Abstract
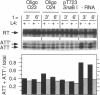
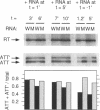
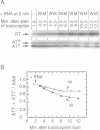
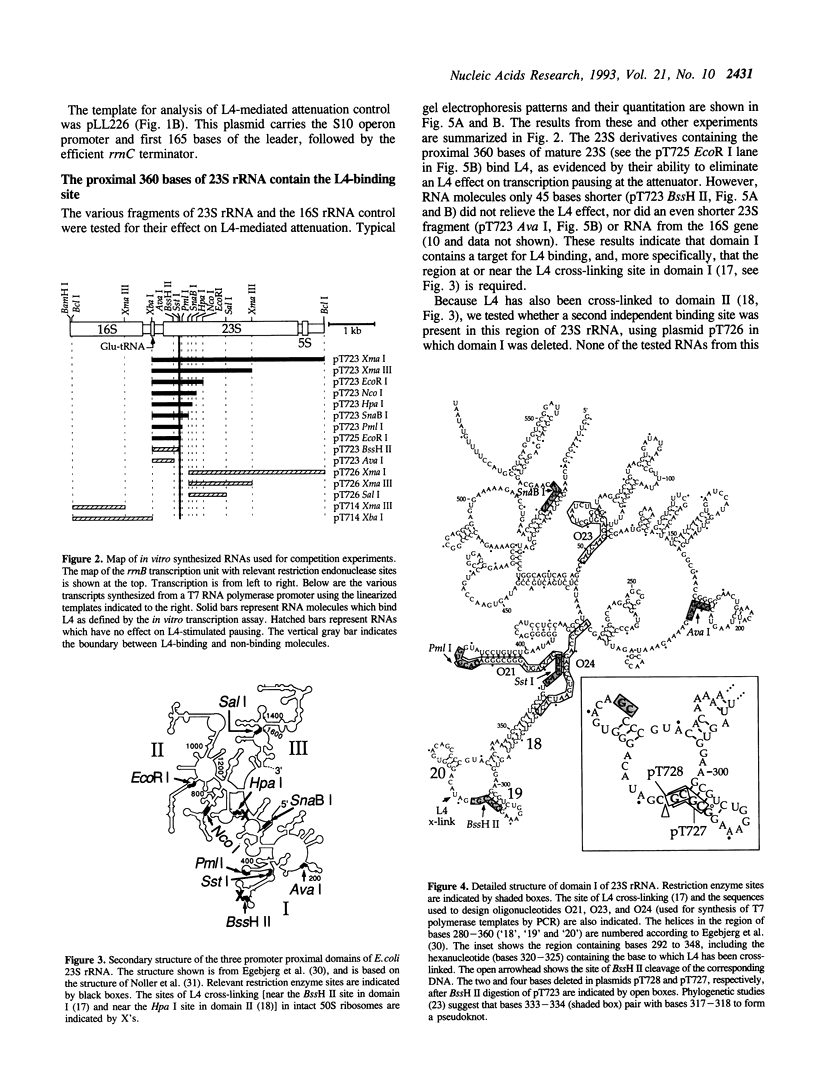
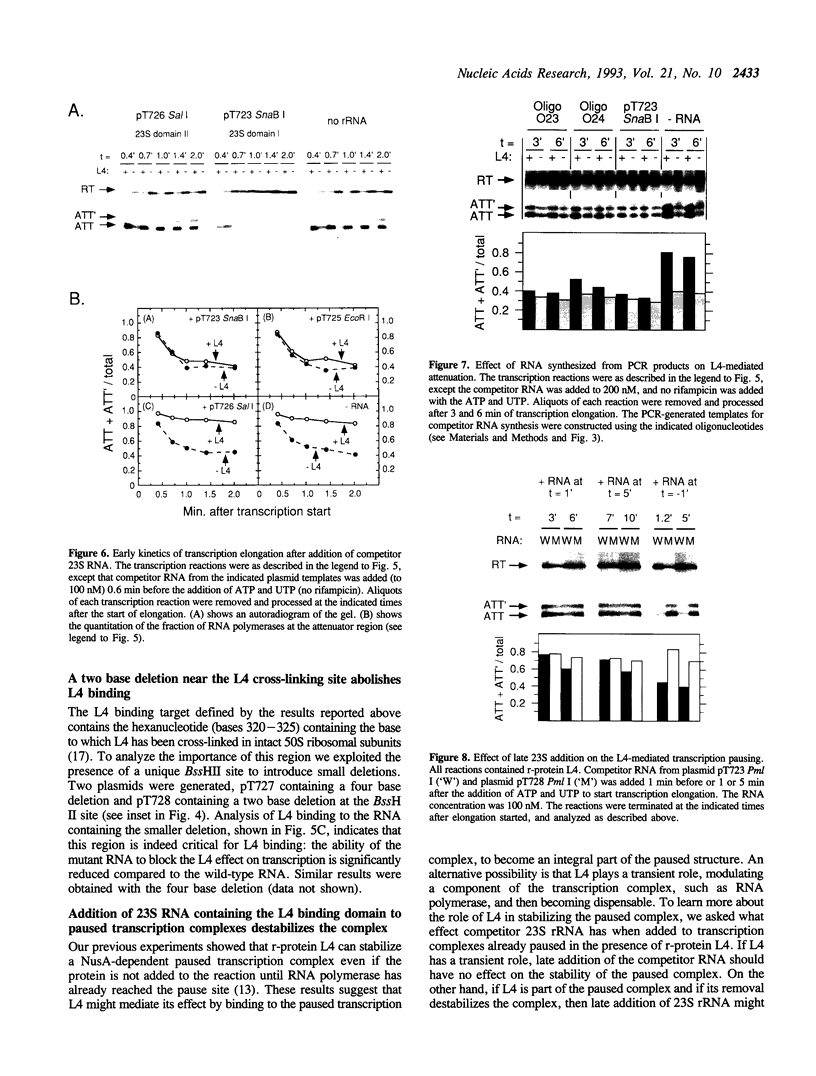
Ribosomal protein L4 of Escherichia coli regulates expression of its own eleven gene S10 operon both by inhibiting translation and by stimulating premature termination of transcription. Both regulatory processes presumably involve L4 recognition of the S10 leader RNA. To help define L4's regulatory target, we have investigated the protein's cognate target on 23S rRNA. Binding of L4 to various fragments of the 23S rRNA was monitored by determining their ability to sequester L4 in an in vitro transcription system and thereby eliminate the protein's effect on transcription. Using this approach we identified a region of about 110 bases within domain I of 23S rRNA which binds L4. A two base deletion within this region, close to the base to which L4 has been cross-linked in intact 50S subunits, eliminates L4 binding. These results also confirm the prediction of the autogenous control model, that L4 bound to its target on rRNA is not active in regulating transcription of the S10 operon.
Full text
PDF
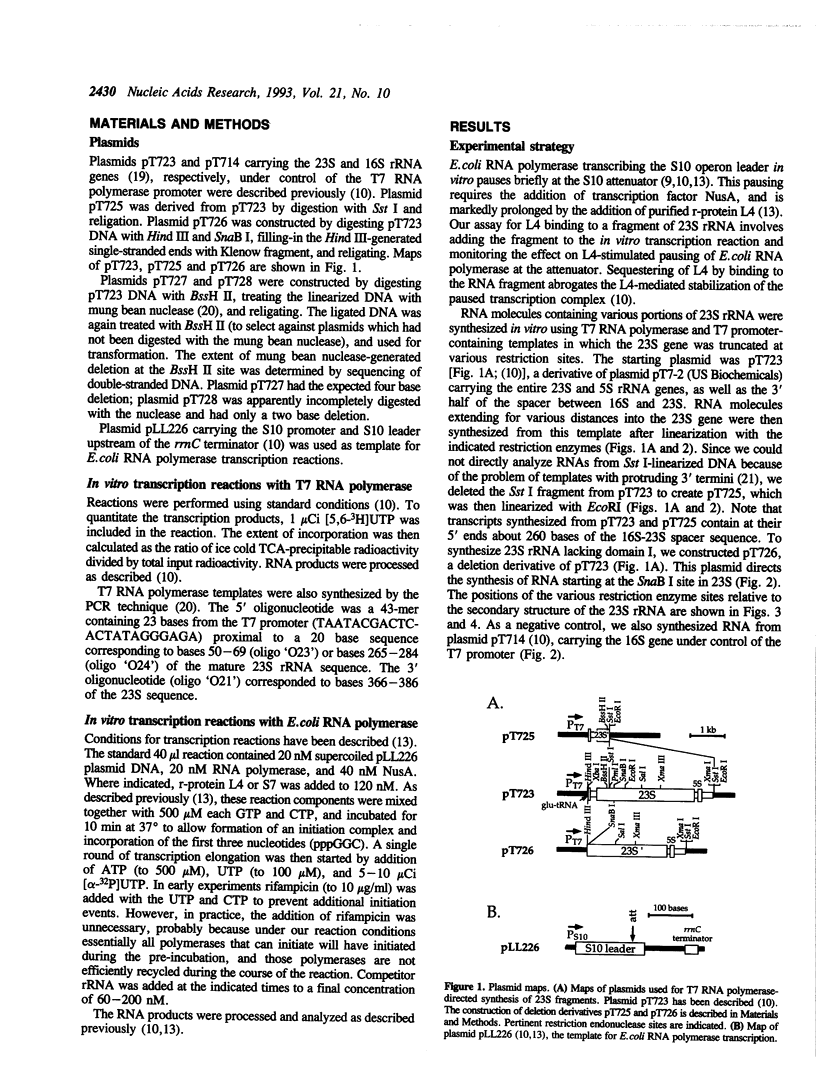



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Egebjerg J., Leffers H., Christensen A., Andersen H., Garrett R. A. Structure and accessibility of domain I of Escherichia coli 23 S RNA in free RNA, in the L24-RNA complex and in 50 S subunits. Implications for ribosomal assembly. J Mol Biol. 1987 Jul 5;196(1):125–136. doi: 10.1016/0022-2836(87)90515-8. [DOI] [PubMed] [Google Scholar]
- Freedman L. P., Zengel J. M., Archer R. H., Lindahl L. Autogenous control of the S10 ribosomal protein operon of Escherichia coli: genetic dissection of transcriptional and posttranscriptional regulation. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6516–6520. doi: 10.1073/pnas.84.18.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayhack E. J., Yang X. J., Lau L. F., Roberts J. W. Phage lambda gene Q antiterminator recognizes RNA polymerase near the promoter and accelerates it through a pause site. Cell. 1985 Aug;42(1):259–269. doi: 10.1016/s0092-8674(85)80121-5. [DOI] [PubMed] [Google Scholar]
- Gulle H., Hoppe E., Osswald M., Greuer B., Brimacombe R., Stöffler G. RNA-protein cross-linking in Escherichia coli 50S ribosomal subunits; determination of sites on 23S RNA that are cross-linked to proteins L2, L4, L24 and L27 by treatment with 2-iminothiolane. Nucleic Acids Res. 1988 Feb 11;16(3):815–832. doi: 10.1093/nar/16.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell R. R., Power A., Hertz G. Z., Putz E. J., Stormo G. D. Identifying constraints on the higher-order structure of RNA: continued development and application of comparative sequence analysis methods. Nucleic Acids Res. 1992 Nov 11;20(21):5785–5795. doi: 10.1093/nar/20.21.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutell R. R., Woese C. R. Higher order structural elements in ribosomal RNAs: pseudo-knots and the use of noncanonical pairs. Proc Natl Acad Sci U S A. 1990 Jan;87(2):663–667. doi: 10.1073/pnas.87.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold M., Nierhaus K. H. Incorporation of six additional proteins to complete the assembly map of the 50 S subunit from Escherichia coli ribosomes. J Biol Chem. 1987 Jun 25;262(18):8826–8833. [PubMed] [Google Scholar]
- Larsen N. Higher order interactions in 23s rRNA. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5044–5048. doi: 10.1073/pnas.89.11.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Archer R. H., McCormick J. R., Freedman L. P., Zengel J. M. Translational coupling of the two proximal genes in the S10 ribosomal protein operon of Escherichia coli. J Bacteriol. 1989 May;171(5):2639–2645. doi: 10.1128/jb.171.5.2639-2645.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Archer R., Zengel J. M. Transcription of the S10 ribosomal protein operon is regulated by an attenuator in the leader. Cell. 1983 May;33(1):241–248. doi: 10.1016/0092-8674(83)90353-7. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Operon-specific regulation of ribosomal protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6542–6546. doi: 10.1073/pnas.76.12.6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maly P., Rinke J., Ulmer E., Zwieb C., Brimacombe R. Precise localization of the site of cross-linking between protein L4 and 23S ribonucleic acid induced by mild ultraviolet irradiation of Escherichia coli 50S ribosomal subunits. Biochemistry. 1980 Sep 2;19(18):4179–4188. doi: 10.1021/bi00559a007. [DOI] [PubMed] [Google Scholar]
- Nierhaus K. H. The assembly of prokaryotic ribosomes. Biochimie. 1991 Jun;73(6):739–755. doi: 10.1016/0300-9084(91)90054-5. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Kop J., Wheaton V., Brosius J., Gutell R. R., Kopylov A. M., Dohme F., Herr W., Stahl D. A., Gupta R. Secondary structure model for 23S ribosomal RNA. Nucleic Acids Res. 1981 Nov 25;9(22):6167–6189. doi: 10.1093/nar/9.22.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny V., Nierhaus K. H. Initiator proteins for the assembly of the 50S subunit from Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7238–7242. doi: 10.1073/pnas.79.23.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins P. O., Nomura M. Regulation of the S10 ribosomal protein operon in E. coli: nucleotide sequence at the start of the operon. Cell. 1981 Oct;26(2 Pt 2):205–211. doi: 10.1016/0092-8674(81)90303-2. [DOI] [PubMed] [Google Scholar]
- Schenborn E. T., Mierendorf R. C., Jr A novel transcription property of SP6 and T7 RNA polymerases: dependence on template structure. Nucleic Acids Res. 1985 Sep 11;13(17):6223–6236. doi: 10.1093/nar/13.17.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P., Zengel J. M., Lindahl L. Secondary structure of the leader transcript from the Escherichia coli S10 ribosomal protein operon. Nucleic Acids Res. 1988 Sep 26;16(18):8905–8924. doi: 10.1093/nar/16.18.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierer P., Zimmerman R. A., Mackie G. A. RNA-protein interactions in the ribosome. Binding of 50-S-subunit proteins to 5' and 3' terminal segments of the 23-S RNA. Eur J Biochem. 1975 Apr 1;52(3):459–468. doi: 10.1111/j.1432-1033.1975.tb04014.x. [DOI] [PubMed] [Google Scholar]
- Spillmann S., Dohme F., Nierhaus K. H. Assembly in vitro of the 50 S subunit from Escherichia coli ribosomes: proteins essential for the first heat-dependent conformational change. J Mol Biol. 1977 Sep 25;115(3):513–523. doi: 10.1016/0022-2836(77)90168-1. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Nomura M. E. coli ribosomal protein L4 is a feedback regulatory protein. Cell. 1980 Sep;21(2):517–522. doi: 10.1016/0092-8674(80)90489-4. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Archer R. H., Freedman L. P., Lindahl L. Role of attenuation in growth rate-dependent regulation of the S10 r-protein operon of E. coli. EMBO J. 1984 Jul;3(7):1561–1565. doi: 10.1002/j.1460-2075.1984.tb02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. M., Lindahl L. Escherichia coli ribosomal protein L4 stimulates transcription termination at a specific site in the leader of the S10 operon independent of L4-mediated inhibition of translation. J Mol Biol. 1990 May 5;213(1):67–78. doi: 10.1016/S0022-2836(05)80122-6. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Lindahl L. Ribosomal protein L4 and transcription factor NusA have separable roles in mediating terminating of transcription within the leader of the S10 operon of Escherichia coli. Genes Dev. 1992 Dec;6(12B):2655–2662. doi: 10.1101/gad.6.12b.2655. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Lindahl L. Ribosomal protein L4 of Escherichia coli: in vitro analysis of L4-mediated attenuation control. Biochimie. 1991 Jun;73(6):719–727. doi: 10.1016/0300-9084(91)90052-3. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Lindahl L. Ribosomal protein L4 stimulates in vitro termination of transcription at a NusA-dependent terminator in the S10 operon leader. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2675–2679. doi: 10.1073/pnas.87.7.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. M., Mueckl D., Lindahl L. Protein L4 of the E. coli ribosome regulates an eleven gene r protein operon. Cell. 1980 Sep;21(2):523–535. doi: 10.1016/0092-8674(80)90490-0. [DOI] [PubMed] [Google Scholar]