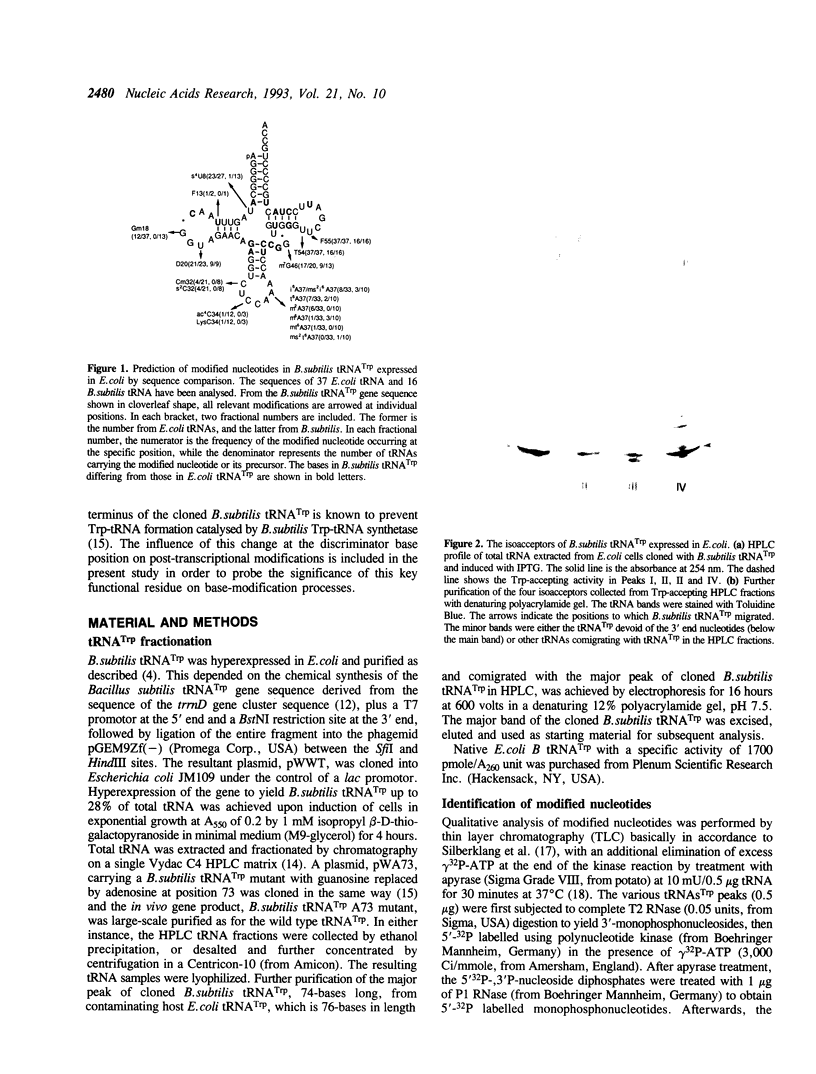
Abstract
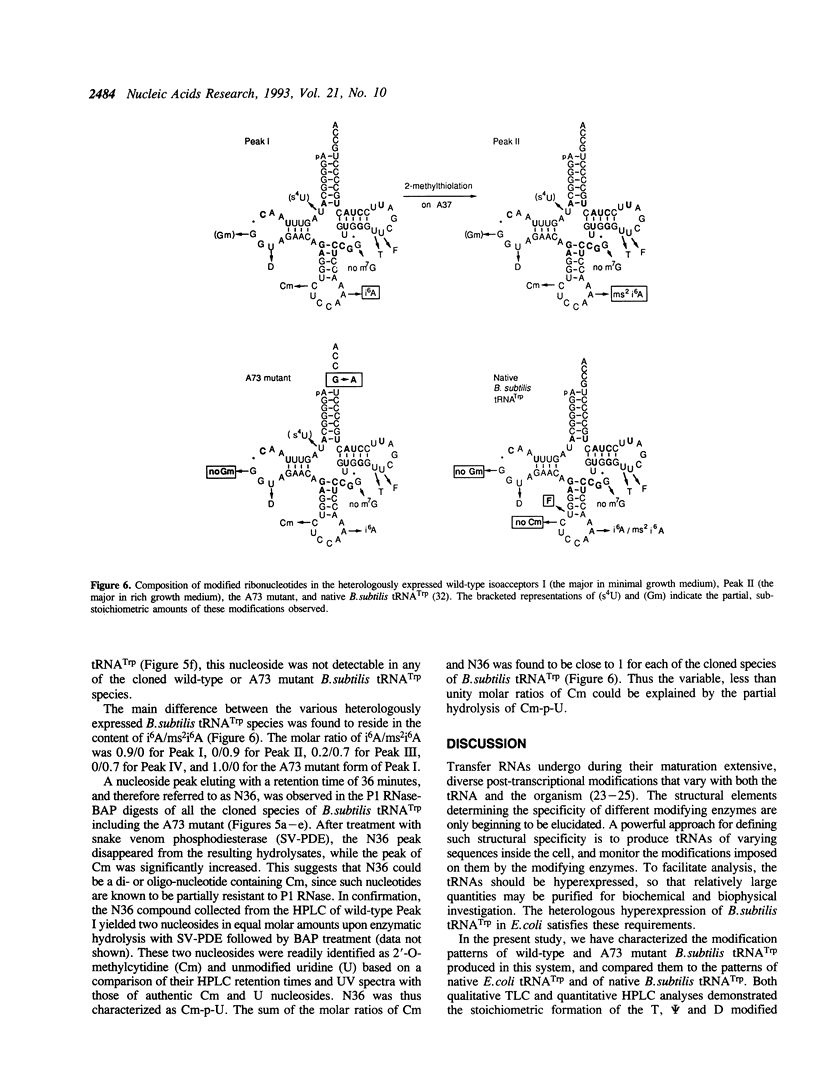
In the present study, modified nucleotides in the B. subtilis tRNA(Trp) cloned and hyperexpressed in E. coli have been identified by TLC and HPLC analyses. The modification patterns of the two isoacceptors of cloned B. subtilis tRNA(Trp) have been compared with those of native tRNA(Trp) from B. subtilis and from E. coli. The modifications of the A73 mutant of B. subtilis tRNA(Trp), which is inactive toward its cognate TrpRS, were also investigated. The results indicate the formation of the modified nucleotides S4U8, Gm18, D20, Cm32, i6A/ms2i6A37, T54 and psi 55 on cloned B. subtilis tRNA(Trp). This modification pattern resembles the pattern of E. coli tRNA(Trp), except that m7G is missing from the cloned tRNA(Trp), probably on account of its short extra loop. In contrast, the pattern departs substantially from that of native B. subtilis tRNA(Trp). Therefore, the cloned B. subtilis tRNA(Trp) has taken on largely the modification pattern of E. coli tRNA(Trp) despite the 26% sequence difference between the two species of tRNA, gaining in particular the Cm32 and Gm18 modifications from the E. coli host. A notable difference between the isoacceptors of the cloned tRNA(Trp) was seen in the extent of modification of A37, which occurred as either the hypomodified i6A or the hypermodified ms2i6A form. Surprisingly, base substitution of guanosine by adenosine at position 73 of the cloned tRNA(Trp) has led to the abolition of the 2'-O-methylation modification of the remote G18 residue.
Full text
PDF
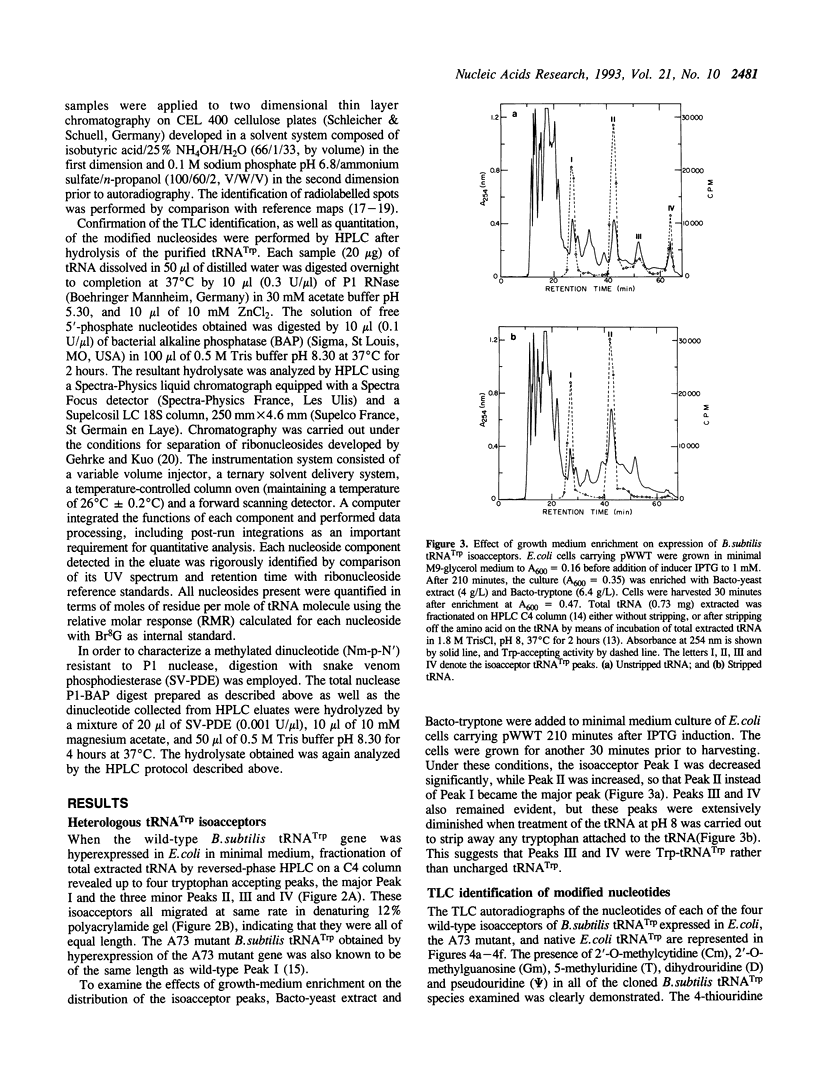

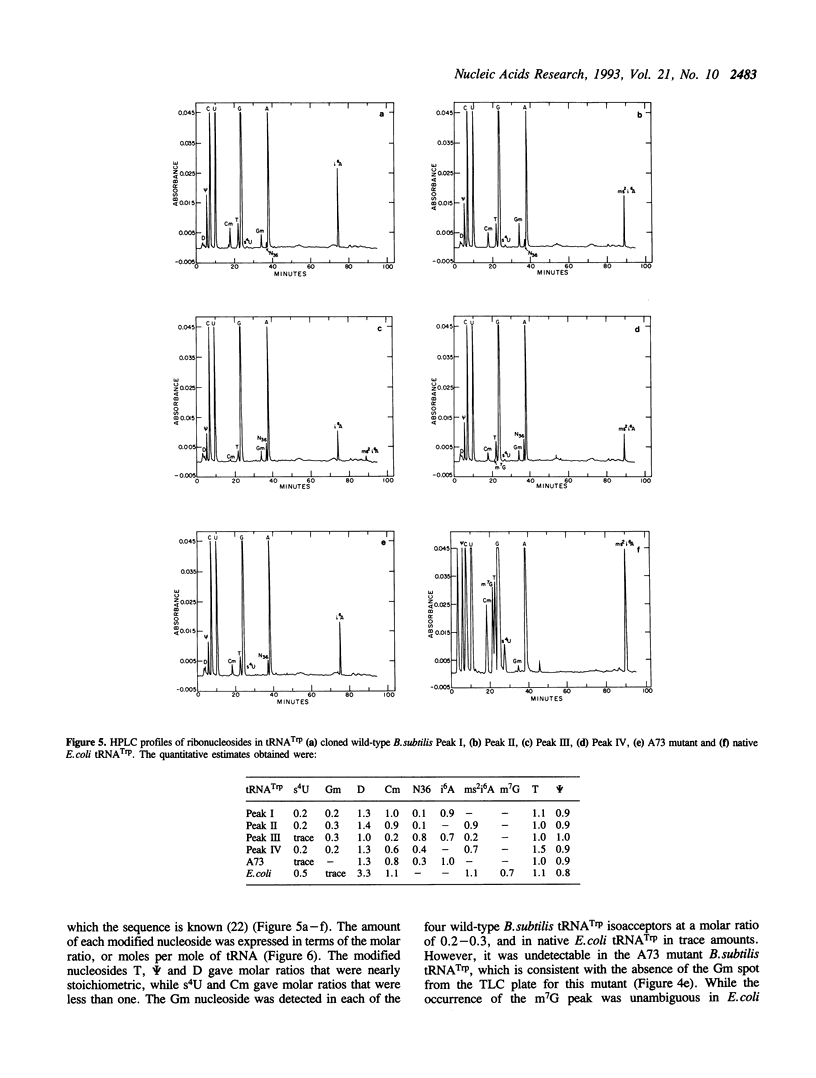


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Koh H., Söll D. The effect of growth temperatures on the in vivo ribose methylation of Bacillus stearothermophilus transfer RNA. Arch Biochem Biophys. 1973 Jan;154(1):277–282. doi: 10.1016/0003-9861(73)90058-1. [DOI] [PubMed] [Google Scholar]
- Arnold H. H., Raettig R. Isoaccepting phenylalanine tRNAs from Bacillus subtilis as a function of growth conditions. Differences in the content of modified nucleosides. FEBS Lett. 1977 Feb 1;73(2):210–214. doi: 10.1016/0014-5793(77)80983-6. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Ericson J. U., Gustafsson C. E., Hagervall T. G., Jönsson Y. H., Wikström P. M. Transfer RNA modification. Annu Rev Biochem. 1987;56:263–287. doi: 10.1146/annurev.bi.56.070187.001403. [DOI] [PubMed] [Google Scholar]
- Buck M., Ames B. N. A modified nucleotide in tRNA as a possible regulator of aerobiosis: synthesis of cis-2-methyl-thioribosylzeatin in the tRNA of Salmonella. Cell. 1984 Feb;36(2):523–531. doi: 10.1016/0092-8674(84)90245-9. [DOI] [PubMed] [Google Scholar]
- Buckingham R. H., Danchin A., Grunberg-Manago M. The effect of an intramolecular cross-link on reversible denaturation in tryptophan transfer ribonucleic acid from Escherichia coli. Biochemistry. 1973 Dec 18;12(26):5393–5399. doi: 10.1021/bi00750a023. [DOI] [PubMed] [Google Scholar]
- Edmonds C. G., Crain P. F., Gupta R., Hashizume T., Hocart C. H., Kowalak J. A., Pomerantz S. C., Stetter K. O., McCloskey J. A. Posttranscriptional modification of tRNA in thermophilic archaea (Archaebacteria). J Bacteriol. 1991 May;173(10):3138–3148. doi: 10.1128/jb.173.10.3138-3148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartland W. J., Sueoka N. Two interconvertible forms of tryptophanyl sRNA in E. coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):948–956. doi: 10.1073/pnas.55.4.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Russell R. L. Role modifications in tyrosine transfer RNA: a modified base affecting ribosome binding. J Mol Biol. 1969 Jan 14;39(1):145–157. doi: 10.1016/0022-2836(69)90339-8. [DOI] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Hori H., Saneyoshi M., Kumagai I., Miura K., Watanabe K. Effects of modification of 4-thiouridine in E. coli tRNA(fMet) on its methyl acceptor activity by thermostable Gm-methylases. J Biochem. 1989 Nov;106(5):798–802. doi: 10.1093/oxfordjournals.jbchem.a122933. [DOI] [PubMed] [Google Scholar]
- Ishida T., Sueoka N. Effect of ambient conditions on conformations of tryptophan transfer ribonucleic acid of Escherichia coli. J Biol Chem. 1968 Oct 25;243(20):5329–5336. [PubMed] [Google Scholar]
- Maxwell I. H., Wimmer E., Tener G. M. The isolation of yeast tyrosine and tryptophan transfer ribonucleic acids. Biochemistry. 1968 Jul;7(7):2629–2634. doi: 10.1021/bi00847a027. [DOI] [PubMed] [Google Scholar]
- McLennan B. D., Buck M., Humphreys J., Griffiths E. Iron-related modification of bacterial transfer RNA. Nucleic Acids Res. 1981 Jun 11;9(11):2629–2640. doi: 10.1093/nar/9.11.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichi B., Heyman T. Study of tyrosine transfer ribonucleic acid modification in relation to sporulation in Bacillus subtilis. J Bacteriol. 1976 Jul;127(1):268–280. doi: 10.1128/jb.127.1.268-280.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. Parameters for the molecular recognition of transfer RNAs. Biochemistry. 1989 Apr 4;28(7):2747–2759. doi: 10.1021/bi00433a001. [DOI] [PubMed] [Google Scholar]
- Schulman L. H. Recognition of tRNAs by aminoacyl-tRNA synthetases. Prog Nucleic Acid Res Mol Biol. 1991;41:23–87. [PubMed] [Google Scholar]
- Sprinzl M., Dank N., Nock S., Schön A. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1991 Apr 25;19 (Suppl):2127–2171. doi: 10.1093/nar/19.suppl.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrousek E. F., Narasimhan N., Hansen J. N. Two large clusters with thirty-seven transfer RNA genes adjacent to ribosomal RNA gene sets in Bacillus subtilis. Sequence and organization of trrnD and trrnE gene clusters. J Biol Chem. 1984 Mar 25;259(6):3694–3702. [PubMed] [Google Scholar]
- Zhang S. B., Bronskill P. M., Wang Q. S., Wong J. T. Separation of tRNA by high-performance liquid chromatography at ambient temperature. J Chromatogr. 1986 Jun 6;360(1):282–287. doi: 10.1016/s0021-9673(00)91676-5. [DOI] [PubMed] [Google Scholar]