Abstract
In bovine adrenal medullary cells synergistically acting type 1 and type 2 angiotensin II (AII) receptors activate the fibroblast growth factor-2 (FGF-2) gene through a unique AII-responsive promoter element. Both the type 1 and type 2 AII receptors and the downstream cyclic adenosine 1′,3′-monophosphate- and protein kinase C-dependent signaling pathways activate the FGF-2 promoter through a novel signal-transducing mechanism. This mechanism, which we have named integrative nuclear FGF receptor-1 signaling, involves the nuclear translocation of FGF receptor-1 and its subsequent transactivation of the AII-responsive element in the FGF-2 promoter.
INTRODUCTION
Stimuli that increase cellular growth or proliferation activate the fibroblast growth factor-2 (FGF-2) and FGF receptor-1 (FGRF1) genes and induce an accumulation of FGF-2 and FGFR1 proteins directly in the cell nucleus (Stachowiak et al., 1994, 1996a,b, 1997a,b; Moffett et al., 1996, 1998; Joy et al., 1997). Overexpressed, recombinant FGF-2 and FGFR1 accumulate in the cell nucleus and stimulate both the transition from G0/G1 to the S phase of the cell cycle and cellular growth in a manner that is independent of cell surface FGFR1 (Stachowiak et al., 1996a, 1997b). In this study we describe the mechanistic basis of this intracrine-nuclear FGF-2/FGFR1 signaling pathway.
Angiotensin II (AII) is a potent growth factor that stimulates gene expression and growth or proliferation in cells of neural, endocrine, cardiovascular, and renal tissues (Huckle and Earp, 1994). AII receptors are divided into AT1 and AT2 subtypes that can act through Gq and Gi proteins, respectively (Jagadesh, 1998). Stimulation of AT1 increases the intracellular concentrations of calcium, stimulates phosphoinositide hydrolysis, and activates protein kinase C (PKC), Src-related kinases, and the JAK-signal transducer activator of transcription and Ras-Raf-mitogen–activated protein kinase signaling pathways. In bovine adrenal medullary chromaffin cells (BAMCs), AII also increases the levels of cyclic adenosine 1′,3′-monophosphate (cAMP) (Boarder et al., 1988).
The activation of these pathways collectively contributes to the ability of AT1 to activate c-fos and related genes. The “AII response element” of c-fos maps to the serum response element (SRE), which interacts with the SRE-binding factor and mitogen-activated protein kinase–targeted p62TCF (Sadoshima and Izumo, 1993; Bhat et al., 1994). A separate, PKC-independent AT1 signal activates the c-fos gene promoter through a STAT3-containing SIFA complex that interacts with a separate, cis-inducible element in the promoter (Bhat et al., 1994).
Because all of the known physiological effects of AII are thought to be mediated by AT1, the function of AT2 receptors remains largely unknown. The AT2 subtype is expressed in the brain and other tissues during development. However, the level of its expression declines with age, and in the adult nervous system, AT2 is expressed only in discrete areas (Grady et al., 1991). AT2 expression also promotes differentiation of cultured neuronal cell lines (Reagan et al., 1990). In some systems, stimulation of AT2 evokes an inhibitory effect in contrast to the stimulatory effect of AT1 activation (Huang et al., 1996)
Evidence is accumulating that AII (Itoh et al., 1993; Fischer et al., 1997; Guo-Hong, 1998) as well as other growth factors (Ali et al., 1993; Moffett et al., 1998) and PKC (Safdar et al., 1994) exerts its growth factor-like effects by up-regulating the endogenous growth modulator FGF-2. In an effort to determine whether FGF-2 could mediate the actions of AII, we found that AII or agents that directly activate its intracellular signaling pathways induce FGF-2 expression (Stachowiak et al., 1994, 1997b). Induction of FGF-2, which lacks a signal peptide, does not lead to its presence in the extracellular medium. Instead, FGF-2 accumulates in the cell nucleus (Stachowiak et al., 1994, 1997b; Moffett et al., 1996; Joy et al., 1997). Induction of nuclear FGF-2 accumulation by AII was observed in neural crest-derived BAMCs, in human astrocytes, and in cultured smooth muscle cells, indicating that it may constitute a common response to AII in a variety of cells (Stachowiak et al., 1994, 1997b). The nuclear accumulation of FGF-2 is a two-stage process. The first stage involves the rapid translocation of FGF-2 from the cytoplasm to the nucleus, and the second stage is driven by new FGF-2 synthesis and is accompanied by an up-regulation of FGF-2 gene activity (Stachowiak et al., 1994, 1996b; Moffett et al., 1998). In this study, we show that the induction of FGF-2 expression by AII in BAMCs results from the synergistic AT1- and AT2-dependent activation of the FGF-2 gene promoter and is mediated by a novel signal transduction mechanism.
MATERIALS AND METHODS
Plasmids
(−1800/+314)FGF-2Luc (numbers depict nucleotides relative to the transcriptional start site) and its deletion mutants were described in Stachowiak et al. (1994), Moffett et al. (1996, 1998), and Joy et al. (1997). pcDNA3.1FGFR1 expressing full-length FGFR1 was described in Stachowiak et al. (1997a). The FGFR1(TK−) mutant, which lacks the tyrosine kinase domain, was generated by deleting the FGFR1 sequence 21 bp downstream from the transmembrane domain. In pcDNA3.1FGFR1(TM−) the amino acids of the transmembrane region (370–400) were deleted. pcDNA3.1FGFR1(SP−) was constructed by deleting the portion of FGFR1 that forms the signal peptide (amino acids 3–19). In FGFR1(SP−/NLS), the signal peptide sequence was replaced with the nuclear localization signal (NLS) from the simian virus 40 large T antigen (PKKKRKV [Dingwall and Laskey, 1991]). All mutations were verified by DNA sequencing.
Cells
Nonproliferating BAMCs were purified and maintained in DMEM/F12 supplemented with 0.25% bovine serum albumin as described in Stachowiak et al. (1990, 1994, 1996a). The TE671 cells were cultured in 5% serum containing DMEM and transfected by using calcium phosphate or lipofectin as described in Kim et al. (1998). Drugs were added 48 h after the transfection. Luciferase activity was expressed in number of light units per microgram of total cellular protein and per picogram of transfected intracellular luciferase DNA (dot-blot hybridization to luciferase cDNA) (Stachowiak et al., 1994; Moffett et al., 1996; Kim et al., 1998). The results obtained with and without DNA normalization were essentially the same. In all experiments (including BAMC time course analyses), all cells were harvested and processed at the same time.
Immunocytochemistry
Cells were fixed and stained by using an FGFR1 polyclonal C-term antibody (Ab) (Hanneken et al., 1995) and Cy3-goat anti-rabbit IgG as described previously (Stachowiak et al., 1996a,b, 1997a). Digitized images of 0.5-μm confocal sections of the immunostained cells were acquired by using a Bio-Rad MRC 1024 confocal microscope with a 15-mW krypton/argon laser. The average nuclear diameter was 3–5 μm. The pinhole diameter was set to prevent the out-of-focus flow of light to the in-focus image. Immunostaining for FGF-2 was done by using monoclonal FGF-2 antibody (Transduction Laboratories, Lexington, KY) as described previously (Stachowiak et al., 1994). The specificity of FGFR1 staining was demonstrated in Stachowiak et al. (1996a,b, 1997a) and of FGF-2 staining in Stachowiak et al. (1994) and Joy et al. (1997).
AII Receptor Binding
Binding of 125I-AII to cell membranes was measured by using 200–300 μg of resuspended membrane protein and 2.4 nM 125I-AII (2200 Ci/mmol) in the presence or absence of unlabeled competing ligands (Reagan et al., 1990); losartan (specific for AT1) (Mizuno et al., 1995; Huang et al.,1996; Laredo et al., 1997; Oauli et al., 1997) and PD-123319 (specific for AT2) (Sasaki et al., 1991; Israel et al., 1995; Jung et al., 1998; Tanabe et al., 1998). Nonspecific radioligand binding was determined in the presence of 1 μM [Sar1, Ile8]-AII.
Western Analyses
Nuclei and the extranuclear (cytoplasmic) fraction were isolated and characterized as described in Stachowiak et al. (1996a,b, 1997a). The nitrocellulose membranes were probed with anti-FGFR1 McAb6 (Hanneken et al., 1995), protein A-purified polyclonal anti-FGF-2 (Gonzalez et al., 1990), FGF-2 monoclonal Ab, or anti-phosphotyrosine PY-20 Ab (Transduction Laboratories) (Stachowiak et al., 1994, 1996a,b). Immune complexes were visualized by using chemiluminescence (Figures 3–5) or 125I-protein A and autoradiography (Figure 1A).
Figure 3.
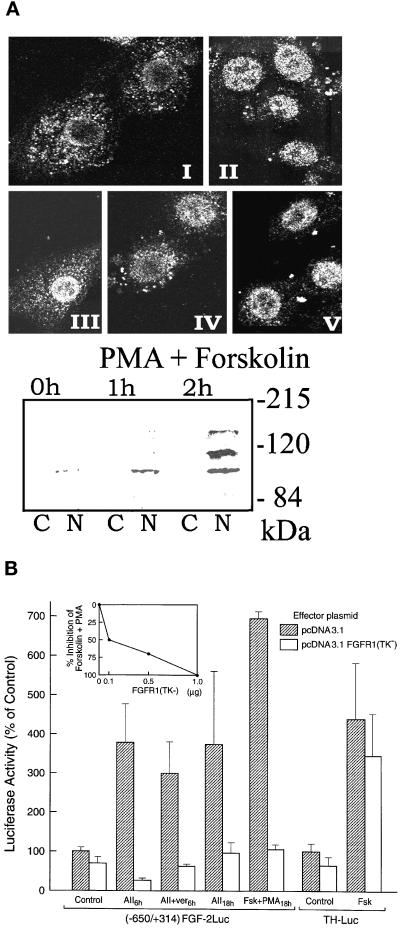
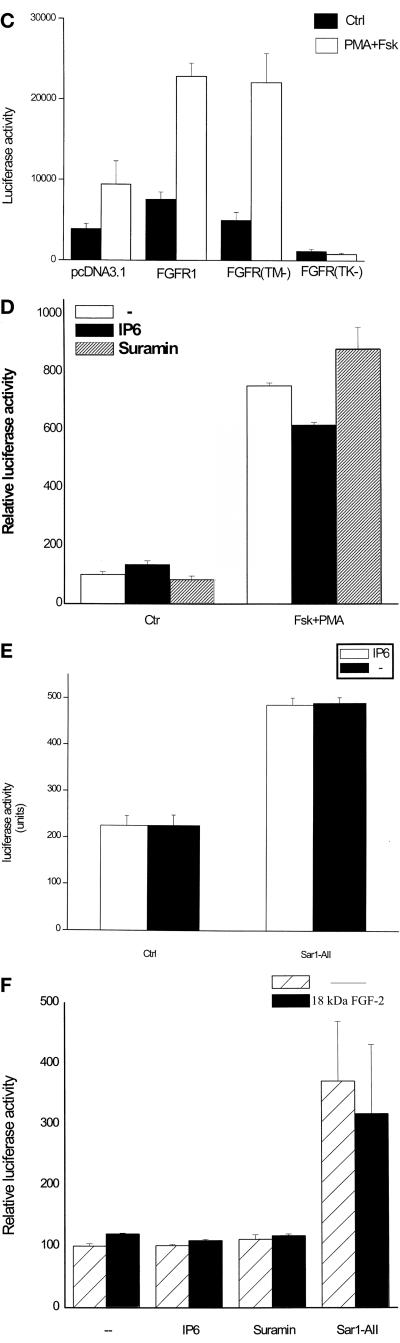
AII or cAMP and PKC activation of the FGF-2 gene promoter is mediated by intracellular FGFR1. (A) Induction of nuclear FGFR1 in BAMC. (Top) Immunofluorescent confocal analysis of endogenous FGFR1 with affinity purified, polyclonal C-term FGFR1 Ab (Hanneken et al., 1995). BAMCs were incubated with sar1-AII or PMA and forskolin or in control serum-free medium: I, control; II, 0.5 h sar1-AII; III, 2 h sar1-AII; IV, 4 h sar1-AII; V, 4 h forskolin + PMA (all treatments were terminated at the same time). Single optical sections approximately through the middle of the BAMC nuclei are shown. (Bottom) Western blot analysis of FGFR1 with McAb6 in the extranuclear (cytoplasmic, C) fraction an in the total nuclear lysate (N) (30 μg of total nuclear or cytoplasmic proteins per lane). Cells were incubated with forskolin and PMA or in control serum-free medium. (B) BAMCs (4 × 105 cells) were cotransfected with (−650/+314)FGF-2Luc or TH promoter-Luc (Kim et al., 1998) and either pcDNA3.1FGFR1(TK−) or control pcDNA3.1 (1 μg each). Two days later BAMCs transfected with (−650/+314)FGF-2Luc were incubated with 1 μM sar1AII, or with 5 μM forskolin + 0.1 μM PMA, and cells transfected with TH-Luc were incubated with 5 μM forskolin. No drugs were added to control cultures. Results are combined from two representative experiments, each with triplicate or quadruplicate cultures. (Inset) Dose-dependent inhibition by FGFR1(TK−) of PMA + forskolin stimulation of (−650/+314)FGF-2Luc. (C) BAMCs were cotransfected with 1 μg (−650/+314)FGF-2Luc and with 1 μg of FGFR1, FGFR1(TM−), FGFR1(TK−), or pcDNA3.1, and were treated with forskolin + PMA or control medium for 12 h. (D-F) BAMCs were transfected with (−650/−314)FGF-2Luc. IP6 (400 μM) or suramin (250 μM) was added 1 h before 18-kDa FGF-2 peptide, forskolin and PMA (Fsk + PMA). IP6 was added 1 h before sar1-AII (AII). Control cultures (Ctr) were not treated with PMA and forskolin or sar1-AII. Bars represent mean ± SEM of four cultures.
Figure 5.
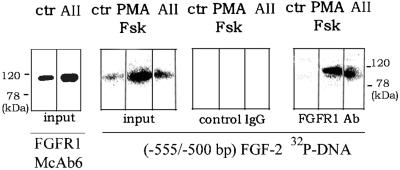
Interaction of FGFR1 with the AII-responsive region of the FGF-2 gene promoter. Nuclei of control or stimulated (2 h) BAMCs were extracted with 0.3 M KCl containing buffer as described for DNA–protein binding reactions (see MATERIALS AND METHODS). Nuclear proteins were subjected to Western analysis (100 μg/lane) with FGFR1 McAb6 or Southwestern analysis (50 μg/lane) with 32P (−555/−500 bp) FGF-2 promoter probe. In addition, 400 μg of extracted nuclear proteins from control, forskolin + PMA, or sar1-AII–treated BAMCs was immunoprecipitated (P) with polyclonal anti-FGFR1 Ab (Santa Cruz Biotechnology) or control polyclonal antibody and was subjected to Southwestern analysis along with the input proteins.
Figure 1.
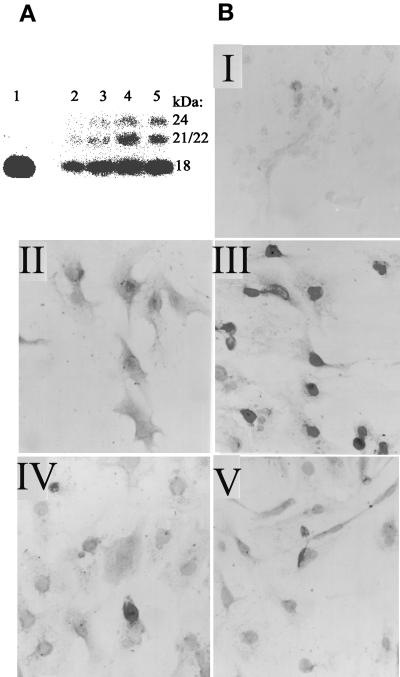
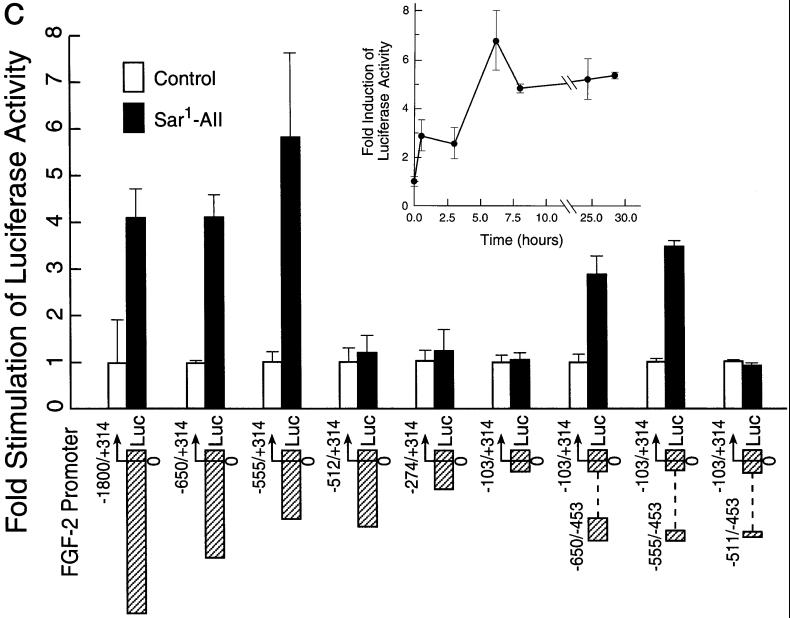
Induction of FGF-2 in BAMCs and identification of the AII-responsive element in FGF-2 gene promoter. (A) Western blot analysis with FGF-2 McAb (lane 1), 5 ng of recombinant 18-kDa FGF-2. Cells were incubated 24 h with sar1-AII (lanes: 2, 0 nM; 3, 1 nM; 4, 10 nM; 5, 100 nM). One hundred micrograms of total cellular proteins was assayed for FGF-2 as described in Stachowiak et al. (1994). (B) BAMCs were incubated24 h in control medium (II), 100 nM sar1-AII (III); 10 μM saralasin (IV); and salaralasin and sar1-AII (V). Cells were immunostained with FGF-2 McAb and horseradish peroxidase-conjugated secondary anti-mouse IgG as in Stachowiak et al. (1994). In I, cells were treated with sar1-AII and FGF-2 McAb was omitted. (C) Deletion analysis of FGF-2 promoter. (Inset) Time-dependent activation of (−1800/+314)FGF-2Luc in BAMC by sar1-AII (0.2 μM). Analysis of variance showed an overall statistically significant effect of sar1-AII (p < 0.0001). Luciferase activity was greater than in the control (0 h) at 6, 24, 30 h (p < 0.05), and at 8 h (p = 0.05) (post hoc test). Constructs, numbers indicate sequences of the FGF-2 gene fused to the luciferase reporter. In (−650/−453)(−103/+314)FGF-2Luc, (−555/−453)(−103/+314)FGF-2Luc, and (−512/−453)(−103/+314)FGF-2Luc, the upstream promoter fragments were ligated directly to the minimal −103/+314 FGF-2 promoter sequence. Forty-eight hours after transfection, BAMCs were incubated for 6 h with 0.2 μM sar1-AII or in control, serum-free medium. The ratio of luciferase activity to transfected DNA was determined, and the values given are expressed as fold increase over the activity in unstimulated cells. Sar1-AII had a statistically significant effect on the expression of (−(1800/+314)FGF-2/Luc (p < 0.01), (−650/+314)FGF-2Luc (p < 0.05), (−555/+314)FGF-2Luc (p < 0.0005), (−650/−453)(−103/+314)FGF-2Luc, and (−555/−453)(−103/+314)FGF-2Luc (p < 0.005) (Tukey's post hoc tests). Bars show mean ± SEM. Results are combined from three to four independent experiments. The expression of luciferase activity by promoterless pGL2Basic was at the background levels and was not affected by sar1-AII (not shown). Sequence of the −555/−512-bp element essential for sar1-AII stimulation: CTGCGTCGTCTAATTCAAGTTAGGTCAGTAAAGGAA AC CTTTT. (D) Effects of AT2 antagonist on activation of (−650/+314)FGF-2Luc by sar1-AII. BAMCs were incubated with 1 μM PD-123319, 30 min before and during a 6-h treatment with 0.2 μM sar1-AII. PD-123319 had no significant effect on basal promoter activity in nonstimulated cells (Table 2).
Electrophoretic Mobility Shift Assay (EMSA), DNase I Footprinting, and Southwestern Blotting of FGF-2 Promoter Binding Proteins
Nuclear proteins were extracted with buffer D (50 μM HEPES pH 7.9, 3 mM MgCl2, 0.3 M KCl, 0.1 mM EDTA, 0.3 mM EGTA, 6% sucrose, 13% glycerol, 0.25 mM spermidine, 0.05 mM spermine, 0.6 mM dithiothreitol, 0.06 mM phenylmethylsulfonyl fluoride, leupeptin, aprotinin, and pepstatin A) and EMSA was performed as in Moffett et al. (1996, 1998). In some DNA–protein binding reactions, double-stranded competitor oligonucleotides were included during the entire incubation. Consensus oligonucleotides AP1, AP2, cAMP responsive element, SP1, TFIID, and nuclear factor-κB (NF-κB) were purchased from Promega (Madison, WI). STAT consensus sequences: 5′-GATCCA TTT(CTGG)AAATG-3′ (STAT1/2), and 5′-GATCCATTT (CCCGT)AAATC-3′ (STAT3/4), with different length spacing sequence (N) and STAT-binding specificity, were taken from Seidel et al. (1995). In other experiments, control antibodies or antibodies against FGFR1 or STAT(1–5) were added and the incubation continued for an additional 8 h at 4°C. Extended incubation with control antibodies had no effect on the formation of protein–DNA complexes. DNase I footprinting of the −650- to −453-bp fragment of the FGF-2 promoter 32P-labeled on the coding strand was performed as described in Kim et al. (1998) and Moffett et al. (1998). Southwestern blotting was performed as described previously (Moffett et al., 1998). In some experiments, 400 μg of nuclear proteins was incubated with 5 μg of anti-phosphotyrosine PY-20, FGFR1 C-term Ab (Santa Cruz Biotechnology, Santa Cruz, CA), C-term FGFR1 Ab (Hanneken et al., 1995), or control IgG overnight at 4°C and then with Sepharose protein G for 1 h on ice. The immunoprecipitates were isolated and washed two times by centrifugation at 4000 × g for 4 min and were electrophoresed along with 50 μg of total nuclear extract, blotted onto nitrocellulose, and probed with FGF-2 promoter DNA.
Scans and micrographs were processed by using Bio-Rad Molecular Analyst and Adobe Photoshop. Confocal images were obtained by using Confocal Assistant.
RESULTS
BAMCs were tested for AII receptor expression by using a quantitative radioligand binding assay on prepared cell membranes. In addition to determining the total level of AII binding, subtype-selective ligands were used to measure the contribution of the two known receptor subtypes, AT1 and AT2, to the total amount of AII binding. The level of AT1 binding was determined by measuring 125I-AII binding in the presence of 1 μM PD-123319, an AT2-selective antagonist with no affinity for the bovine AT1 at that concentration (Sasaki et al., 1991; Jung et al., 1998; Tanabe et al., 1998); conversely, the level of AT2 binding was determined in the presence of 10 μM losartan, an AT1-selective antagonist with no demonstrable affinity for the bovine AT2 at that concentration (Mizuno et al., 1995; Laredo et al., 1997; Oauli et al., 1997). BAMC membrane preparations showed an average of 41.0 ± 16.0 fmol/mg protein of AII binding activity (Table 1). The use of subtype-selective antagonists revealed that AT1 makes the largest contribution (84.7 ± 5.7%), whereas AT2 makes a comparatively smaller contribution (17.9 ± 3.8%) to the overall total amount of AII binding in the BAMCs.
Table 1.
Binding of 2.4 nM 125I-AII to BAMC membranes in the presence or absence of antagonists
| % of Total binding activitya | |
|---|---|
| Total specific binding | 100.0b |
| 1 μM PD 123319 (AT1 binding) | 84.7 ± 4.9b |
| 10 μM losartan (AT2 binding) | 18.0 ± 3.3b |
| PD-123319+ losartan | 4.6 ± 2.0b |
Binding was performed as described in MATERIALS AND METHODS.
The specific 125I-AII binding in the presence of competing ligands in each experiment was expressed as a percentage of the total specific binding measured (100%). The percentages of total specific binding activity presented are the means ± SEM from four independent experiments (mean total specific binding of 125I-AII was 41.0 ± 16.0 fmol/mg protein).
Differences in 125I-AII binding between the four conditions were determined to be statistically significant by using the Tukey compromise post hoc test (p < 0.05).
Incubation of BAMCs with the stable AII analog sar1-AII induced the expression of high-molecular weight (HMW, 21/22 and 24 kDa) FGF-2 and a 1.5- to 3-fold increase in the total cellular content of 18-kDa FGF-2 in a concentration-dependent manner (Figure 1A). These effects were observed with 1 nM sar1-AII, indicating that they were mediated by high-affinity AII receptors. Consistent with the nuclear localization of high-molecular weight FGF-2 in BAMCs (Stachowiak et al., 1994) and in other cells (Florkiewicz et al., 1991), FGF-2 immunoreactivity accumulated predominantly in the nuclei of all sar1-AII-treated BAMCs (Figure 1B). Saralasin, an inactive AII analog and an AII receptor antagonist, had no effect on basal FGF-2 immunoreactivity but it prevented the sar1-AII–induced increase in nuclear FGF-2 staining.
We used transfection of a (−1800/+314 bp) FGF-2 promoter-luciferase reporter construct to examine whether the induction of FGF-2 by AII results from the transcriptional activation of the FGF-2 gene and is mediated by regulatory sequences upstream from the FGF-2 coding region. In transiently transfected BAMCs, sar1-AII increased luciferase activity in a time-dependent manner (Figure 1C, inset). A 2-fold stimulation was observed by 0.5 h, was maximal (5- to 8-fold) after 6 h, and remained elevated for at least 30 h.
A (−650/+314)FGF-2Luc plasmid displayed a similar level of sar1-AII stimulation as the (−1800/+314)FGF-2 plasmid (Figure 1C). The (−650/+314)FGF-2Luc construct lacks an AP-1–like sequence (TTACTCA, −937/−944 bp; Stachowiak et al., 1994), indicating that this sequence does not participate in sar1-AII stimulation of the FGF-2 gene promoter in transfected BAMCs. Earlier studies indicated that the core FGF-2 promoter (−20/+50 bp) was sufficient to support basal expression of the chloramphenicol acetyltransferase reporter gene and its regulation by p53 and Egr1 (Biesiada et al., 1996). In the present study, however, a short FGF-2 promoter fragment (−103/+314 bp) did not respond to sar1-AII stimulation (Figure 1C). In contrast, AII stimulation was restored to levels similar to that seen with (−650/+314)FGF-2Luc when an upstream promoter region (−650/−453 bp or 555/−453 bp but not −512/−453 bp) was ligated directly to the inactive −103/+314-bp minimal promoter (Figure 1C). Further deletions of the 5′ end of the wild-type FGF-2 promoter revealed that sar1-AII stimulation was abolished when the −555/−513-bp sequence was deleted. The lack of stimulation of (−512/+314)FGF-2Luc and shorter promoter constructs (Figure 1C) excluded posttranscriptional modification of the luciferase activity by sar1-AII treatment. The −555/−512-bp AII-responsive element also confers a high level of basal activity on the downstream FGF-2 promoter (Moffett et al., 1998).
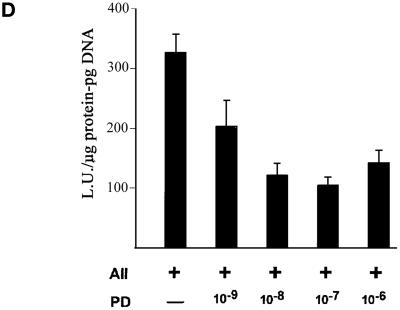
To determine which AII receptor subtype mediates FGF-2 promoter activation, BAMCs transfected with the FGF-2-luciferase reporter plasmid were incubated with 10 μM losartan or 1 μM PD-123319. Losartan or PD-123319 significantly reduced (p < 0.0001) sar1-AII stimulation from 4.67-fold to a statistically insignificant 1.3-fold (Table 2). Thus, the concurrent stimulation of both the AT1 and AT2 receptors is required for the activation of the FGF-2 promoter by sar1-AII. Concentration-dependent inhibition of promoter activation by PD-123319 is illustrated in Figure 1D. Approximately half-maximal inhibition was observed at a concentration of 1 nM and maximal inhibition was reached at 0.1 μM PD-123319.
Table 2.
Effects of AT1 and AT2 antagonists on activation of (−650/+314)FGF-2Luc by sar1-AII
| Control | sar1-AII | Losartan | Losartan + sar1-AII | PD-123319 | PD-123319+ sar1-AII | Losartan +PD-123319 | Losartan +PD-123319+ sar1-AII |
|---|---|---|---|---|---|---|---|
| (Fold FGF-2 promoter stimulation by sar1-AII) | |||||||
| 1.00 ± 0.09a (31) | 4.67 ± 0.54c (32) | 1 ± 0.17a (9) | 1.33 ± 0.16a (21) | 1.00 ± 0.16a (11) | 1.27 ± 0.12a (18) | 1.00 ± 0.25a (6) | 0.92 ± 0.24a (23) |
BAMCs were incubated with 1 μM PD-123319, 10 μM losartan, or both, 30 min before and during a 6-h treatment with 0.2 μM sar1-AII. In each group, luciferase activity is expressed relative to cells that were not treated with sar1-AII. Losartan and PD-123319 had no significant effect on basal promoter activity in nonstimulated cells. Numbers show mean ± SEM of (n) samples. Three-way ANOVA showed a significant interaction between AII and PD-123319 as well as between AII and losartan (p < 0.007).
Post hoc analysis: difference from sar1-AII (p < 0.05).
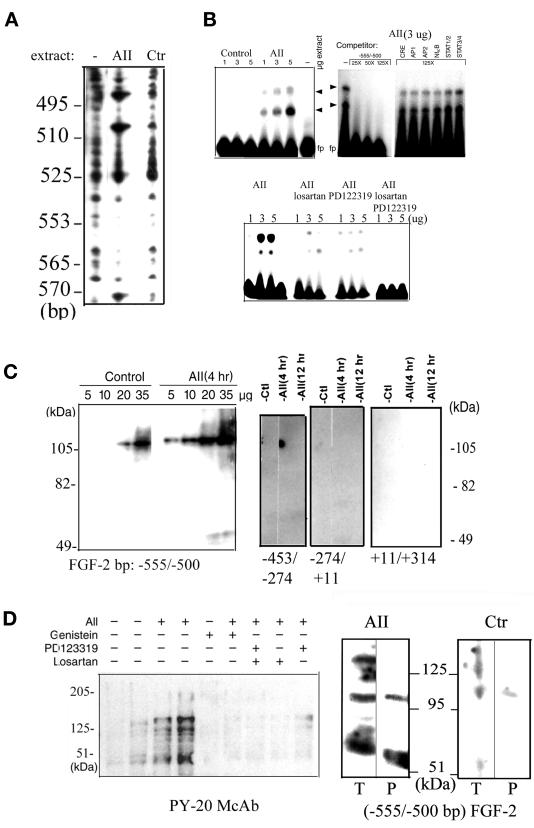
DNase I footprinting revealed a protein binding region between −555 and −535 nucleotides (nt) and DNase I hypersensitivity downstream of the protected sequence at −512 and −500 nt (Figure 2A). Nuclear extracts from cells incubated with sar1-AII showed increased protection of the DNA. A BLAST-assisted search revealed no obvious homology between the −555/−512-bp promoter fragment and the target sequences for known trans-acting factors, including cAMP- and PKC-responsive elements (cAMP responsive element, AP-2, AP-1, NF-κB, SRE), STAT proteins (Seidel et al., 1995) or Smads (Massague, 1998). Therefore, the proteins that bind to the AII-responsive region were further investigated by using EMSA (Figure 2B). A −555/−500-bp fragment of the FGF-2 promoter, which contains the protein-binding and DNase I-hypersensitive sites, was used as a probe. Extracts from unstimulated BAMCs showed little DNA-binding activity. Treatment of BAMC with sar1-AII markedly increased the DNA binding. Two major retarded bands were detected (Figure 2B, top left). Their formation was nearly completely inhibited by either losartan or PD-123319 and completely abolished by combined treatment with these two antagonists (Figure 2B, bottom). An excess of unlabeled probe but not of unrelated binding sequences for the common cAMP or PKC transactivators (CREB/ATF, AP1, AP2, or NF-κB) prevented protein binding to the −555/−500-bp fragment (Figure 2B, top right). Thus, these common transactivators may not interact with the atypical sequences in the FGF-2 promoter. Western analysis revealed that nuclear extracts of BAMCs contain STAT1, STAT2, STAT3, and STAT5 (our unpublished observations). However, consistent with the absence of a STAT-binding sequence in the AII-responsive element, preincubation of nuclear extracts with an excess of STAT consensus oligonucleotides had no effect on protein binding to the −555/−500-bp promoter probe (Figure 2B, top right). In the c-fos gene promoter, the AII-induced STAT3-containing SIFA complex is disrupted by incubation with STAT3 antibody (Bhat et al., 1994). In contrast, STAT1, STAT2, STAT3, or STAT5 antibodies added to the binding reactions for up to 24 h did not affect BAMC nuclear protein binding to the −555/−500-bp FGF-2 promoter region (our unpublished observations).
Figure 2.
Protein binding to the AII-responsive element. (A) DNase I footprinting of FGF-2 promoter region (coding strand) involved in AII stimulation. Nuclear extracts obtained from control or 0.2 μM sar1-AII–treated BAMCs were incubated with 32P-labeled FGF-2 promoter DNA followed by limited digestion with DNase I (“ −” no protein). The products were electrophoresed on 7% sequencing gels. The numbers represent location of bases within the FGF-2 gene promoter determined by using sequencing ladder (not shown). (B) EMSA with [32P]ATP and T4 kinase labeled −555/−500-bp FGF-2 promoter fragment (1–5 μg protein/lane). Arrows indicate two major DNA-protein complexes and “fp” free probe. (Top left) Nuclear extracts from control or 0.2 μM sar1-AII (AII)-treated BAMCs (4 h). (Top right) DNA–protein binding reactions were performed in the absence (−) or presence of DNA competitors (25, 50, or 125× molar excess of unlabeled −555/−500-bp target DNA or 125-fold excess of double-stranded target oligonucleotides for STAT1/2, STAT3/4, AP-1, AP-2, or NF-κB). A 25-fold molar excess of unlabeled promoter fragment completely abolished binding). (Bottom) BAMCs were treated with 0.2 μM sar1-AII in the presence or absence of 10 μM losartan, 1 μM PD-123319, or both. Treatment of control cells with losartan and PD-123319 had no effect on protein binding (not shown). (C) Southwestern analysis, nuclear proteins from control or 0.2 μM sar1-AII–treated BAMCs were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and probed with a 32P-labeled (1 × 106 cpm/ml) FGF-2 promoter fragments. The migration of prelabeled molecular weight standards (not shown) is indicated. (D) Binding of tyrosine-phosphorylated proteins to the FGF-2 promoter DNA. (Left) Western analysis with PY-20 anti-phosphotyrosine antibody. Nuclear extracts were prepared from BAMCs treated as indicated (+) with 0.2 μM sar1-AII, 20 μM genistein, 1 μM PD-123319, or 1 μM losartan for 60 min. Twenty-five micrograms of nuclear proteins was used in all lanes except lanes 2 and 4 (50 μg). (Right) Four hundred micrograms of nuclear proteins from sar1-AII–treated or control untreated BAMCs was immunoprecipitated (P) with PY-20 antibody and subjected to Southwestern analysis with −555/−500-bp FGF-2 promoter probe along with total nuclear extracts (T, 50 μg of protein). When PY-20 was replaced with control IgG, no binding of precipitated proteins to FGF-2 promoter DNA was detected (Figure 5).
To characterize the factors that interact with the AII-responsive element, nuclear proteins from control or sar1-AII-treated BAMCs were subjected to Southwestern analysis by using a 32P-labeled −555/−500-bp fragment of the FGF-2 promoter as a probe. In control extracts, we detected a protein band migrating between 100 and 110 kDa (Figure 2C). Treatment of cells with sar1-AII increased the amount of DNA binding to this band and, in longer exposure, additional bands of ∼120 and 140 kDa and bands of 50/55 kDa became visible. No binding was detected with the cytoplasmic extracts (our unpublished observations). Also, the binding of these nuclear proteins was not observed with other regions of the FGF-2 promoter, indicating that the interaction with the −555/−500-bp region is sequence specific (Figure 2C).
Stimulation of AII receptors increases tyrosine phosphorylation in a variety of cells (Jagadesh, 1998). To determine whether stimulation of the FGF-2 gene promoter by sar1-AII requires tyrosine phosphorylation, we used the general tyrosine kinase inhibitor genistein. In the absence of genistein, sar1-AII induced a 5.26 ± 0.34-fold increase in the expression of (−650/+314)FGF-2Luc (p < 0.0005). Genistein alone (20 μM) had no statistically significant effect on promoter activity. However, when genistein was added 30 min before sar1-AII, promoter stimulation was reduced to an insignificant level (1.15 ± 0.14; n = 20). In BAMCs treated with sar1-AII for 60 min, Western blot analysis by using an anti-phosphotyrosine antibody (PY-20) revealed increases in the phosphotyrosine content of several nuclear proteins (Figure 2D, left). These increases were detected within 15 min and were not associated with increases in the abundance of the proteins as determined by staining with Coomassie blue (our unpublished observations). Thus, the increase in the binding of PY-20 reflects the increased phosphorylation of existing proteins. Similar to the activation of the FGF-2 gene promoter, the sar1-AII–induced tyrosine phosphorylation of nuclear proteins was prevented by treatment of BAMCs with genistein, PD-123319, or losartan (Figure 2D, left). To determine whether the activation of AII receptors induces the tyrosine phosphorylation of nuclear proteins that interact with the AII-responsive region, nuclear extracts were immunoprecipitated with PY-20 and subjected to Southwestern analysis with the −555/−500-kb promoter fragment in parallel with total nuclear extracts (Figure 2D, right). The FGF-2 promoter DNA bound to proteins of ∼100 and 55 kDa that were immunoprecipitated with PY-20 Ab from the nuclear extracts of sar1-AII–stimulated BAMCs. In contrast, little or no FGF-2 promoter binding activity was immunoprecipitated from the nuclear extracts prepared from control unstimulated cells (Figure 2D, right).
One candidate tyrosine kinase for the activation of the FGF-2 promoter and the phosphorylation of proteins that interact with the AII-responsive element is FGF receptor-1 (FGFR1). In BAMCs, both the cell surface and the nucleus contain high-affinity binding sites for FGF-2 (Stachowiak et al., 1996a). The number of sites in the nucleus is >10-fold greater than on the cell surface or in the cytoplasm. FGFR1 is the only high-affinity FGF receptor type expressed by BAMCs and it accounts for the high-affinity FGF-2 binding sites in both locations (Stachowiak et al., 1996a). Stimulation of BAMCs results in the rapid nuclear accumulation of FGF-2 and FGFR1 (shown both by Western analysis of subcellular fractions and immunocytochemistry), and the activation of FGFR1 tyrosine kinase activity and FGFR1 phosphorylation (Stachowiak et al., 1994, 1996a, 1997b). In the present report, the subcellular localization of FGFR1 as a function of AII stimulation was examined by immunocytochemistry and confocal microscopy (Figure 3A). We used a polyclonal C-term FGFR1 Ab (Hanneken et al., 1995) that recognizes predominantly a hypoglycosylated form of FGFR1 migrating as a single, ∼100-kDa band (Stachowiak et al., 1996a,b, 1997a). In control cells, FGFR1 immunoreactivity was predominantly cytoplasmic with a perinuclear localization. Sar1-AII induced the nuclear accumulation of FGFR1 within 30 min of treatment. In the subsequent hours, FGFR1 continued to accumulate around the nuclear membrane and remained within the nucleus (Figure 3A), as confirmed by viewing of individual confocal sections (our unpublished observations). BAMCs treated with phorbol 12-myristate 13-acetate (PMA) and forskolin also showed a nuclear accumulation of FGFR1 (Figure 3A). Staining with the C-term FGFR1 Ab was abolished by preincubating the antibody with an excess of its cognate peptide (Stachowiak et al., 1996a,b).
Nuclear accumulation of FGFR1 in stimulated BAMCs was confirmed by Western blot analysis of nuclear lysates with FGFR1 McAb6. McAb6 (Hanneken et al., 1995) recognizes the N-terminal portion of FGFR1 and detects bands at ∼130, 110, and 100 kDa (Figure 3A) that represent different degrees of FGFR1 glycosylation (Stachowiak et al., 1997b). In different BAMC preparations, the level of nuclear FGFR1 in control cells was either below (Figure 3A) or slightly above (our unpublished observations) the detection limit of our assay. Forskolin and PMA reproducibly increased the levels of all nuclear FGFR1 isoforms with maximal increases observed in 110-kDa FGFR1. Similar increases were observed in BAMCs treated with sar1-AII (our unpublished observations). Consistent with the earlier FGF-2 binding experiments (Stachowiak et al., 1996) and the immunocytochemistry (Figure 3A), extranuclear material contained only traces of FGFR1 protein (Figure 3A). Analysis of the isolated nuclei by phase-contrast microscopy showed no contamination with cytoplasmic membranes and organelle. The nuclei contained <5% of the total cellular activity of 5′nucleotidase (plasma membrane marker), <2% of total activity of acid phosphatase (lysosomal marker), and nearly 90% of the total trichloroacetic acid-precipitable DNA (Stachowiak et al. 1996a). Also, the absence of surface receptor in the nuclear fraction was demonstrated by treating cells with N-hydroxysuccinimide (NHS)-sulfobiotin. Biotinylated FGFR1 was detected in the extranuclear fraction but was absent from the nuclear fraction (Stachowiak et al., 1998). These observations and the relative absence of FGFR1 in the extranuclear material of BAMCs demonstrate that the presence of FGFR1 in the isolated nuclei was not artifactual.
Signaling by high-affinity FGF receptors can be specifically blocked by expression of a dominant negative FGFR1 mutant with a deleted tyrosine kinase domain [FGFR1(TK−)] (Ueno et al., 1992; Li et al., 1994; Peters et al., 1994; Campochiaro et al., 1996; Saffell et al., 1997; Stolen and Griep, 2000). To test whether FGFR1 could be involved in the stimulation of the FGF-2 gene by AII receptors, BAMCs were cotransfected with (−650/+314)FGF-2Luc and a plasmid expressing FGFR1(TK−). Both the early (6-h) and the long-lasting (18-h) elevation of luciferase expression induced by continuous treatment with sar1-AII were completely prevented by FGFR1(TK−) (Figure 3B). This inhibition was not overcome by cotreatment of BAMCs with sar1-AII and the depolarizing agent veratridine (Figure 3B), a potent gene coactivator in BAMCs (Stachowiak et al., 1990). In BAMCs, AII stimulates both PKC- and cAMP signaling pathways (Boarder et al., 1988; Stachowiak et al., 1990), an effect that can be mimicked by direct stimulation of PKC with phorbol ester (0.1 μM PMA) and adenylate cyclase with 10 μM forskolin (Stachowiak et al., 1990). To determine whether FGFR1(TK−) blocked FGF-2 gene stimulation up- or downstream from PKC and adenylate cyclase, cells were cotransfected with (−650/+314)FGF-2-Luc and pcDNAFGFR1(TK−) and treated with forskolin and PMA. The 7-fold increase in promoter activity induced by PMA and forskolin was completely prevented by FGFR1(TK−) (Figure 3B), indicating that this stimulation was mediated by FGFR1. FGFR1(TK−) also caused a reproducible reduction in basal FGF-2 promoter activity compared with empty vector. However, FGFR1(TK−) did not cause a generalized inhibition of transcriptional activation because stimulation of a tyrosine hydroxylase (TH) promoter–luciferase construct by forskolin was not significantly affected by FGFR1(TK−) (Figure 3B).
To determine whether signaling through cell surface or intracellular FGFR1 may be involved in the transactivation of FGF-2 gene we used an FGFR1 mutant with deleted transmembrane domain [FGFR1(TM−)]. FGFR1 lacking its transmembrane domain can be released and competes with membrane FGFR for the extracellular ligand (Guillonneau et al., 1998, 2000; Wang et al., 2000). In BAMCs maintained in control serum-free medium transfected FGFR1(TM−) had only a minimal effect (one-third of the wild-type FGFR1 effect) on FGF-2 promoter activity (Figure 3C). However, FGFR1(TM−) did not inhibit promoter stimulation by forskolin and PMA. In fact, this stimulation was enhanced compared with BAMCs transfected with control pcDNA3.1 plasmid.
The increase in FGF-2Luc expression in BAMCs caused by PMA and forskolin or sar1-AII (Figure 3, D and E) was not prevented by inositol hexakis phosphate (IP6), an extracellularly acting FGFR antagonist (Sherman et al., 1993; Morrison et al., 1994). Similarly, suramin, an agent that blocks cell surface receptors for FGFs and other peptide growth factors and hormones (Dai and Peng, 1995), had no effect on the stimulation of FGF-2-Luc by PMA and forskolin (Figure 3D). Furthermore, addition of exogenous 18-kDa FGF-2 to BAMC cultures (Figure 3F) transfected with (−650/+314)FGF-2Luc induced only a slight (20%) elevation in luciferase activity. This stimulation was not observed when suramin or IP6 was included in the culture medium. Because AII up-regulated both nuclear and cytoplasmic FGFR1, we examined whether the response of the FGF-2 promoter to extracellular FGF-2 may be affected sar1-AII. As in our other experiments, Sar1-AII increased luciferase expression, but exogenous FGF-2 had no significant additional effect on promoter activity (Figure 3F). Thus, activation of the FGF-2 gene by AII or downstream PKC- and cAMP signaling pathways is unlikely to be mediated by extracellular FGFs interacting with surface FGFR1.
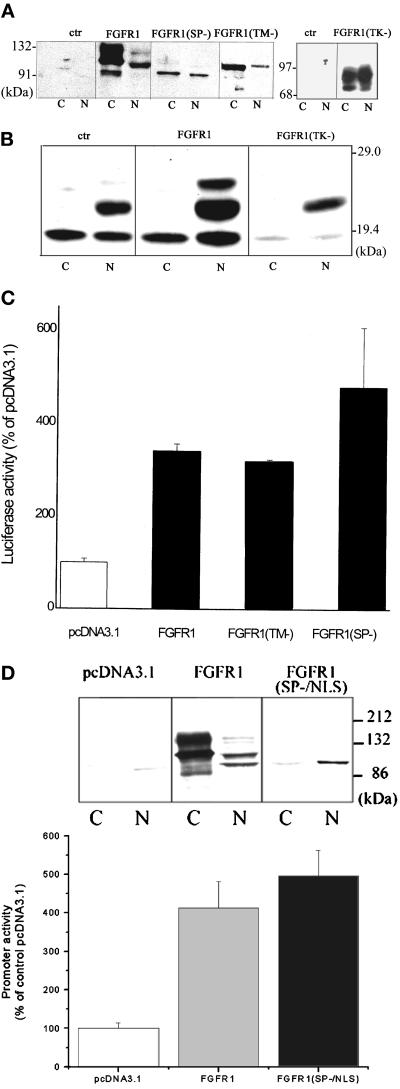
Next, we examined whether induction of FGFR1 is sufficient to activate the FGF-2 gene promoter and whether normal membrane insertion or association is necessary for the nuclear accumulation and promoter transactivation by FGFR1. For these studies we used TE671 medulloblastoma cells that express low levels of endogenous FGFR1 (Stachowiak et al., 1997a) and can be efficiently transfected (30 to 50% cells) in vitro. In control, vector-transfected TE671 cells we observed only trace amounts of the cytoplasmic or nuclear FGFR1. In cells transfected with FGFR1, the different FGFR1 glycosylation isoforms (Stachowiak et al., 1997b) were detected in both the nuclear (∼105- and 120-kDa FGFR1) and cytoplasmic fractions (90-, 110-, and 130-kDa FGFR1). Transfected FGFR1(TK−) was also detected in both the cytoplasmic and nuclear fractions. Transfected FGFR1(TM−) was detected as a single, ∼100-kDa band in the cytoplasmic fraction and in the nucleus (Figure 4A). To prevent the insertion of FGFR1 into the cellular membranes (endoplasmic reticulum and plasma membranes), we deleted the sequence encoding its 16 amino acid signal peptide (SP). FGFR1(SP−) was expressed both in the cytoplasmic and nuclear fractions (Figure 4A). The 90-kDa molecular mass of FGFR1(SP−) is consistent with the size of nonglycosylated receptor (Stachowiak et al., 1997). The levels of FGFR1(SP−) in the nucleus were 2- to 4-fold lower than in the extranuclear fraction. Nevertheless, nuclear accumulation of FGFR1(SP−) was consistently detected in several independent experiments. Thus, the deletion of the receptor transmembrane domain or its leader sequence did not prevent the nuclear accumulation of FGFR1. Unlike endogenous or transfected wild-type FGFR1, FGFR1(SP−) was not biotinylated in cells labeled with membrane insoluble NHS-sulfobiotin, thus confirming that FGFR1(SP−) does not reach the cell surface (not shown). FGFR1(TM−), unlike FGFR1(SP−), was detected outside the cell (our unpublished observations).
Figure 4.
Induction of intracellular FGFR1 is sufficient to transactivate the FGF-2 gene promoter. (A) TE671 cells were transfected with pcDNA3.1FGFR1, pcDNA3.1FGFR1(TK−), pcDNA3.1FGFR1(TM−), pcDNAFGFR1(SP−), or with control pcDNA3.1. Nuclear (N) and cytoplasmic (C) fractions (30 μg of protein per lane) were purified 48 h later and were analyzed with FGFR1 McAb6. (B) TE671 cells were transfected with pcDNA3.1FGFR1, pcDNA3.1FGFR1(TK−), or control pcDNA3.1 (1 μg each). Nuclear (N) and cytoplasmic (C) extracts were prepared 48 h later and analyzed by Western blot with polyclonal FGF-2 Ab. (C) (−650/+314)FGF-2Luc (1 μg) was cotransfected into the TE671 cells with one of the following effector plasmids (1 μg): FGFR1, FGFR1(TM−), FGFR1(SP−), or control pcDNA3.1. The luciferase activity was measured 48 h later. The experiments were repeated two to four times. (D) Expression and transactivation of FGF-2 promoter by FGFR1(SP−/NLS). (Inset) TE671 cells were transfected with pcDNA3.1FGFR1, pcDNA3.1FGFR1(SP−/NLS), or with control pcDNA3.1. Nuclear (N) and cytoplasmic (C) fractions (Western blot with FGFR1 McAb6, 60 μg of protein per lane). Bar graph (−650/+314)FGF-2Luc (1 μg) was cotransfected with (1 μg) pcDNAFGFR1, pcDNAFGFR1(SP−/NLS), or control pcDNA3.1. The luciferase activity was measured 48 h later.
The effects of transfected FGFR1 on the expression of endogenous FGF-2 by TE671 cells are shown in Figure 4B. In general, little or no FGF-2 can be detected in TE671 maintained in serum-free medium (our unpublished observations). In the present study, all experiments were performed with serum containing TE671 cultures. Control, vector-transfected cells showed the presence of 18-kDa FGF-2 in the cytoplasm and nucleus. In the nucleus, 22-kDa FGF-2 isoform was also detected. In cells transfected with FGFR1, we observed a marked induction of the nuclear 24-kDa FGF-2 and increases in the levels of 22- and 18-kDa FGF-2. The levels of cytoplasmic FGF-2 were unchanged. In contrast, transfection with FGFR1(TK−) reduced both cytoplasmic and nuclear 18-kDa FGF-2 as well as the level of the 22-kDa isoform in the nucleus (Figure 4B).
The effects of FGFR1 and FGFR1(TK−) on the cotransfected FGF-2 promoter–luciferase construct were essentially the same as the effects on the endogenous FGF-2 gene. Transfected FGFR1 increased (−650/+314)FGF-2Luc activity 3- to 4-fold relative to pcDNA3.1 transfected controls (Figure 4C). In contrast, FGFR1(TK−) reduced basal promoter activity and completely blocked transactivation by cotransfected FGFR1, thus confirming that FGFR1(TK−) acts as a dominant negative inhibitor of gene transactivation by wild-type FGFR1 (our unpublished observations). IP6 had no effect on promoter transactivation by FGFR1 (not shown). Similar to the BAMC (Figure 3F), addition of exogenous 18-kDa FGF-2 to the TE671 cells increased FGF-2Luc expression by only 20% and this increase was not observed in cells treated with IP6 or suramin (our unpublished observations). Also, in cells transfected with FGFR1, exogenous FGF-2 had no additional effect on the 4-fold increase in luciferase expression induced by FGFR1 (our unpublished observations). Receptor mutants, FGFR1(TM−) and FGFR1(SP−), increased basal promoter activity 3.4- and 4.6-fold, respectively, in TE671 cells. Transfected FGFR1(SP−) accumulated both in the nucleus and in the cytoplasm (Figure 4A). Therefore, to ascertain whether nuclear FGFR1 alone can transactivate the FGF-2 gene promoter, we made the construct FGFR1(SP−/NLS) in which the signal peptide was replaced with the NLS of the simian virus 40 large T antigen. FGFR1(SP−/NLS) or wild-type FGFR1 were transfected into TE671 cells (Figure 4D). Wild-type FGFR1 accumulated in the cytoplasmic fraction predominantly as 110- and 130-kDa bands and in the nucleus as 100- and 120-kDa bands. In contrast, FGFR1(SP−/NLS) migrated as a single 100-kDa band detected almost exclusively in the nucleus. Nonetheless, FGFR1(SP−/NLS) activated the FGF-2 gene promoter at least as effectively as wild-type FGFR1. Thus, the specific accumulation of FGFR1 in the cell nucleus is sufficient to activate transcription from the FGF-2 gene promoter.
We next examined whether nuclear FGFR1 participates directly in the activation of the FGF-2 gene. The intermediate glycosylated FGFR1 (105–110-kDa) isoform was the most abundant (Figure 3A) and the most heavily phosphorylated (Stachowiak et al., 1996a) FGFR1 isoform found in nuclear lysates of stimulated BAMCs. This intermediate FGFR1 isoform was also detected by Western analysis of the 0.3 M KCl nuclear extracts prepared for DNA–protein binding reactions (see MATERIALS AND METHODS). The fact that other isoforms were usually not detected in this extract could reflect their lower levels in the nucleus. Also, they could be less effectively extracted by this method. The 105–110-kDa FGFR1 band comigrated with the band recognized by the 32P (−555/−500 bp) FGF-2 promoter probe on 10% SDS polyacrylamide gels (Figure 5).
To determine whether this promoter binding protein is FGFR1, nuclear extracts from control, forskolin + PMA, or sar1-AII–treated BAMCs were incubated with a C-term polyclonal FGFR1Ab (Santa Cruz Biotechnology) and the immune complexes were precipitated with protein G Sepharose beads. Figure 5 shows a Southwestern blot of the input material and the immunoprecipitated proteins. The DNA binding protein (∼110 kDa) detected in the input extracts of forskolin and PMA- or sar1-AII–treated cells was also found in the corresponding FGFR1 Ab immunoprecipitates. This band was not detected in immunoprecipitates with control rabbit serum.
DISCUSSION
We show here that the induction of FGF-2 by AII reflects, at least in part, the increased transcription of the FGF-2 gene and is mediated through a unique −555/−512 regulatory sequence, ∼500 bp upstream from the earlier identified FGF-2 core promoter. This regulation is not mediated by common transacting factors. Instead the candidate trans-activator is an ∼105–110-kDa nuclear protein that binds to the AII responsive element in a manner dependent upon AII receptors or cAMP/PKC signaling. Our experiments indicate that this protein is phosphorylated, partially glycosylated FGFR1.
We show that tyrosine kinase activity is essential for AII signaling and that the FGF-2 promoter binding factors are among the proteins that are phosphorylated on tyrosine following AII receptor activation. Protein phosphorylation similar to promoter activation requires the synergistic action of both AT1 and AT2 receptor and is prevented by genistein. Our experiments with FGFR1(TK−) strongly implicate the tyrosine kinase activity of FGFR1 in the activation of the FGF-2 gene by AII receptors, cAMP, and PKC. FGFR1(TK−) is a dominant negative receptor that specifically forms nonphosphorylated, inactive dimers with FGFR and, in the case of BAMCs, with FGFR1, the only type of FGFR expressed by these cells. The inhibition of FGF-2 promoter activation by FGFR1(TK−) demonstrates that FGFR1 signaling is essential for the activation of the FGF-2 gene by AII receptors and by common intracellular regulators such as cAMP and PKC. Unlike FGFR1(TK−), wild-type full-length receptor or mutants that retain the TK domain act as FGF-2 promoter transactivators (acting through AII-responsive element). Thus, the induction of FGFR1 constitutes the stimulus that transmits signals generated by AII, cAMP, and PKC to the FGF-2 gene, and the tyrosine kinase portion of FGFR1 is essential for its transactivating function.
In BAMCs, the nuclear accumulation of FGFR1 and the activation of the FGF-2 gene can be induced by heterologous stimuli such as activated AII or acetylcholine receptors, adenylate cyclase, and PKC (Stachowiak et al., 1994, 1996a; present study), but not by incubation with 18-kDa FGF-2 (Stachowiak et al., 1996a). Also, FGFR1-mediated activation of the FGF-2 gene by heterologous stimuli is not prevented by secreted FGFR1(TM−) or extracellular-acting FGFR antagonists (IP6 or suramin). Thus, the activation the FGF-2 gene is not mediated by stimulation of surface FGFR1.
Studies in our laboratory have provided good evidence for the localization of full-length, functional FGFR1 in the nuclei of BAMCs, astrocytes, and glioma cells, and in sympathetic neurons. The nuclear accumulation of endogenous or transfected FGFR1 was shown by using Western and far Western assays with several antibodies that recognize distinct FGFR1 epitopes (Stachowiak et al., 1996a,b, 1997a,b; present study). The nuclear localization of transfected, epitope-tagged FGFR1 was also detected with epitope tagged-specific antibodies (our unpublished observations). Whether FGFR1 can accumulate in the nuclear interior has been a matter of some controversy (Prudovsky et al., 1994; Maher, 1996, Stachowiak et al., 1996a,b, 1997), perhaps due to its brief occurrence during cell stimulation or just before entry into the S phase of the cell cycle (Stachowiak et al., 1997a). We have shown the localization of FGFR1 within the nuclear interior by using immunocytochemistry with confocal or electron microscopy and antibodies that recognize the C- or N-terminal portions of FGFR1 (Stachowiak et al., 1996a,b, 1997a; Figure 3A) and in living cells transfected with FGFR1 fused to green fluorescent protein (our unpublished observations). The presence of FGFR1 in the cell nucleus has also been reported in substantia nigra neurons, in Swiss 3T3 cells, and NIH 3T3 fibroblasts (reviewed in Stachowiak et al., 1997b). In a separate study, we found that after a 30-min labeling of surface proteins with NHS-sulfobiotin in cells transfected with FGFR1, the surface FGFR1 becomes biotinylated, whereas the nuclear receptor remains unlabeled for at least 4 h (our unpublished observations). Thus, the FGFR1 that enters the nucleus (and activates the FGF-2 gene, see discussion below) may not represent internalized cell surface FGFR1. Still unlike FGFR1(SP−), wild-type, nuclear FGFR1 is glycosylated, suggesting that it is initially processed through the endoplasmic reticulum/Golgi before it enters the nucleus. FGFR1 lacks a typical NLS so its nuclear uptake may require an interaction with NLS-containing proteins. Candidate chaperones are the 21–24-kDa FGF-2 isoforms that contain a functional NLS (Courdec et al., 1991), even though its small size allows FGF-2 to diffuse freely into the nucleus [i.e., NLS-lacking 18-kDa FGF-2 is also found in the nucleus of BAMCs (Stachowiak et al., 1994) and other cells (Florkiewicz et al., 1991)]. Indeed, in stimulated BAMCs, astrocytes (Stachowiak et al., 1996a,b), and in TE671 transfected with pcDNA3.1FGFR (our unpublished observations), FGFR1 translocates into the nucleus in parallel with cytosolic FGF-2.
Experiments using FGFR1(SP−) demonstrate that insertion of the receptor into the plasma membrane and glycosylation are not essential for either nuclear entry or the transactivating function of FGFR1. The addition of an NLS to FGFR1, which drives a signal peptide-deficient form of FGFR1 into the nucleus, generated fully active, transactivating receptor despite its absence from the cytoplasm. These findings allow us to dissociate two functions of FGFR1: 1) paracrine or autocrine signaling by the plasma membrane receptor, which might be continued following receptor internalization into the cytoplasm; and 2) intracrine signaling by nuclear FGFR1 and demonstrate for the first time the regulation of gene transcription by nuclear FGFR1.
By associating with the nuclear matrix, FGFR1 (Stachowiak et al., 1996a,b) is strategically positioned to be directly involved in the regulation of gene expression. In nuclear extracts of stimulated BAMCs, Western blotting with McAb6 detected three major FGFR1 bands with apparent molecular masses between 95 and 130 kDa (Figure 3A). These glycosylation isoforms are immunoprecipitated with a C-term FGFR1 Ab and can be detected with FGFR1 McAb6 in a far Western assay and by autophosphorylation with [32P]ATP (Stachowiak et al., 1996a). In the present study, we show that one of these C-term FGFR1Ab-immunoprecipitated FGFR1 isoforms (the most abundant, intermediate FGFR1) can bind to the AII-responsive promoter element in a sequence-specific manner and that this binding correlates with promoter activity. We also show that this promoter binding protein is recognized by the anti-phosphotyrosine antibody PY-20. This is consistent with the observation that this FGFR1 isoform incorporates the largest amount of 32P (Stachowiak et al., 1996a). Binding of the less abundant FGFR1 isoforms to the promoter may be below the detection limit of our assay.
At present, the specific molecular mechanisms through which nuclear FGFR1 increases the transcription of the FGF-2 gene are unknown. Such mechanisms could involve the tyrosine phosphorylation of transcriptional factors, or histones and/or their interaction with phosphorylated receptor. As shown in this study, AII stimulated the tyrosine phosphorylation of several nuclear proteins in addition to FGFR-1. Their nature, and whether they are phosphorylated by FGFR1 remains to be elucidated.
Although the majority of AII binding in BAMCs is due to the AT1 receptor subtype, we show that a small but detectable level of AT2 is present as well. AT1 and AT2 activate the FGF-2 promoter in a synergistic manner, suggesting their localization on the same cells. AT2 and AT1 also coexist in several regions of the nervous system (Höhle et al., 1995). By regulating FGF-2 gene expression together with AT1, AT2 could have an important function in the trophic effects of AII in neuroendocrine cells. The nuclear accumulation of FGFR1 and the activity of the FGF-2 gene can be stimulated also by acetylcholine in BAMCs (Stachowiak et al., 1994, 1996a), by epidermal growth factor (EGF) and FGF-2 in astrocytes (Stachowiak et al., 1997b; Moffett et al., 1998), by Bone Morphogenetic Protein-7 in human mesencephalic neurons (our unpublished observations), and by serum, cAMP, and PKC in all of these cells. Also, other genes in addition to FGF-2 may be regulated by nuclear FGFR1 (our unpublished observations). Thus, by being induced by a variety of heterologous signals, the integrative nuclear FGFR1 signaling described here may constitute a novel common pathway through which growth factors, hormones, neurotransmitters, cell–cell interactions, and second messengers execute control over genetic programs for cellular growth, differentiation, and survival.
ACKNOWLEDGMENTS
We thank Dr. Ronald Smith (DuPont/Merck, Wilmington, DE) for losartan, Dr. David Dudley (Parke-Davis, Ann Arbor, MI) for PD-123319, Dr. Wade Sigurdson and Gabriel Martins (3D Imaging, School of Medicine, State University of New York at Buffalo) for their help with confocal microscopy, and Dr. Robert Z. Florkiewicz (Scripps Research Institute, La Jolla, CA) for help with FGF-2 assay in BAMC. This study was supported by the National Science Foundation (IBN-9728923), the National Institutes of Health (HL-49376) (to M.K.S.), and by the Arizona Disease Control Research Commission (1-209) (to D.C.B. and M.K.S.). P.A.M. was supported by the National Institutes of Health (NS-28121).
Abbreviations used:
- AII
angiotensin II
- AT1
AT2, type 1 and 2 AII receptors
- BAMC
bovine adrenal medullary chromaffin cell
- EGF
epidermal growth factor
- EMSA
electrophoretic mobility shift assay
- FGF-2
fibroblast growth factor-2
- FGFR1
FGF receptor 1
- HMW
high molecular weight
- NLS
nuclear localization signal
- PMA
phorbol 12-myristate 13-acetate
- PBS
phosphate-buffered saline
- PD-123319
(S)-1-[4-(dimethylamino)-3-methylphenyl]methyl-5-(diphenylacetyl)-4,5,6,7-tetrahydro-1H-imidazo(4,5-C)pyridine-6-carboxylic acid
- PKC
protein kinase C
- SRE
serum responsive element
- STAT
signal transducer activator of transcription
REFERENCES
- Ali S, Becker MW, Dorn GW., II Thromboxane A2 stimulates vascular smooth muscle hypertrophy by up-regulating the synthesis and release of endogenous basic fibroblast growth factor. J Biol Chem. 1993;268:17397–17403. [PubMed] [Google Scholar]
- Bhat GJ, Thekkumkara TJ, Thomas WG, Conrad KM, Baker KM. Angiotensin II stimulates cis-inducing factor-like DNA binding activity. Evidence that the AT1A receptor activates transcription factor-Stat91 and/or a related protein. J Biol Chem. 1994;269:31443–31449. [PubMed] [Google Scholar]
- Biesiada E, Razanadi M, Levin ER. Egr-1 activates basic fibroblast growth factor transcription. J Biol Chem. 1996;271:18576–18581. doi: 10.1074/jbc.271.31.18576. [DOI] [PubMed] [Google Scholar]
- Boarder MR, Plevin R, Marriot DD. Angiotensin II potentiates prostaglandin stimulation of cyclic AMP levels in intact bovine adrenal medulla cells but not adenylate cyclase in permeabilized cells. J Biol Chem. 1988;263:15319–15324. [PubMed] [Google Scholar]
- Campochiaro PA, Chang M, Ohsato M, Vinores SA, Nie Z, Hjelmemland L, Mansukhani A, Basilico C, Zack DJ. Retinal degeneration in transgenic mice with photoreceptor-specified expression of a dominant-negative fibroblast growth factor receptor. J Neurosci. 1996;16:1679–1688. doi: 10.1523/JNEUROSCI.16-05-01679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courdec B, Prats H, Bayard F, Almaric F. Potential oncogenic effects of basic fibroblast growth factor requires cooperation between CUG and AUG-initiated forms. Cell Regul. 1991;2:709–718. doi: 10.1091/mbc.2.9.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Peng HB. Presynaptic differentiation induced in cultured neurons by local application of basic fibroblast growth factor. J Neurosci. 1995;15:5466–5475. doi: 10.1523/JNEUROSCI.15-08-05466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C, Laskey RA. Nuclear targeting sequences–a consensus? Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Fischer TA, Ungureanu-Longrois D, Singh K, De Zengoita J, De Ugarte D, Alali A, Gadbut AP, Lee MA, Balligand JL, Kifor I, Smith TW, Kelly RA. Regulation of bFGF expression and ANG II secretion in cardiac myocytes and microvascular endothelial cells. Am J Physiol. 1997;272:H958–H968. doi: 10.1152/ajpheart.1997.272.2.H958. [DOI] [PubMed] [Google Scholar]
- Florkiewicz RZ, Baird A, Gonzalez AM. Multiple forms of bFGF: differential nuclear and cell surface localization. Growth Factors. 1991;4:265–275. doi: 10.3109/08977199109043912. [DOI] [PubMed] [Google Scholar]
- Gonzalez A-M, Buscaglia M, Ong M, Baird A. Distribution of basic fibroblast growth factor in the 18-day rat fetus: localization in the basement membrane of diverse tissues. J Cell Biol. 1990;110:753–765. doi: 10.1083/jcb.110.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JF. Expression of AT2 receptors in the developing rat fetus. J Clin Invest. 1991;88:921–933. doi: 10.1172/JCI115395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillonneau X, Regnier-Ricard F, Jeanny JC, Thomasseau S, Courtois Y, Mascarelli F. Regulation of FGF soluble receptor type 1 (SR1) expression and distribution in developing, degenerating, and FGF-2-treated retina. Dev Dyn. 2000;217:24–36. doi: 10.1002/(SICI)1097-0177(200001)217:1<24::AID-DVDY3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Guillonneau X, Reigner-Ricard F, Laplace O, Jonet L, Bryckaert M, Courtois Y, Mascarelli F. Fibroblast growth factor (FGF) soluble receptor 1 acts as a natural inhibitor of FGF2 neurotrophic activity during retinal degeneration. Mol Biol Cell. 1998;9:2785–802. doi: 10.1091/mbc.9.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo-Hong Li. Inhibitory effects of antisense basic fibroblast growth factor oligonucleotides on proliferation of cultured aortic smooth muscle cells induced by angiotensin II in SHR rats. Acta Pharmacol Sin. 1998;19:132–135. [PubMed] [Google Scholar]
- Hanneken A, Maher PA, Baird A. High affinity immunoreactive FGF receptors in the extracellular matrix of vascular endothelial cells - implications for the modulation of FGF-2. J Cell Biol. 1995;128:1221–1228. doi: 10.1083/jcb.128.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhle S, Blume A, Lebrun C, Culman J, Unger T. Angiotensin receptors in the brain. Pharmacol Toxicol. 1995;77:306–315. doi: 10.1111/j.1600-0773.1995.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Huang XC, Richards EM, Sumners C. Mitogen-activated protein kinases in rat brain neuronal cultures are activated by angiotensin II type 1 receptors and inhibited by angiotensin II type 2 receptors. J Biol Chem. 1996;271:15635–15641. doi: 10.1074/jbc.271.26.15635. [DOI] [PubMed] [Google Scholar]
- Huckle WR, Earp HS. Regulation of cell proliferation and growth by angiotensin II. Prog Growth Factor Res. 1994;5:177–194. doi: 10.1016/0955-2235(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Israel A, Stromberg C, Tsutsumi K, Del Rosario Carrido M, Torres M, Saavedra JM. Angiotensin II receptor subtypes and phosphoinositide hydrolysis in rat adrenal medulla. Brain Res Bull. 1995;38:441–446. doi: 10.1016/0361-9230(95)02011-f. [DOI] [PubMed] [Google Scholar]
- Itoh H, Mukoyama M, Pratt RE, Gibbons GH, Dzau VJ. Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin II. J Clin Invest. 1993;91:2268–2274. doi: 10.1172/JCI116454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadesh G. Angiotensin II-receptor antagonists, molecular biology, and signal transduction. Indian J Exp Biol. 1998;36:1171–1194. [PubMed] [Google Scholar]
- Joy A, Moffett J, Neary K, Shapiro J, Coons S, Mordechai E, Stachowiak E, Stachowiak MK. Nuclear accumulation of FGF-2 is associated with proliferation of human astrocytes and glioma cells. Oncogene. 1997;14:171–183. doi: 10.1038/sj.onc.1200823. [DOI] [PubMed] [Google Scholar]
- Jung E-M, Betancourt-Calle S, Mann-Blakeney R, Foushee T, Isales CM, Bollag WB. Sustained phospholipase D activation in response to angiotensin II but not carbachol in bovine adrenal glomerulosa cells. Biochem J. 1998;330:445–451. doi: 10.1042/bj3300445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EL, Peng H, Esparza FM, Maltchenko SZ, Stachowiak MK. Cruciform-extruding regulatory element controls cell-specific activity of the tyrosine hydroxylase gene promoter. Nucleic Acids Res. 1998;26:1793–1800. doi: 10.1093/nar/26.7.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laredo J, Shah JR, Lu Z-R, Hamilton BP, Hamlyn JM. Angiotensin II stimulates secretion of endogenous ouabain from bovine adrenocortical cells via angiotensin type 2 receptors. Hypertension. 1997;29:401–407. doi: 10.1161/01.hyp.29.1.401. [DOI] [PubMed] [Google Scholar]
- Li Y, Basilico C, Mansukhanin A. Transformation by fibroblast growth factors can be suppressed by truncated fibroblast growth factor receptors. Mol Cell Biol. 1994;14:7660–7669. doi: 10.1128/mcb.14.11.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher PA. Nuclear translocation of fibroblast growth factor (FGF) receptors in response to FGF-2. J Cell Biol. 1996;134:529–536. doi: 10.1083/jcb.134.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Sada T, Ikeda M, Fukuda N, Miyamoto M, Yanagisawa H, Koike H. Pharmacology of CS-866, a novel nonpeptide angiotensin II receptor antagonist. Eur J Pharmacol. 1995;285:181–188. doi: 10.1016/0014-2999(95)00401-6. [DOI] [PubMed] [Google Scholar]
- Moffett J, Kratz E, Florkiewicz R, Stachowiak MK. Promoter regions involved in density-dependent regulation of basic fibroblast growth factor gene expression in human astrocytic cells. Proc Natl Acad Sci USA. 1996;93:2470–2475. doi: 10.1073/pnas.93.6.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett J, Kratz E, Myers J, Stachowiak EK, Florkiewicz RZ, Stachowiak MK. Transcriptional regulation of fibroblast growth factor-2 expression in human astrocytes: implications for cell plasticity. Mol Biol Cell. 1998;9:2269–2285. doi: 10.1091/mbc.9.8.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RS, Shi E, Kan M, Yamaguchi F, McKeehan W, Rudnicka-Nawrot M, Palczewski K. Inositolhexakisphosphate (INSP6): an antagonist of fibroblast growth factor receptor binding and activity in vitro. Cell Dev Biol. 1994;30A:783–789. doi: 10.1007/BF02631302. [DOI] [PubMed] [Google Scholar]
- Oauli R, Berthelon M-C, Bégeot M, Saez JM. Angiotensin II receptor subtypes AT1 and AT2 are down-regulated by angiotensin II through AT1 receptor by different mechanisms. Endocrinology. 1997;138:725–733. doi: 10.1210/endo.138.2.4952. [DOI] [PubMed] [Google Scholar]
- Peters K, Werner S, Liao X, Wert S, Whitsett J, Williams L. Targeted expression of a dominant negative FGF receptor blocks branching morphogenesis and epithelial differentiation of the mouse lung. EMBO J. 1994;13:3296–3301. doi: 10.1002/j.1460-2075.1994.tb06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudovsky I, Savion N, Zhang X, Friesel R, Xu J, Hou J, McKeehan WL, Maciag T. Intact and functional fibroblast growth factor (FGF) receptor-1 traffics near the nucleus in response to FGF-1. J Biol Chem. 1994;269:31720–31724. [PubMed] [Google Scholar]
- Reagan LP, Ye X, Mir R, DePalo LR, Fluharty SJ. Up-regulation of angiotensin II receptors by in vitro differentiation of murine N1E-115 neuroblastoma cells. Mol Pharmacol. 1990;38:878–886. [PubMed] [Google Scholar]
- Sadoshima J, Izumo S. Signal transduction pathways of angiotensin II-induced c-fos gene expression in cardiac myocytes in vitro. Roles of phospholipid-derived second messengers. Circ Res. 1993;73:424–438. doi: 10.1161/01.res.73.3.424. [DOI] [PubMed] [Google Scholar]
- Safdar A, Becker MW, Davis MG, Dorn GW. Dissociation of vasoconstrictor stimulated basic fibroblast growth factor expression from hypertrophic growth in cultured vascular smooth muscle cells: relevant roles of protein kinase C. Circ Res. 1994;75:836–843. doi: 10.1161/01.res.75.5.836. [DOI] [PubMed] [Google Scholar]
- Saffell JL, Williams EJ, Mason IJ, Walsh FS, Doherty P. Expression of dominant negative FGF receptor inhibits axonal growth and FGF receptor phosphorylation stimulated by CAMs. Neuron. 1997;18:213–242. doi: 10.1016/s0896-6273(00)80264-0. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Yamano Y, Bardhan S, Iwai N, Murray JJ, Hasegawa M, Matsuda Y, Inagami T. Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type-1 receptor. Nature. 1991;351:230–233. doi: 10.1038/351230a0. [DOI] [PubMed] [Google Scholar]
- Seidel HM, Milocco LH, Lamb P, Darnell JE, Stein RB, Rosen J. Spacing of palindromic half sites as a determinant of selective STAT (signal transducers and activators of transcription) DNA binding and transcriptional activity. Proc Natl Acad Sci USA. 1995;92:3041–3045. doi: 10.1073/pnas.92.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman K, Stocker KM, Morrison R, Ciment G. Basic fibroblast growth factor (bFGF) acts intracellularly to cause the differentiation of avian neural crest-derived Schwann cell precursors into melanocytes. Development. 1993;118:1313–1326. doi: 10.1242/dev.118.4.1313. [DOI] [PubMed] [Google Scholar]
- Stachowiak MK, Jiang HK, Poisner AM, Tuominen RK, Hong JS. Short and long term regulation of catecholamine biosynthetic enzymes by angiotensin in cultured adrenal medullary cells. Molecular mechanisms and nature of second messenger system. J Biol Chem. 1990;265:4694–4702. [PubMed] [Google Scholar]
- Stachowiak MK, Maher PA, Joy A, Mordechai E, Stachowiak EK. Nuclear accumulation of fibroblast growth factor receptors is regulated by multiple signals in adrenal medullary cells. Mol Biol Cell. 1996a;7:1299–1317. doi: 10.1091/mbc.7.8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak MK, Maher PA, Joy A, Mordechai E, Stachowiak EK. Nuclear localization of functional FGF receptor 1 in human astrocytes suggests a novel mechanism for growth factor action. Mol Brain Res. 1996b;38:161–165. doi: 10.1016/0169-328x(96)00010-1. [DOI] [PubMed] [Google Scholar]
- Stachowiak EK, Maher PA, Tucholski J, Mordechai E, Joy A, Moffett J, Coons S, Stachowiak MK. Nuclear accumulation of fibroblast growth factor receptors in human glial cells–association with cell proliferation. Oncogene. 1997a;14:2201–2211. doi: 10.1038/sj.onc.1201057. [DOI] [PubMed] [Google Scholar]
- Stachowiak MK, Moffet J, Joy A, Puchacz EK, Florkiewicz R, Stachowiak EK. Regulation of bFGF gene expression and subcellular distribution of bFGF protein in adrenal medullary cells. J Cell Biol. 1994;127:203–223. doi: 10.1083/jcb.127.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak MK, Moffett J, Maher PA, Tucholski J, Stachowiak EK. Growth factor regulation of cell growth and proliferation in the nervous system. A new intracrine nuclear mechanism. Mol Neurobiol. 1997b;15:1–27. doi: 10.1007/BF02740663. [DOI] [PubMed] [Google Scholar]
- Stolen CM, Griep AE. Disruption of lens fiber differentiation and survival at multiple stages by region-specific expression of truncated FGF receptors. Dev Biol. 2000;217:205–220. doi: 10.1006/dbio.1999.9557. [DOI] [PubMed] [Google Scholar]
- Tanabe A, Naruse M, Arai K, Naruse K, Yoshimoto T, Seki T, Imaki T, Kobayashi M, Miyazaki H, Demura H. Angiotensin II stimulates both aldosterone secretion and DNA synthesis via type 1 but not type 2 receptors in bovine adrenocortical cells. J Endocrinol Invest. 1998;21:668–672. doi: 10.1007/BF03350796. [DOI] [PubMed] [Google Scholar]
- Ueno H, Gunn M, Dell K, Tseng A, Jr, Williams L. A truncated form of fibroblast growth factor receptor 1 inhibits signal transduction by multiple types of fibroblast growth factor receptor. J Biol Chem. 1992;267:1470–1476. [PubMed] [Google Scholar]
- Wang JF, Shen M, Fong GH, Hill DJ. A soluble fibroblast growth factor receptor is released from HL-60 promyelocytic leukemia cells: implications for paracrine growth control. Growth Factors. 2000;17:203–214. doi: 10.3109/08977190009001069. [DOI] [PubMed] [Google Scholar]