Abstract
Objectives:
To investigate changes in muscular activity and strength of subjects diagnosed with lateral epicondylitis (LE). To assess the appropriateness of these measures in the patient’s follow-up.
Methods:
Twenty-four subjects (11 men and 13 women) with LE, were evaluated at baseline and after 5 weeks of an experimental treatment. Measurements included: the (1) pain-free grip (PFG), (2) maximal isometric strength, (3) surface electromyography (EMG) of forearm muscle (healthy and affected), (4) a visual analogue scale (VAS), and (5) the Patient Rated Tennis Elbow Evaluation (PRTEE) (Canadian-French version).
Results:
All subjects showed improvement in VAS and PRTEE. The maximal isometric strength during flexion and extension of the wrist and the EMG analysis failed to discriminate the affected from the healthy elbow during the initial assessment. Only the PFG measured with the elbow in extension could discriminate elbows with LE from the healthy ones.
Conclusions:
The use of the PFG with the elbow in extension seems to be the most indicated strength measurement to monitor the recovery of patients with LE. The EMG acquisition protocol used in this research was not adequate to monitor effectively the recovery of LE.
Keywords: lateral epicondylitis, tennis elbow, pain-free grip, electromyography, measurements
Abstract
Objectifs :
Étudier les changements de force et d’activité musculaire de sujets avec un diagnostic d’épicondylite latérale (LE). Évaluer la pertinence de ces mesures dans le suivi de patients.
Méthodes :
Vingt-quatre sujets (11 hommes et 13 femmes) avec LE, ont été évalués au départ et après 5 semaines d’un traitement expérimental. Les mesures comprenaient: (1) la préhension maximale sans douleur (PFG), (2) la force isométrique maximale, (3) l’électromyographie de surface (EMG) des muscles des avant-bras (sains et affectés), (4) une échelle visuelle analogue (VAS) et (5) le questionnaire Patient Rated Tennis Elbow Evaluation (PRTEE) (version française canadienne).
Résultats :
Tous les sujets ont démontré une amélioration du VAS et du PRTEE. La force isométrique maximale lors de la flexion et l’extension du poignet et de l’analyse EMG n’ont pas permis de discriminer les coudes affectés des coudes sains lors de l’évaluation initiale. Seuls la PFG mesurée avec le coude en extension a réussi à discriminer les coudes avec LE des sains.
Conclusions :
L’utilisation de la PFG avec le coude en extension semble être la mesure de force la plus indiquée pour effectuer le suivi de patient avec LE. Le protocole d’acquisition EMG utilisé dans cette recherche n’était pas adéquat pour effectuer le suivi de patients avec LE.
Keywords: épicondylite latérale, préhension maximale sans douleur, électromyographie, évaluation
Introduction
Lateral epicondylitis (LE) or “tennis elbow” is an injury at the insertion of the extensor carpi radialis brevis and the extensor digitorum. It is characterized by pain at the external aspect of the elbow exacerbated during extension of the elbow with the wrist in flexion or during resisted extension of the wrist with the elbow in extension.1 Grip strength is affected and simple activities such as simply taking a cup of coffee may become painful. This is the most common condition diagnosed in the elbow and it affects between 1% and 3% of the population.2–4 Smoking (Odds Ratio (OR) = 1,3),4 forceful work (OR = 3,1)5 and the combination of repetitive movements of the arm and forceful activities (OR = 5,6)4 are associated with the occurrence of LE. LE naturally resolves over a period of 1 to 2 years in 80 to 90% of cases.6 It is thought to be a self-limiting condition as pain limits the function of the elbow thus protecting the insertion of the extensor muscles of mechanical stresses during the healing process.7 It should be noted that the term “tennis elbow” is inappropriate because tennis players represent only 5 to 10% of cases, however the practice of racket sports increases the risk of developing LE (OR = 2,8)6 and 40 to 50% of players may develop this condition.8 The term tendinitis is also inappropriate to describe the chronic presentation of this disease because no histological inflammatory reaction has been found in patients treated surgically for chronic LE.9 The term tendinosis should be utilized preferentially since it refers to degenerative tendinopathy (angiofibroblastic hyperplasia)10 as seen in this condition.
Recent literature reviews7,11 have listed more than 40 different treatment methods for this condition. The majority of studies reported inconsistent results and no therapeutic modality seems to stand out or alter the natural history of the disease. Surprisingly, despite the multitude of studies, there is not enough evidence to currently recommend the use of one treatment modality over another. This can be explained by the limited usage (fewer than the half of the experimental studies) of assessment instruments, with adequate psychometric properties (valid, reliable, and sensitive to change), that limit the power and validity of studies on lateral epicondylitis.12 The Patient-rated Tennis Elbow Evaluation (PRTEE) is a questionnaire that has demonstrated sufficient psychometric properties.13–16 Since the PRTEE depends on the patient rating of 15 items, there is still a need to find measures that can objectively monitor the progress of patients with LE.
Grip strength is commonly measured to quantify the progression of LE. Several variations of grip strength testing are found in the literature. Healthy subjects demonstrate stronger maximal grip when measured with the elbow bent at 90 degrees than when measured with the elbow extended.17 Patients with LE showed no difference between the elbow positions for the healthy arm, while the affected arm showed a significant lower strength when measured with the elbow in extension.18 An 8% decrease of grip strength when measured with the elbow in flexion compared to extension was sufficient to distinguish the affected elbow from the healthy one. These variations have diagnostic implications for both researchers and clinicians. Although maximal grip has an adequate interobserver reproducibility (ICC = 0.97)19 many researchers prefer to quantify the progress of LE using the Pain-Free Grip (PFG).19,20 In addition to the advantageous interobserver reliability,19 PFG shows a better correlation with common pain scales.20 Since PFG is always measured with the elbow in extension, we decided to compare the two positions (flexion / extension) in order to identify the more appropriate position in which to monitor progress in patients with LE.
Electromyography (EMG) has also been used to investigate the function of forearm muscle in healthy and LE individuals. Rojas had identified muscle asymmetry characterized by a decrease of the activation of the extensor carpi radialis, a compensatory increase in the activation of extensor carpi ulnaris and higher muscle fatigue index in LE subjects compared to control subjects.21 A similar study documented a reduction in the activation of the extensor carpi radialis, without increased activation of the extensor carpi ulnaris, or modification of the fatigue index.22 They also have noted a weakness of the affected upper limb when compared to a control group. Another study was able to compare a group of healthy subjects, a LE group, and a group of recovered LE patients (no pain for 6 months).23 The EMG results showed a decrease in the activation of the extensor carpi radialis in LE subjects and an increased activation in recovered subjects, despite persistent weakness of the upper limb. The decreased activation of the extensor carpi radialis is in accordance with the pain adaptation model24 that predicts a reduced activity of agonist muscles in the presence of chronic pain. Small increases in the level of activity of the antagonist could also be caused by pain. As a consequence of these changes, force production as well as the range and velocity of movement of the affected body part are often reduced. While this is not commonly used, we believe that the use of the co-activation percentage (ratio of : activity of a muscle acting as an antagonist/activity when acting as an agonist) should show a modulation of motor activity in a context of chronic elbow pain. According to the pain adaptation model,24 pain causes a reduction in the activity of agonist muscles and an increase in the antagonist activity which should lead to an increase in the percentage of co-activation. We compared the affected elbow to the unaffected elbow since we believe that this is preferable to between-subjects comparisons done in previous studies.
Since the number of adequate assessment instruments for LE is limited, the purpose of this study was to investigate change in EMG and dynamometry of subjects diagnosed with LE to assess the appropriateness of these measures in the patient initial functional assessment and follow-up.
Methodology
Subjects
This study was conducted at the biomechanics laboratory of the University. Subjects were recruited through an e-mail sent to University employees that were concurrently enrolled in a project comparing the effect of two treatments on LE. The recruitment period extended from February to April 2007. The subjects were accepted if they met the criteria for inclusions (Table 1). These criteria included those frequently used in studies on LE25–27 and those recommended for the experimental treatment administered (augmented soft tissue mobilization). This study was approved by the Institutional Review Board of the University (number ERC-06-114-06.02). All potential subjects (n = 34) during the recruitment period were considered. Five did not meet the inclusion criteria, two refused to participate and three were lost to follow-up so their initial information was not utilized. Twenty-four subjects completed both the initial evaluation (week 1) and the final evaluation after 5 weeks of treatment (week 6). Table 2 summarizes the clinical presentation of the subjects.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Table 2.
Characteristics of subjects
| Age (years) | 46 ± 10 |
| Sex (male/female) | 11 / 13 |
| Affected elbow (Dominant / Non-dominant) | 13 / 11 |
| Onset of the LE (months) | 29 ± 38 |
LE : lateral epicondylitis
The initial evaluation included: the Patient Rated Tennis Elbow Evaluation (PRTEE) cross-culturally adapted to Canadian French,28 a visual analogue scale (VAS), PFG, maximal isometric strength of the wrist and surface EMG. The first half of the group received ten treatments of augmented soft tissue mobilisation (a variation of deep friction massage) for a five week period, and the second received home exercises (stretching of the forearm). One week after the last treatment (week 6), all initial tests were repeated. All the analyses presented in this manuscript are for both treatment groups as a summary, they are not separated (this occurs in another manuscript).29
Dependant variables
The VAS was measured with a scaled line from zero to one hundred millimetres.30 The question asked was: « Indiquez par un X l’endroit sur la ligne ci-dessous qui représente le mieux l’intensité de votre douleur aujourd’hui » which translates to: “Mark with an X the location on the line below which best represents the intensity of your pain today.”
The PRTEE (cross-culturally adapted to Canadian French28) with a score from zero to one hundred13–16 was used. This questionnaire provides a brief, uncomplicated, and standardised quantitative description of pain and functional disability.
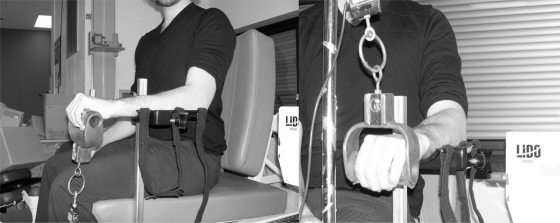
PFG, commonly used to quantify the progression of LE,19,30,31 was measured using a dynamometer JAMAR model 08940189 (Preston, Jackson, MI), whose dynamic range is 981 N. The test was performed with the subjects in two standard positions: the arm along the body with the elbow bent at 90 degrees17 and the elbow in full extension along with the body (Figure 1). The subjects also received standardized instructions: they were to squeeze the dynamometer slowly until they began to feel discomfort. The PFG was measured 3 times, with a 20-second rest interval between each measurement. The results were averaged for better reproducibility.19
Figure 1.
The PFG with the elbow in extension and the elbow bent at 90 degrees
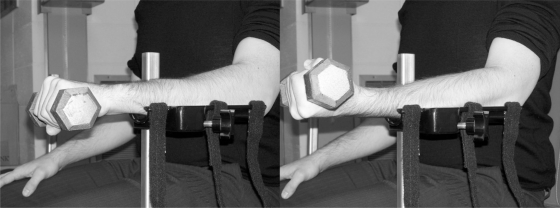
The surface EMG of the extensor carpi radialis, the extensor digitorum and the superficial flexor digitorum were recorded during maximum and sub-maximal isometric contractions of five seconds in flexion and extension of the wrist. During all EMG acquisition sessions, the sampling frequency was set at 1000 Hz. Disposable bipolar surface electrodes Ag-AgCl (BORTEC Biomedical, Calgary, Alberta, Canada) were applied bilaterally according to literature recommendations.32,33 The electrode placement site was first shaved and scrubbed with alcohol. The reference electrode (ground) was placed on the olecranon. The subjects were seated, with their elbow bent at 90°. With their hand initially in a prone position, they were asked to hold a U-shaped handle linked to a strain gauge to measure the maximal voluntary contraction (Figure 2). The subjects had their forearm fixed and were producing maximal flexion and extension of the wrist during verbal reinforcement. The sub-maximal EMG signals of muscles were collected during the hold against gravity of a 5 lbs dumbbell with the hand both in pronation and supination (Figure 3). Each contraction was followed by a two minute pause to reduce the risk of fatigue. Each measurement was made three times on both forearms for comparison. The signals were pre-amplified at the source (gain = 500), to eliminate motion artifacts, before a second amplification (AMT-8, BORTEC Biomedical). The EMG signals were filtered according to a seventh order Butterworth (bandwidth of 10 to 450 Hz), rectified in absolute values and smoothed (a moving average including 10 points was used to filter the data) to determine the maximal value of each curve. The average of three maximal values was calculated and used for each condition. In order to quantify the level of muscle activity, the average of sub-maximal values is expressed as a percentage of the average of the maximal values according to the following equation:
The co-activation percentage was represented by the EMG activity of a muscle when acting as antagonist expressed as a percentage of when acting as agonist. These percentages were obtained using the mean of maximal isometric tasks as in the following example:
Figure 2.
Maximal isometric contraction during wrist extension (left picture) and wrist flexion (right picture).
Figure 3.
Sub-maximal isometric contraction during wrist extension (left picture) and wrist flexion (right picture).
During maximum isometric contractions in flexion and extension of the wrist (Figure 2), the strength signals were collected using a strength gauge (NTEP-87-057A3 class III, Artech, Riverside, CA). For each test, the force was recorded at the same sampling frequency as the EMG (1000 Hz) and then filtered in a second-order Butterworth (cut-off frequency up to 8 Hz). The maximal strength was measured in each attempt and the average of the three trials was calculated.
Statistics
The paired t-test (a = 0.05) comparing the initial and the week 6 evaluation was performed for the VAS and the PRTEE. The two-way or three-way ANOVAs with repeated measures (a = 0.05) on all factors were used to determine whether statistically significant differences were present with the other variable. When appropriate, post hoc Fisher LSD test (a = 0.05) was performed. The status of the elbow (healthy or affected) was the independent variable while the PFG, EMG and isometric strength values were considered dependent variable. The experimental plan of analysis is represented by: A × Br for the results of EMG and isometric strength, and A × Br × C for PFG, where:
A has 2 levels represented by the healthy and affected elbow;
B has 2 levels respresented by the initial evaluation and the week 6 evaluation;
C has 2 levels represented by the position of the elbow during PFG in extension and flexion.
Results
Patient rated outcomes
Patient progress was evaluated using the PRTEE (Canadian-French version) and the VAS. Table 3 summarizes the results of these two scales. Both the PRTEE and the VAS showed a significant decrease of the score after 6 weeks (P < 0,001) which represents an improvement of the subject condition.
Table 3.
Patient rated outcomes
Mean ± standard deviation
Significant decrease of the score from baseline
Strength
Isometric strength was measured in four specific conditions: flexion and extension of the wrist and PFG with the elbow in flexion and extension. Table 4 shows the average maximal obtained.
Table 4.
Isometric strength and PFG (kg)
| Baseline | Week 6 | |||
|---|---|---|---|---|
| Elbow | Healthy | Affected | Healthy | Affected |
| Wrist strength in extension | 12 ± 5 | 12 ± 5 | 13* ± 5 | 13* ± 5 |
| Wrist strength in flexion | 39 ± 17 | 37 ± 19 | 40 ± 22 | 40 ± 22 |
| PFG (extension position)a | 30 ± 11 | 26a ± 14 | 33b ± 11 | 28ab ± 13 |
| PFG (flexion position) | 31 ± 11 | 29 ± 13 | 34b ± 12 | 31b ± 14 |
Mean ± standard deviation
Significant increase of the score from baseline (main effect of time, P = 0.022)
PFG significantly lower than the measure with the elbow in flexion and the healthy elbows (interaction elbow status-position, P = 0.011)
PFG significantly higher than the score from baseline (main effect of time, P = 0.021)
The maximal isometric wrist flexion strength revealed no main effect of elbow status or time.
The maximal isometric wrist extension strength during extension showed a main effect of time (P = 0,022), result of a significant increase of strength in both elbows after 6 weeks. This measure of strength showed no significant difference between the healthy and affected elbow.
The PFG showed main effects of time (P = 0,021) and elbow (P = 0,014) in addition to an interaction between the elbow and its position (P = 0,011). The post hoc analyses demonstrated:
A significant increase of the PFG after six weeks in both arms and both positions.
A significantly lower PFG in the affected elbows compared to healthy elbow when measured with the elbows in extension.
A significant lower PFG of the affected elbows measured in extension compared to flexion.
Surface EMG
Table 5 shows the percentages of muscle activation and co-activation. For the muscle activation, the statistical analysis demonstrated main effect of elbow status and/or time only for the percentage of activation during flexion of the extensor digitorum and the superficial flexor digitorum.
Table 5.
Percentages of muscle activation and co-activation
| Elbow | Baseline | Week 6 | ||
|---|---|---|---|---|
| Affected | Healthy | Affected | Healthy | |
| % activation during extension | ||||
| Extensor carpi radialis | 27 ± 13 | 25 ± 12 | 25 ± 14 | 25 ± 10 |
| Extensor digitorum | 37 ± 23 | 37 ± 16 | 32 ± 17 | 31 ± 9 |
| Superficial flexor digitorum | 43 ± 24 | 45 ± 35 | 58 ± 37 | 45 ± 24 |
| % activation during flexion | ||||
| Extensor carpi radialis | 34 ± 22 | 34 ± 28 | 36 ± 27 | 27 ± 17 |
| Extensor digitorum | 36 ± 29 | 31 ± 23 | 22a ± 15 | 20a ± 10 |
| Superficial flexor digitorum | 16 ± 11 | 14 ± 8 | 21b ± 19 | 20b ± 17 |
| % co-activation | ||||
| Extensor carpi radialis | 13 ± 7 | 15 ± 6 | 15 ± 6 | 16 ± 5 |
| Extensor digitorumcd | 23c ± 10 | 30 ± 21 | 41cd ± 18 | 43d ± 18 |
| Superficial flexor digitorum | 63 ± 77 | 68 ± 56 | 45 ± 75 | 34 ± 19 |
Mean ± standard deviation
The 6th week follow up percentage are significantly lower than the baseline week (main effect of time, P = 0.006)
The 6th week follow up percentage are significantly higher than the baseline week (main effect of time, P = 0.007)
The affected elbows percentage are significantly lower than the healthy one (main effect of elbow status, P = 0.048)
The 6th week follow up percentage are significantly higher than the baseline week (main effect of time, P = 0.003)
The activation percentage of the extensor digitorum during flexion showed a main effect of time (P = 0.006), resulting from a significant decrease in the percentage of muscle activation of both forearms.
The activation percentage of the superficial flexor digitorum during flexion showed a main effect of time (P = 0.007), caused by a significant increase in muscle activation after 6 weeks in both forearms.
For muscle co-activation, the statistical analysis demonstrated main effect of elbow status and/or time only for the co-activation percentage of the extensor digitorum.
The co-activation percentage of the extensor digitorum showed a main effect of elbow status (P = 0.048) and time (P = 0.003), result of a significant increase of the co-activation percentage after 6 weeks in both forearms and a co-activation percentage significantly lower in affected elbows.
Discussion
Patient rated outcomes
Since both the VAS and the PRTEE showed significant improvement after six weeks, it is reasonable to consider that the subject’s LE improved during that period of time. The Canadian French version of the PRTEE has demonstrated good acceptability, construct validity, internal consistency and responsiveness.28 In 2005, the first English version of the PRTEE, the Patient-rated Forearm Evaluation Questionnaire (PRFEQ), was considered to be the most reliable (Interclass Correlation Coefficient (ICC) = 0.96; Standard error of measure (SEM) = 1.0) and responsive (Standardized response means (SRM) = 1.0) questionnaire utilized in patients suffering from lateral epicondylitis (compared to the Visual Analogue Scale (VAS), the Disabilities of the Arm, Shoulder, and Hand questionnaire (DASH), the Medical Outcomes Study 36-item Short Form Health Survey and the Pain-Free Grip (PFG)).13 In 2005, the PRFEQ was updated to the actual PRTEE to accommodate findings from different research groups and to improve its clarity.15 In 2007, the comparison of this updated version with results of the VAS, the DASH Questionnaire, the Roles and Maudsley score, and the Upper Extremity Function Scale (UEFS), showed excellent reliability (r2 = 0.87) and internal consistency (Cronbach’s alpha = 0.94).16 SRM was higher in the PRTEE (SRM = 2.1) than in the other outcome measures (1.5 – 1.7).16 The questionnaire provides a brief, uncomplicated, and standardised quantitative description of pain and functional disability.14 It can be completed in less than five minutes. The use of PRTEE as a standard outcome measure in research may help determine best practice approaches for lateral epicondylitis.
Strength
In order to quantify the functional capacity of subjects, several measures of strength involving the forearm muscles were used. Measuring the maximal isometric strength of the wrist during flexion and extension seemed logical, but our results showed no significant difference between the healthy and the affected elbows. Only an increase in the maximum isometric strength during extension of both elbows was observed after 6 weeks. Since an improvement of the affected elbow should demonstrate an interaction between time and elbow status, the increased strength of both elbows could be attributed to test-retest effects, because subjects often exert more force in a retest. The absence of difference between the affected and healthy elbows of LE subjects has been observed in the literature,22 but the affected arm usually presented a lower strength when compared with a group of healthy subjects. LE typically affects the dominant arm and a decreased strength of the dominant arm could make the strength of both arms comparables. Hand dominance of the subject could be a confounding variable since 13 of our subjects had LE on the dominant arm and 11 on the non-dominant arm. Nevertheless, these measures cannot effectively discriminate the healthy and affected elbow (both arms showed comparable improvement even after a significant decrease in pain), they show little interest for initial assessment and follow-up in clinical practice.
Our results showed an increase of PFG for the two positions after 6 weeks. Similarly to the maximal isometric strength during wrist extension, the increase PFG of both elbows could be attributed to test-retest effects. But, the PFG was significantly lower among affected elbows when measured with the elbow in extension rather than in flexion. In addition, the PFG in the extension position was the only strength measure that could discriminate healthy elbows from the affected. The elbow extension, as in the provocation test of resisted wrist extension,1 put greater stress on the extensor carpi radialis and produces pain at lower grip strength. This greater stress of the injured muscles allows better discrimination of affected and healthy elbows. According to this data, the PFG with the elbow in extension is the best measure of strength for the assessment of LE in research and practice.
Surface EMG
The only EMG measure that was able to discriminate the affected elbows from the healthy ones was the co-activation percentage of the extensor digitorum. These results are different from previous studies that described a decrease in the activation of extensor carpi radialis in LE subjects.21,22,23 The fact that our results did not confirm this difference between healthy and affected elbows could indicate a methodological difference in the EMG acquisition. In previous studies, EMG data were acquired at 50% maximal voluntary isometric contraction during grip, and analysed using normalized median frequency slope as a fatigue index.22,23,34 Our experimental task carried out during the acquisition of EMG signals during wrist extension combined the grip of a U-shape handle in addition to a resisted extension while previous research only included gripping22,23,34 or a resisted wrist extension.21 It has been suggested that the extensor carpi radialis brevis acts as a flexor muscle stabilizer for gripping during pronation.35 Combining gripping in pronation and resisted extension could possibly cause an increase activation of extensor carpi radialis during our experiment. Another difference between our methodology and the one of previous studies is that our experimental design did not allow us to assess fatigue index. It is also possible that the lack of difference between healthy and affected elbows is due to the choice of non-affected elbows of the same subjects as a source of comparison. All previous studies used a group of healthy subjects.
Our experiment showed a decreased activation of the extensor digitorum of both forearms after six weeks. The co-activation percentage of that muscle is lower in the affected arm and increased in both arms after six weeks. These differences are opposite to those we expected under the pain adaptation model24 since in the presence of a decrease of pain (VAS) a decreased percentage of muscle co-activation was expected. The pain adaptation model applies to the agonist-antagonist activity of different muscles, and not to the agonist-antagonist activity of the affected muscle. Once again, the increased co-activation percentage of both arms could be attributed to the test-retest effect. The same phenomenon was observed with the superficial flexor digitorum that showed an increase of activation in both arms after six week. One of the limitations of our research design is that we have not assessed the test-retest reliability of our EMG protocol. However, we have used a procedure that should increase test-retest reliability: using the average of 3 measures and expressing the level of muscle activity as a percentage of maximal values (standardisation). Even if Alizadehkhaiyat et al. claim that their EMG procedure is reliable for measuring muscle imbalance in the wrist-forearm-shoulder-chain,33 they did not assess the test-retest reliability between two sessions. The discrepancy between our results and those found in previous studies21–23 comes from changes in the EMG acquisition and analysis procedures.
According to our results and those from previous studies, the best way to monitor the recovery of LE in both clinical trials and clinical practice is to use the PRTEE13–16,28 and the PFG with the elbow in extension.19,30,31 Even if the method proposed by Alizadehkhaiyat et al. seems more promising than our’s, future research using EMG data should assess the test-retest reliability of their protocol before it could be used in clinical trials. Since co-activation percentage of the extensor digitorum, was the only EMG measure that discriminated the affected elbows from the healthy ones in this study, more investigation should be done on co-activation of forearm muscle during other experimental tasks before it could be used as a clinical or research outcome.
Conclusion
The use of the PFG with the elbow in extension seems to be the most indicated strength measurement to monitor the recovery of patients with LE. The EMG acquisition protocol used in this research was not adequate to monitor effectively the recovery of LE patient. Future research should address the lack of significant difference between the affected and healthy elbows during EMG measurement.
Acknowledgments
The authors thank Dr. Chloé Moussaoui, MD and Dr. Julie-Marthe Grenier, DC for the revision of the manuscript, M. Pierre Black MSc for the technical support and the Foundation de recherche Chiropratique du Québec for their financial support.
References
- 1.Evans RC. Illustrated Orthopedic Physical Assessment. second ed. St Louis, Missouri, USA: Mosby; 2001. [Google Scholar]
- 2.Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145–153. doi: 10.3109/03009747409097141. [DOI] [PubMed] [Google Scholar]
- 3.Verhaar JA. Tennis elbow. Anatomical, epidemiological and therapeutic aspects. Int Orthop. 1994 Oct;18(5):263–267. doi: 10.1007/BF00180221. [DOI] [PubMed] [Google Scholar]
- 4.Shiri R, Viikari-Juntura E, Varonen H, Heliovaara M. Prevalence and determinants of lateral and medial epicondylitis: a population study. Am J Epidemiol. 2006 Dec 1;164(11):1065–1074. doi: 10.1093/aje/kwj325. [DOI] [PubMed] [Google Scholar]
- 5.Haahr JP, Andersen JH. Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med. 2003 May;60(5):322–329. doi: 10.1136/oem.60.5.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mens JM, Stoeckart R, Snijders CJ, Verhaar JA, Stam HJ. Tennis elbow, natural course and relationship with physical activities: an inquiry among physicians. J Sports Med Phys Fitness. 1999 Sep;39(3):244–248. [PubMed] [Google Scholar]
- 7.Boisaubert B, Brousse C, Zaoui A, Montigny JP. [Nonsurgical treatment of tennis elbow] Ann Readapt Med Phys. 2004 Aug;47(6):346–355. doi: 10.1016/j.annrmp.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Gruchow HW, Pelletier D. An epidemiologic study of tennis elbow. Incidence, recurrence, and effectiveness of prevention strategies. Am J Sports Med. 1979 Jul-Aug;7(4):234–238. doi: 10.1177/036354657900700405. [DOI] [PubMed] [Google Scholar]
- 9.Alfredson H, Ljung BO, Thorsen K, Lorentzon R. In vivo investigation of ECRB tendons with microdialysis technique--no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand. 2000 Oct;71(5):475–479. doi: 10.1080/000164700317381162. [DOI] [PubMed] [Google Scholar]
- 10.Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999 Feb;81(2):259–278. [PubMed] [Google Scholar]
- 11.Goguin JP, Rush F. Lateral epicondylitis. What is it really? Current Orthopaedics. 2003;17(5):386–389. [Google Scholar]
- 12.Hong QN, Durand MJ, Loisel P. Treatment of lateral epicondylitis: where is the evidence? Joint Bone Spine. 2004 Sep;71(5):369–373. doi: 10.1016/j.jbspin.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Newcomer KL, Martinez-Silvestrini JA, Schaefer MP, Gay RE, Arendt KW. Sensitivity of the Patient-rated Forearm Evaluation Questionnaire in lateral epicondylitis. J Hand Ther. 2005 Oct-Dec;18(4):400–406. doi: 10.1197/j.jht.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Overend TJ, Wuori-Fearn JL, Kramer JF, MacDermid JC. Reliability of a patient-rated forearm evaluation questionnaire for patients with lateral epicondylitis. J Hand Ther. 1999 Jan-Mar;12(1):31–37. doi: 10.1016/s0894-1130(99)80031-3. [DOI] [PubMed] [Google Scholar]
- 15.Macdermid J. Update: The Patient-rated Forearm Evaluation Questionnaire is now the Patient-rated Tennis Elbow Evaluation. J Hand Ther. 2005 Oct-Dec;18(4):407–410. doi: 10.1197/j.jht.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Rompe JD, Overend TJ, MacDermid JC. Validation of the Patient-rated Tennis Elbow Evaluation Questionnaire. J Hand Ther. 2007 Jan-Mar;20(1):3–10. doi: 10.1197/j.jht.2006.10.003. quiz 11. [DOI] [PubMed] [Google Scholar]
- 17.Mathiowetz V, Rennells C, Donahoe L. Effect of elbow position on grip and key pinch strength. J Hand Surg [Am] 1985 Sep;10(5):694–697. doi: 10.1016/s0363-5023(85)80210-0. [DOI] [PubMed] [Google Scholar]
- 18.Dorf ER, Chhabra AB, Golish SR, McGinty JL, Pannunzio ME. Effect of elbow position on grip strength in the evaluation of lateral epicondylitis. J Hand Surg [Am] 2007 Jul-Aug;32(6):882–886. doi: 10.1016/j.jhsa.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Smidt N, van der Windt DA, Assendelft WJ, et al. Interobserver reproducibility of the assessment of severity of complaints, grip strength, and pressure pain threshold in patients with lateral epicondylitis. Arch Phys Med Rehabil. 2002 Aug;83(8):1145–1150. doi: 10.1053/apmr.2002.33728. [DOI] [PubMed] [Google Scholar]
- 20.Stratford PW, Levy DR. Assessing Valid Change over Time in Patients with Lateral Epicondylitis at the Elbow. [Article] Clinical Journal of Sport Medicine. 1994 Apr;4(2):88–91. [Google Scholar]
- 21.Rojas M, Mananas MA, Muller B, Chaler J. Activation of forearm muscles for wrist extension in patients affected by lateral epicondylitis. Conf Proc IEEE Eng Med Biol Soc. 2007;1:4858–4861. doi: 10.1109/IEMBS.2007.4353428. [DOI] [PubMed] [Google Scholar]
- 22.Alizadehkhaiyat O, Fisher AC, Kemp GJ, Vishwanathan K, Frostick SP. Upper limb muscle imbalance in tennis elbow: a functional and electromyographic assessment. J Orthop Res. 2007 Dec;25(12):1651–1657. doi: 10.1002/jor.20458. [DOI] [PubMed] [Google Scholar]
- 23.Alizadehkhaiyat O, Fisher AC, Kemp GJ, Vishwanathan K, Frostick SP. Assessment of functional recovery in tennis elbow. J Electromyogr Kinesiol. Mar 13, 2008. [DOI] [PubMed]
- 24.Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991 May;69(5):683–694. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- 25.Korthals-de Bos IB, Smidt N, van Tulder MW, et al. Cost effectiveness of interventions for lateral epicondylitis: results from a randomised controlled trial in primary care. Pharmacoeconomics. 2004;22(3):185–195. doi: 10.2165/00019053-200422030-00004. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Silvestrini JA, Newcomer KL, Gay RE, Schaefer MP, Kortebein P, Arendt KW. Chronic lateral epicondylitis: comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J Hand Ther. 2005 Oct-Dec;18(4):411–419. doi: 10.1197/j.jht.2005.07.007. quiz 420. [DOI] [PubMed] [Google Scholar]
- 27.Smidt N, van der Windt DA, Assendelft WJ, Deville WL, Korthals-de Bos IB, Bouter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet. 2002 Feb 23;359(9307):657–662. doi: 10.1016/S0140-6736(02)07811-X. [DOI] [PubMed] [Google Scholar]
- 28.Blanchette MA, Normand MC. Cross-cultural Adaptation of the Patient-rated Tennis Elbow Evaluation to Canadian French. J Hand Ther. Apr 18, [DOI] [PubMed]
- 29.Blanchette MA, Normand MC. Augmented soft tissue mobilisation vs natural history in the treatment of lateral epicondylitis: a pilot study. J Manip Physiol Ther. 2011;34(2):123–130. doi: 10.1016/j.jmpt.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Stratford PW, Levy DR, Gauldie S, Levy K, Miseferi D. Extensor carpi radialis tendonitis: a validation of selected outcome measures. Physiother Can. 1987;39:250–255. [Google Scholar]
- 31.Stratford PW, Levy DR, Gowland C. Evaluative properties of measures used to assess patients with lateral epicondylitis at the elbow. Physiother Can. 1993;45:160–164. [Google Scholar]
- 32.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000 Oct;10(5):361–374. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 33.Delagi EFP, Aldo . Anatomic guide for the electromyographer : the limbs. second ed. Springfield, Illinois, USA: Charles C. Thomas; 1980. [Google Scholar]
- 34.Alizadehkhaiyat O, Fisher AC, Kemp GJ, Frostick SP. Strength and fatigability of selected muscles in upper limb: assessing muscle imbalance relevant to tennis elbow. J Electromyogr Kinesiol. 2007 Aug;17(4):428–436. doi: 10.1016/j.jelekin.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 35.O’Sullivan LW, Gallwey TJ. Upper-limb surface electromyography at maximum supination and pronation torques: the effect of elbow and forearm angle. J Electromyogr Kinesiol. 2002 Aug;12(4):275–285. doi: 10.1016/s1050-6411(02)00014-7. [DOI] [PubMed] [Google Scholar]