Read G. S. 1989. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J. Virol. 63:1897–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Page H. G., Read G. S. 2010. The virion host shutoff endonuclease (UL41) of herpes simplex virus interacts with the cellular cap-binding complex eIF4F. J. Virol. 84:6886–6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pasieka T. J., et al. 2009. Host responses to wild-type and attenuated herpes simplex virus infection in the absence of Stat1. J. Virol. 83:2075–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pasieka T. J., et al. 2008. Herpes simplex virus virion host shutoff attenuates establishment of the antiviral state. J. Virol. 82:5527–5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pasieka T. J., Lu B., Leib D. A. 2008. Enhanced pathogenesis of an attenuated herpes simplex virus for mice lacking Stat1. J. Virol. 82:6052–6055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pelletier J., Sonenberg N. 1985. Insertion mutagenesis to increase secondary structure within the 5′ noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell 40:515–526 [DOI] [PubMed] [Google Scholar]
- 38. Perkins K. D., Gregonis J., Borge S., Rice S. A. 2003. Transactivation of a viral target gene by herpes simplex virus ICP27 is posttranscriptional and does not require the endogenous promoter or polyadenylation site. J. Virol. 77:9872–9884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Read G. S., Frenkel N. 1983. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J. Virol. 46:498–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Read G. S., Karr B. M., Knight K. 1993. Isolation of a herpes simplex virus type 1 mutant with a deletion in the virion host shutoff gene and identification of multiple forms of the vhs (UL41) polypeptide. J. Virol. 67:7149–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rogers G. W., Jr., Richter N. J., Lima W. F., Merrick W. C. 2001. Modulation of the helicase activity of eIF4A by eIF4B, eIF4H, and eIF4F. J. Biol. Chem. 276:30914–30922 [DOI] [PubMed] [Google Scholar]
- 42. Rogers G. W., Jr., Richter N. J., Merrick W. C. 1999. Biochemical and kinetic characterization of the RNA helicase activity of eukaryotic initiation factor 4A. J. Biol. Chem. 274:12236–12244 [DOI] [PubMed] [Google Scholar]
- 43. Roizman B., Knipe D. M., Whitley R. J. 2007. Herpes simplex viruses, p. 2501–2601 In Knipe D. M., Howley P. M. (ed.), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 44. Saffran H. A., Read G. S., Smiley J. R. 2010. Evidence for translational regulation by the herpes simplex virus virion host shutoff protein. J. Virol. 84:6041–6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Samady L., et al. 2003. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: potential of vhs− HSV vectors for dendritic cell-mediated immunotherapy. J. Virol. 77:3768–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Samuel C. E. 1993. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J. Biol. Chem. 268:7603–7606 [PubMed] [Google Scholar]
- 47. Sarma N., Agarwal D., Shiflett L. A., Read G. S. 2008. Small interfering RNAs that deplete the cellular translation factor eIF4H impede mRNA degradation by the virion host shutoff protein of herpes simplex virus. J. Virol. 82:6600–6609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sciortino M. T., et al. 2007. Replication-competent herpes simplex virus 1 isolates selected from cells transfected with a bacterial artificial chromosome DNA lacking only the UL49 gene vary with respect to the defect in the UL41 gene encoding host shutoff RNase. J. Virol. 81:10924–10932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sciortino M. T., Taddeo B., Poon A. P., Mastino A., Roizman B. 2002. Of the three tegument proteins that package mRNA in herpes simplex virions, one (VP22) transports the mRNA to uninfected cells for expression prior to viral infection. Proc. Natl. Acad. Sci. U. S. A. 99:8318–8323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sedlackova L., et al. 2010. Identification of an ICP27-responsive element in the coding region of a herpes simplex virus type 1 late gene. J. Virol. 84:2707–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shahbazian D., et al. 2010. Control of cell survival and proliferation by mammalian eukaryotic initiation factor 4B. Mol. Cell. Biol. 30:1478–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smibert C. A., Popova B., Xiao P., Capone J. P., Smiley J. R. 1994. Herpes simplex virus VP16 forms a complex with the virion host shutoff protein vhs. J. Virol. 68:2339–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Smibert C. A., Smiley J. R. 1990. Differential regulation of endogenous and transduced beta-globin genes during infection of erythroid cells with a herpes simplex virus type 1 recombinant. J. Virol. 64:3882–3894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smiley J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith R. W., Graham S. V., Gray N. K. 2008. Regulation of translation initiation by herpesviruses. Biochem. Soc. Trans. 36:701–707 [DOI] [PubMed] [Google Scholar]
- 56. Smith T. J., Morrison L. A., Leib D. A. 2002. Pathogenesis of herpes simplex virus type 2 virion host shutoff (vhs) mutants. J. Virol. 76:2054–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Strelow L., Smith T., Leib D. 1997. The virion host shutoff function of herpes simplex virus type 1 plays a role in corneal invasion and functions independently of the cell cycle. Virology 231:28–34 [DOI] [PubMed] [Google Scholar]
- 58. Suzutani T., et al. 2000. The role of the UL41 gene of herpes simplex virus type 1 in evasion of non-specific host defence mechanisms during primary infection. J. Gen. Virol. 81:1763–1771 [DOI] [PubMed] [Google Scholar]
- 59. Svitkin Y. V., et al. 2001. The requirement for eukaryotic initiation factor 4A (elF4A) in translation is in direct proportion to the degree of mRNA 5′ secondary structure. RNA 7:382–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Taddeo B., Roizman B. 2006. The virion host shutoff protein (UL41) of herpes simplex virus 1 is an endoribonuclease with a substrate specificity similar to that of RNase A. J. Virol. 80:9341–9345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Taddeo B., Zhang W., Roizman B. 2006. The U(L)41 protein of herpes simplex virus 1 degrades RNA by endonucleolytic cleavage in absence of other cellular or viral proteins. Proc. Natl. Acad. Sci. U. S. A. 103:2827–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wylie K. M., Schrimpf J. E., Morrison L. A. 2009. Increased eIF2alpha phosphorylation attenuates replication of herpes simplex virus 2 vhs mutants in mouse embryonic fibroblasts and correlates with reduced accumulation of the PKR antagonist ICP34.5. J. Virol. 83:9151–9162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zelus B. D., Stewart R. S., Ross J. 1996. The virion host shutoff protein of herpes simplex virus type 1: messenger ribonucleolytic activity in vitro. J. Virol. 70:2411–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.