Abstract
Posttraumatic stress disorder (PTSD) is associated with elevated catecholamines and increased sympathetic arousal. However, it is unknown whether this condition is a preexisting vulnerability factor for PTSD or an acquired result of either trauma exposure or the development of PTSD symptoms. We sought to examine if salivary 3-methoxy-4-hydroxy-phenylglycol (MHPG) in response to a laboratory stressor prior to critical incident exposure predicts the development of PTSD symptoms and if early childhood trauma influences this relationship. In a prospective cohort study, 349 urban police officers were assessed during academy training (baseline) and 243 were reassessed 12 months after the start of active duty (follow-up). At baseline, participants observed a video consisting of police critical incidents. Salivary MHPG was measured before and immediately after the challenge, and after 20 minutes recovery. At follow-up, peritraumatic distress and PTSD symptoms were assessed in relationship to the worst critical incident during the past year. Participants with childhood trauma showed a trend towards higher MHPG increase to the challenge. Higher MHPG levels after 20 minutes recovery were associated with both higher levels of peritraumatic distress and PTSD symptoms at follow-up. In a path analysis, elevated MHPG levels predicted higher peritraumatic distress which in turn predicted higher levels of PTSD symptoms while the direct effect of elevated MHPG levels on PTSD symptoms was no longer significant. Prolonged elevation of salivary MHPG in response to a laboratory stressor marks a predisposition to experience higher levels of peritraumatic distress and subsequently more PTSD symptoms following critical incident exposure.
Keywords: Neuroendocrinology, Posttraumatic Stress Disorder, Stress Test, Salivary MHPG, Prospective Study, Police
Introduction
Our understanding of the biology of PTSD has major limitations because of a lack of data about characteristics of trauma survivors before they were exposed to a traumatic event. Such studies are difficult to conduct for several reasons. First, a naturalistic prospective study would require studying very large groups of people for long periods of time because of the difficulty in predicting who will be exposed to a traumatic incident in the future. Second, exposing people to trauma as an experiment is unethical. Third, the approach of collecting biological data immediately after exposure does not provide an understanding of the pre-existing biological baseline. One promising approach is to study special populations who are healthy at the time of study enrollment but have a high likelihood of being exposed to traumatic incidents in the near future such as first responders to critical incidents. We are currently conducting a prospective longitudinal cohort study of police academy recruits to examine if baseline characteristics assessed during training predict the subsequent development of posttraumatic stress symptoms following exposure to traumatic stress during active police duty (Marmar et al., 2006).
A meta-analysis found subjectively perceived peritraumatic distress to be a strong predictor of PTSD (Ozer et al., 2003). It has been hypothesized that the magnitude of peritraumatic distress depends on autonomic arousal at the time of trauma (Pitman et al., 2000). Greater arousal responses may result from pre-existing vulnerabilities to anxious arousal under threat, higher levels of exposure during the critical incident, or both (Yehuda & LeDoux, 2007).
There is also some evidence from prospective studies that higher autonomic arousal and larger catecholamine response to the traumatic event assessed in the emergency room predict the development of PTSD: Several studies (Bryant et al., 2008; Bryant et al., 2000; Kuhn et al., 2006; Zatzick et al., 2005) – though not all (Ehring et al., 2008; Shalev et al., 1998) – linked increased heart rate as an indicator of sympathetic arousal in the emergency room with the development of PTSD. Urinary epinephrine predicted the development of acute PTSD symptoms in a study of children (Delahanty et al., 2005) but not in adults (Delahanty et al., 2000). Another study measuring both plasma and urinary norepinephrine could not predict the development of PTSD (Videlock et al., 2008). However, it is well known that the role of norepinephrine in the stress response is quite complex and that different stressors activate the multiple stress response systems of the body in specific ways (Cryer, 1980; Goldstein & Kopin, 2007; Pacak & Palkovits, 2001; Pacak et al., 1998; Robertson et al., 1979; Young et al., 1984).
We choose salivary MHPG as a measure of the stress response, as saliva is easy to collect without causing additional stress such as blood drawing. MHPG is a major metabolite of norepinephrine, which functions as a neurotransmitter in the central and sympathetic nervous system and as a stress hormone in the periphery. Salivary MHPG correlates with plasma MHPG, which increases in response to acute stressors e.g. physical exercise or mental stress and is unaffected by beta-blockade, suggesting that it is a measure of central noradrenergic activity (Drici et al., 1991; Hamer et al., 2007). Salivary MHPG also correlates strongly with MHPG in cerebrospinal fluid, a second reason to see it as a good measure of the central noradrenergic metabolism (Reuster et al., 2002).
In a previous analysis of a subsample of 76 subjects drawn from the current sample we had found that participants with childhood trauma responded with a larger MHPG increase compared to participants without childhood trauma (Otte et al., 2005).
The aim of the current study is to determine the predictive value of the salivary MHPG response to a laboratory challenge paradigm prior to critical incident exposure as a potential biological marker of peritraumatic reactions and subsequent development of PTSD symptoms and the influence of childhood trauma on this relationship. We hypothesized that childhood trauma will be associated with greater salivary MHPG response to the video challenge, that this response to the challenge will predict increased peritraumatic distress following critical incidents during police service, and this in turn will predict the development of higher levels of PTSD symptoms.
Methods
This study is part of a prospective longitudinal cohort study of risk and resilience factors for PTSD in police officers. Police recruits were assessed at baseline prior to any professional critical incident exposure and are followed annually over seven years. This design allows differentiating risk factors assessed before trauma exposure and trauma induced changes. A variety of biological, psychological and social factors are studied, with earlier findings from this study already published (Inslicht et al., 2009; Maguen et al., 2009; McCaslin et al., 2009; Otte et al., 2005; Pole et al., 2009).
Participants
Participants were recruited from four urban police departments (New York Police Department (NYPD), Oakland (OPD), San Francisco (SFPD) and San Jose (SJPD)) during academy training. Academy trainees were introduced to the study by study personnel during academy classes and provided contact information to reach the study team. Exclusion criteria were previous employment in law enforcement, other emergency services or the military.
For this evaluation we had video challenge data collected during academy training from 349 participants and follow-up data after 12 months of active police duty from 243 participants. The study was approved by the University of California San Francisco Committee on Human Research and the Clinical Research Review Committee of the San Francisco VA Medical Center. Participants gave written informed consent to participate after receiving a complete description of the study.
Procedures and Measures
Baseline Assessments
Baseline assessments were conducted during police academy training in which participants completed a clinical interview and a battery of self-report questionnaires. The interview included the Clinician Administered PTSD Scale (CAPS), the Structured Clinical Interview for DSM disorders (SCID) and the Life Stressor Checklist (LSC). Then participants underwent startle testing and video challenge as described earlier (Otte et al., 2005; Pole et al., 2009; Pole et al., 2007). They also were assessed for use of prescription and over the counter medication, and no food, drink or smoking was allowed two hours prior to the video challenge test.
Life stressor Checklist-R (LSC-R)
The Life stressor checklist assesses 21 stressful life events and determines at which age they happened and if the individual experienced intense emotions (Wolfe et al., 1996). We used a dichotomous score and considered participants as having experienced a childhood trauma, if they reported a life event with serious threat to life or physical harm to the self during which they experienced intensive fear before the age of 14. This variable was chosen to be consistent with our previous analysis (Otte et al., 2005).
Baseline Video Challenge Task
Participants observed consecutively a neutral travelogue video for 10 min, a critical incidents video for 20 min, and then another travelogue video for 20 min which constituted the recovery period. The critical incidents video contained real-life footage of 14 incidents depicting police-related scenes such as a suicide, a homicide, an officer being hit by a car, an autopsy, and an officer being killed by a detonated bomb. These scenes were edited into one continuous 20 min tape. After the video, participants were asked to rate the subjective distress they had experienced while watching this video on an analogue scale from zero to ten. They were also asked to select the single video vignette among the 14 incidents that was most stressful to observe and to rate their distress level during this vignette on the same scale.
MHPG Assessment
The saliva collections for MHPG followed the methods described by Yang and colleagues (Goenjian et al., 1996; Yang et al., 1997). Saliva was collected in Salivette nylon swabs at each of three time points: Time 1 - immediately prior to the critical incidents video, Time 2 - immediately following the 20 min critical incidents video, and Time 3 – after 20 min of recovery. Immediately after the collection, the swabs were placed in the Salivette tubes and stored at 4 °C un til centrifugation. The tubes were centrifuged at 1000g for 2 minutes and the filtrate was transferred to separate polypropylene tubes and stored at − 70 °C. Then th e tubes were shipped on dry ice to the Neuroendocrinology Laboratory at the Bronx Veterans Affairs Medical Center and at this facility MHPG was assayed by technicians in Dr. Yehuda’s laboratory by High Pressure Liquid Chromatography (Yang et al., 1997). The intra- and interassay coefficients of variation for salivary MHPG were 5.3% and 10.0%, respectively. Studies have shown high correlations between salivary and plasma MHPG (r=0.70) (Goenjian et al., 1996).
12 Month Follow-up Assessments
The follow-up assessments were conducted after twelve months of active police service. The participants were administered self-report questionnaires to assess traumatic experiences during their first year of active duty and PTSD symptoms.
Peritraumatic Distress Inventory (PDI)
The PDI is a thirteen item self-report measure, which assesses and quantifies the level of emotional distress during and immediately after a traumatic event (Brunet et al., 2001). It is a measure of the A2 criterion for the diagnosis of PTSD as listed in the DSM-IV including additional items for emotional and physical reactions. The items are rated from 0 to 4 (0=not at all, 1=slightly, 2=somewhat, 3=very, and 4=extremely true). The total score is obtained by determining the mean response across all 13 items. The PDI is internally consistent with good test-retest reliability and good validity (Brunet et al., 2001).
PTSD Checklist (PCL)
The PCL is a seventeen item self-report measure of the symptoms of PTSD as described in the DSM-IV (Blanchard et al., 1996; Weathers et al., 1993). This study used the PCL-S version which asks about a specific “stressful experience”. Police officers were asked to choose the most stressful incident in their police career and to rate their symptoms during the last week with respect to this “critical incident”. The items can be rated from 1 to 5 (1=not at all, 2=a little bit, 3=moderately, 4=quite a bit, 5=extremely). The PCL was scored as a total of all item ratings. The PCL-S has excellent internal consistency and test-retest reliability (Adkins et al., 2008; Norris & Hamblen, 2004).
Statistical analyses
First, the relation between childhood trauma and MHPG levels was analyzed by a linear mixed model fitting childhood trauma as a between-groups fixed effect, time (at the end of the video and after recovery) as a within-subjects repeated effect and the MHPG level at the start of the video as a covariate. Group by time interactions were included into the model. Second, bivariate correlations between childhood trauma, MHPG, peritraumatic distress and PTSD symptoms were performed and the variables which were significantly correlated with PTSD symptoms at 12 months were selected for a path analysis using the maximum likelihood method of parameter estimation. Path analysis is an extension of a multiple linear regression analysis with independent, intermediary and dependent variables. It allows estimates of the magnitude and significance of hypothesized causal connections among these variables (Kline, 1998). Direct and indirect effects were estimated predicting PTSD symptoms at 12 months of police service. Variables entered into the model were: MHPG 20 minutes after recovery period, peritraumatic distress measured by the PDI and PTSD symptoms measured by the PCL-S. The PCL-S score was skewed and therefore logarithmically transformed. In the last set of analyses, the three subcategories of PTSD symptoms, re-experiencing, avoidance and hyperarousal were used instead of the total score of PTSD symptoms and the same path analyses as described above were performed to test if any and which of these subcategories were predicted by the MHPG response to a video challenge. The statistical software packages SPSS16, AMOS16 and Stata 11 were used for the analyses.
Results
The demographics and assessment results for the whole sample and the one-year follow-up subsample are shown in Table 1. As would be expected for police academy recruits, our participants were a relatively young cohort with a mean age of 27 years, ethnically diverse and predominantly male. Most of the participants had a college education. None of the participants met criteria for a current psychiatric diagnosis including PTSD. Three participants reported a history of PTSD, one participant reported a history of panic disorder, and fifteen reported a history of depression. Twenty two percent of all participants reported a childhood trauma.
Table 1.
Demographics and test results of 349 police officers
| Whole sample n=349 | Subsample after 1 year n=243 | Difference between samples | ||||
|---|---|---|---|---|---|---|
| Mean (Std. deviation) or % | Range | Mean (Std. deviation) or % | Range | (t-test or chi2- test | ||
| Age | 27.3 (4.9) | 21 – 46 | 27.1 (4.7) | 21 – 43 | ns | |
| Gender | Female | 14 % | 12 % | ns | ||
| Male | 86 % | 88 % | ||||
| Ethnicity | Caucasian | 36 % | 42 % | p=0.037 | ||
| Asian | 16 % | 13 % | ||||
| Hispanic | 24 % | 23 % | ||||
| Afro-American | 13 % | 13 % | ||||
| Other | 11 % | 9 % | ||||
| Education | High school | 11 % | 10 % | ns | ||
| 2 years college | 35 % | 36 % | ||||
| 4 years college | 50 % | 48 % | ||||
| postgrad. degree | 4 % | 5 % | ||||
| Childhood trauma | 22 % | 22 % | 1.6 – 22.4 | ns | ||
| MHPG at video start | 5.64 (2.4) | 5.56 (2.6) | ns | |||
| MHPG at video end | 5.91 (2.6) | 5.78 (2.5) | 1.8 –20.0 | ns | ||
| MHPG after recovery | 5.87 (2.6) | 5.73 (2.5) | 1.5 – 21.2 | ns | ||
| Peritraumatic distress (PDI)# | .55 (.48) | 0 – 2.5 | ||||
| Reexperience symptoms (PCL) # | 5.5 (1.7) | 5 – 24 | ||||
| Avoidance symptoms (PCL) # | 8.0 (2.4) | 7 – 27 | ||||
| Hyperarousal symptoms (PCL) # | 6.1 (2.0) | 5 – 17 | ||||
| PTSD symptoms (PCL) # | 19.6 (5.1) | 17 – 63 | ||||
ns: the difference between the groups is not significant in the t-test or chi2-test.
measured after one year of police service
Responses to the Baseline Video Challenge
The participants’ mean subjective distress level in response to the 20 minute video was 4.4 (SD 2.6; range = 0–10). The single most stressful video vignette was rated 5.5 on average (SD 2.8; range = 0–10) on the same distress scale. The mean MHPG increased about 5% during the challenge video, which was statistically significant, and decreased about 1% in the twenty minutes recovery period (Table 1).
Regression analysis testing for the effects of childhood trauma
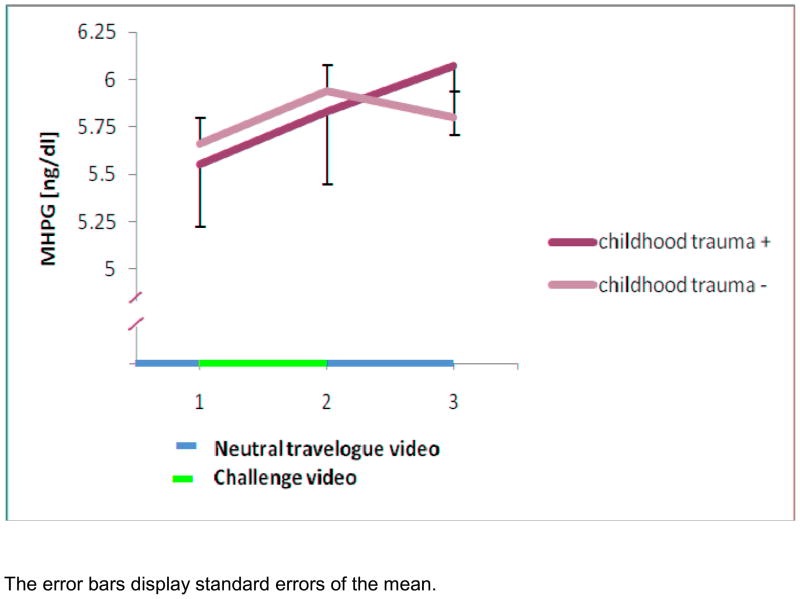
In the whole sample the main effects of group (childhood trauma positive versus negative) (z = 0.62, p = 0.5) and of time (z = 1.31, p = 0.2) were non-significant. However, the interaction of group by time showed a trend (z = −1.84, p = 0.07) indicating that the group with childhood trauma had a higher MHPG increase during the recovery period (Figure 2). In contrast to the earlier analysis on a subsample (Otte et al., 2005) we did not find more subjective distress to the challenge in participants with childhood trauma compared to those without childhood trauma (t(1,342) = −0.27, p = 0.8).
Figure 2.
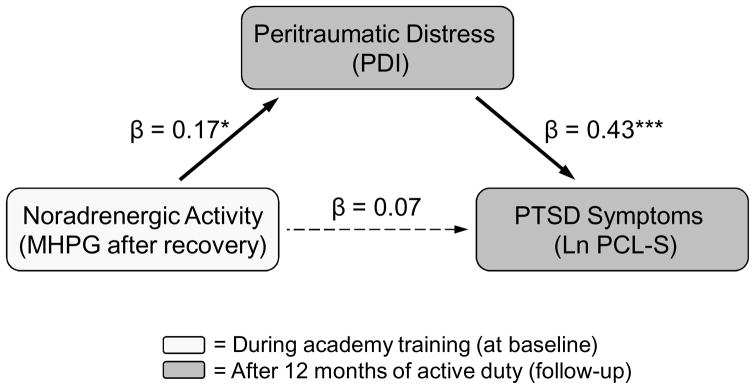
Path analysis of noradrenergic activity, peritraumatic distress and PTSD symptoms
The subsample of 243 participants who came to the follow-up examination differed significantly on ethnicity from the subsample who dropped out (χ2=10.2, p=0.037), with the proportion of “Caucasian” being higher in the follow-up sample. There was no significant difference in the other demographics and baseline results (Table 1).
Symptoms related to Critical Incident Stressors during Police Service
At follow-up 78% of the participants reported a critical incident during the first year of police service. The mean PDI score was 0.6 (SD 0.5, range 0 to 2.5) and mean PCL-S score was 20 (SD 5, range 17–63) for the self-identified most distressing critical incident. All follow-up participants were included in the subsequent analyses, independent of the report of a critical incident.
Correlational analyses
Table 2 shows strong correlations among MHPG measurements at the three time points. MHPG levels at the start of the video and after 20 minutes recovery period were positively correlated with PDI scores. Both higher MHPG levels after the recovery period and greater PDI scores were positively associated with greater PTSD symptom scores on the PCL-S after one year of police service. Childhood trauma was not significantly associated with the PDI or the PCL-S score and therefore was not included in the predictive model.
Table 2.
Bivariate correlations among childhood trauma, MHPG levels, PDI score and PCL-S score
| Childhood trauma | MHPG at video start | MHPG at video end | MHPG after recovery | Peritraumatic Distress (PDI) | Reexperiencing (ln transformed PCL) | Avoiding (ln transformed PCL) | Hyperarousal (ln transformed PCL) | PTSD symptoms (ln transformed PCL) | |
|---|---|---|---|---|---|---|---|---|---|
| MHPG at video start | 0.03 | 1.00 | |||||||
| MHPG at video end | −0.01 | .79** | 1.00 | ||||||
| MHPG after recovery | 0.07 | .71** | .77** | 1.00 | |||||
| Peritraumatic Distress (PDI) | 0.00 | .16* | .11 | .14* | 1.00 | ||||
| Reexperiencing (ln transformed PCL) | 0.03 | .08 | .07 | .09 | .38** | 1.00 | |||
| Avoiding (ln transformed PCL) | 0.03 | .11 | .05 | .15* | .40** | .67** | 1.00 | ||
| Hyperarousal (ln transformed PCL) | 0.06 | .10 | .01 | .08 | .38** | .45** | .61** | 1.00 | |
| PTSD symptoms (ln transformed PCL) | 0.05 | .12 | .05 | .13* | .45** | .78** | .91** | .84** | 1.00 |
correlation is significant at the p<0.05 level,
significant at the p<0.01 level (two-tailed).
Not corrected for multiple testing.
Path Analysis
The path analysis demonstrated a direct effect of MHPG levels after 20 minutes recovery on PDI scores as well as a direct effect of PDI scores on the log-transformed PCL-S score. However, with the inclusion of PDI scores, the direct effect of MHPG after recovery on PCL-S was no longer significant (Fig. 3). Peritraumatic distress levels fully mediated the relationship between MHPG and PTSD symptoms after 12 months of police service. Twenty one percent of the variance of the PCL-S score was predicted by this model.
The division of PTSD symptoms into the three subscales, re-experiencing, avoidance and hyperarousal, showed that both MHPG after recovery and peritraumatic distress were positively associated with avoidance scores on the PCL-S at 12 months. Using the avoidance subscale as the outcome measure, the path model explained 17% of the variance.
Discussion
This study yielded two main findings: First, the level of salivary MHPG after 20 minutes of recovery correlated positively with the PCL-S score after 12 months of police service, so that participants who had prolonged elevations in response to a video challenge during police academy training were at a greater risk to develop PTSD symptoms. Second, this relationship was fully mediated by the degree of peritraumatic emotional distress experienced during the critical incident.
The video challenge evoked both subjective distress and a small, but statistically significant increase of the mean salivary MHPG level as expected from other psychological stress challenge tests (Okamura et al., 2010; Schommer et al., 2003). The possible source of the salivary MHPG increase is complex, as MHPG is the major metabolite of norepinephrine, which is involved as a neurotransmitter in the central nervous system and in the peripheral sympathetic nervous system, and as a stress hormone in the adreno-medullary system. The interpretation of plasma norepinephrine is very complex, depending on site of collection, balance of release and clearance, or metabolism (Goldstein, 1995; McFall et al., 1990). Salivary MHPG might represent an increase in post-ganglionic sympathetic input to the salivary glands reflecting a systemic increase in peripheral sympathetic activity. Salivary MHPG levels are also highly correlated with plasma (Drebing et al., 1989; Yajima et al., 2001; Yang et al., 1997) and with cerebrospinal fluid levels (Reuster et al., 2002). MHPG as a glycol can easily pass the blood-brain barrier (Goldstein, 1995). Although the metabolism of peripheral norepinephrine or the clearance of MHPG from saliva may influence measured levels, salivary MHG is seen as an indicator of the central “noradrenergic activity” (Drici et al., 1991; Hamer et al., 2007; Reuster et al., 2002). It also has been shown that salivary MHPG levels do not fluctuate with diurnal rhythms or with salivary flow (Yajima et al., 2001).
Salivary MHPG is easy to obtain but it might not be the most sensitive measure for sympathetic activity. Therefore in future studies it might be interesting to compare salivary MHPG with other measures of anxious arousal, e.g., neuropeptide Y, corticotrophin releasing factor, plasma epinephrine or norepinephrine, heart rate, or blood pressure, to investigate which variable responds most robustly to experimental stressors and might be a sensitive marker for discriminating individuals with vulnerability to PTSD symptoms following trauma exposure.
In this analysis the 83 subjects reporting childhood trauma on average showed a continued rise in MHPG levels during the challenge video and the recovery period, whereas those without childhood trauma returned to baseline during the recovery period. The finding in this sample missed the statistical significance level but has the same direction as the previous analysis of a subsample of n=76 including 16 subjects with childhood trauma (Otte et al., 2005). An increase in heterogeneity of this larger sample and an overestimation of the effect size in the previous subsample are possible explanations for the loss of statistical significance.
Several studies have shown that experience of childhood trauma increases the risk for anxiety disorders in adulthood (Bremner et al., 1993; Kendler et al., 1992; Kessler et al., 1997; Yehuda, 2004). A putative mechanism for this correlation is longer duration of anxious arousal with elevated catecholamine levels in response to stress in individuals with a history of childhood trauma. Sustained anxiety reactions at the time of trauma exposure and associated increased noradrenergic activity in the brain are thought to increase the risk of PTSD by enhancing memory encoding (Krystal & Neumeister, 2009; McGaugh, 2000; O’Donnell et al., 2004; Orr et al., 2000; Southwick et al., 2002) and over-consolidation of traumatic memories (McGaugh, 1989; Roozendaal et al., 1997; Southwick et al., 1999; van Stegeren, 2008). Elevated levels of norepinephrine in the cerebrospinal fluid of patients with chronic PTSD and their correlation with symptom severity suggests that noradrenergic activity is also involved in the maintenance of PTSD symptoms (Geracioti et al., 2001).
We found a trend for the relationship between more childhood trauma and prolonged elevation of MHPG levels in response to the laboratory stressor and a significant relationship between prolonged elevation of MHPG levels and higher PTSD symptoms, supporting the hypothesis described above. However, we did not find a direct correlation between childhood trauma and the development of PTSD symptoms in this study. A probable cause is that the participants in our study are at an early stage of their career and have low levels of PTSD symptoms after one year of service, reducing the power to detect this association. A complementary explanation could be that participants choosing a career in police despite the experience of childhood trauma are especially resilient.
As expected, we found that higher peritraumatic emotional distress as reported by higher scores in the PDI questionnaire, predicted higher levels of PTSD symptoms. In the path analysis, peritraumatic distress was found to fully mediate the effect of prolonged elevation of salivary MHPG on the later development of PTSD symptoms. Interestingly, the delayed off-switch rather than the acute response was predictive. These results suggest that prolonged arousal measured by salivary MHPG in response to a laboratory stressor is an individual vulnerability factor which is associated with greater emotional distress at the time of trauma and the subsequent development of PTSD symptoms. This finding is congruent with earlier findings from this prospective longitudinal cohort study focusing on individual differences in acoustic startle testing during academy training (Pole et al., 2009). We found that elevated sympathetic nervous system reactivity to acoustic startle in the context of explicit threat and a lack of habituation to repeated startle stimuli are vulnerability factors which predicted greater PTSD symptom severity following critical incident exposure.
This finding also agrees with results from Guthrie and Bryant who found that post-startle eye blink and skin conductance responses during academy training predicted later PTSD symptoms in firefighters (Guthrie & Bryant, 2005). Similarly, Morgan et al. had concluded that biological differences may exist before the index trauma exposure by showing that under uncontrollable stress Special Forces soldiers demonstrated a greater capacity for norepinephrine and neuropeptide Y release with a rapid return to baseline levels at recovery compared to other soldiers (Morgan et al., 2001). Together, these findings add to growing evidence that prolonged arousal in response to a stress challenge that does not rapidly return to baseline following cessation of the stressor is a vulnerability factor for PTSD predating the trauma exposure in adulthood.
It has been suggested that the neurotransmitter norepinephrine is mainly involved in the hyperarousal and re-experiencing symptoms of PTSD (O’Donnell et al., 2004; Southwick et al., 1999), however we found that avoidance, a typical anxiety behavior, was the most relevant PTSD symptom cluster in the path analysis. This supports the results from a review article which concluded that avoidance and numbing symptoms appear to be the most specific for the identification of PTSD (North et al., 2009). As MHPG’s parent compound norepinephrine is involved in the neural circuitry of anxiety (Hughes et al., 2004; Itoi, 2008; van West et al., 2008), the tendency to react to stressors, both laboratory challenges and real life critical incidents, with longer duration of anxious arousal may lead to greater fear conditioning and memory consolidation with pathogenic beliefs about danger and therefore avoidant behavior.
Prior studies have utilized video challenges to provoke stress responses and measure the catecholamine response. For example, Takai measured the increase of salivary amylase in healthy participants and found that salivary amylase significantly increased during a challenge video and correlated with the score of the State-Trait Anxiety Inventory (Takai et al., 2004; Takai et al., 2007). McFall found that veterans with PTSD had an increased autonomic and plasma epinephrine – but not plasma norepinephrine - response after watching a combat video compared to a video depicting a stressful car accident and also compared to healthy controls (McFall et al., 1990). Geracioti found an increase of norepinephrine in the cerebrospinal fluid of PTSD patients in response to a trauma related video challenge but no increase in response to a neutral video (Geracioti et al., 2008). However, to our knowledge the current study is unique in examining MHPG response to a video challenge paradigm prior to critical incident exposure using a prospective longitudinal cohort design.
Several limitations of this study have to be considered: The main limitation of this study is the measurement of MHPG peripherally in saliva, because this collection method is non-invasive and feasible. Salivary MHPG may be an imperfect proxy for norepinephrine neurotransmission in the brain. Second, generalizability may be limited in the highly selected, young, healthy, and well educated population studied. Third, we report PTSD symptoms after one year of active service when the participants are at a very early stage of their police career. At this time most officers have not yet been repeatedly exposed to severe critical incidents and present with relatively low levels of PTSD symptoms. As there is already a pattern recognizable in this early stage, prolonged salivary MHPG increase and peritraumatic distress may prove distinctive later in those participants who develop full PTSD after exposure to repeated severe traumatic stressors. It will be important to model the MHPG response to the video challenge as a predictor of PTSD symptoms as cumulative exposure increases over the years. Another limitation of this study may be that the video challenge, although using real life footage, is a laboratory test and a situation in real life may be far more stressful. Fifth, the peritraumatic distress caused by the worst incident during this time was assessed retrospectively and may be biased by memory fading and symptom recovery. An important limitation is the fact that PTSD is a complex disorder with a multifactorial causality including environmental, genetic and psychological factors, and this model explains only one facet of it (Yehuda, 2002).
In conclusion, prolonged elevations of salivary MHPG in response to an experimental stressor prior to duty related critical incident exposure, predicted the later development of PTSD symptoms. This relationship was mediated by peritraumatic distress, capturing perceived life threat and physical reactions during and immediately after critical incident exposure. Our data indicate that longer duration of anxious arousal in response to experimental stress could be useful in identifying individuals at risk for developing PTSD. This merits further investigation and replication in other samples.
Figure 1.
Mean MHPG levels during the video challenge test for participants with and without childhood trauma
The error bars display standard errors of the mean.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins JW, Weathers FW, McDevitt-Murphy M, Daniels JB. Psychometric properties of seven self-report measures of posttraumatic stress disorder in college students with mixed civilian trauma exposure. Journal of Anxiety Disorders. 2008;22:1393–1402. doi: 10.1016/j.janxdis.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL) Behaviour Research and Therapy. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in vietnam veterans. The American Journal of Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Brunet A, Weiss DS, Metzler TJ, Best SR, Neylan TC, Rogers C, Fagan J, Marmar CR. The peritraumatic distress inventory: A proposed measure of PTSD criterion A2. The American Journal of Psychiatry. 2001;158:1480–1485. doi: 10.1176/appi.ajp.158.9.1480. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Creamer M, O’Donnell M, Silove D, McFarlane AC. A multisite study of initial respiration rate and heart rate as predictors of posttraumatic stress disorder. The Journal of Clinical Psychiatry. 2008;69:1694–1701. doi: 10.4088/jcp.v69n1104. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG, Guthrie RM, Moulds ML. A prospective study of psychophysiological arousal, acute stress disorder, and posttraumatic stress disorder. The Journal of Abnormal Psychology. 2000;109:341–344. [PubMed] [Google Scholar]
- Cryer PE. Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. The New England Journal of Medicine. 1980;303:436–444. doi: 10.1056/NEJM198008213030806. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30:121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biological Psychiatry. 2000;48:940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- Drebing CJ, Freedman R, Waldo M, Gerhardt GA. Unconjugated methoxylated catecholamine metabolites in human saliva. Quantitation methodology and comparison with plasma levels. Biomedical Chromatography. 1989;3:217–220. doi: 10.1002/bmc.1130030509. [DOI] [PubMed] [Google Scholar]
- Drici MD, Roux M, Candito M, Rimailho A, Morand P, Lapalus P. Influence of beta-blockade on circulating plasma levels of 3-methoxy-4-hydroxy phenylethylene glycol (mhpg) during exercise in moderate hypertension. Clinical and Experimental Pharmacology and Physiology. 1991;18:807–811. doi: 10.1111/j.1440-1681.1991.tb01399.x. [DOI] [PubMed] [Google Scholar]
- Ehring T, Ehlers A, Cleare AJ, Glucksman E. Do acute psychological and psychobiological responses to trauma predict subsequent symptom severities of PTSD and depression? Psychiatry Research. 2008;161:67–75. doi: 10.1016/j.psychres.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE, Jr, Kasckow JW. Csf norepinephrine concentrations in posttraumatic stress disorder. The American Journal of Psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Baker DG, Kasckow JW, Strawn JR, Jeffrey Mulchahey J, Dashevsky BA, Horn PS, Ekhator NN. Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology. 2008;33:416–424. doi: 10.1016/j.psyneuen.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Goenjian AK, Yehuda R, Pynoos RS, Steinberg AM, Tashjian M, Yang RK, Najarian LM, Fairbanks LA. Basal cortisol, dexamethasone suppression of cortisol, and mhpg in adolescents after the 1988 earthquake in armenia. The American Journal of Psychiatry. 1996;153:929–934. doi: 10.1176/ajp.153.7.929. [DOI] [PubMed] [Google Scholar]
- Goldstein DS. Clinical assessment of sympathetic responses to stress. Annals of the New York Academy of Sciences. 1995;771:570–593. doi: 10.1111/j.1749-6632.1995.tb44711.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Kopin IJ. Evolution of concepts of stress. Stress. 2007;10:109–120. doi: 10.1080/10253890701288935. [DOI] [PubMed] [Google Scholar]
- Guthrie RM, Bryant RA. Auditory startle response in firefighters before and after trauma exposure. The American Journal of Psychiatry. 2005;162:283–290. doi: 10.1176/appi.ajp.162.2.283. [DOI] [PubMed] [Google Scholar]
- Hamer M, Tanaka G, Okamura H, Tsuda A, Steptoe A. The effects of depressive symptoms on cardiovascular and catecholamine responses to the induction of depressive mood. Biological Psychology. 2007;74:20–25. doi: 10.1016/j.biopsycho.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. Journal of Psychosomatic Research. 2004;57:353–358. doi: 10.1016/j.jpsychores.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Inslicht SS, McCaslin SE, Metzler TJ, Henn-Haase C, Hart SL, Maguen S, Neylan TC, Marmar CR. Family psychiatric history, peritraumatic reactivity, and posttraumatic stress symptoms: A prospective study of police. Journal of Psychiatric Research. 2009 doi: 10.1016/j.jpsychires.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoi K. Ablation of the central noradrenergic neurons for unraveling their roles in stress and anxiety. Annals of the New York Academy of Sciences. 2008;1129:47–54. doi: 10.1196/annals.1417.012. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Childhood parental loss and adult psychopathology in women. A twin study perspective. Archives of General Psychiatry. 1992;49:109–116. doi: 10.1001/archpsyc.1992.01820020029004. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the us national comorbidity survey. Psychological Medicine. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Neumeister A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Research. 2009;1293:13–23. doi: 10.1016/j.brainres.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E, Blanchard EB, Fuse T, Hickling EJ, Broderick J. Heart rate of motor vehicle accident survivors in the emergency department, peritraumatic psychological reactions, asd, and PTSD severity: A 6-month prospective study. Journal of Traumatic Stress. 2006;19:735–740. doi: 10.1002/jts.20150. [DOI] [PubMed] [Google Scholar]
- Maguen S, Metzler TJ, McCaslin SE, Inslicht SS, Henn-Haase C, Neylan TC, Marmar CR. Routine work environment stress and PTSD symptoms in police officers. The Journal of Nervous and Mental Disease. 2009;197:754–760. doi: 10.1097/NMD.0b013e3181b975f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmar CR, McCaslin SE, Metzler TJ, Best S, Weiss DS, Fagan J, Liberman A, Pole N, Otte C, Yehuda R, Mohr D, Neylan T. Predictors of posttraumatic stress in police and other first responders. Annals of the New York Academy of Sciences. 2006;1071:1–18. doi: 10.1196/annals.1364.001. [DOI] [PubMed] [Google Scholar]
- McCaslin SE, de Zoysa P, Butler LD, Hart S, Marmar CR, Metzler TJ, Koopman C. The relationship of posttraumatic growth to peritraumatic reactions and posttraumatic stress symptoms among sri lankan university students. Journal of Traumatic Stress. 2009;22:334–339. doi: 10.1002/jts.20426. [DOI] [PubMed] [Google Scholar]
- McFall ME, Murburg MM, Ko GN, Veith RC. Autonomic responses to stress in vietnam combat veterans with posttraumatic stress disorder. Biological Psychiatry. 1990;27:1165–1175. doi: 10.1016/0006-3223(90)90053-5. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Involvement of hormonal and neuromodulatory systems in the regulation of memory storage. Annual Review of Neuroscience. 1989;12:255–287. doi: 10.1146/annurev.ne.12.030189.001351. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Wang S, Rasmusson A, Hazlett G, Anderson G, Charney DS. Relationship among plasma cortisol, catecholamines, neuropeptide y, and human performance during exposure to uncontrollable stress. Psychosomatic Medicine. 2001;63:412–422. doi: 10.1097/00006842-200105000-00010. [DOI] [PubMed] [Google Scholar]
- Norris FH, Hamblen JL. Standardized self-report measures of civilian trauma and PTSD. In: Wilson JP, Keane TM, Martin T, editors. Assessing psychological trauma and PTSD. New York: Guilford Press; 2004. pp. 63–102. [Google Scholar]
- North CS, Suris AM, Davis M, Smith RP. Toward validation of the diagnosis of posttraumatic stress disorder. The American Journal of Psychiatry. 2009;166:34–41. doi: 10.1176/appi.ajp.2008.08050644. [DOI] [PubMed] [Google Scholar]
- O’Donnell T, Hegadoren KM, Coupland NC. Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology. 2004;50:273–283. doi: 10.1159/000080952. [DOI] [PubMed] [Google Scholar]
- Okamura H, Tsuda A, Yajima J, Mark H, Horiuchi S, Toyoshima N, Matsuishi T. Short sleeping time and psychobiological responses to acute stress. International Journal of Psychophysiology. 2010;78:209–214. doi: 10.1016/j.ijpsycho.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. The Journal of Abnormal Psychology. 2000;109:290–298. [PubMed] [Google Scholar]
- Otte C, Neylan TC, Pole N, Metzler T, Best S, Henn-Haase C, Yehuda R, Marmar CR. Association between childhood trauma and catecholamine response to psychological stress in police academy recruits. Biological Psychiatry. 2005;57:27–32. doi: 10.1016/j.biopsych.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychological Bulletin. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocrine Reviews. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: A test of selye’s doctrine of nonspecificity. American Journal of Physiology. 1998;275:R1247–1255. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- Pitman R, Shalev A, Orr S. Posttraumatic stress disorder: Emotion, conditioning, and memory. In: Corbetta M, Gazzaniga M, editors. The new cognitive neurosciences. 2. New York: Plenum Press; 2000. pp. 687–700. [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biological Psychiatry. 2009;65:235–240. doi: 10.1016/j.biopsych.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Metzler TJ, Best SR, Henn-Haase C, Marmar CR. Associations between childhood trauma and emotion-modulated psychophysiological responses to startling sounds: A study of police cadets. The Journal of Abnormal Psychology. 2007;116:352–361. doi: 10.1037/0021-843X.116.2.352. [DOI] [PubMed] [Google Scholar]
- Reuster T, Rilke O, Oehler J. High correlation between salivary mhpg and csf mhpg. Psychopharmacology (Berlin) 2002;162:415–418. doi: 10.1007/s00213-002-1125-z. [DOI] [PubMed] [Google Scholar]
- Robertson D, Johnson GA, Robertson RM, Nies AS, Shand DG, Oates JA. Comparative assessment of stimuli that release neuronal and adrenomedullary catecholamines in man. Circulation. 1979;59:637–643. doi: 10.1161/01.cir.59.4.637. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Stress-activated hormonal systems and the regulation of memory storage. Annals of the New York Academy of Sciences. 1997;821:247–258. doi: 10.1111/j.1749-6632.1997.tb48284.x. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosomatic Medicine. 2003;65:450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Sahar T, Freedman S, Peri T, Glick N, Brandes D, Orr SP, Pitman RK. A prospective study of heart rate response following trauma and the subsequent development of posttraumatic stress disorder. Archives of General Psychiatry. 1998;55:553–559. doi: 10.1001/archpsyc.55.6.553. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biological Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Davis M, Horner B, Cahill L, Morgan CA, 3rd, Gold PE, Bremner JD, Charney DC. Relationship of enhanced norepinephrine activity during memory consolidation to enhanced long-term memory in humans. The American Journal of Psychiatry. 2002;159:1420–1422. doi: 10.1176/appi.ajp.159.8.1420. [DOI] [PubMed] [Google Scholar]
- Takai N, Yamaguchi M, Aragaki T, Eto K, Uchihashi K, Nishikawa Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Archives of Oral Biology. 2004;49:963–968. doi: 10.1016/j.archoralbio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Takai N, Yamaguchi M, Aragaki T, Eto K, Uchihashi K, Nishikawa Y. Gender-specific differences in salivary biomarker responses to acute psychological stress. Annals of the New York Academy of Sciences. 2007;1098:510–515. doi: 10.1196/annals.1384.014. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH. The role of the noradrenergic system in emotional memory. Acta Psychologica (Amsterdam) 2008;127:532–541. doi: 10.1016/j.actpsy.2007.10.004. [DOI] [PubMed] [Google Scholar]
- van West D, Claes S, Sulon J, Deboutte D. Hypothalamic-pituitary-adrenal reactivity in prepubertal children with social phobia. Journal of Affective Disorders. 2008;111:281–290. doi: 10.1016/j.jad.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Videlock EJ, Peleg T, Segman R, Yehuda R, Pitman RK, Shalev AY. Stress hormones and post-traumatic stress disorder in civilian trauma victims: A longitudinal study. Part ii: The adrenergic response. The International Journal of Neuropsychopharmacology. 2008;11:373–380. doi: 10.1017/S1461145707008139. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD checklist: Reliability, validity and diagnostic utility. Annual Meeting of the International Society for Traumatic Stress Studies; San Antonio, Texas. 1993. [Google Scholar]
- Yajima J, Tsuda A, Yamada S, Tanaka M. Determination of saliva free-3-methoxy-4-hydroxy-phenylglycol in normal volunteers using gas chromatography mass spectrometry. Biogenic Amines. 2001;16:173–183. [Google Scholar]
- Yang RK, Yehuda R, Holland DD, Knott PJ. Relationship between 3-methoxy-4-hydroxyphenylglycol and homovanillic acid in saliva and plasma of healthy volunteers. Biological Psychiatry. 1997;42:821–826. doi: 10.1016/s0006-3223(97)00055-3. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Post-traumatic stress disorder. The New England Journal of Medicine. 2002;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Risk and resilience in posttraumatic stress disorder. The Journal of Clinical Psychiatry. 2004;65 (Suppl 1):29–36. [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. Response variation following trauma: A translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Young JB, Rosa RM, Landsberg L. Dissociation of sympathetic nervous system and adrenal medullary responses. American Journal of Physiology. 1984;247:E35–40. doi: 10.1152/ajpendo.1984.247.1.E35. [DOI] [PubMed] [Google Scholar]
- Zatzick DF, Russo J, Pitman RK, Rivara F, Jurkovich G, Roy-Byrne P. Reevaluating the association between emergency department heart rate and the development of posttraumatic stress disorder: A public health approach. Biological Psychiatry. 2005;57:91–95. doi: 10.1016/j.biopsych.2004.10.005. [DOI] [PubMed] [Google Scholar]