Abstract
Background: Phenylbutyrate is a drug used in patients with urea cycle disorder to elicit alternative pathways for nitrogen disposal. However, phenylbutyrate administration decreases plasma branched-chain amino acid (BCAA) concentrations, and previous research suggests that phenylbutyrate administration may increase leucine oxidation, which would indicate increased protein degradation and net protein loss.
Objective: We investigated the effects of phenylbutyrate administration on whole-body protein metabolism, glutamine, leucine, and urea kinetics in healthy and ornithine transcarbamylase–deficient (OTCD) subjects and the possible benefits of BCAA supplementation during phenylbutyrate therapy.
Design: Seven healthy control and 7 partial-OTCD subjects received either phenylbutyrate or no treatment in a crossover design. In addition, the partial-OTCD and 3 null-OTCD subjects received phenylbutyrate and phenylbutyrate plus BCAA supplementation. A multitracer protocol was used to determine the whole-body fluxes of urea and amino acids of interest.
Results: Phenylbutyrate administration reduced ureagenesis by ≈15% without affecting the fluxes of leucine, tyrosine, phenylalanine, or glutamine and the oxidation of leucine or phenylalanine. The transfer of 15N from glutamine to urea was reduced by 35%. However, a reduction in plasma concentrations of BCAAs due to phenylbutyrate treatment was observed. BCAA supplementation did not alter the respective baseline fluxes.
Conclusions: Prolonged phenylbutyrate administration reduced ureagenesis and the transfer of 15N from glutamine to urea without parallel reductions in glutamine flux and concentration. There were no changes in total-body protein breakdown and amino acid catabolism, which suggests that phenylbutyrate can be used to dispose of nitrogen effectively without adverse effects on body protein economy.
INTRODUCTION
Phenylbutyrate and its active metabolite phenylacetate have been used as an alternative route for nitrogen disposal in patients with urea cycle disorder (UCD) (1–3). Phenylbutyrate is β-oxidized to phenylacetate, and in primates, phenylbutyrate is further conjugated with glutamine in the liver and kidney and excreted as phenylacetylglutamine in the urine (4, 5). In this fashion, 2 nitrogen atoms are disposed for each conjugated glutamine molecule, which, thus, relieves the urea cycle and reduces plasma concentrations of ammonia and glutamine. In addition, novel secondary metabolites of phenylbutyrate (comprising glucuronides and phenylbutyrate β-oxidation side products) have been identified (6).
However, the actions of phenylbutyrate and phenylacetate are not limited to the conjugation and excretion of glutamine. Both drugs have been shown to inhibit DNA methylation, histone deacetylation, and protein isoprenylation (7) and to cause the growth arrest of a variety of neoplastic cells, including human breast cancer (8). The effects of phenyl compounds on the enzyme activity have been known for some time (9), and it has been shown that phenylbutyrate increases the expression of certain enzymes of the urea cycle, such as arginase I (10). Despite the additional effects of phenylbutyrate, the long-term prescription of phenylbutyrate has been proven to be relatively safe in UCD patients (3). However, improper dosage in the treatment of hyperammonemia has resulted in metabolic acidosis and cerebral edema that may have fatal consequences (11).
Phenylbutyrate has been shown to reduce plasma glutamine and ammonia concentrations and the concentration of branched-chain amino acids (BCAAs) (12–14). Both glutamine and leucine have been implicated in the regulation of protein synthesis. Glutamine further regulates cell hydration and cell volume, which affect cell function, including protein metabolism (15). Leucine is an anabolic agent (16), which, at physiologic concentrations, provides a signal that stimulates muscle protein synthesis (17). Therefore, it is possible that chronic phenylbutyrate administration may increase the disposal of leucine that will lead to a reduction in protein synthesis and a net loss of body protein.
This study was undertaken to investigate the effects of prolonged (72 h) phenylbutyrate administration in healthy subjects and ornithine transcarbamylase–deficient (OTCD) patients on whole-body protein metabolism, glutamine and leucine kinetics, and ureagenesis.
SUBJECTS AND METHODS
Subjects
The recruitment of subjects began before 1 July 2008, and the study was carried out at the General Clinical Research Center (GCRC) of the Texas Children's Hospital, Houston, Texas. The protocol received prior approval from the Institutional Review Board for Investigations in Human Subjects of the Baylor College of Medicine (protocol H-9281), and informed consent was obtained from each subject before enrollment. Subjects received monetary compensation for participation in the investigation.
Stable-isotope infusion protocol
Seven healthy subjects (control subjects), 7 subjects with partial-OTCD, and 3 subjects with null-OTCD participated in the study (Table 1). Control subjects were admitted twice to the GCRC (protocol number 0713) where they received either sodium phenylbutyrate (Buphenyl; Ucyclyd Pharma, Scottsdale, AZ; 10 g phenylbutyrate · m−2· d−1) treatment or no treatment (control treatment). Partial-OTCD subjects (who were naive to phenylbutyrate) were admitted 3 times to the GCRC where they received sodium phenylbutyrate, no treatment, or phenylbutyrate plus BCAA supplementation (20 mg leucine · kg−1 · d−1, 10 mg isoleucine · kg−1 · d−1, and 10 mg valine · kg−1 · d−1). null-OTCD patients were admitted twice to the GCRC, where they received phenylbutyrate or phenylbutyrate plus BCAAs. null-OTCD patients received phenylbutyrate and citrulline supplementation as their regular therapy for the ornithine transcarbamylase (OTC) deficiency. At least 1 mo elapsed between admissions.
TABLE 1.
Characteristics of subjects who participated in the study1
| Subjects | Sex | Age | Weight | Height |
| y | kg | cm | ||
| Control | ||||
| 1 | F | 24 | 61.2 | 163 |
| 2 | F | 24 | 62.6 | 165 |
| 3 | F | 35 | 68.0 | 163 |
| 4 | F | 38 | 68.0 | 180 |
| 5 | M | 23 | 77.6 | 191 |
| 6 | M | 38 | 75.3 | 166 |
| 7 | M | 26 | 60.0 | 168 |
| Partial-OTCD | ||||
| 8 | F | 6 | 20.9 | 114 |
| 9 | F | 14 | 60.3 | 169 |
| 10 | F | 15 | 51.1 | 161 |
| 11 | F | 15 | 54.3 | 166 |
| 12 | F | 35 | 69.4 | 159 |
| 13 | F | 52 | 61.5 | 162 |
| 14 | F | 71 | 70.3 | 159 |
| Null-OTCD | ||||
| 15 | F | 11 | 43.0 | 133 |
| 16 | M | 12 | 54.5 | 148 |
| 17 | M | 23 | 59.7 | 181 |
OTCD, ornithine transcarbamylase deficient.
Upon admission, informed consent was obtained and blood was drawn from subjects for baseline measurements of complete blood counts and concentrations of circulating hepatic enzymes, plasma amino acids, and plasma ammonia. A urine pregnancy test was performed in all female subjects to rule out pregnancy. Subjects were fed a controlled diet for the 3-d duration of the study that supplied 0.6 g protein · kg−1 · d−1 and adequate nonprotein energy (32 kcal · kg−1 · d−1). Phenylbutyrate was administered orally in 4 equal doses every 6 h after admission (at 0600, 1200, 1800, and 2400).
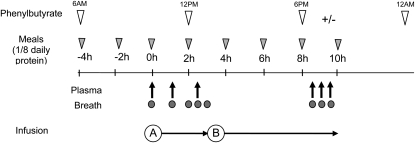
On day 3 of admission, after an overnight fast, catheters were placed in either the antecubital or superficial hand veins of both arms. A baseline blood sample was drawn for the determination of plasma amino acids, ammonia concentrations, and background isotopic enrichments. The total daily dietary allowance was divided into 8 portions, and the subjects were fed every 2 h starting at 4 h before the initiation of the infusion (Figure 1). A priming dose of [13C18O]urea (16 μmol/kg), [5-15N]glutamine (13.6 μmol/kg), [ring-d5]phenylalanine (9.6 μmol/kg), and [ring 3,5-d2]tyrosine (1.6 μmol/kg) was given over 10 min in a total volume of 1.0 mL/kg. The priming dose was followed immediately by a 10-h continuous infusion of [13C18O]urea (1.6 μmol · kg−1 · h−1), [5-15N]glutamine (13.6 μmol · kg−1 · h−1), [ring-d5]phenylalanine (9.6 μmol · kg−1 · h−1), and [ring 3,5-d2]tyrosine (1.6 μmol · kg−1 · h−1). For the first 3 h of the infusion, Na13CHO3 was infused to determine the production rate of CO2. After discontinuing the bicarbonate infusion, a priming dose of [113C]leucine (0.6 μmol/kg) followed by the continuous infusion of this tracer (0.6 μmol · kg−1 · h−1) was conducted for the remaining 7 h of the infusion protocol (Figure 1). Blood samples (7 mL) were taken from the second catheter at 0, 2.5, 3, 8.5, 9, and 9.5 h of the infusion. Plasma was obtained by centrifugation at 4°C and immediately stored at −80°C until analysis. Breath samples were obtained at 0, 2, 2.5, 3, 8.5, 9, and 9.5 h.
FIGURE 1.
Stable-isotope infusion protocol and sampling schedule. A priming dose of [13C18O]urea, [5-15N]glutamine, [2,3,3,4,4 d5]glutamine, [ring-d5]phenylalanine, and [ring 3,5-d2]tyrosine was given at time zero followed by a 10-h continuous infusion of the same tracers. For the first 3 h of the infusion (A), Na13CHO3 was infused to determine the production rate of CO2. After discontinuing the bicarbonate infusion, a primed-continuous infusion of [13C]leucine was given for the remaining 7 h of the infusion protocol (B).
Sample analyses
Plasma ammonia concentrations were measured by reductive amination of 2-oxoglutarate and oxidation of NADPH with a clinical analyzer (Cobas Fara; Roche Products, Indianapolis, IN). Plasma amino acid concentrations were measured by reverse-phase HPLC (Waters Technologies, Milford, MA) as phenylisothiocyanate derivatives.
[5-15N]glutamine enrichment was determined on its dansyl derivative by the single reaction monitoring of the transition m/z 380–363 and 381–363 with a TSQ Quantum Ultra System (Thermo Finnigan, San Jose, CA). To measure the isotopic enrichments of plasma phenylalanine, tyrosine and leucine plasma protein was precipitated with ice-cold sulfosalicylic acid (10% wt:vol solution), and the supernatant fluid was passed over a cation-exchange column (Dowex AG 50W-8×, 100–200 mesh H+ form; Bio-Rad Laboratories, Richmond, CA). Amino acids were eluted with 2 M NH4OH and the eluate was dried under a vacuum with a Speed Vac (Savant Instruments, Farmingdale, NY). Amino acids were derivatized to their N-heptafluorobutyryl isopropyl ester derivative and the isotope ratio were determined by negative chemical ionization gas chromatography–mass spectrometry monitoring m/z ions 383 and 388, 595, 597 and 599, and 349 and 350, for phenylalanine, tyrosine, and leucine, respectively.
Plasma urea isotopic enrichment was determined by electron impact gas chromatography–mass spectrometry after the urea was derivatized to the tert-butyldimethylsilyl derivative. A total of 20 μL plasma protein was precipitated with 100 μL ice-cold acetone, and the supernatant fluid that contained the urea was obtained after centrifugation at 1500 × g for 15 min at 4°C. The supernatant fluid was evaporated under a gentle stream of nitrogen gas at 80°C, and the sample was derivatized with 25 μL of a 1:1 mixture of N-methyl-N-(tert-butyldimethylsilyl) trifluoroacetamide (Sigma-Aldrich Inc, St Louis, MO) and acetonitrile at 80°C for 20 min in tightly capped V-Vials (Wheaton Science International, Millville, NJ). The analysis was performed in a 5973 Agilent GC MSD (Agilent Technologies, Santa Clara, CA) in selective ion monitoring mode and with monitoring of m/z ions 231, 232, and 234.
Calculation of fluxes from continuous infusion of tracer
Urea, leucine, glutamine, phenylalanine, tyrosine, and bicarbonate fluxes were calculated from the isotopic dilution of the infused tracer at plateau enrichment, as
where QM is the flux of the metabolite M (μmol · kg−1 · h−1), i is the infusion rate of the tracer (μmol · kg−1 · h−1), EM is the enrichment of M at the plateau, and Ei is the enrichment of the infusate.
The rate of conversion of phenylalanine to tyrosine and leucine to CO2 and the transfer of the 15N label from glutamine to urea were determined as follows (18)
where Qa and Qb are the fluxes of the precursor and product, respectively, determined from the steady state enrichments of the infused tracers ([ring-d5]phenylalanine, [13C]leucine, and [13C18O]urea), Ea and Eb are the respective plasma enrichments of the precursors and products ([ring-d5]phenylalanine and [ring-d4]-tyrosine, [13C]leucine, and 13CO2, [13C18O]urea and [15N]urea); and ia is the rate of infusion of the labeled precursor.
Data analysis
Two 2 × 2 factorial arrangements of treatments were conducted. In the first arrangement, the effect of phenylbutyrate was studied in control and partial-OTCD subjects (2 genotypes, control and partial-OTCD subjects; 2 treatments, control and phenylbutyrate). In the second arrangement, the effect of BCAA supplementation on OTCD was studied (2 genotypes, partial- and null-OTCD; 2 treatments, phenylbutyrate and phenylbutyrate plus BCAA). Data were analyzed statistically with the PROC MIXED procedure of SAS (version 9.1; SAS Institute Inc, Cary, NC), with the subject as the random effect of the model. Data are expressed as means ± SEMs. The level of significance was set at P ≤ 0.05. If a significant interaction was obtained (P ≤ 0.05), the post hoc Tukey-Kramer procedure for multiple pairwise comparisons was also applied.
RESULTS
Isotopic plasma enrichments had reached steady state for all infused tracers (see supplemental Figures 1 and 2 under “Supplemental data” in the online issue). The phenylbutyrate administration significantly reduced (P < 0.012) the production of urea by ≈14% and 23% compared with the control treatment in control and partial-OTCD subjects (Table 2). This administration was associated with a reduction in the transfer of 15N from glutamine to urea (P < 0.001). Ureagenesis was reduced (P < 0.017) in partial-OTCD compared with control subjects. The phenylbutyrate treatment did not affect the flux of any of the amino acids measured. There were no significant differences between control and phenylbutyrate treatments in either the oxidation of leucine or the conversion of phenylalanine into tyrosine (Table 2).
TABLE 2.
Amino acids, urea, and carbon dioxide kinetics in healthy control and partial–ornithine transcarbamylase–deficient (partial-OTCD) subjects who received either control or sodium phenylbutyrate (10 g · m−2 · d−1) treatment1
| Control subjects (n = 7)2 |
Partial-OTCD subjects (n = 7)2 |
P values |
||||||
| Item | Control | Phenylbutyrate | Control | Phenylbutyrate | Pooled SEM | TRT | Gen | TRT × Gen |
| Flux (μmol · kg−1 · h−1) | ||||||||
| Urea | 180 | 156 | 157 | 121 | 11.0 | <0.012 | <0.017 | <0.600 |
| Phenylalanine | 49 | 50 | 46 | 43 | 2.9 | <0.778 | <0.062 | <0.503 |
| Tyrosine | 44 | 51 | 47 | 45 | 3.0 | <0.358 | <0.738 | <0.169 |
| Leucine | 114 | 128 | 114 | 127 | 8.7 | <0.467 | <0.432 | <0.371 |
| Glutamine | 266 | 299 | 281 | 263 | 19.3 | <0.709 | <0.587 | <0.201 |
| CO2 (×100) | 143 | 135 | 140 | 134 | 13.2 | <0.607 | <0.895 | <0.932 |
| Clearance (mL plasma/min) | ||||||||
| Phenylalanine | 10.5 | 11.7 | 10.1 | 9.2 | 0.77 | <0.800 | <0.180 | <0.014 |
| Tyrosine | 11.5 | 11.7 | 9.7 | 8.5 | 1.2 | <0.583 | <0.129 | <0.426 |
| Leucine | 14.5 | 23.7 | 17.2 | 25.0 | 2.69 | <0.001 | <0.539 | <0.736 |
| Glutamine | 7.8 | 9.2 | 6.6 | 6.7 | 0.65 | <0.082 | <0.051 | <0.116 |
| Rate of conversion (μmol · kg−1 · h−1) | ||||||||
| Gln→urea | 45a | 33a,b | 51a | 22b | 4.6 | <0.001 | <0.711 | <0.011 |
| Phe→Tyr | 7 | 8 | 8 | 8 | 0.6 | <0.980 | <0.377 | <0.403 |
| Leu→CO2 | 43 | 45 | 46 | 44 | 3.7 | <0.903 | <0.816 | <0.578 |
TRT, treatment (control compared with phenylbutyrate treatments); Gen, genotype (control compared with partial-OTCD subjects); TRT × Gen, interaction of main effects; Gln→urea, rate of transfer of the amido group of glutamine to urea; Phe→Tyr, rate of conversion of phenylalanine to tyrosine; Leu→CO2, rate of conversion of leucine to carbon dioxide. Data were analyzed by using mixed models (fixed effects for TRT, Gen, and TRT × Gen; random effects for subject). Values with different superscript letters differed (Tukey-Kramer–adjusted P < 0.05).
Values are means.
A significant reduction (P < 0.001) in plasma concentrations of the 3 BCAAs was observed as a result of the phenylbutyrate treatment (Table 3). This was also accompanied by a significant reduction in plasma ammonia concentrations (P < 0.002) without a reduction of plasma glutamine concentrations (P = 0.081). The clearance of leucine, but not of glutamine, tyrosine, or phenylalanine, increased (P < 0.001) in control and partial-OTCD subjects who received phenylbutyrate treatment (Table 2). As expected, partial-OTCD subjects had higher plasma glutamine (P < 0.017), but not ammonia (P = 0.193), concentrations than did control subjects. This result reflects the fact that all patients were in stable metabolic control upon admission. Some differences were observed in other amino acids, notably in the significant reduction (P < 0.001) in plasma citrulline concentrations because of the phenylbutyrate treatment.
TABLE 3.
Plasma amino acid concentrations in healthy control and partial–ornithine transcarbamylase–deficient (partial-OTCD) subjects who received either control or sodium phenylbutyrate (10 g · m−2 · d−1) treatment1
| Control subjects (n = 7)2 |
Partial-OTCD subjects (n = 7)2 |
P values |
||||||
| Amino acid | Control | Phenylbutyrate | Control | Phenylbutyrate | Pooled SEM | TRT | Gen | TRT × Gen |
| μmol/L | μmol/L | μmol/L | ||||||
| Leucine | 135 | 94 | 130 | 95 | 10.8 | <0.001 | <0.869 | <0.608 |
| Isoleucine | 61 | 45 | 67 | 53 | 5.5 | <0.001 | <0.364 | <0.852 |
| Valine | 216 | 161 | 225 | 164 | 14.2 | <0.001 | <0.751 | <0.653 |
| Methionine | 29 | 28 | 36 | 30 | 1.9 | <0.007 | <0.074 | <0.067 |
| Cysteine | 30a | 22b | 22a,b | 25a,b | 3.7 | <0.264 | <0.583 | <0.006 |
| Phenylalanine | 80 | 72 | 76 | 79 | 3.9 | <0.422 | <0.771 | <0.063 |
| Tyrosine | 66 | 80 | 83 | 93 | 6.1 | <0.022 | <0.063 | <0.617 |
| Lysine | 204 | 195 | 210 | 193 | 14.3 | <0.047 | <0.935 | <0.524 |
| Arginine | 79 | 81 | 67 | 64 | 6.3 | <0.905 | <0.081 | <0.539 |
| Ornithine | 49 | 43 | 56 | 47 | 5.4 | <0.006 | <0.451 | <0.519 |
| Citrulline | 27 | 23 | 27 | 19 | 3.7 | <0.001 | <0.683 | <0.099 |
| Glutamate | 30 | 40 | 55 | 68 | 8.7 | <0.019 | <0.026 | <0.669 |
| Glutamine | 571 | 531 | 720 | 680 | 44.6 | <0.081 | <0.017 | <0.985 |
| Aspartate | 4 | 2 | 5 | 6 | 1.2 | <0.519 | <0.085 | <0.227 |
| Asparagine | 73 | 84 | 83 | 68 | 8.0 | <0.675 | <0.764 | <0.033 |
| Ammonia | 12 | 7 | 33 | 20 | 9.2 | <0.002 | <0.193 | <0.131 |
TRT, treatment (control compared with phenylbutyrate treatments); Gen, genotype (control compared with partial-OTCD subjects); TRT × Gen, interaction of main effects. Data were analyzed by using mixed models (fixed effects for TRT, Gen, and TRT × Gen; random effects for subject). Values with different superscript letters differed (Tukey-Kramer–adjusted P < 0.05).
Values are means.
BCAA supplementation did not have a significant effect on either the concentrations or kinetics of any amino acid in partial-OTCD and null-OTCD subjects who received phenylbutyrate (Tables 4 and 5). A trend (P < 0.054) toward higher plasma leucine concentrations was observed only in partial-OTCD subjects who were supplemented with BCAAs. null-OTCD subjects had higher (P < 0.009) plasma ammonia concentrations than did partial-OTCD subjects, and there were significant differences in plasma methionine, phenylalanine, tyrosine, and lysine concentrations between partial- and null-OTCD subjects. Plasma citrulline and arginine concentrations were also significantly elevated in null-OTCD subjects because they received citrulline supplementation as part of their normal drug therapy. Likewise, a slower rate of leucine oxidation, but not of phenylalanine disposal, was observed in null-OTCD subjects than in partial-OTCD subjects.
TABLE 4.
Amino acids, urea, and carbon dioxide kinetics in partial– and null–ornithine transcarbamylase–deficient (partial- and null-OTCD) subjects who received treatment with sodium phenylbutyrate (10 g · m−2 · d−1) or phenylbutyrate plus branched-chain amino acid (BCAA) supplementation1
| Partial-OTCD subjects (n = 7)2 |
Null-OTCD subjects (n = 3)2 |
P values |
||||||
| Item | Phenylbutyrate | BCAA | Phenylbutyrate | BCAA | Pooled SEM3 | TRT | Gen | TRT × Gen |
| Flux (μmol · kg−1 · h−1) | ||||||||
| Urea | 121 | 117 | 99 | 82 | 21.7 | <0.575 | <0.145 | <0.736 |
| Phenylalanine | 43 | 44 | 41 | 41 | 2.8 | <0.550 | <0.805 | <0.794 |
| Tyrosine | 45 | 46 | 49 | 41 | 3.4 | <0.466 | <0.815 | <0.344 |
| Leucine | 127 | 162 | 106 | 147 | 16.5 | <0.096 | <0.407 | <0.902 |
| Glutamine | 263 | 298 | 302 | 298 | 26.7 | <0.286 | <0.242 | <0.947 |
| CO2 (×100) | 134 | 138 | 131 | 132 | 15.8 | <0.900 | <0.829 | <0.963 |
| Rate of conversion (μmol · kg−1 · h−1) | ||||||||
| Gln→urea | 19 | 26 | 27 | 31 | 6.5 | <0.376 | <0.290 | <0.796 |
| Phe→Tyr | 8 | 8 | 8 | 7 | 1.3 | <0.753 | <0.852 | <0.510 |
| Leu→CO2 | 44 | 56 | 28 | 39 | 4.6 | <0.093 | <0.026 | <0.923 |
TRT, treatment (phenylbutyrate compared with phenylbutyrate plus BCAA treatments); Gen, genotype (partial-OTCD compared with null-OTCD subjects); TRT × Gen, interaction of main effects; Gln→urea, rate of transfer of the amido group of glutamine to urea; Phe→Tyr, rate of conversion of phenylalanine to tyrosine; Leu→CO2, rate of conversion of leucine to carbon dioxide. Data were analyzed by using mixed models (fixed effects for TRT, Gen, and TRT × Gen; random effects for subject).
Values are means.
On the basis of n = 7.
TABLE 5.
Plasma amino acid concentrations in partial– and null–ornithine transcarbamylase–deficient (partial- and null-OTCD) subjects who received treatment with either sodium phenylbutyrate (10 g · m−2 · d−1) or phenylbutyrate plus branched-chain amino acid (BCAA) supplementation1
| Partial-OTCD subjects (n = 7)2 |
Null-OTCD subjects (n = 3)2 |
P values | ||||||
| Amino acid | Phenylbutyrate | BCAA | Phenylbutyrate | BCAA | Pooled SEM3 | TRT | Gen | TRT × Gen |
| μmol/L | μmol/L | μmol/L | ||||||
| Leucine | 95 | 117 | 69 | 74 | 15.2 | <0.210 | <0.205 | <0.400 |
| Isoleucine | 52 | 60 | 34 | 38 | 8.2 | <0.274 | <0.164 | <0.714 |
| Valine | 164 | 176 | 122 | 110 | 19.4 | <0.996 | <0.126 | <0.221 |
| Methionine | 30 | 34 | 18 | 16 | 4.5 | <0.849 | <0.035 | <0.593 |
| Cysteine | 17 | 17 | 25 | 33 | 5.1 | <0.198 | <0.207 | <0.217 |
| Phenylalanine | 79 | 74 | 58 | 53 | 4.5 | <0.145 | <0.009 | <0.928 |
| Tyrosine | 93 | 82 | 49 | 51 | 6.5 | <0.231 | <0.003 | <0.077 |
| Lysine | 193 | 178 | 111 | 110 | 18.3 | <0.241 | <0.032 | <0.279 |
| Arginine | 64a,b | 58b | 71a,b | 93a | 6.2 | <0.110 | <0.056 | <0.008 |
| Ornithine | 47 | 46 | 62 | 64 | 9.7 | <0.864 | <0.337 | <0.780 |
| Citrulline | 19 | 22 | 121 | 178 | 25.8 | <0.290 | <0.004 | <0.349 |
| Glutamate | 68 | 61 | 107 | 76 | 11.7 | <0.018 | <0.203 | <0.129 |
| Glutamine | 679 | 610 | 424 | 497 | 76.2 | <0.938 | <0.192 | <0.023 |
| Aspartate | 6 | 7 | 4 | 2 | 1.5 | <0.804 | <0.227 | <0.306 |
| Asparagine | 68 | 72 | 46 | 46 | 7.8 | <0.670 | <0.096 | <0.697 |
| Ammonia | 10 | 11 | 36 | 27 | 4.9 | <0.347 | <0.009 | <0.299 |
TRT, treatment (phenylbutyrate compared with phenylbutyrate plus BCAA treatments); Gen, genotype (partial-OTCD compared with null-OTCD subjects); TRT × Gen, interaction of main effects. Data were analyzed by using mixed models (fixed effects for TRT, Gen, and TRT × Gen; random effects for subject). Values with different superscript letters differed (Tukey-Kramer–adjusted P < 0.05).
Values are means.
On the basis of n = 7.
DISCUSSION
Alternative pathways for the excretion of nitrogen in UCD patients have been used for the past 30 y (1). In the current study, phenylbutyrate administration reduced ureagenesis by ≈13% and 24% in control and partial-OTCD subjects, respectively, which relieved the urea cycle and, even more clinically relevant, reduced plasma ammonia concentrations. The reduction in the disposal of the 15N label from glutamine was consistent with an alternative pathway for the disposal of glutamine nitrogen (ie, the conjugation with phenylacetate). However, the reduction in the disposal of the 15N label was not associated with a reduction in plasma glutamine concentrations. Phenylbutyrate has been used to deplete the glutamine pool in humans (12, 13), with reductions of ≤25% in the circulating glutamine concentration after the ingestion of a single dose of 0.36 g phenylbutyrate/kg (12). At this dose, the amount of glutamine, which is presumably trapped by phenylbutyrate over a 24-h period, is equivalent to 32% of the estimated glutamine content of skeletal muscle (12). Even when taking into account that the recovery of phenylacetylglutamine in urine is ≈80% of the phenylbutyrate dose (2, 19), the glutamine trapped as phenylacetylglutamine would be ≈25% of the muscle glutamine pool (which constitutes the main component of the glutamine whole-body pool) at the 0.36 g phenylbutyrate/kg dose or ≈35% at the dose used in this study (≈0.50 g phenylbutyrate/kg). Because no difference in the flux of glutamine was shown in the current study or was previously reported (12, 13), at this rate of depletion, the extensive intracellular glutamine pool [≈20 mmol glutamine/L intracellular water (20)] would be completely depleted after 3–4 d of phenylbutyrate therapy. This is difficult to reconcile with the successful use of phenylbutyrate as a long-term therapy in UCD patients and the lack of glutamine depletion observed in the current study. A difference between this study and previous reports in the literature is that subjects were medicated for 72 h before the study was conducted, whereas previous work was performed after a single dose of phenylbutyrate (12, 13). The fact that a modest reduction in glutamine concentration (7%) was observed by the same group of researchers (21) when subjects were supplemented with phenylbutyrate for 24 h further supported our conclusions.
In agreement with other studies (12–14, 22), we observed a ≈25% reduction in plasma BCAA concentrations in response to phenylbutyrate therapy. This reduction of plasma BCAA concentrations was a cause of concern because of the role of leucine in the mammalian target of rapamycin activation in protein synthesis (23), which, together with low-protein diets fed to OTCD patients, might limit the protein deposition and increase the amino acid disposal in OTCD patients who received phenylbutyrate therapy (14). However, there was no difference in the flux of leucine or phenylalanine in response to the phenylbutyrate administration at the amount of protein intake used in the current study. Because the intake of protein was identical between control and phenylbutyrate treatments, it seems that the phenylbutyrate administration did not affect proteolysis in these control and partial-OTCD subjects at steady state protein and drug intakes. Similarly, the phenylbutyrate administration did not increase the leucine flux in fasted subjects (12, 13). Finally, whereas these previous studies reported faster leucine oxidation rates, which provided an indication of the net protein loss, we were unable to detect differences in leucine oxidation or phenylalanine conversion to tyrosine. These differences might be related to the difference in the nutritional status in subjects in the current study compared with the nutritional status in subjects in previous studies or to the prolonged phenylbutyrate administration in the current study.
BCAA supplementation did not significantly alter measures of proteolysis or leucine oxidation, although a nonsignificant increase in plasma leucine concentrations was observed. Differences in concentrations of some amino acid and the leucine oxidation between partial- and null-OTCD subjects may have been because of differences in age and physiologic stages between these 2 groups. In addition, null-OTCD subjects received citrulline as part of their therapy; hence, the higher plasma citrulline (and arginine) concentrations reported.
It is important to keep in mind that all subjects studied were stable and were in good metabolic control. It is unknown whether BCAA depression may cause patients to be less stable and more prone to a catabolic state, for example, in the face of combined environmental stressors such as viral infections. Similarly, this study did not address whether BCAA depression might worsen the course of a metabolic decompensation marked by hyperammonemia and catabolism. Anecdotally, patients who experience periods of poor metabolic control with frequent hyperammonemia often also have depressed BCAAs (24). Whether BCAA supplementation in this context would be beneficial remains to be confirmed. A prospective study assessing the effects of BCAA supplementation during metabolic decompensation would be needed.
The actual mechanism that underlies the reduction in BCAA concentrations remains controversial, but it is probably caused by activation of the branched-chain α-keto acid dehydrogenase complex (BCKDC) that catalyzes the irreversible oxidative decarboxylation of the BCAA α-ketoacids. This enzymatic complex is common to the 3 BCAAs and is the rate-limiting enzyme in their catabolism. The reduction in glutamine concentrations seen in previous studies has been proposed as the mechanism that may trigger the up-regulation and increased BCKDC activity (13). However, despite a marked reduction in plasma BCAA concentrations, we failed to find a reduction in glutamine concentrations between control and phenylbutyrate-treated subjects, which did not support the hypothesis proposed by Le Bacquer et al (13). We recently confirmed that phenylbutyrate prevented the phosphorylation of E1α and activated the E1 decarboxylase activity and BCKDC overall activity, which provided a molecular explanation for the effect of phenylbutyrate on BCAA concentrations and increased leucine clearance (25). The reduction in citrulline concentrations seen by us and others (21) because of phenylbutyrate supplementation raised some concerns in the use of phenylbutyrate in OTCD patients. However, the 3 null-OTCD patients studied were receiving citrulline supplementation as part as their normal therapy and had high plasma citrulline concentrations.
In conclusion, phenylbutyrate administration decreased plasma BCAA concentrations without affecting the whole-body proteolysis or net protein catabolism in steady state, metabolically stable control and OTCD subjects. Phenylbutyrate administration reduced the transfer of 15N from labeled glutamine to urea and ureagenesis but did not reduce plasma glutamine concentrations. These results were consistent with the clinical observation that phenylbutyrate is beneficial in UCD by decreasing the dependence on the urea cycle for nitrogen disposal. No change in whole-body proteolysis or net protein catabolism was shown in control and OTCD subjects because an increase in these processes might have hindered the medication effectiveness by increasing nitrogen turnover. Whether decreased BCAA concentrations may increase susceptibility to catabolism is unknown, and whether BCAA supplementation in the context of acute hyperammonemia may reverse an associated catabolic state is unclear. The reduction in plasma citrulline concentrations warrants further studies to determine whether this reduction is due to reduced citrulline synthesis and to investigate citrulline supplementation during phenylbutyrate therapy.
Supplementary Material
Acknowledgments
We thank Susan Carter, Mary Mullins, Alyssa Tran, Janice Stuff, and the GCRC nursing staff for their clinical and administrative support.
The authors' responsibilities were as follows—JCM: designed the study, analyzed samples and data, and prepared the report; BCL and FS: implemented the study and prepared the report; WEO and QS: analyzed samples and data; PJG: designed the study and prepared the report; FJ: designed the study, analyzed samples, and prepared the report; and BL: designed the study, analyzed data, and prepared the report. BL received research grant support from Ucyclyd Pharma Inc. JCM, BCL, FS, WEO, QS, PJG, and FJ received no financial support from nor had any personal interest in any company or organization that sponsored the research.
REFERENCES
- 1.Batshaw ML, MacArthur RB, Tuchman M. Alternative pathway therapy for urea cycle disorders: twenty years later. J Pediatr 2001;138:S46–54 [DOI] [PubMed] [Google Scholar]
- 2.Brusilow SW. Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. Pediatr Res 1991;29:147–50 [DOI] [PubMed] [Google Scholar]
- 3.Burlina AB, Ogier H, Korall H, Trefz FK. Long-term treatment with sodium phenylbutyrate in ornithine transcarbamylase-deficient patients. Mol Genet Metab 2001;72:351–5 [DOI] [PubMed] [Google Scholar]
- 4.James MO, Smith RL, Williams RT, Reidenberg M. The conjugation of phenylacetic acid in man, sub-human primates and some non-primate species. Proc R Soc Lond B Biol Sci 1972;182:25–35 [DOI] [PubMed] [Google Scholar]
- 5.Moldave K, Meister A. Synthesis of phenylacetylglutamine by human tissue. J Biol Chem 1957;229:463–76 [PubMed] [Google Scholar]
- 6.Kasumov T, Brunengraber LL, Comte B, et al. New secondary metabolites of phenylbutyrate in humans and rats. Drug Metab Dispos 2004;32:10–9 [DOI] [PubMed] [Google Scholar]
- 7.Gore SD, Samid D, Weng LJ. Impact of the putative differentiating agents sodium phenylbutyrate and sodium phenylacetate on proliferation, differentiation, and apoptosis of primary neoplastic myeloid cells. Clin Cancer Res 1997;3:1755–62 [PubMed] [Google Scholar]
- 8.Sawatsri S, Samid D, Malkapuram S, Sidell N. Inhibition of estrogen-dependent breast cell responses with phenylacetate. Int J Cancer 2001;93:687–92 [DOI] [PubMed] [Google Scholar]
- 9.Shama Bhat C, Ramasarma T. Inhibition of rat liver mevalonate pyrophosphate decarboxylase and mevalonate phosphate kinase by phenyl and phenolic compounds. Biochem J 1979;181:143–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kern RM, Yang Z, Kim PS, Grody WW, Iyer RK, Cederbaum SD. Arginase induction by sodium phenylbutyrate in mouse tissues and human cell lines. Mol Genet Metab 2007;90:37–41 [DOI] [PubMed] [Google Scholar]
- 11.Praphanphoj V, Boyadjiev SA, Waber LJ, Brusilow SW, Geraghty MT. Three cases of intravenous sodium benzoate and sodium phenylacetate toxicity occurring in the treatment of acute hyperammonaemia. J Inherit Metab Dis 2000;23:129–36 [DOI] [PubMed] [Google Scholar]
- 12.Darmaun D, Welch S, Rini A, Sager BK, Altomare A, Haymond MW. Phenylbutyrate-induced glutamine depletion in humans: effect on leucine metabolism. Am J Physiol 1998;274:E801–7 [DOI] [PubMed] [Google Scholar]
- 13.Le Bacquer O, Mauras N, Welch S, Haymond M, Darmaun D. Acute depletion of plasma glutamine increases leucine oxidation in prednisone-treated humans. Clin Nutr 2007;26:231–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scaglia F, Carter S, O'Brien WE, Lee B. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol Genet Metab 2004;81(suppl 1):S79–85 [DOI] [PubMed] [Google Scholar]
- 15.Waldegger S, Busch GL, Kaba NK, et al. Effect of cellular hydration on protein metabolism. Miner Electrolyte Metab 1997;23:201–5 [PubMed] [Google Scholar]
- 16.Matthews DE. Observations of branched-chain amino acid administration in humans. J Nutr 2005;135:1580S–4S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr 2005;135:1553S–6S [DOI] [PubMed] [Google Scholar]
- 18.Thompson GN, Pacy PJ, Merritt H, et al. Rapid measurement of whole body and forearm protein turnover using a [2H5]phenylalanine model. Am J Physiol 1989;256:E631–9 [DOI] [PubMed] [Google Scholar]
- 19.Piscitelli SC, Thibault A, Figg WD, et al. Disposition of phenylbutyrate and its metabolites, phenylacetate and phenylacetylglutamine. J Clin Pharmacol 1995;35:368–73 [DOI] [PubMed] [Google Scholar]
- 20.Bergstrom J, Furst P, Noree LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol 1974;36:693–7 [DOI] [PubMed] [Google Scholar]
- 21.Rouge C, Des Robert C, Robins A, et al. Manipulation of citrulline availability in humans. Am J Physiol 2007;293:G1061–7 [DOI] [PubMed] [Google Scholar]
- 22.Tuchman M, Lee B, Lichter-Konecki U, et al. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Mol Genet Metab 2008;94:397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stipanuk MH. Leucine and protein synthesis: mTOR and beyond. Nutr Rev 2007;65:122–9 [DOI] [PubMed] [Google Scholar]
- 24.Lee B, Singh RH, Rhead WJ, King LS, Smith W, Summar ML. Considerations in the difficult-to-manage urea cycle disorder patient. Crit Care Clin 2005;21(4 suppl):S19–25. [DOI] [PubMed] [Google Scholar]
- 25.Brunetti-Pierri N, Lanpher B, Erez A, et al. Phenylbutyrate therapy for maple syrup urine disease. Hum Mol Genet 2011;20:631–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.