Abstract
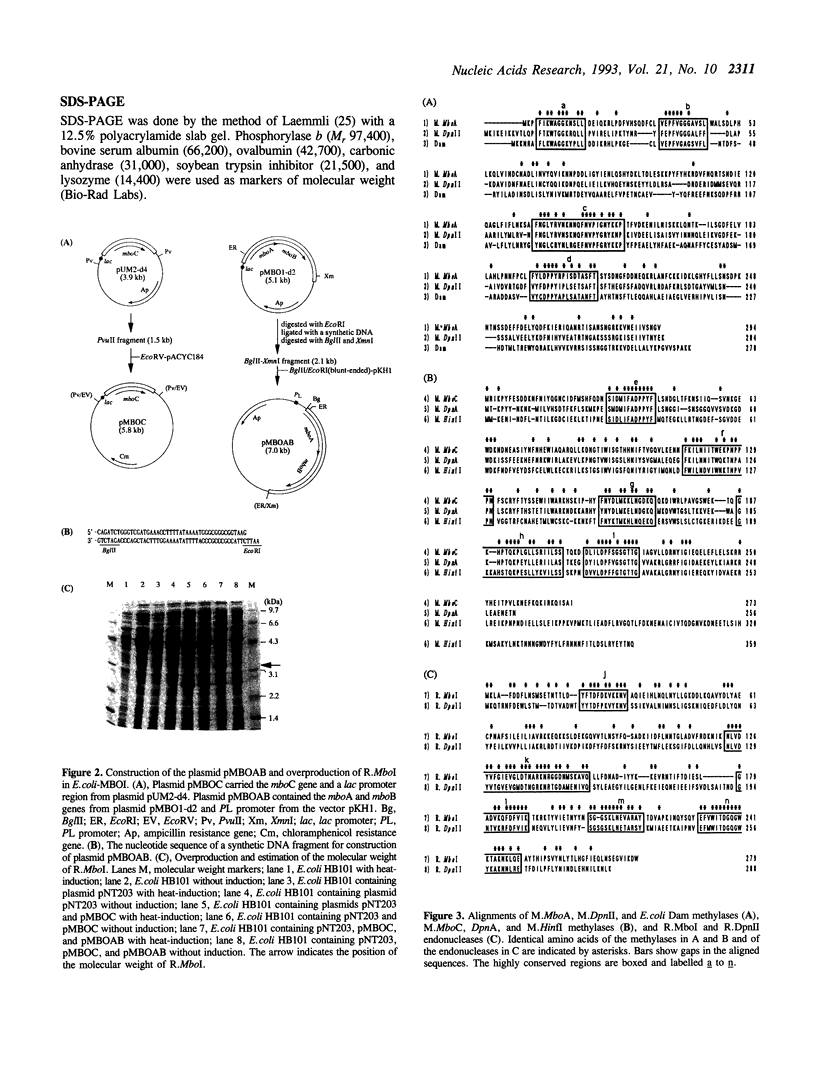
The genes from Moraxella bovis encoding the MboI restriction--modification system were cloned and expressed in Escherichia coli. Three open reading frames were found in the sequence containing the genes. These genes, which we named mboA, mboB, and mboC, had the same orientation in the genome. Genes mboA and mboC encoded MboI methyltransferases (named M.MboA and M.MboC) with 294 and 273 amino acid residues, respectively. The mboB gene coded for MboI restriction endonuclease (R.MboI) with 280 amino acid residues. Recombinant E.coli-MBOI, which contained the whole MboI system, overproduced R.MboI. R.MboI activity from E.coli-MBOI was 480-fold that of M.bovis. The amino acid sequences deduced from these genes were compared with those of other restriction--modification systems. The protein sequences of the MboI system had 38-49% homology with those of the DpnII system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocklage H., Heeger K., Müller-Hill B. Cloning and characterization of the MboII restriction-modification system. Nucleic Acids Res. 1991 Mar 11;19(5):1007–1013. doi: 10.1093/nar/19.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bougueleret L., Schwarzstein M., Tsugita A., Zabeau M. Characterization of the genes coding for the Eco RV restriction and modification system of Escherichia coli. Nucleic Acids Res. 1984 Apr 25;12(8):3659–3676. doi: 10.1093/nar/12.8.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brooks J. E., Blumenthal R. M., Gingeras T. R. The isolation and characterization of the Escherichia coli DNA adenine methylase (dam) gene. Nucleic Acids Res. 1983 Feb 11;11(3):837–851. doi: 10.1093/nar/11.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasegaran S., Lunnen K. D., Smith H. O., Wilson G. G. Cloning and sequencing the HinfI restriction and modification genes. Gene. 1988 Oct 30;70(2):387–392. doi: 10.1016/0378-1119(88)90210-7. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas R. E., Myers P. A., Roberts R. J. Two sequence-specific endonucleases from Moraxella bovis. J Mol Biol. 1977 Jul;114(1):169–179. doi: 10.1016/0022-2836(77)90290-x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Ito H., Sadaoka A., Kotani H., Hiraoka N., Nakamura T. Cloning, nucleotide sequence, and expression of the HincII restriction-modification system. Nucleic Acids Res. 1990 Jul 11;18(13):3903–3911. doi: 10.1093/nar/18.13.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Shimato H., Sadaoka A., Kotani H., Kimizuka F., Kato I. Cloning and expression of the HpaI restriction-modification genes. Nucleic Acids Res. 1992 Feb 25;20(4):705–709. doi: 10.1093/nar/20.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimasauskas S., Timinskas A., Menkevicius S., Butkienè D., Butkus V., Janulaitis A. Sequence motifs characteristic of DNA[cytosine-N4]methyltransferases: similarity to adenine and cytosine-C5 DNA-methylases. Nucleic Acids Res. 1989 Dec 11;17(23):9823–9832. doi: 10.1093/nar/17.23.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacks S. A., Mannarelli B. M., Springhorn S. S., Greenberg B. Genetic basis of the complementary DpnI and DpnII restriction systems of S. pneumoniae: an intercellular cassette mechanism. Cell. 1986 Sep 26;46(7):993–1000. doi: 10.1016/0092-8674(86)90698-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauster R. Close relationship between the HinfI and DpnA DNA-methyltransferase. Nucleic Acids Res. 1989 Jun 12;17(11):4402–4402. doi: 10.1093/nar/17.11.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauster R. Evolution of type II DNA methyltransferases. A gene duplication model. J Mol Biol. 1989 Mar 20;206(2):313–321. doi: 10.1016/0022-2836(89)90481-6. [DOI] [PubMed] [Google Scholar]
- Lauster R., Trautner T. A., Noyer-Weidner M. Cytosine-specific type II DNA methyltransferases. A conserved enzyme core with variable target-recognizing domains. J Mol Biol. 1989 Mar 20;206(2):305–312. doi: 10.1016/0022-2836(89)90480-4. [DOI] [PubMed] [Google Scholar]
- Maki H., Horiuchi T., Sekiguchi M. Structure and expression of the dnaQ mutator and the RNase H genes of Escherichia coli: overlap of the promoter regions. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7137–7141. doi: 10.1073/pnas.80.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagman S. L., Hattman S. Molecular cloning of a functional dam+ gene coding for phage T4 DNA adenine methylase. Gene. 1983 May-Jun;22(2-3):139–156. doi: 10.1016/0378-1119(83)90098-7. [DOI] [PubMed] [Google Scholar]
- Seeber S., Kessler C., Götz F. Cloning, expression and characterization of the Sau3AI restriction and modification genes in Staphylococcus carnosus TM300. Gene. 1990 Sep 28;94(1):37–43. doi: 10.1016/0378-1119(90)90465-4. [DOI] [PubMed] [Google Scholar]
- Shigesada K., Tsurushita N., Matsumoto Y., Imai M. Overproduction of transcription termination factor Rho in Escherichia coli. Gene. 1984 Jul-Aug;29(1-2):199–209. doi: 10.1016/0378-1119(84)90180-x. [DOI] [PubMed] [Google Scholar]
- Wilson G. G., Murray N. E. Restriction and modification systems. Annu Rev Genet. 1991;25:585–627. doi: 10.1146/annurev.ge.25.120191.003101. [DOI] [PubMed] [Google Scholar]
- Wilson G. G. Organization of restriction-modification systems. Nucleic Acids Res. 1991 May 25;19(10):2539–2566. doi: 10.1093/nar/19.10.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- de la Campa A. G., Kale P., Springhorn S. S., Lacks S. A. Proteins encoded by the DpnII restriction gene cassette. Two methylases and an endonuclease. J Mol Biol. 1987 Aug 5;196(3):457–469. doi: 10.1016/0022-2836(87)90024-6. [DOI] [PubMed] [Google Scholar]



